Valorization of Posidonia oceanica Biomass Extract as an Elicitor to Mitigate Aphid-Induced Stress in Sweet Pepper Plants
Abstract
1. Introduction
2. Results
2.1. Characterization of the Posidonia oceanica Extracts
2.2. Effect of Posidonia oceanica Extracts on Aphid-Infected Sweet Pepper Plant Growth
2.3. Effect of Posidonia oceanica Extracts on Oxidative Stress of Sweet Pepper Plants
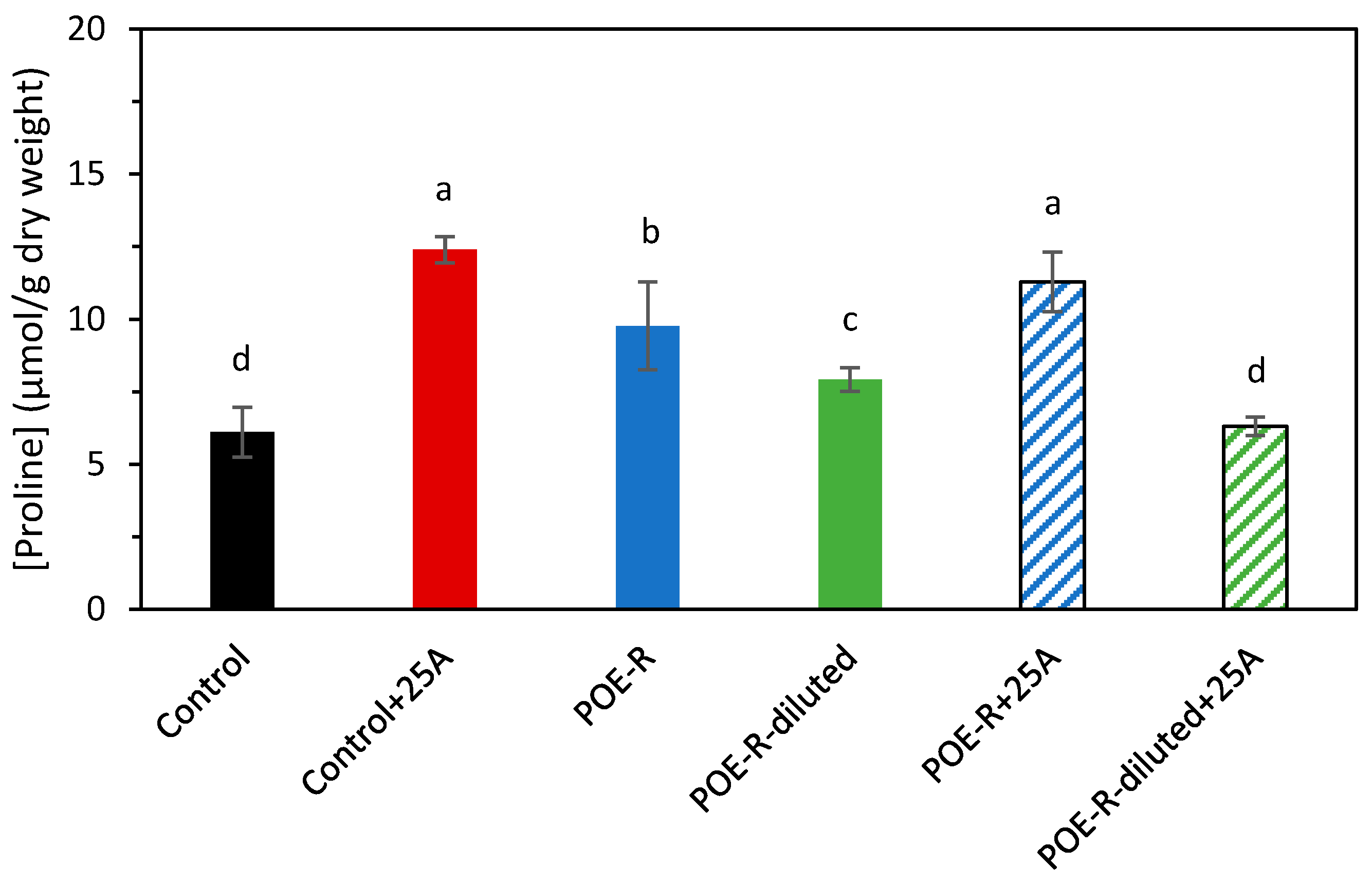
2.4. Effect of Posidonia oceanica Extracts on Proline Concentration of Sweet Pepper Plants
2.5. Effect of Posidonia oceanica Extracts on Population Growth of Myzus persicae
3. Discussion
4. Materials and Methods
4.1. Extraction and Characterization of Extracts from Posidonia oceanica
4.2. Green Peach Aphids
4.3. Plant Material, Cultural Conditions and Treatments
- (i)
- Control: plants developed under normal conditions;
- (ii)
- Control+25A: plants infected with 25 aphids of M. persicae (Sulzer);
- (iii)
- POE-R: plants treated with P. oceanica extract from water reflux method;
- (iv)
- POE-R-diluted: plants treated with 1:1 (%v/v) dilution with distilled water of P. oceanica extract from water reflux method;
- (v)
- POE-R+25A: plants infected with 25 aphids of M. persicae (Sulzer) and treated with P. oceanica extract from water reflux method;
- (vi)
- POE-R-diluted+25A: plants infected with 25 aphids of M. persicae (Sulzer) and treated with 1:1 (%v/v) dilution with distilled water of P. oceanica extract from water reflux method.
4.4. Effect of Posidonia Oceanica Extracts on Population Growth of Mizus persicae
- (i)
- Control+1A: plants infected with 1 aphid of M. persicae (Sulzer);
- (ii)
- POE-R+1A: plants infected with 1 aphid of M. persicae (Sulzer) and treated with P. oceanica extract from water reflux method;
- (iii)
- POE-R-diluted+1A: plants infected with 1 aphid of M. persicae (Sulzer) and treated with 1:1 (v/v) dilution with distilled water of P. oceanica extract from water reflux method.
4.5. Physical Parameters of Sweet Pepper Plants
4.6. Physiological Parameters in Sweet Pepper Plants
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial Use of Pepper (Capsicum annum L.) Derived Products: Technological Benefits and Biological Advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Sarath Babu, B.; Pandravada, S.R.; Prasada Rao, R.D.V.J.; Anitha, K.; Chakrabarty, S.K.; Varaprasad, K.S. Global Sources of Pepper Genetic Resources against Arthropods, Nematodes and Pathogens. Crop Prot. 2011, 30, 389–400. [Google Scholar] [CrossRef]
- Waweru, B.W.; Rukundo, P.; Kilalo, D.C.; Miano, D.W.; Kimenju, J.W. Effect of Border Crops and Intercropping on Aphid Infestation and the Associated Viral Diseases in Hot Pepper (Capsicum sp.). Crop Prot. 2021, 145, 105623. [Google Scholar] [CrossRef]
- Özgökçe, M.S.; Chi, H.; Atlıhan, R.; Kara, H. Demography and Population Projection of Myzus persicae (Sulz.) (Hemiptera: Aphididae) on Five Pepper (Capsicum annuum L.) Cultivars. Phytoparasitica 2018, 46, 153–167. [Google Scholar] [CrossRef]
- Cai, H.; Yang, L.; Zuo, Z.; Liao, W.; Yang, Z. Resistance Status of Myzus Persicae to Pesticide and Its Relationship with Enzymes. Agron. J. 2021, 113, 806–819. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; Ramesh, R.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Bhutia, T.L.; Devi, S.H.; Thounaojam, A.S.; et al. Role of Biostimulants in Mitigating the Effects of Climate Change on Crop Performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Erb, M.; Lou, Y.; Turlings, T.C.J. The Prospect of Applying Chemical Elicitors and Plant Strengtheners to Enhance the Biological Control of Crop Pests. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20120283. [Google Scholar] [CrossRef]
- Guru, A.; Dwivedi, P.; Kaur, P.; Pandey, D.K. Exploring the Role of Elicitors in Enhancing Medicinal Values of Plants under in Vitro Condition. S. Afr. J. Bot. 2022, 149, 1029–1043. [Google Scholar] [CrossRef]
- Wiesel, L.; Newton, A.C.; Elliott, I.; Booty, D.; Gilroy, E.M.; Birch, P.R.J.; Hein, I. Molecular Effects of Resistance Elicitors from Biological Origin and Their Potential for Crop Protection. Front. Plant Sci. 2014, 5, 655. [Google Scholar] [CrossRef]
- Humbal, A.; Pathak, B. Influence of Exogenous Elicitors on the Production of Secondary Metabolite in Plants: A Review (“VSI: Secondary Metabolites”). Plant Stress 2023, 8, 100166. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and Plant Responses to Salinity Stress: A Review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Van Bockhaven, J.; De Vleesschauwer, D.; Höfte, M. Towards Establishing Broad-Spectrum Disease Resistance in Plants: Silicon Leads the Way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A. ROS and RNS in Plant Physiology: An Overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Csiszár, J.; Horváth, E.; Váry, Z.; Gallé, Á.; Bela, K.; Brunner, S.; Tari, I. Glutathione Transferase Supergene Family in Tomato: Salt Stress-Regulated Expression of Representative Genes from Distinct GST Classes in Plants Primed with Salicylic Acid. Plant Physiol. Biochem. 2014, 78, 15–26. [Google Scholar] [CrossRef]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic Compounds as Indicators of Drought Resistance in Shrubs from Patagonian Shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef]
- Antonić, D.; Milošević, S.; Cingel, A.; Lojić, M.; Trifunović-Momčilov, M.; Petrić, M.; Subotić, A.; Simonović, A. Effects of Exogenous Salicylic Acid on Impatiens walleriana L. Grown in Vitro under Polyethylene Glycol-Imposed Drought. S. Afr. J. Bot. 2016, 105, 226–233. [Google Scholar] [CrossRef]
- Lambers, H.; Oliveira, R.S. Mineral Nutrition. In Plant Physiological Ecology; Springer International Publishing: Cham, Switzerland, 2019; pp. 301–384. [Google Scholar]
- Tripathi, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; De Corato, U.; Sansinenea, E. Plant Mineral Nutrition and Disease Resistance: A Significant Linkage for Sustainable Crop Protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The Molecular–Physiological Functions of Mineral Macronutrients and Their Consequences for Deficiency Symptoms in Plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef]
- González-Correa, J.M.; Bayle, J.T.; Sánchez-Lizaso, J.L.; Valle, C.; Sánchez-Jerez, P.; Ruiz, J.M. Recovery of Deep Posidonia oceanica Meadows Degraded by Trawling. J. Exp. Mar. Biol. Ecol. 2005, 320, 65–76. [Google Scholar] [CrossRef]
- Guillén, J.E.; Sánchez Lizaso, J.L.; Jiménez, S.; Martínez, J.; Codina, A.; Montero, M.; Triviño, A.; Soler, G.; Zubcoff, J.J. Evolution of Posidonia oceanica Seagrass Meadows and Its Implications for Management. J. Sea Res. 2013, 83, 65–71. [Google Scholar] [CrossRef]
- Simeone, S.; De Falco, G. Morphology and Composition of Beach-Cast Posidonia oceanica Litter on Beaches with Different Exposures. Geomorphology 2012, 151–152, 224–233. [Google Scholar] [CrossRef]
- Fernández-Torquemada, Y.; Sánchez-Lizaso, J.L. Effects of Salinity on Seed Germination and Early Seedling Growth of the Mediterranean Seagrass Posidonia oceanica (L.) Delile. Estuar. Coast. Shelf Sci. 2013, 119, 64–70. [Google Scholar] [CrossRef]
- Klap, V.; Hemminga, M.; Boon, J. Retention of Lignin in Seagrasses: Angiosperms That Returned to the Sea. Mar. Ecol. Prog. Ser. 2000, 194, 1–11. [Google Scholar] [CrossRef]
- Benito-González, I.; López-Rubio, A.; Galarza-Jiménez, P.; Martínez-Sanz, M. Multifunctional Cellulosic Aerogels from Posidonia oceanica Waste Biomass with Antioxidant Properties for Meat Preservation. Int. J. Biol. Macromol. 2021, 185, 654–663. [Google Scholar] [CrossRef]
- Benito-González, I.; Cucharero, J.; Al Haj, Y.; Hänninen, T.; Lokki, T.; Martínez-Sanz, M.; López-Rubio, A.; Martínez-Abad, A.; Vapaavuori, J. Waste Biomass Valorisation for the Development of Sustainable Cellulosic Aerogels and Their Sound Absorption Properties. Adv. Sustain. Syst. 2022, 6, 2200248. [Google Scholar] [CrossRef]
- Benito-González, I.; Göksen, G.; Pérez-Bassart, Z.; López-Rubio, A.; Sánchez, R.; Alonso, J.M.; Gavara, R.; Gallur, M.; Martínez-Sanz, M. Pilot Plant Scale-up of the Production of Optimized Starch-Based Biocomposites Loaded with Cellulosic Nanocrystals from Posidonia oceanica Waste Biomass. Food Packag. Shelf Life 2021, 30, 100730. [Google Scholar] [CrossRef]
- Asimakopoulos, G.; Baikousi, M.; Salmas, C.; Bourlinos, A.B.; Zboril, R.; Karakassides, M.A. Advanced Cr(VI) Sorption Properties of Activated Carbon Produced via Pyrolysis of the “Posidonia oceanica” Seagrass. J. Hazard. Mater. 2021, 405, 124274. [Google Scholar] [CrossRef]
- Moltó, J.; Montalbán, M.G.; Núñez, S.S.; Jordá, J.D. Revalorization of Posidonia oceanica Waste for the Thermochemical Production of Biochar. Appl. Sci. 2022, 12, 7422. [Google Scholar] [CrossRef]
- Spyrou, A.V.; Tantis, I.; Baikousi, M.; Bourlinos, A.B.; Salmas, C.E.; Zboril, R.; Karakassides, M.A. The Use of Activated Bio-Carbon Derived from “Posidonia oceanica” Sea-Waste for Lithium-Sulfur Batteries Development. Sustain. Energy Technol. Assess. 2022, 53, 102748. [Google Scholar] [CrossRef]
- Peruzzi, E.; Macci, C.; Doni, S.; Zelari, L.; Masciandaro, G. Co-Composting as a Management Strategy for Posidonia Oceanica Residues and Dredged Sediments. Waste Biomass Valorization 2020, 11, 4907–4919. [Google Scholar] [CrossRef]
- D’Imperio, M.; Montesano, F.F.; Montemurro, N.; Parente, A. Posidonia Natural Residues as Growing Substrate Component: An Ecofriendly Method to Improve Nutritional Profile of Brassica Microgreens. Front. Plant Sci. 2021, 12, 580596. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Proestos, C. Food Preservation: Challenges and Efforts for the Future. Foods 2020, 9, 391. [Google Scholar] [CrossRef]
- Ferrández-Gómez, B.; Jordá, J.D.; Cerdán, M.; Sánchez, A. Valorization of Posidonia oceanica Biomass: Role on Germination of Cucumber and Tomato Seeds. Waste Manag. 2023, 171, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Astudillo-Pascual, M.; Domínguez, I.; Aguilera, P.A.; Frenich, A.G. New Phenolic Compounds in Posidonia oceanica Seagrass: A Comprehensive Array Using High Resolution Mass Spectrometry. Plants 2021, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Heglmeier, A.; Zidorn, C. Secondary Metabolites of Posidonia oceanica (Posidoniaceae). Biochem. Syst. Ecol. 2010, 38, 964–970. [Google Scholar] [CrossRef]
- Hernán, G.; Ortega, M.J.; Tomas, F. Specialized Compounds across Ontogeny in the Seagrass Posidonia oceanica. Phytochemistry 2022, 196, 113070. [Google Scholar] [CrossRef]
- Ozbil, E.; Ilktac, M.; Ogmen, S.; Isbilen, O.; Duran Ramirez, J.M.; Gomez, J.; Walker, J.N.; Volkan, E. In Vitro Antibacterial, Antibiofilm Activities, and Phytochemical Properties of Posidonia oceanica (L.) Delile: An Endemic Mediterranean Seagrass. Heliyon 2024, 10, e35592. [Google Scholar] [CrossRef]
- Agostini, S.; Desjobert, J.-M.; Pergent, G. Distribution of Phenolic Compounds in the Seagrass Posidonia oceanica. Phytochemistry 1998, 48, 611–617. [Google Scholar] [CrossRef]
- Boukhari, M.; Asencio-Vicedo, R.; Cerdán, M.; Sánchez-Sánchez, A.; Jordá, J.D.; Ferrández-Gómez, B. Foliar Application of Equisetum Arvense Extract Enhances Growth, Alleviates Lipid Peroxidation and Reduces Proline Accumulation in Tomato Plants Under Salt Stress. Plants 2025, 14, 488. [Google Scholar] [CrossRef]
- Zidorn, C. Secondary Metabolites of Seagrasses (Alismatales and Potamogetonales; Alismatidae): Chemical Diversity, Bioactivity, and Ecological Function. Phytochemistry 2016, 124, 5–28. [Google Scholar] [CrossRef]
- Gokce, G.; Haznedaroglu, M.Z. Evaluation of Antidiabetic, Antioxidant and Vasoprotective Effects of Posidonia oceanica Extract. J. Ethnopharmacol. 2008, 115, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Box, A.; Terrados, J.; Deudero, S.; Pons, A. Antioxidant Response of the Seagrass Posidonia oceanica When Epiphytized by the Invasive Macroalgae Lophocladia lallemandii. Mar. Environ. Res. 2008, 66, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, T.; Feng, B.; Peng, S.; Tao, L.; Fu, G. Respiration, Rather Than Photosynthesis, Determines Rice Yield Loss Under Moderate High-Temperature Conditions. Front. Plant Sci. 2021, 12, 678653. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Carbo, M.; Taylor, N.L.; Giles, L.; Busquets, S.; Finnegan, P.M.; Day, D.A.; Lambers, H.; Medrano, H.; Berry, J.A.; Flexas, J. Effects of Water Stress on Respiration in Soybean Leaves. Plant Physiol. 2005, 139, 466–473. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Chaoxing, H. Effect of Arbuscular Mycorrhizal Fungi on Growth, Mineral Nutrition, Antioxidant Enzymes Activity and Fruit Yield of Tomato Grown under Salinity Stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Wei, H.; Zhikuan, J.; Qingfang, H. Effects of Herbivore Stress by Aphis medicaginis Koch on the Malondialdehyde Contents and the Activities of Protective Enzymes in Different Alfalfa Varieties. Acta Ecol. Sin. 2007, 27, 2177–2183. [Google Scholar] [CrossRef]
- Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of Plant Secondary Metabolites in Defence and Transcriptional Regulation in Response to Biotic Stress. Plant Stress 2023, 8, 100154. [CrossRef]
- Trejo-Téllez, L.I.; García-Jiménez, A.; Escobar-Sepúlveda, H.F.; Ramírez-Olvera, S.M.; Bello-Bello, J.J.; Gómez-Merino, F.C. Silicon Induces Hormetic Dose-Response Effects on Growth and Concentrations of Chlorophylls, Amino Acids and Sugars in Pepper Plants during the Early Developmental Stage. PeerJ 2020, 8, e9224. [Google Scholar] [CrossRef]
- Al-aghabary, K.; Zhu, Z.; Shi, Q. Influence of Silicon Supply on Chlorophyll Content, Chlorophyll Fluorescence, and Antioxidative Enzyme Activities in Tomato Plants Under Salt Stress. J. Plant Nutr. 2005, 27, 2101–2115. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.-H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and Salinity: Crosstalk in Crop-Mediated Stress Tolerance Mechanisms. Front. Plant Sci. 2019, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Savvas, D.; Ntatsi, G. Biostimulant Activity of Silicon in Horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Kong, J.; Dong, Y.; Xu, L.; Liu, S.; Bai, X. Effects of Exogenous Salicylic Acid on Alleviating Chlorosis Induced by Iron Deficiency in Peanut Seedlings (Arachis hypogaea L.). J. Plant Growth Regul. 2014, 33, 715–729. [Google Scholar] [CrossRef]
- Blokhina, O. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- Ni, X.; Quisenberry, S.S.; Heng-Moss, T.; Markwell, J.; Higley, L.; Baxendale, F.; Sarath, G.; Klucas, R. Dynamic Change in Photosynthetic Pigments and Chlorophyll Degradation Elicited by Cereal Aphid Feeding. Entomol. Exp. Appl. 2002, 105, 43–53. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Benrey, B. Effects of Plant Metabolites on the Behavior and Development of Parasitic Wasps. Écoscience 1998, 5, 321–333. [Google Scholar] [CrossRef]
- Satar, S.; Kersting, U.; Uygun, N. Effect of Temperature on Population Parameters of Aphis gossypii Glover and Myzus persicae (Sulzer) (Homoptera: Aphididae) on Pepper. J. Plant Dis. Prot. 2008, 115, 69–74. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of Silicon in Plant Stress Tolerance: Opportunities to Achieve a Sustainable Cropping System. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef]
- Pavela, R. Effectiveness of Some Botanical Insecticides against Spodoptera littoralis Boisduvala (Lepidoptera: Noctudiae), Myzus persicae Sulzer (Hemiptera: Aphididae) and Tetranychus urticae Koch (Acari: Tetranychidae). Plant Prot. Sci. 2009, 45, 161–167. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Garrido, I.; Casimiro, I.d.J.; Casero, P.J.; Espinosa, F.; García-Romera, I.; Aranda, E. Defence Response of Tomato Seedlings to Oxidative Stress Induced by Phenolic Compounds from Dry Olive Mill Residue. Chemosphere 2012, 89, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, I.J.; White, P.F. Simple Estimation of Intrinsic Increase Rates for Aphids and Tetranychid Mites. J. Appl. Ecol. 1977, 14, 757. [Google Scholar] [CrossRef]
- Abadía, J.; Monge, E.; Montañés, L.; Heras, L. Extraction of Iron from Plant Leaves by Fe (II) Chelators. J. Plant Nutr. 1984, 7, 777–784. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ikinci, A. An experiment to investigate ameliorative effects of potassium sulphate on salt and alkalinity stressed vegetable crops. J. Plant Nutr. 2002, 25, 2545–2558. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate Metabolism of Three Submersed Aquatic Angiosperms during Ageing. Aquat. Bot. 1982, 12, 345–354. [Google Scholar] [CrossRef]
- Shu, X.; Yin, L.; Zhang, Q.; Wang, W. Effect of Pb Toxicity on Leaf Growth, Antioxidant Enzyme Activities, and Photosynthesis in Cuttings and Seedlings of Jatropha curcas L. Environ. Sci. Pollut. Res. 2012, 19, 893–902. [Google Scholar] [CrossRef]
- Magné, C.; Larher, F. High Sugar Content of Extracts Interferes with Colorimetric Determination of Amino Acids and Free Proline. Anal. Biochem. 1992, 200, 115–118. [Google Scholar] [CrossRef]

| Parameter | Posidonia oceanica | Sig. 1 | ||
|---|---|---|---|---|
| Magnetic Stirring | Soxhlet Extraction | Water Reflux | ||
| Na (mg/L) | 110.4 ± 1.5 a | 64.4 ± 0.2 b | 41.4 ± 0.5 c | *** |
| K (mg/L) | 13.3 ± 1.2 a | 4.8 ± 1.5 c | 8.6 ± 0.7 b | *** |
| Ca (mg/L) | 100.3 ± 0.9 a | 68.2 ± 0.6 b | 44.7 ± 0.2 c | *** |
| Mg (mg/L) | 34.3 ± 0.4 c | 51.0 ± 0.4 a | 38.9 ± 0.2 b | *** |
| Fe (mg/L) | 0.23 ± 0.07 a | 0.15 ± 0.03 b | 0.30 ± 0.07 a | ** |
| Zn (mg/L) | 0.051 ± 0.005 a | 0.036 ± 0.004 b | 0.062 ± 0.06 a | ** |
| Mn (mg/L) | 0.023 ± 0.006 a | 0.004 ± 0.002 b | 0.013 ± 0.008 a | ** |
| Cu (mg/L) | 0.014 ± 0.003 c | 0.029 ± 0.008 b | 0.053 ± 0.005 a | *** |
| Si (mM) | 0.11 ± 0.02 b | 0.28 ± 0.09 a | 0.30 ± 0.05 a | *** |
| TPC (mM) | 0.18 ± 0.04 c | 0.35 ± 0.04 b | 0.56 ± 0.06 a | *** |
| Parameter | Control | Control+25A | POE-R | POE-R-Diluted | POE-R+25A | POE-R-Diluted+25A | Sig. 1 |
|---|---|---|---|---|---|---|---|
| Fresh weight (g) | 35.1 ± 0.1 a | 19.9 ± 0.6 d | 29.6 ± 0.4 b | 34.5 ± 0.8 a | 24.7 ± 0.7 c | 27 ± 2 b | *** |
| Dry weight (g) | 3.36 ± 0.04 a | 1.98 ± 0.02 d | 2.73 ± 0.1 b | 3.38 ± 0.03 a | 1.96 ± 0.03 d | 2.05 ± 0.02 c | *** |
| Total chlorophylls (mg/g FW) | 1.46 ± 0.05 a | 0.99 ± 0.08 c | 1.10 ± 0.04 c | 1.5 ± 0.1 a | 1.05 ± 0.01 c | 1.29 ± 0.05 b | ** |
| Carotenoids (mg/g FW) | 0.45 ± 0.03 a | 0.27 ± 0.01 c | 0.38 ± 0.03 b | 0.41 ± 0.04 b | 0.29 ± 0.07 c | 0.33 ± 0.04 c | ** |
| Weight Loss (mg/g) | Control | Control+25A | POE-R | POE-R-Diluted | POE-R+25A | POE-R-Diluted+25A | Sig. 1 |
|---|---|---|---|---|---|---|---|
| Peak 270 °C | 3.6 ± 0.1 a | 2.8 ± 0.1 c | 3.4 ± 0.1 ab | 3.2 ± 0.2 b | 3.4 ± 0.2 ab | 3.4 ± 0.4 ab | *** |
| Peak 400 °C | 3.9 ± 0.1 ab | 3.6 ± 0.3 b | 4.1 ± 0.1 a | 3.9 ± 0.1 ab | 3.8 ± 0.2 b | 3.7 ± 0.4 b | *** |
| Treatment | Md | d | Rm |
|---|---|---|---|
| Control+1A | 48 ± 4 a | 6.7 ± 0.5 | 0.43 ± 0.03 a |
| POE-R+1A | 31 ± 3 c | 7.3 ± 0.5 | 0.35 ± 0.02 b |
| POE-R-diluted+1A | 40 ± 3 b | 6.8 ± 0.4 | 0.40 ± 0.2 a |
| Sig. 1 | *** | ns | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrández-Gómez, B.; Cerdán, M.; Jordá, J.D.; Sánchez-Sánchez, A. Valorization of Posidonia oceanica Biomass Extract as an Elicitor to Mitigate Aphid-Induced Stress in Sweet Pepper Plants. Plants 2025, 14, 3002. https://doi.org/10.3390/plants14193002
Ferrández-Gómez B, Cerdán M, Jordá JD, Sánchez-Sánchez A. Valorization of Posidonia oceanica Biomass Extract as an Elicitor to Mitigate Aphid-Induced Stress in Sweet Pepper Plants. Plants. 2025; 14(19):3002. https://doi.org/10.3390/plants14193002
Chicago/Turabian StyleFerrández-Gómez, Borja, Mar Cerdán, Juana D. Jordá, and Antonio Sánchez-Sánchez. 2025. "Valorization of Posidonia oceanica Biomass Extract as an Elicitor to Mitigate Aphid-Induced Stress in Sweet Pepper Plants" Plants 14, no. 19: 3002. https://doi.org/10.3390/plants14193002
APA StyleFerrández-Gómez, B., Cerdán, M., Jordá, J. D., & Sánchez-Sánchez, A. (2025). Valorization of Posidonia oceanica Biomass Extract as an Elicitor to Mitigate Aphid-Induced Stress in Sweet Pepper Plants. Plants, 14(19), 3002. https://doi.org/10.3390/plants14193002







