Suppression Efficacy of Clubroot on Cruciferous Crops Through Application of the Humic Acid Material
Abstract
1. Introduction
2. Results and Discussion
2.1. Regression Analysis by a General Liner Mixed Model (GLMM)
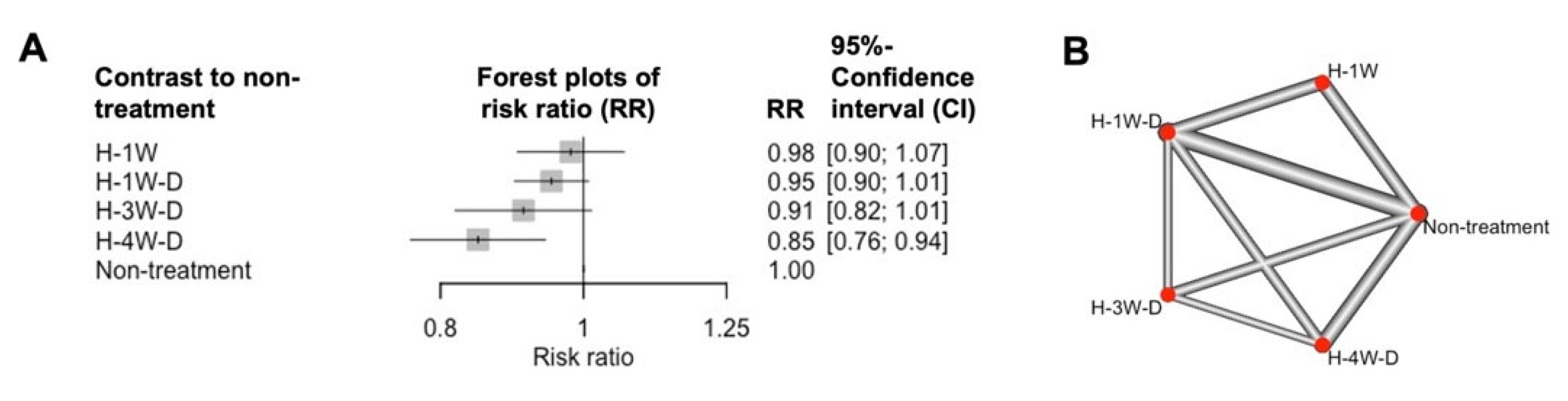
2.2. Evaluating the Control Effect of HAM by a Network Meta-Analysis (NMA)
2.3. Field Trials
3. Materials and Methods
3.1. Greenhouse Experiments
3.2. Regression Analysis
3.3. Network Meta-Analysis (NMA)
3.4. Field Experiments
3.5. Disease Assessment Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakanishi, M.; Mori, M. Relationship between Physical and Chemical Properties of Soil and Clubroot Disease Severity of Broccoli in Kagawa Prefecture. Jpn. J. Soil. Sci. Plant Nutr. 2016, 87, 458–461. (In Japanese) [Google Scholar] [CrossRef]
- MAFF (Ministry of Agriculture, Forestry and Fisheries, Japan). MIDORI Strategy for Sustainable Food Systems. 2021. Available online: https://www.maff.go.jp/e/policies/env/env_policy/midori.html (accessed on 25 August 2025).
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the Role of Humic Acids on Crop Performance and Soil Health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Okorokova-Facanha, A.L.; Facanha, A.R. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.G.; Lupu, C.; Constantinescu-Aruxandei, D.; Oancea, F. Humic substances as microalgal biostimulants—Implications for microalgal biotechnology. Mar. Drugs 2022, 20, 327. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, A.; Kirino, N.; Inoue, K. Biological control for grapevine crown gall through soil injection with Allorhizobium vitis strain ARK-1. Eur. J. Plant Pathol. 2024, 170, 479–489. [Google Scholar] [CrossRef]
- Lu, H.; McClung, C.R.; Zhang, C. Tick Tock: Circadian Regulation of Plant Innate Immunity. Annu. Rev. Phytopathol. 2017, 55, 287–311. [Google Scholar] [CrossRef] [PubMed]
- Struck, C.; Rüsch, S.; Strehlow, B. Control strategies of clubroot disease caused by Plasmodiophora brassicae. Microorganisms 2022, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.F.F.; García, A.C.; Sátiro, J.N.d.O.; de Lima, B.R.; Fernandes, M.S.; Berbara, R.L.L.; Santos, L.A. Humic Acid Regulates Root Growth through ROS-Dependent Pathway and Hormone Signaling in Rice. J. Agric. Food Chem. 2025, 73, 20081–20093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, J.; Zhang, S.; Zhang, S.; Li, F.; Strelkov, S.E.; Sun, R.; Hwang, S. Resistance to Plasmodiophora brassicae in Brassica rapa and Brassica juncea genotypes from China. Plant Dis. 2015, 99, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Koopmann, B.; Tiedemann, A.V. Methods for assessment of viability and germination of Plasmodiophora brassicae resting spores. Front. Microbiol. 2022, 12, 823051. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, A.; Sone, T.; Ochi, S.; Matsushita, Y.; Noutoshi, Y.; Nita, M. Origin of pathogens of grapevine crown gall disease in Hokkaido in Japan as characterized by molecular epidemiology of Allorhizobium vitis strains. Life 2021, 11, 1265. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, A.; Kirino, N.; Inoue, K. Biological control for grapevine crown gall evaluated by a network meta-analysis. Plants 2023, 12, 572. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Information Theory: Proceedings of the 2nd International Symposium; Petrov, B.N., Caski, F., Eds.; Akadimiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Network Meta-Analysis. In Meta-Analysis with R, 1st ed.; Schwarzer, G., Carpenter, J.R., Rücker, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 187–216. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Nemoto, M.; Ochi, S.; Matsushita, Y.; Sato, T.; Sone, T. Insight into the population dynamics of pathogenic bacteria causing grapevine crown gall in snowfall areas: Snow cover protects the proliferation of pathogenic bacteria. Front. Plant Sci. 2023, 14, 1198710. [Google Scholar] [CrossRef] [PubMed]

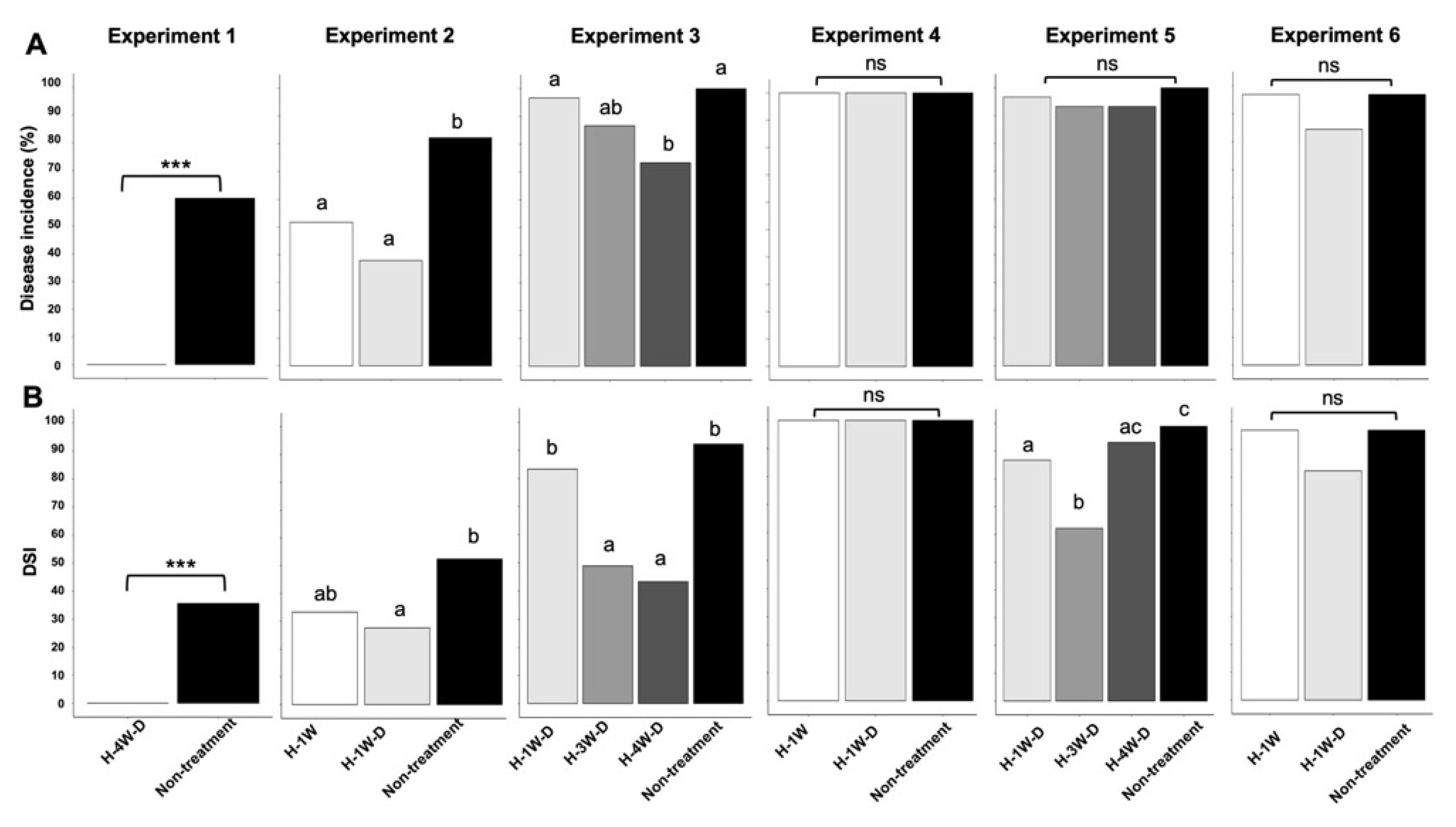
| Experiment ID | Treatment ID a | Amount of Humic Acid Material (HAM1) for Pouring onto Soil b | Category of HAM1 Amount | Timing for Planting (Weeks After Treatment) | Category of Timing for Planting | Avarage of Day-Length (h) | Average of Sunshine Duration (h) | No. of Total Plants | No. of Plants per Pot | No. of Pots per Treatment | Disease Incidence (%) | Risk Ratio (RR) c | No. of Plants in Each Disease Severity Scale d | Disease Severity Index (DSI) e | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||||||||||||
| 1 | H-4W-D | Double the standard amount | 2 | 4 weeks | 3 | 12.3 | 5.3 | 15 | 5 | 3 | 0 | 0 | 15 | 0 | 0 | 0 | 0 |
| Non-treatment | - | 0 | - | 0 | 15 | 5 | 3 | 60.0 | - | 6 | 5 | 1 | 3 | 35.6 | |||
| 2 | H-1W | The standard amount | 1 | 1 week | 1 | 10.5 | 5.4 | 29 | 4 to 5 | 6 | 51.7 | 0.63 | 14 | 4 | 8 | 3 | 33.3 |
| H-1W-D | Double the standard amount | 2 | 1 week | 1 | 29 | 4 to 5 | 6 | 37.9 | 0.46 | 18 | 2 | 5 | 4 | 27.6 | |||
| Non-treatment | - | 0 | - | 0 | 28 | 4 to 5 | 6 | 82.1 | - | 5 | 7 | 11 | 5 | 52.4 | |||
| 3 | H-1W-D | Double the standard amount | 2 | 1 week | 1 | 11.3 | 4.7 | 30 | 5 | 6 | 96.7 | 0.97 | 1 | 2 | 8 | 19 | 83.3 |
| H-3W-D | Double the standard amount | 2 | 3 weeks | 2 | 30 | 5 | 6 | 86.7 | 0.87 | 4 | 14 | 6 | 6 | 48.9 | |||
| H-4W-D | Double the standard amount | 2 | 4 weeks | 3 | 30 | 5 | 6 | 73.3 | 0.73 | 8 | 9 | 9 | 4 | 43.3 | |||
| Non-treatment | - | 0 | - | 0 | 30 | 5 | 6 | 100 | - | 0 | 1 | 5 | 24 | 92.2 | |||
| 4 | H-1W | The standard amount | 1 | 1 week | 1 | 14.2 | 4.9 | 30 | 5 | 6 | 100 | 1 | 0 | 0 | 0 | 30 | 100 |
| H-1W-D | Double the standard amount | 2 | 1 week | 1 | 30 | 5 | 6 | 100 | 1 | 0 | 0 | 0 | 30 | 100 | |||
| Non-treatment | - | 0 | - | 0 | 30 | 5 | 6 | 100 | - | 0 | 0 | 0 | 30 | 100 | |||
| 5 | H-1W-D | Double the standard amount | 2 | 1 week | 1 | 14.0 | 7.9 | 30 | 5 | 6 | 96.7 | 0.97 | 2 | 0 | 6 | 22 | 86.7 |
| H-3W-D | Double the standard amount | 2 | 3 weeks | 2 | 30 | 5 | 6 | 93.3 | 0.93 | 2 | 7 | 14 | 7 | 62.2 | |||
| H-4W-D | Double the standard amount | 2 | 4 weeks | 3 | 30 | 5 | 6 | 93.3 | 0.93 | 1 | 0 | 3 | 25 | 93.1 | |||
| Non-treatment | - | 0 | - | 0 | 30 | 5 | 6 | 100 | - | 0 | 0 | 1 | 29 | 98.9 | |||
| 6 | H-1W | The standard amount | 1 | 1 week | 1 | 13.8 | 9.3 | 32 | 5 to 6 | 6 | 96.9 | 1 | 1 | 0 | 0 | 31 | 96.9 |
| H-1W-D | Double the standard amount | 2 | 1 week | 1 | 32 | 5 to 6 | 6 | 84.4 | 0.87 | 5 | 0 | 2 | 25 | 82.3 | |||
| Non-treatment | - | 0 | - | 0 | 32 | 5 to 6 | 6 | 96.9 | - | 1 | 0 | 0 | 31 | 96.9 | |||
| Objective Variable | Explanatory Variable | Parameter Estimate b | Standard Error | z Value | p Value | Significance c |
|---|---|---|---|---|---|---|
| Risk ratio (RR) | y-Intercept a | 4.122 | 1.152 | 3.579 | 3.5 × 10−5 | *** |
| Humic acid material (HAM) treatment | −1.109 | 0.538 | −2.061 | 0.039 | * | |
| Interaction effect between the amount of HAM1 and planting timing | −0.371 | 0.114 | −3.260 | 1.1 × 10−3 | ** |
| Trial ID | Planting Date | Rating Date | HAM Product a | Plot ID | Timing for Planting (Weeks After Treatment) | No. of Total Plants | No. of Plants in Each Disease Severity Scale b | Disease Incidence (%) | Risk Ratio (RR) c | p Value (Fisher’s Exact Test) c | Disease Severity Index (DSI) d | Wilcoxon Rank Sum Test e | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||||||||||||
| 1 | 27 September 2023 | 16 January 2024 | HAM2 | 1 | 3 weeks | 30 | 8 | 4 | 3 | 14 | 1 | 0 | 73.3 | 37.3 | |||
| 2 | 3 weeks | 30 | 4 | 9 | 7 | 8 | 2 | 0 | 86.7 | 36.7 | |||||||
| 3 | 3 weeks | 30 | 3 | 12 | 3 | 9 | 3 | 0 | 90.0 | 38.0 | |||||||
| Average | - | 83.3 | 0.93 | p = 0.2728 ns | 37.3 | p = 0.0002 *** | |||||||||||
| Non-treatment | 1 | 3 weeks | 30 | 3 | 5 | 4 | 13 | 5 | 0 | 90.0 | 48.0 | ||||||
| 2 | 3 weeks | 30 | 6 | 7 | 8 | 8 | 1 | 0 | 80.0 | 34.0 | |||||||
| 3 | 3 weeks | 30 | 0 | 0 | 0 | 13 | 16 | 1 | 100 | 72.0 | |||||||
| Average | - | 90.0 | - | 51.3 | |||||||||||||
| 2 | 25 March 2025 | 5 June 2025 | HAM1 | 1 | 4 weeks | 30 | 0 | 5 | 8 | 12 | 5 | 0 | 100 | 51.3 | |||
| 2 | 4 weeks | 30 | 0 | 0 | 0 | 12 | 18 | 0 | 100 | 72.0 | |||||||
| 3 | 4 weeks | 30 | 1 | 4 | 9 | 16 | 0 | 0 | 96.7 | 46.7 | |||||||
| Average | - | 98.9 | 0.99 | - | 56.7 | a | |||||||||||
| HAM2 | 1 | 4 weeks | 30 | 0 | 0 | 0 | 5 | 25 | 0 | 100 | 76.7 | ||||||
| 2 | 4 weeks | 30 | 0 | 0 | 0 | 11 | 19 | 0 | 100 | 72.7 | |||||||
| 3 | 4 weeks | 30 | 0 | 6 | 7 | 16 | 1 | 0 | 100 | 48.0 | |||||||
| Average | - | 100 | 1.00 | - | 65.8 | b | |||||||||||
| Non-treatment | 1 | 4 weeks | 30 | 0 | 0 | 1 | 4 | 25 | 0 | 100 | 76.0 | ||||||
| 2 | 4 weeks | 30 | 0 | 0 | 0 | 2 | 28 | 0 | 100 | 78.7 | |||||||
| 3 | 4 weeks | 30 | 0 | 0 | 0 | 15 | 15 | 0 | 100 | 70.0 | |||||||
| Average | - | 100 | - | 74.9 | c | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitabayashi, S.; Nishimura, F.; Katayama, T.; Yoshida, M.; Mori, M.; Saba, M.; Inoue, Y.; Kawaguchi, A. Suppression Efficacy of Clubroot on Cruciferous Crops Through Application of the Humic Acid Material. Plants 2025, 14, 3035. https://doi.org/10.3390/plants14193035
Kitabayashi S, Nishimura F, Katayama T, Yoshida M, Mori M, Saba M, Inoue Y, Kawaguchi A. Suppression Efficacy of Clubroot on Cruciferous Crops Through Application of the Humic Acid Material. Plants. 2025; 14(19):3035. https://doi.org/10.3390/plants14193035
Chicago/Turabian StyleKitabayashi, Shoya, Fumihiro Nishimura, Takahiro Katayama, Miyu Yoshida, Mitsutaka Mori, Masafumi Saba, Yasuhiro Inoue, and Akira Kawaguchi. 2025. "Suppression Efficacy of Clubroot on Cruciferous Crops Through Application of the Humic Acid Material" Plants 14, no. 19: 3035. https://doi.org/10.3390/plants14193035
APA StyleKitabayashi, S., Nishimura, F., Katayama, T., Yoshida, M., Mori, M., Saba, M., Inoue, Y., & Kawaguchi, A. (2025). Suppression Efficacy of Clubroot on Cruciferous Crops Through Application of the Humic Acid Material. Plants, 14(19), 3035. https://doi.org/10.3390/plants14193035






