Preliminary Insights into the Non-Volatile Constituents of Commiphora ornifolia (Balf.f.) J.B.Gillett Oleogum Resin from Socotra Island
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction of Secondary Metabolites and Isolation of Yangambin
2.2. Extraction and Analyses of Monosaccharides
3. Materials and Methods
3.1. Plant Material
3.2. Solvents, Reference Compounds, and Chromatographic Materials
3.3. Instruments and Analytical Methods
3.4. Extraction and Isolation of the Main Components
3.4.1. Column Chromatography and Isolation of Yangambin from RC7EE
3.4.2. Monosaccharide Composition Analysis of Polysaccharide
3.5. HPLC-DAD Quantitative Analyses of Yangambin in RC7EE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EE | Ethanol extract |
| EPA | Environment Protection Authority |
| GC-MS | Gas chromatography–mass spectrometry |
| HPLC | High-performance liquid chromatography |
| HPLC-DAD | High-performance liquid chromatography with diode array detector |
| HPTLC | High-performance thin-layer chromatography |
| HRMS | High-resolution mass spectrometry |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| NMR | Nuclear magnetic resonance |
| PAF | Platelet-activating factor |
| PLC | Preparative liquid chromatography |
| PTFE | Polytetrafluoroethylene |
| RC7EE | Ethanolic extract of RC7 |
| SPME | Solid-phase microextraction |
| TFA | Trifluoroacetic acid |
References
- Cao, B.; Wei, X.-C.; Xu, X.-R.; Zhang, H.-Z.; Luo, C.-H.; Feng, B.; Xu, R.-C.; Zhao, S.-Y.; Du, X.-J.; Han, L.; et al. Seeing the Unseen of the Combination of Two Natural Resins, Frankincense and Myrrh: Changes in Chemical Constituents and Pharmacological Activities. Molecules 2019, 24, 3076. [Google Scholar] [CrossRef] [PubMed]
- Sarup, P.; Bala, S.; Kamboj, S. Pharmacology and Phytochemistry of Oleo-Gum Resin of Commiphora wightii (Guggulu). Scientifica 2015, 2015, 138039. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, X.; Peng, C.; Wei, J.; Yang, X. The Genus Commiphora: An Overview of Its Traditional Uses, Phytochemistry, Pharmacology, and Quality Control. Pharmaceuticals 2024, 17, 1524. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Oesch, F. Anti-inflammatory and anti-cancer activities of frankincense: Targets, treatments and toxicities. Semin. Cancer Biol. 2022, 80, 39–57. [Google Scholar] [CrossRef]
- Batiha, G.E.; Wasef, L.; Teibo, J.O.; Shaheen, H.M.; Zakariya, A.M.; Akinfe, O.A.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbee, A.I.; Alexiou, A.; et al. Commiphora myrrh: A phytochemical and pharmacological update. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 405–420. [Google Scholar] [CrossRef]
- Ulrich, J.; Stiltz, S.; St-Gelais, A.; El Gaafary, M.; Simmet, T.; Syrovets, T.; Schmiech, M. Phytochemical Composition of Commiphora Oleogum Resins and Their Cytotoxicity against Skin Cancer Cells. Molecules 2022, 27, 3903. [Google Scholar] [CrossRef]
- WHO. The World Flora Online. Available online: https://www.worldfloraonline.org/ (accessed on 28 August 2025).
- Attorre, F.; Van Damme, K. Twenty years of biodiversity research and nature conservation in the Socotra Archipelago (Yemen). Rend. Lincei Sci. Fis. Nat. 2020, 31, 563–569. [Google Scholar] [CrossRef]
- La Montagna, D.; Attorre, F.; Hamdiah, S.; Maděra, P.; Malatesta, L.; Vahalík, P.; Van Damme, K.; De Sanctis, M. Climate change effects on the potential distribution of the endemic Commiphora species (Burseraceae) on the island of Socotra. Front. For. Glob. Change 2023, 6, 1183858. [Google Scholar] [CrossRef]
- Gillett, J.B. Burseraceae. In Flora of Tropical East Africa; Polhill, R.M., Ed.; A.A. Balkema: Rotterdam, The Netherlands, 1991; pp. 1–95. [Google Scholar]
- Mothana, R.A.; Lindequist, U.; Gruenert, R.; Bednarski, P.J. Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island Soqotra. BMC Complement. Med. Ther. 2009, 9, 7. [Google Scholar] [CrossRef]
- Maděra, P.; Paschová, Z.; Ansorgová, A.; Vrškový, B.; Lvončík, S.; Habrová, H. Volatile compounds in oleo-gum resin of Socotran species of Burseraceae. Acta Univ. Agric. Silvic. Mendel. Brun. 2017, 65, 73–90. [Google Scholar] [CrossRef]
- Mothana, R.A.; Al-Rehaily, A.J.; Schultze, W. Chemical Analysis and Biological Activity of the Essential Oils of Two Endemic Soqotri Commiphora Species. Molecules 2010, 15, 689–698. [Google Scholar] [CrossRef] [PubMed]
- La Montagna, D.; De Vita, D.; Frezza, C.; Garzoli, S.; Attorre, F. HS-SPME-GC/MS analysis of the volatile components of the resins of different Commiphora Jacq. Species collected in Socotra Island. Biochem. Syst. Ecol. 2025, 120, 104965. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Vargas, M.R.W.; Silva, I.G.; França, I.S.; Morals, L.C.S.L.; Da Cunha, E.V.L.; Da-Silva, M.S.; Agra, M.F. Ocotea duckei: Exceptional source of yangambin and other furofuran lignans. Anais Acad. Brasil. Ci. 1999, 71, 231–238. [Google Scholar]
- Solís, P.N.; Olmedo, D.; Nakamura, N.; Calderón, Á.I.; Hattori, M.; Gupta, M.P. A new larvicidal lignan from Piper fimbriulatum. Pharm. Biol. 2005, 43, 378–381. [Google Scholar] [CrossRef]
- Abd El-Razek, M.H.; Eissa, I.H.; Al-Karmalawy, A.A.; Elrashedy, A.A.; El-Desoky, A.H.; Aboelmagd, M.; Mohamed, T.A.; Hegazy, M.F. epi-Magnolin, a tetrahydrofurofuranoid lignan from the oleo-gum resin of Commiphora wightii, as inhibitor of pancreatic cancer cell proliferation, in-vitro and in-silico study. J. Biomol. Struct. Dyn. 2025, 43, 5151–5163. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Ando, S.; Oominami, H.; Murakami, T.; Kimura, I.; Yoshikawa, M. Absolute stereostructures of polypodane- and octanordammarane-type triterpenes with nitric oxide production inhibitory activity from guggul-gum resins. Bioorganic Med. Chem. 2004, 12, 3037–3046. [Google Scholar] [CrossRef]
- El-Mekkawy, S.; Meselhy, M.R.; Nkobole, N.; Lall, N. Three new α-glucosidase inhibitors from guggul, the oleogum resin of Commiphora wightii. Nat. Prod. Res. 2012, 27, 146–154. [Google Scholar] [CrossRef]
- Verma, R.K.; Ibrahim, M.; Fursule, A.; Mitra, R.; Sastry, J.L.N.; Ahmad, S. Metabolomic profiling of Commiphora wightii (Arn.) Bhandari bark, oleogum-resin, and stem collected from different geographical regions of India. S. Afr. J. Bot. 2022, 149, 211–221. [Google Scholar] [CrossRef]
- Alsharidah, A.; Alehaideb, Z.; Alghamdi, S.S.; Suliman, R.S.; Althenayyan, S.; Almourfi, F.; Hazazi, B.; Almogren, A.; Al Tuwaijri, A.; Boudjelal, M.; et al. Commiphora myrrha resin extract-modulated cytochrome P-450 2C9 enzyme expression in cultured Hep G2 cells is associated with resin extract-derived metabolites binding to Pregnane X receptor. BMC Complement. Med. Ther. 2025, 25, 25247. [Google Scholar] [CrossRef]
- Shi, X.; Jie, L.; Wu, P.; Mao, J.; Wang, P.; Liu, Z.; Yin, S. Comprehensive network pharmacological analysis and in vitro verification reveal the potential active ingredients and potential mechanisms of frankincense and myrrh in knee osteoarthritis. Nat. Prod. Commun. 2022, 17, 1934578X221116984. [Google Scholar] [CrossRef]
- Francis, J.A.; Raja, S.N.; Nair, M.G. Bioactive terpenoids and guggulusteroids from Commiphora mukul gum resin of potential anti-inflammatory interest. Chem. Biodivers. 2004, 1, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Wang, Y.H.; Ali, Z.; Smillie, T.J.; Khan, I.A. HPLC method for chemical fingerprinting of Guggul (Commiphora wightii)—Quantification of E-and Z-guggulsterones and detection of possible adulterants. Planta Med. 2012, 82, 356–361. [Google Scholar] [CrossRef]
- El-Razek, A.; Mohamed, T.A.; Abdel-Halim, S.; Bata, S.M.; Kubacy, T.M. Comprehensive NMR reassignments of lignans derived from Commiphora myrrha. Egypt. J. Chem. 2023, 66, 45–57. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Nørskov, N.; Bolvig, A.K.; Hedemann, M.S.; Lærke, H.N. Lignans. In Dietary Polyphenols: Their Metabolism and Health Effects; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 365–406. [Google Scholar]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef]
- Xu, W.H.; Zhao, P.; Wang, M.; Liang, Q. Naturally occurring furofuran lignans: Structural diversity and biological activities. Nat. Prod. Res. 2019, 33, 1357–1373. [Google Scholar] [CrossRef]
- Ražná, K.; Nôžková, J.; Vargaová, A.; Harenčár, Ľ.; Bjelková, M. Biological functions of lignans in plants. Agriculture 2021, 67, 155–165. [Google Scholar] [CrossRef]
- Pei, Y.; Cao, W.; Yu, W.; Peng, C.; Xu, W.; Zuo, Y.; Wu, W.; Hu, Z. Identification and functional characterization of the dirigent gene family in Phryma leptostachya and the contribution of PlDIR1 in lignan biosynthesis. BMC Plant Biol. 2023, 23, 291. [Google Scholar] [CrossRef]
- Wang, G.K.; Lin, B.B.; Rao, R.; Zhu, K.; Qin, X.Y.; Xie, G.Y.; Qin, M.J. A new lignan with anti-HBV activity from the roots of Bombax ceiba. Nat. Prod. Res. 2013, 27, 1348–1352. [Google Scholar] [CrossRef]
- Xu, X.Y.; Wang, D.Y.; Li, Y.P.; Deyrup, S.T.; Zhang, H.J. Plant-derived lignans as potential antiviral agents: A systematic review. Phytochem. Rev. 2022, 21, 239–289. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and Their Derivatives from Plants as Antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kim, M.-Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Wilairatana, P.; Chowdhury, R.; Rakib, A.I.; Kamli, H.; Shaikh, A.; Coutinho, H.D.M.; Islam, M.T. Anticancer Potentials of the Lignan Magnolin: A Systematic Review. Molecules 2023, 28, 3671. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Massri, M.; Nasrallah, G.K. A comprehensive review on the anti-cancer properties and mechanisms of action of sesamin, a lignan in sesame seeds (Sesamum indicum). Eur. J. Pharmacol. 2017, 815, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, F.; Guo, T.; Han, S.; Wang, H.; Lin, Q. Targeting NF-κB pathway by dietary lignans in inflammation: Expanding roles of gut microbiota and metabolites. Crit. Rev. Food Sci. 2023, 63, 5967–5983. [Google Scholar] [CrossRef]
- Han, N.; Wen, Y.; Liu, Z.; Zhai, J.; Li, S.; Yin, J. Advances in the roles and mechanisms of lignans against Alzheimer’s disease. Front. Pharmacol. 2022, 13, 960112. [Google Scholar] [CrossRef]
- Isah, M.B.; Tajuddeen, N.; Yusuf, A.; Mohammed, A.; Ibrahim, M.A.; Melzig, M.; Zhang, X. The antidiabetic properties of lignans: A comprehensive review. Phytomedicine 2025, 141, 156717. [Google Scholar] [CrossRef]
- Chu, Z.; Hu, Z.; Luo, Y.; Zhou, Y.; Yang, F.; Luo, F. Targeting gut-liver axis by dietary lignans ameliorate obesity: Evidences and mechanisms. Crit. Rev. Food Sci. 2025, 65, 243–264. [Google Scholar] [CrossRef]
- Tibiriçá, E. Cardiovascular properties of yangambin, a lignan isolated from Brazilian plants. Cardiovasc. Drug Rev. 2001, 19, 313–328. [Google Scholar] [CrossRef]
- Araújo, I.G.A.; Silva, D.F.; Do Carmo de Alustau, M.; Dias, K.L.G.; Cavalcante, K.V.M.; Veras, R.C.; Barbosa-Filho, J.M.; Neto, M.D.A.; Bendhack, L.M.; De Azevedo Correia, N.; et al. Calcium Influx Inhibition is Involved in the Hypotensive and Vasorelaxant Effects Induced by Yangambin. Molecules 2014, 19, 6863–6876. [Google Scholar] [CrossRef]
- Serra, M.F.; Diaz, B.L.; Barreto, E.; Pereira, A.P.B.; Lima, M.C.R.; Barbosa-Filho, J.M.; Cordeiro, R.S.B.; Martins, M.A.; De Silva, P.M.R. Anti-allergic properties of the natural PAF antagonist yangambin. Planta Med. 1997, 63, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Hausott, B.; Greger, H.; Marian, B. Naturally occurring lignans efficiently induce apoptosis in colorectal tumor cells. Cancer Res. Clin. Oncol. 2003, 129, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.; Pratap, K.; Verma, P.K.; Singh, B.; Padwad, Y. Validation of ethnomedicinal potential of Tinospora cordifolia for anticancer and immunomodulatory activities and quantification of bioactive molecules by HPTLC. J. Ethnopharmacol. 2015, 175, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Monte Neto, R.L.; Barbosa Filho, J.M.; Sousa, L.M.A.; Athayde Filho, P.F.; Dias, C.S.; Oliveira, M.R. Crude ethanolic extract, lignoid fraction and yangambin from Ocotea duckei (Lauraceae) show antileishmanial activity. Z. Naturforsch. C 2007, 62, 348–352. [Google Scholar] [CrossRef]
- Neto, R.L.M.; Sousa, L.M.; Dias, C.S.; Barbosa Filho, J.M.; Oliveira, M.R.; Figueiredo, R.C. Morphological and physiological changes in Leishmania promastigotes induced by yangambin, a lignan obtained from Ocotea duckei. Exp. Parasitol. 2011, 127, 215–221. [Google Scholar] [CrossRef]
- Rebouças-Silva, J.; Santos, G.F.; Filho, J.M.B.; Berretta, A.A.; Marquele-Oliveira, F.; Borges, V.M. In vitro leishmanicidal effect of Yangambin and Epi-yangambin lignans isolated from Ocotea fasciculata (Nees) Mez. Front. Cell. Infect. Microbiol. 2023, 12, 1045732. [Google Scholar] [CrossRef]
- De Sousa, F.C.F.; Pereira, B.A.; Lima, V.T.M.; Lacerda, C.D.G.; Melo, C.T.V.; Barbosa-Filho, J.M.; Vasconcelos, S.M.M.; Viana, G.S.B. Central nervous system activity of yangambin from Ocotea duckei Vattimo (Lauraceae) in mice. Phytother. Res. 2005, 19, 282–286. [Google Scholar] [CrossRef]
- Boual, Z.; Pierre, G.; Kemassi, A.; Mosbah, S.; Benaoun, F.; Delattre, C.; Ould El Hadj, M.D. Chemical composition and biological activities of water-soluble polysaccharides from Commiphora myrrha (Nees) Engl. Gum. Analele Univ. Oradea Fasc. Biol. 2020, 27, 50–55. [Google Scholar]
- Hwang, Y.-H.; Lee, A.; Kim, T.; Jang, S.-A.; Ha, H. Anti-Osteoporotic Effects of Commiphora Myrrha and Its Poly-Saccharide via Osteoclastogenesis Inhibition. Plants 2021, 10, 945. [Google Scholar] [CrossRef]
- Liu, D.; Tang, W.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Monosaccharide composition analysis of polysaccharides from natural sources: Hydrolysis condition and detection method development. Food Hydrocoll. 2021, 116, 106641. [Google Scholar] [CrossRef]
- Madia, V.N.; De Vita, D.; Messore, A.; Toniolo, C.; Tudino, V.; De Leo, A.; Pindinello, I.; Ialongo, D.; Saccoliti, F.; D’Ursi, A.M.; et al. Analytical Characterization of an Inulin-Type Fructooligosaccharide from Root-Tubers of Asphodelus ramosus L. Pharmaceuticals 2021, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, J.; Zhang, Q.Q.; Zhu, M.H.; Hua, M.L.; Xu, Y.H. Monosaccharide analysis and fingerprinting identification of polysaccharides from Poria cocos and Polyporus umbellatus by HPLC combined with chemometrics methods. Chin. Herb. Med. 2019, 11, 406–411. [Google Scholar] [CrossRef]
- Uhliariková, I.; Matulová, M.; Capek, P. Optimizing acid hydrolysis for monosaccharide compositional analysis of Nostoc cf. linckia acidic exopolysaccharide. Carbohydr. Res. 2021, 508, 108400. [Google Scholar] [CrossRef] [PubMed]
- Dahi, A.; Abdellahi, B.M.L.; Deida, M.F.; Hucher, N.; Malhiac, C.; Renou, F. Chemical and physicochemical characterizations of the water-soluble fraction of the Commiphora Africana exudate. Food Hydrocoll. 2008, 86, 2–10. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Řezanka, T.; Dembitsky, V.M.; Moussaieff, A. Myrrh-commiphora chemistry. Biomed Pap. 2005, 149, 3–28. [Google Scholar] [CrossRef]
- Yang, C.; Guan, J.; Zhang, J.S.; Li, S.P. Use of HPTLC to differentiate among the crude polysaccharides in six traditional Chinese medicines. JPC-J. Planar Chromat. 2010, 23, 46–49. [Google Scholar] [CrossRef]
- Baskaran, K.; Craft, D.L.; Eghbalnia, H.R.; Gryk, M.R.; Hoch, J.C.; Maciejewski, M.W.; Wedell, J.; Wilburn, C.W. Merging NMR data and computation facilitates data-centered research. Front. Mol. Biosci. 2022, 8, 817175. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The human metabolome database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Wishart, D.S.; Sajed, T.; Pin, M.; Poynton, E.F.; Goel, B.; Lee, B.L.; Guo, A.C.; Saha, S.; Sayeeda, Z.; Han, S.; et al. The natural products magnetic resonance database (NP-mrd) for 2025. Nucleic Acids Res. 2025, 53, D700–D708. [Google Scholar] [CrossRef]
- Hough, L.; Jones, J.K.N.; Wadman, W.H. 144. Some observations on the constitution of gum myrrh. J. Chem. Soc. (Resumed) 1952, 796–800. [Google Scholar] [CrossRef]
- MacRae, W.D.; Towers, G.N. Non-alkaloidal constituents of Virola elongata bark. Phytochemistry 1985, 24, 561–566. [Google Scholar] [CrossRef]
- Jesus-Morais, C.M.; Assis, E.F.; Cordeiro, R.S.; Barbosa-Filho, J.M.; Lima, W.T.; Silva, Z.L.; Bozza, P.T.; Castro-Faria-Neto, H.C. Yangambin, a lignan obtained from Ocotea duckei, differentiates putative PAF receptor subtypes in the gastrointestinal tract of rats. Planta Med. 2000, 66, 211–216. [Google Scholar] [CrossRef][Green Version]

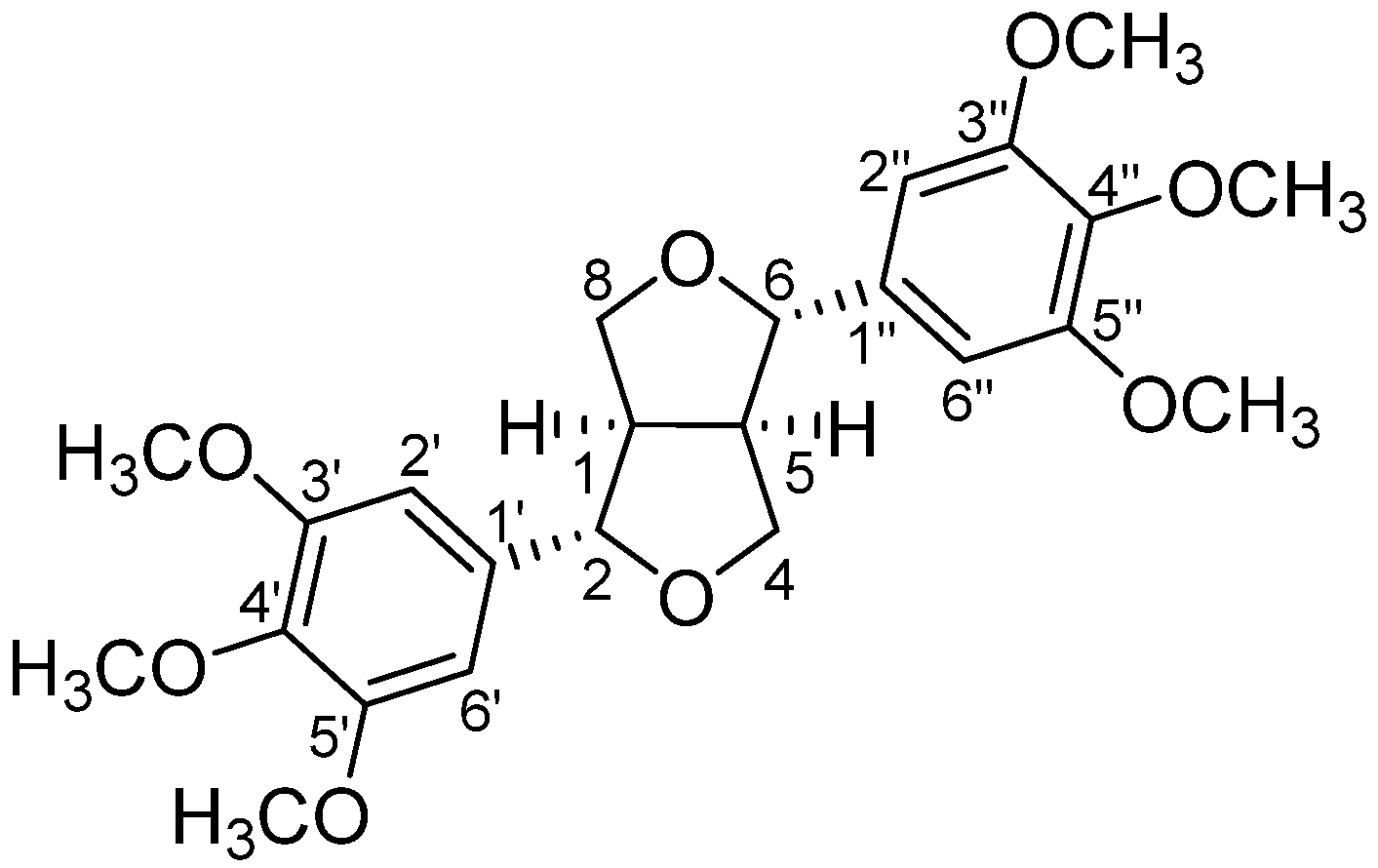
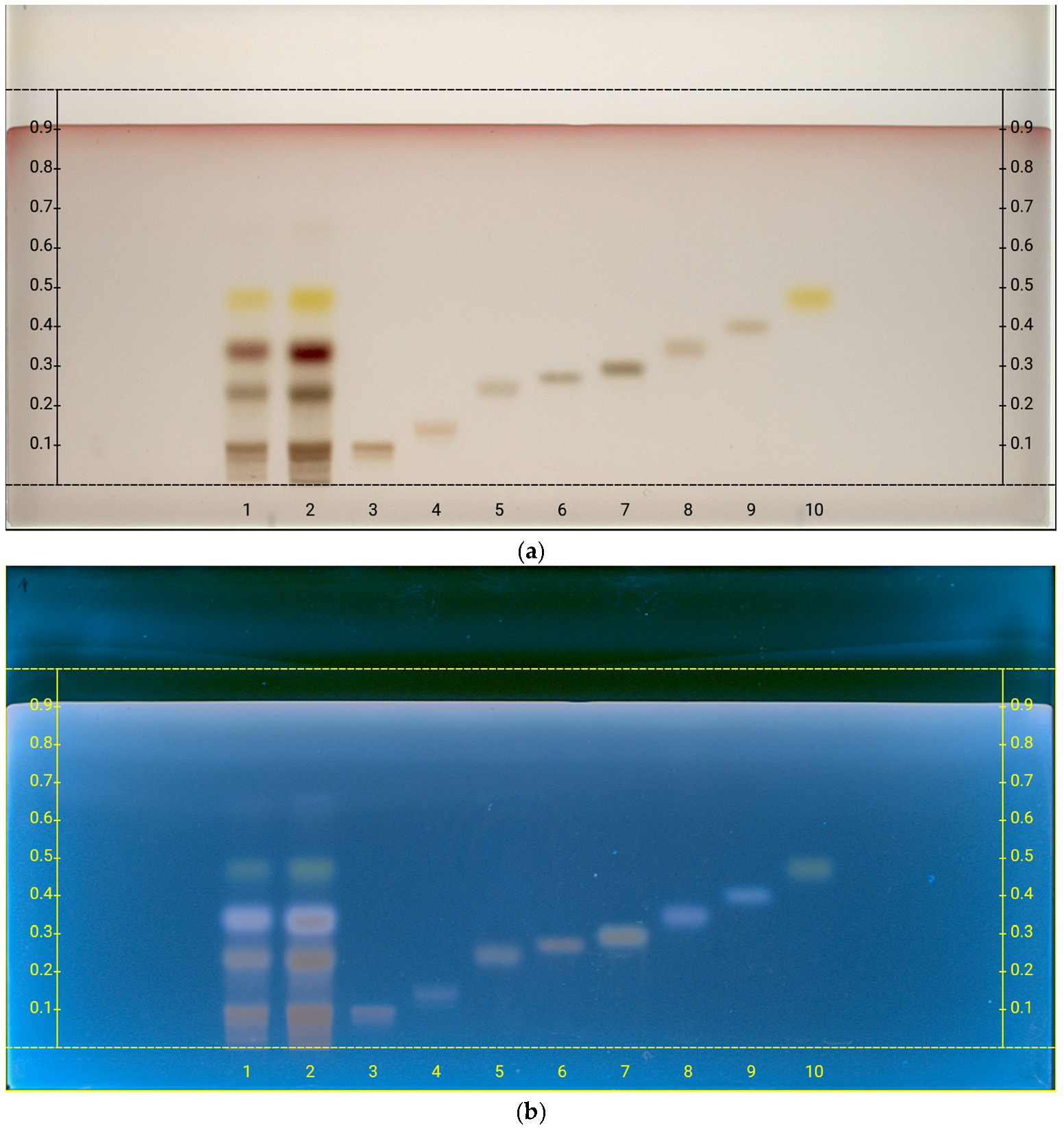
| C. ornifolia Sample | w/w % ± S.D. |
|---|---|
| RC1 | 6.07 ± 0.06 |
| RC2 | 6.58 ± 0.42 |
| RC3 | 5.84 ± 0.08 |
| RC4 | 8.86 ± 0.12 |
| RC5 | 4.08 ± 0.06 |
| RC6 | 3.50 ± 0.02 |
| RC7 | 9.05 ± 0.19 |
| RC8 | 4.23 ± 0.16 |
| Compound | 1H δ (ppm) | J (Hz) | Assignment |
|---|---|---|---|
| Arabinopiranose | 5.22 | 3.6 | α-CH |
| 4.50 | 7.8 | β-CH | |
| Arabinofuranose | 5.23 | 3.2 | α-CH |
| 4.51 | 9.3 | β-CH | |
| Galactose | 5.25 | 3.5 | α-CH |
| 4.57 | 7.9 | β-CH | |
| Galacturonic acid | 5.28 | 4.5 | α-CH |
| 4.58 | 7.9 | β-CH | |
| Rhamnose | 5.10 | 1.7 | α-CH |
| 4.85 | NaN | β-CH |
| Monosaccharide | Ratio to Rhamnose |
|---|---|
| Total arabinose | 2.3 |
| Galactose | 0.8 |
| Galacturonic acid | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortolami, M.; La Montagna, D.; Toniolo, C.; Sciubba, F.; Patriarca, A.; Moretti, T.; Serafini, I.; Mura, F.; Cocco, E.; Maděra, P.; et al. Preliminary Insights into the Non-Volatile Constituents of Commiphora ornifolia (Balf.f.) J.B.Gillett Oleogum Resin from Socotra Island. Plants 2025, 14, 2999. https://doi.org/10.3390/plants14192999
Bortolami M, La Montagna D, Toniolo C, Sciubba F, Patriarca A, Moretti T, Serafini I, Mura F, Cocco E, Maděra P, et al. Preliminary Insights into the Non-Volatile Constituents of Commiphora ornifolia (Balf.f.) J.B.Gillett Oleogum Resin from Socotra Island. Plants. 2025; 14(19):2999. https://doi.org/10.3390/plants14192999
Chicago/Turabian StyleBortolami, Martina, Dario La Montagna, Chiara Toniolo, Fabio Sciubba, Adriano Patriarca, Tiziana Moretti, Ilaria Serafini, Francesco Mura, Emma Cocco, Petr Maděra, and et al. 2025. "Preliminary Insights into the Non-Volatile Constituents of Commiphora ornifolia (Balf.f.) J.B.Gillett Oleogum Resin from Socotra Island" Plants 14, no. 19: 2999. https://doi.org/10.3390/plants14192999
APA StyleBortolami, M., La Montagna, D., Toniolo, C., Sciubba, F., Patriarca, A., Moretti, T., Serafini, I., Mura, F., Cocco, E., Maděra, P., Van Damme, K., Garzoli, S., Santi, L., Attorre, F., & De Vita, D. (2025). Preliminary Insights into the Non-Volatile Constituents of Commiphora ornifolia (Balf.f.) J.B.Gillett Oleogum Resin from Socotra Island. Plants, 14(19), 2999. https://doi.org/10.3390/plants14192999














