Mild Drought Promotes Biomass Accumulation and Increases Diosgenin Content in Rhizomes of Dioscorea nipponica
Abstract
1. Introduction
2. Results
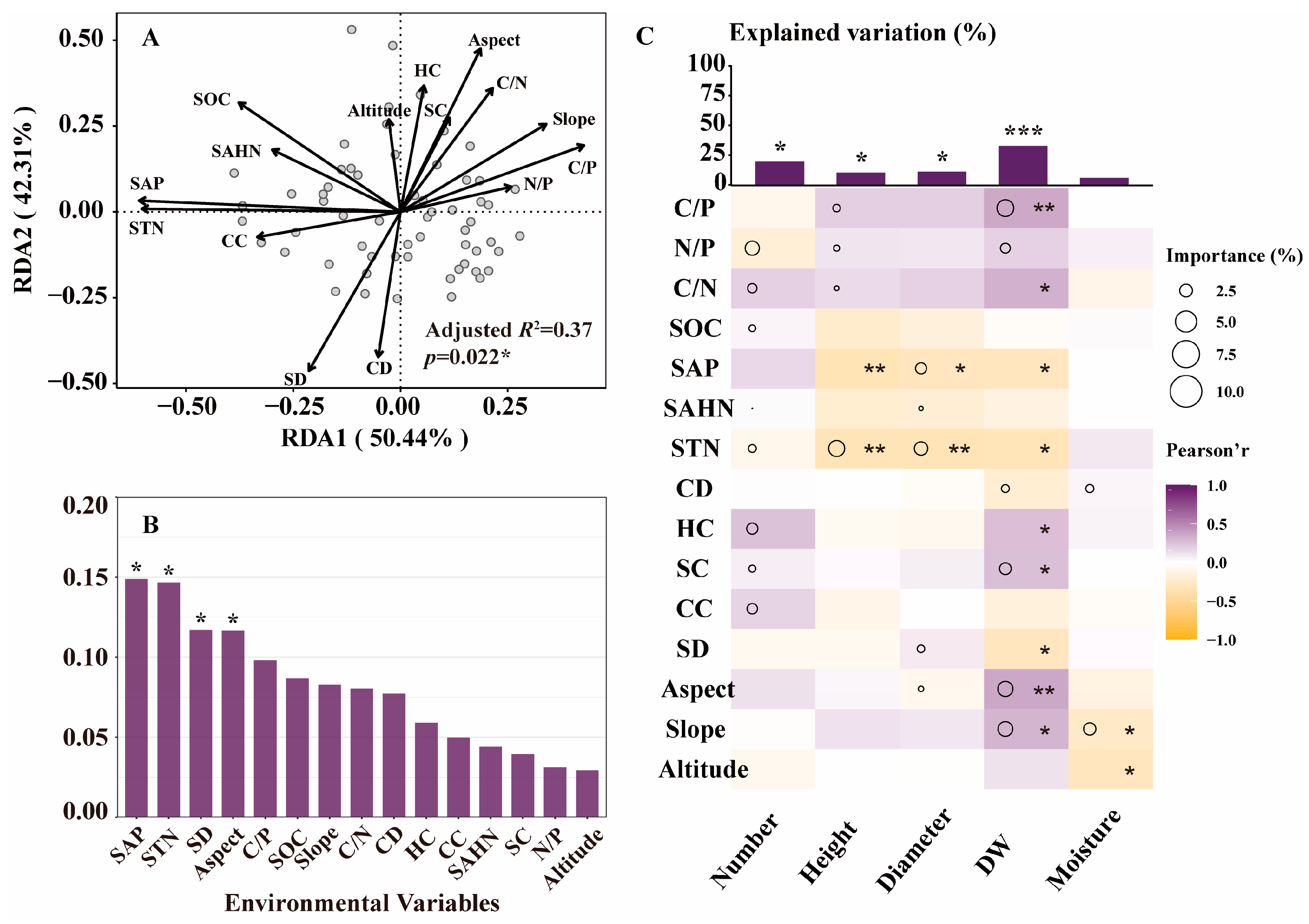
2.1. Effects of Environment on Growth and Diosgenin Content of D. nipponica
2.1.1. Effects of Environmental Factors on Growth
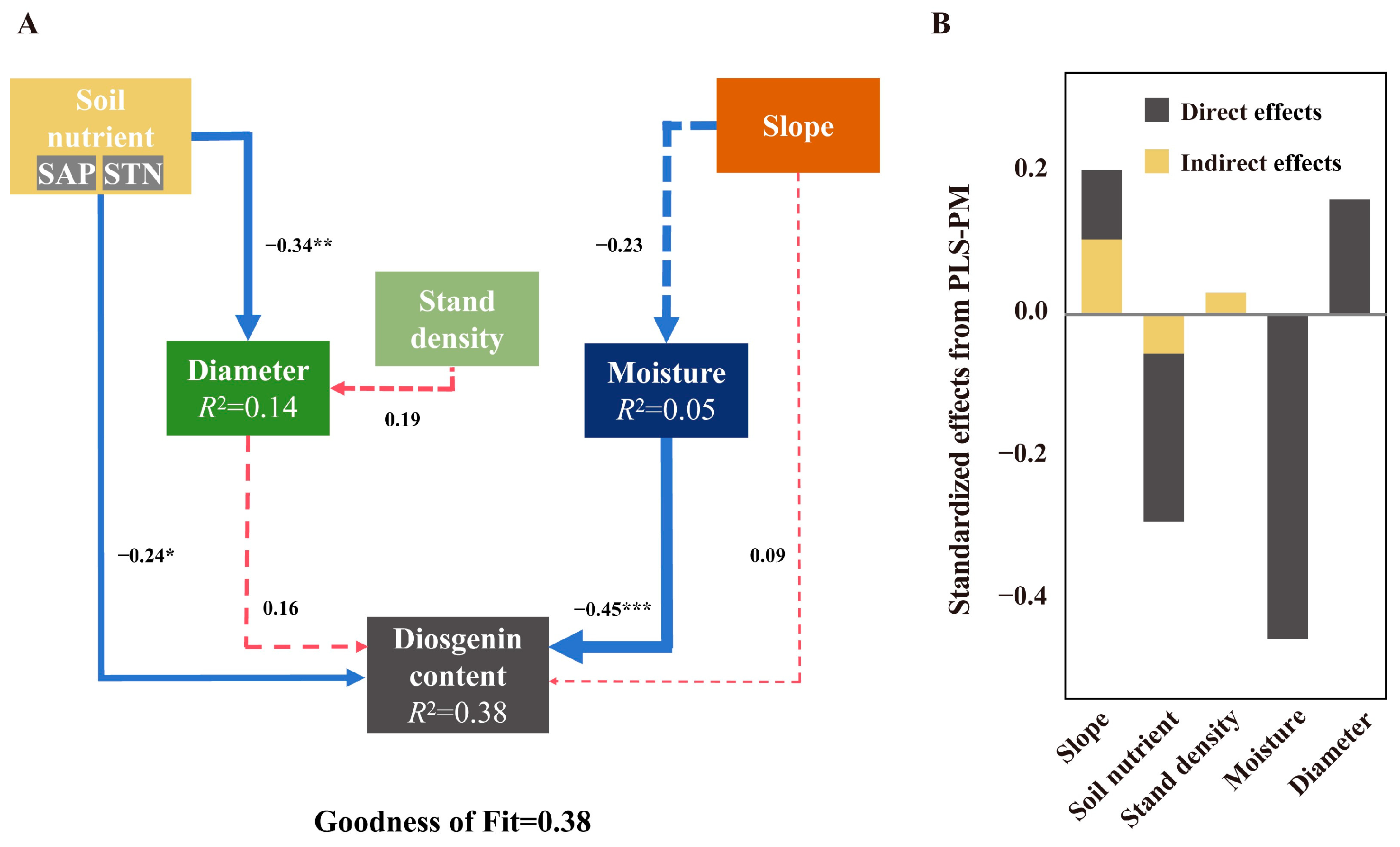
2.1.2. Effects of Environmental Factors on Diosgenin
2.2. Analysis on the Difference of Indexes of D. nipponica Under Different Drought Stresses
2.2.1. Effects of Drought Stress on Growth
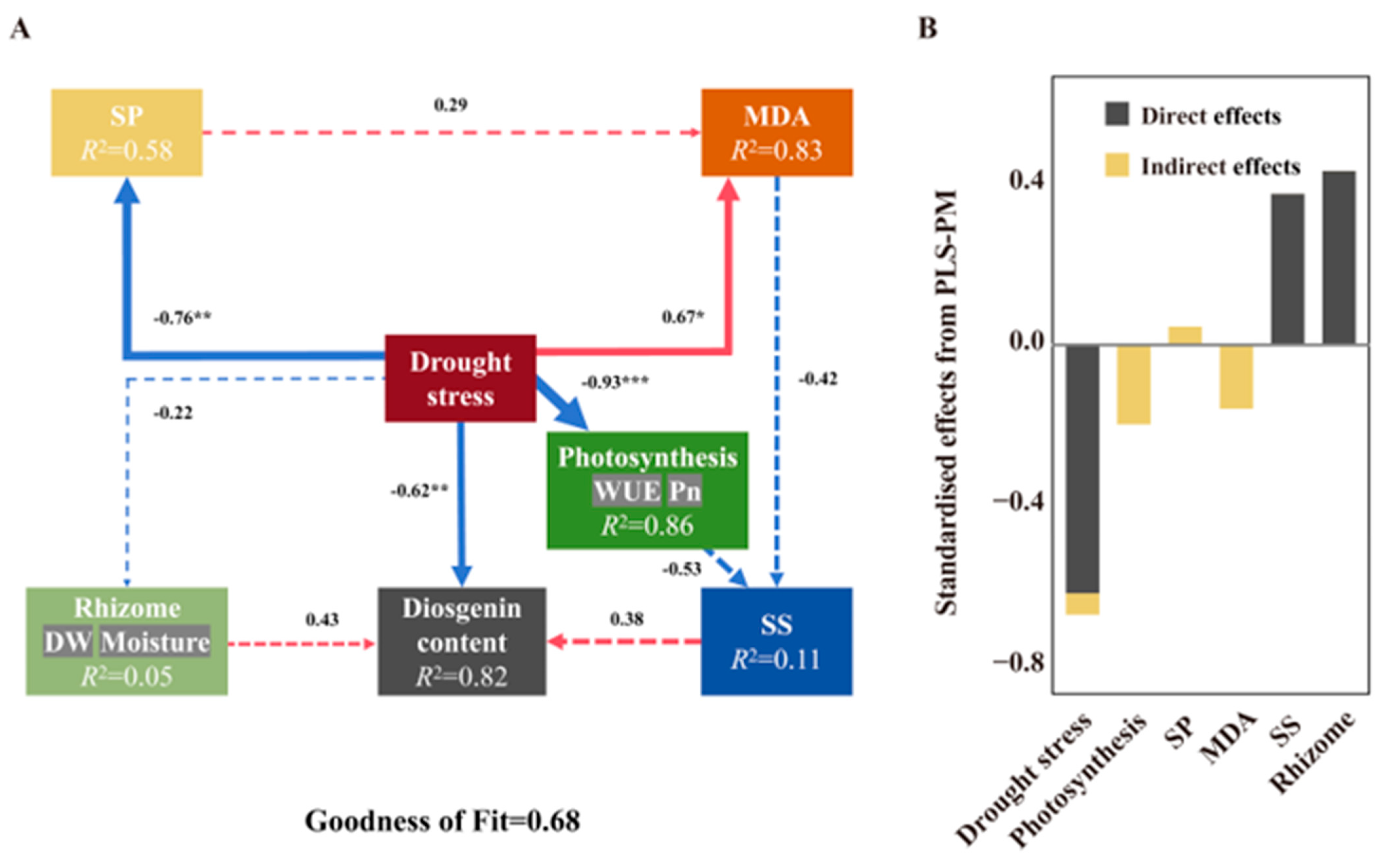
2.2.2. Effects of Drought Stress on Physiological Parameters
2.2.3. Effects of Drought Stress on Rhizomes
3. Discussion
3.1. The Key Factors Affecting the Growth and Diosgenin Content of Wild D. nipponica
3.2. Effect of Drought Stress on D. nipponica
3.2.1. Effects of Drought Stress on Growth
3.2.2. Effects of Drought Stress on Diosgenin
4. Materials and Methods
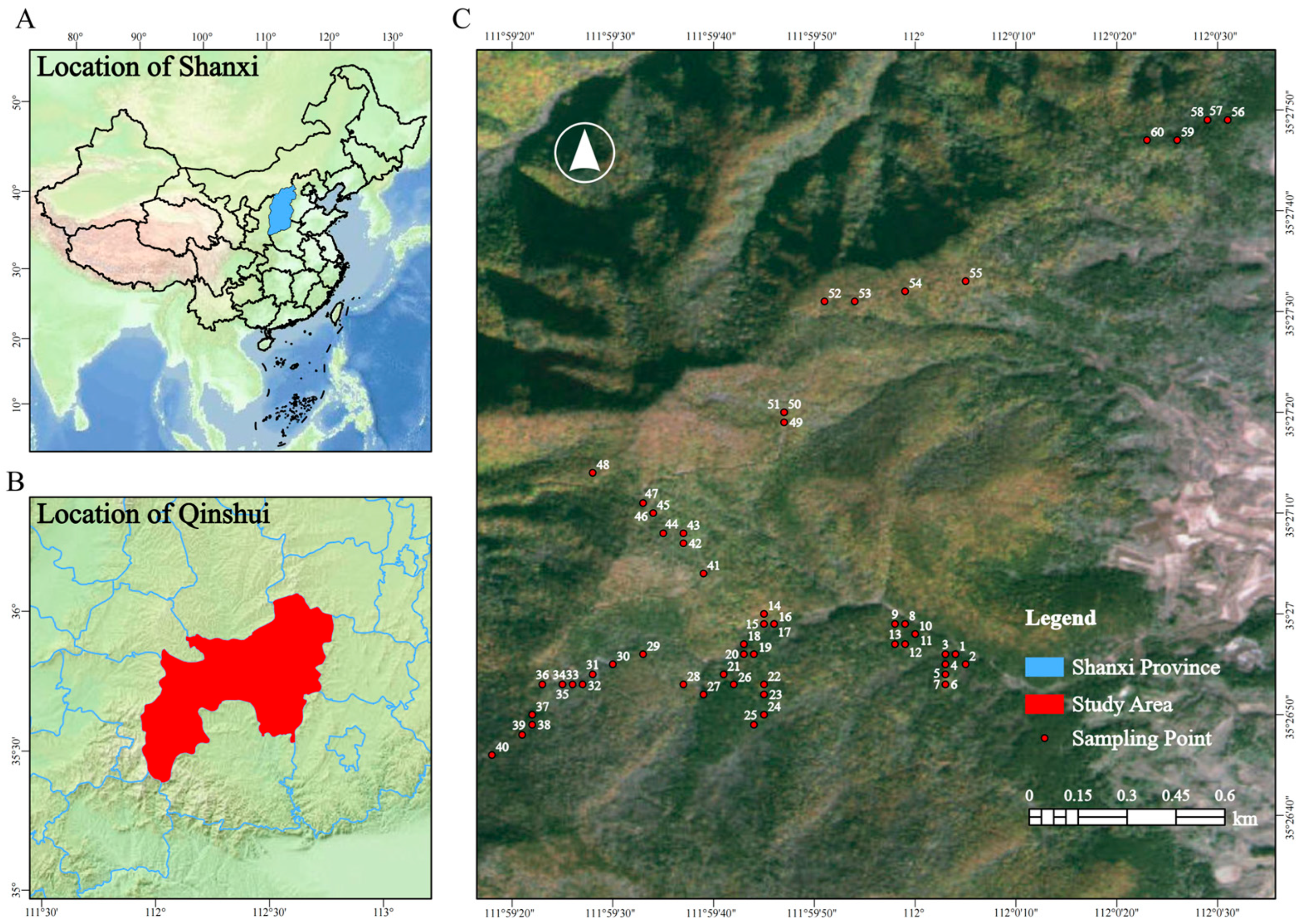
4.1. Study Areas and Sample Collection
4.2. Drought Stress Experimental Design
4.3. Measurements
4.3.1. Environmental Factors
4.3.2. Physiological Factors
- 1.
- Measurement of gas exchange and pigment content
- 2.
- Measurement of antioxidant enzyme activities
- 3.
- Measurement of malondialdehyde contents
- 4.
- Measurement of soluble protein, soluble sugar and proline contents
4.3.3. Diosgenin
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Position | Slope position |
| STN | Soil total nitrogen |
| SAHN | Soil ammonium hydroxide nitrogen |
| SAP | Soil available phosphorus |
| SOC | Soil organic carbon |
| C/N | Carbon to nitrogen ratio |
| N/P | Nitrogen to phosphorus ratio |
| C/P | Carbon to phosphorus ratio |
| Type | Community type |
| SD | Stand density |
| CC | Canopy closure |
| SC | Shrub cover |
| HC | Herbaceous cover |
| Diversity | Community diversity |
| Count | Community stem count |
| Height | Plant height |
| Diameter | Ground diameter |
| DW | Dry weight of rhizome |
| Moisture | Moisture content of rhizome |
| Dio | Diosgenin content of rhizome |
| SR | Seedling survival rate |
| PR | Rhizome proliferation rate |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| MDA | Malonaldehyde |
| Pro | Proline |
| SS | Soluble sugar |
| SP | Soluble protein |
| Chl | Chlorophyll |
| Pn | Net photosynthetic rate |
| Tr | Transpiration rate |
| Gs | Stomatal conductivity |
| Ci | CO2 concentration between cells |
| WUE | Water use efficiency |
References
- Lei, L.; Zhang, W.; Gao, Z.; Liu, L.; Yu, X. Preparation of diosgenin from Dioscorea zingiberensis C.H. Wright by stepwise biocatalysis-foam separation-preparative high-performance liquid chromatography (P-HPLC). Eur. Food Res. Technol. 2018, 244, 1447–1452. [Google Scholar] [CrossRef]
- Liu, W.; Huang, W.; Sun, W.; Zhu, Y.; Ni, J. Production of diosgenin from yellow ginger (Dioscorea zingiberensis C. H. Wright) saponins by commercial cellulase. World J. Microbiol. Biotechnol. 2009, 26, 1171–1180. [Google Scholar] [CrossRef]
- Al-Habori, M.; Raman, A.; Lawrence, M.J.; Skett, P. In vitro effect of fenugreek extracts on intestinal sodium-dependent glucose uptake and hepatic glycogen phosphorylase A. Int. J. Exp. Diabetes Res. 2001, 2, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Moalic, S.; Liagre, B.; Corbière, C.; Bianchi, A.; Dauça, M.; Bordji, K.; Beneytout, J.L. A plant steroid, diosgenin, induces apoptosis, cell cycle arrest and COX activity in osteosarcoma cells. FEBS Lett. 2001, 506, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Nibbering, C.P.; Groen, A.K.; Ottenhoff, R.; Brouwers, J.F.H.M.; vanBerge-Henegouwen, G.P.; Erpecum, K.J.V. Regulation of biliary cholesterol secretion is independent of hepatocyte canalicular membrane lipid composition: A study in the diosgenin-fed rat model. J. Hepatol. 2001, 35, 164–169. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Luo, L.; Hu, H.; Meng, X.; Li, X.; Chen, S. Predicting the potential global distribution of diosgenin-contained Dioscorea species. Chin. Med. 2018, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Shi, X.; Ma, Z.; Li, Y.; Wen, J. Physiological responses to drought in three provenances of Discorea nipponica Makino. J. Appl. Bot. Food Qual. 2018, 91, 261–270. [Google Scholar] [CrossRef]
- Ou-yang, S.-H.; Jiang, T.; Zhu, L.; Yi, T. Dioscorea nipponica Makino: A systematic review on its ethnobotany, phytochemical and pharmacological profiles. Chem. Cent. J. 2018, 12, 57. [Google Scholar] [CrossRef]
- Chen, F.-Q.; Fu, Y.; Wang, D.-L.; Gao, X.; Wang, L. The Effect of Plant Growth Regulators and Sucrose on the Micropropagation and Microtuberization of Dioscorea nipponica Makino. J. Plant Growth Regul. 2007, 26, 38–45. [Google Scholar] [CrossRef]
- Xue, Y.; Cao, B.; Liang, H.; Yang, J.; Gao, P.; Mao, M.; Li, G.; Bai, C. Environmental shifts have important impacts on the functional traits and bioactive products of medicinal crop Cornus officinalis. Ind. Crops Prod. 2021, 162, 113304. [Google Scholar] [CrossRef]
- Hou, L.; Li, S.; Tong, Z.; Yuan, X.; Xu, J.; Li, J. Geographical variations in fatty acid and steroid saponin biosynthesis in Dioscorea zingiberensis rhizomes. Ind. Crops Prod. 2021, 170, 113779. [Google Scholar] [CrossRef]
- Wang, W.; Hou, L.; Li, S.; Li, J. The Functional Characterization of DzCYP72A12-4 Related to Diosgenin Biosynthesis and Drought Adaptability in Dioscorea zingiberensis. Int. J. Mol. Sci. 2023, 24, 8430. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, C.; Li, Z.; Guo, J.; Li, S.; Chen, X.; Wang, C.; Dai, X.; Yang, H.; Song, W.; et al. The genome of Dioscorea zingiberensis sheds light on the biosynthesis, origin and evolution of the medicinally important diosgenin saponins. Hortic. Res. 2022, 9, uhac165. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Radunz, A.; He, P.; Schmid, G.H. Influence of different light intensities on the content of diosgenin, lipids, carotenoids and fatty acids in leaves of Dioscorea zingiberensis. Z. Naturforschung C 2002, 57, 135–143. [Google Scholar] [CrossRef]
- Arabasadi, M.; Ebrahimi, A.; Amerian, M.-R.; Ebrahimibasabi, E.; Azadvari, E. The amelioration of salt stress-induced damage in fenugreek through the application of cold plasma and melatonin. Plant Physiol. Biochem. 2024, 207, 108382. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Gharanjik, S.; Azadvari, E.; Rashidi-Monfared, S. Characterizing reference genes for high-fidelity gene expression analysis under different abiotic stresses and elicitor treatments in fenugreek leaves. Plant Methods 2024, 20, 40. [Google Scholar] [CrossRef]
- Sheikhi, S.; Ebrahimi, A.; Heidari, P.; Amerian, M.R.; Rashidi-Monfared, S.; Alipour, H. Exogenous 24-epibrassinolide ameliorates tolerance to high-temperature by adjusting the biosynthesis of pigments, enzymatic, non-enzymatic antioxidants, and diosgenin content in fenugreek. Sci. Rep. 2023, 13, 6661. [Google Scholar] [CrossRef]
- Liang, Y.; Wei, G.; Ning, K.; Li, M.; Zhang, G.; Luo, L.; Zhao, G.; Wei, J.; Liu, Y.; Dong, L.; et al. Increase in carbohydrate content and variation in microbiome are related to the drought tolerance of Codonopsis pilosula. Plant Physiol. Biochem. 2021, 165, 19–35. [Google Scholar] [CrossRef]
- Qian, H.; Xu, Z.; Cong, K.; Zhu, X.; Zhang, L.; Wang, J.; Wei, J.; Ji, P. Transcriptomic responses to drought stress in Polygonatum kingianum tuber. BMC Plant Biol. 2021, 21, 537. [Google Scholar] [CrossRef]
- Su, Q.; Yao, B.; Yang, W.; Wu, C.; Sang, S.; Gao, X.; Liu, C. Transcriptional regulation of polyphyllin biosynthesis of Paris polyphylla in response to soil moisture stress. Ind. Crops Prod. 2025, 230, 121108. [Google Scholar] [CrossRef]
- Lee, J.; Mudge, K.W. Water deficit affects plant and soil water status, plant growth, and ginsenoside contents in American ginseng. Hortic. Environ. Biotechnol. 2014, 54, 475–483. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, H.; Zhang, Z.; Sun, H.; Li, M.; Shao, C.; Liang, H.; Wu, H.; Zhang, Y. Physiological and transcriptomic analyses of roots from Panax ginseng C. A. Meyer under drought stress. Ind. Crops Prod. 2023, 191, 115858. [Google Scholar] [CrossRef]
- Tang, J.; Yue, X.; Yang, Q.; Liang, J.; Wang, H. Coupling effects of irrigation level and terrain slope on disease, yield and quality of Panax notoginseng under micro-sprinkler irrigation. Agric. Water Manag. 2024, 299, 108871. [Google Scholar] [CrossRef]
- Zang, Z.; Liang, J.; Yang, Q.; Zhou, N.; Li, N.; Liu, X.; Liu, Y.; Tan, S.; Chen, S.; Tang, Z. An adaptive abiotic stresses strategy to improve water use efficiency, quality, and economic benefits of Panax notoginseng: Deficit irrigation combined with sodium chloride. Agric. Water Manag. 2022, 274, 107923. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Zhen, J. Photosynthetic, antioxidant activities, and osmoregulatory responses in winter wheat differ during the stress and recovery periods under heat, drought, and combined stress. Plant Sci. 2023, 327, 111557. [Google Scholar] [CrossRef]
- Fang, J.; Ma, J.; Wen, T.; Niu, G.; Wei, S.; Su, S.; Yi, L.; Cheng, Y.; Yuan, J.; Zhao, X.; et al. Cry for help from rhizosphere microbiomes and self-rescue strategies cooperatively alleviate drought stress in spring wheat. Soil Biol. Biochem. 2025, 206, 109813. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Zhen, J.; Song, T. Individual and combined effects of heat and drought and subsequent recovery on winter wheat (Triticum aestivum L.) photosynthesis, nitrogen metabolism, cell osmoregulation, and yield formation. Plant Physiol. Biochem. 2023, 196, 222–235. [Google Scholar] [CrossRef]
- Dang, S.; Gao, R.; Zhang, Y.; Feng, Y. In vitro regeneration and its histological characteristics of Dioscorea nipponica Makino. Sci. Rep. 2022, 12, 18436. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bai, Z.; Luo, Y.; Zhang, Y.; Wang, Y.; Liu, H.; Luo, M.; Huang, X.; Chen, A.; Ma, L.; et al. Multiomics dissection of Brassica napus L. lateral roots and endophytes interactions under phosphorus starvation. Nat. Commun. 2024, 15, 9732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Wang, T.; Ma, H.; Zhou, G. Integrated microbiological and metabolomic analyses reveal the mechanism by which P addition affects the quality of Anisodus tanguticus. Ind. Crops Prod. 2025, 228, 120956. [Google Scholar] [CrossRef]
- Morelli, L.; Paulišić, S.; Qin, W.; Iglesias-Sanchez, A.; Roig-Villanova, I.; Florez-Sarasa, I.; Rodriguez-Concepcion, M.; Martinez-Garcia, J.F. Light signals generated by vegetation shade facilitate acclimation to low light in shade-avoider plants. Plant Physiol. 2021, 186, 2137–2151. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Fu, Z.; Wang, K.; Chen, H. Characterizing rapid infiltration processes on complex hillslopes: Insights from soil moisture response to rainfall events. J. Hydrol. 2024, 644, 132110. [Google Scholar] [CrossRef]
- Liu, H.; Gu, H.; Ye, C.; Guo, C.; Zhu, Y.; Huang, H.; Liu, Y.; He, X.; Yang, M.; Zhu, S. Planting Density Affects Panax notoginseng Growth and Ginsenoside Accumulation by Balancing Primary and Secondary Metabolism. Front. Plant Sci. 2021, 12, 628294. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska, D.; Grudkowska, M.; Zagdańska, B. Drought-Responsive Antioxidant Enzymes in Potato (Solanum tuberosum L.). Potato Res. 2010, 53, 373–382. [Google Scholar] [CrossRef]
- Ayub, M.; Ashraf, M.Y.; Kausar, A.; Saleem, S.; Anwar, S.; Altay, V.; Ozturk, M. Growth and physio-biochemical responses of maize (Zea mays L.) to drought and heat stresses. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2020, 155, 535–542. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Holtum, J.A.M. Facultative crassulacean acid metabolism (CAM) plants: Powerful tools for unravelling the functional elements of CAM photosynthesis. J. Exp. Bot. 2014, 65, 3425–3441. [Google Scholar] [CrossRef]
- Winter, K.; Holtum, J.A.M.; Smith, J.A.C. Crassulacean acid metabolism: A continuous or discrete trait? New Phytol. 2015, 208, 73–78. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, J.; Zhou, J.; Qiao, J.; Li, Y.; Quan, R.; Huang, R. Abscisic acid promotes auxin biosynthesis to inhibit primary root elongation in rice. Plant Physiol. 2023, 191, 1953–1967. [Google Scholar] [CrossRef]
- Giolai, M.; Laine, A.-L. A trade-off between investment in molecular defense repertoires and growth in plants. Science 2024, 386, 677–680. [Google Scholar] [CrossRef]
- Luo, W.; Muraina, T.O.; Griffin-Nolan, R.J.; Te, N.; Qian, J.; Yu, Q.; Zuo, X.; Wang, Z.; Knapp, A.K.; Smith, M.D.; et al. High below-ground bud abundance increases ecosystem recovery from drought across arid and semiarid grasslands. J. Ecol. 2023, 111, 2038–2048. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta--analyses of interspecific variation and environmental control. New Phytol. 2011, 193, 30–50. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Wang, Y.; Zhou, J.; Hua, Y.; Zhou, X.; Zheng, H.; Ma, X.; Ge, Y.; Qiu, B.; et al. Multi-omics to hormonal signaling networks: Decoding growth-defense trade-offs underlying growth-retardation in two-year-old cultivated ginseng (Panax ginseng C.A. Mey.). Ind. Crops Prod. 2025, 236, 121873. [Google Scholar] [CrossRef]
- Ba, C.; Zhai, S.; Qian, J.; Liu, B.; Zhu, J.; Liu, Z.; Roscher, C. Trade-offs in growth and reproduction of rhizomatous clonal plant Phragmites communis in response to aeolian processes. J. Plant Ecol. 2024, 17, 1. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Li, Q.; Zhang, J.; Hou, R.; Wang, Z.; Zhu, Q.; Zhou, Y.; Chen, Y.; Huang, J. Integrated transcriptome and metabolome analysis provide insights into the mechanism of saponin biosynthesis and its role in alleviating cadmium-induced oxidative damage in Ophiopogon japonicum. Plant Physiol. Biochem. 2024, 210, 108634. [Google Scholar] [CrossRef] [PubMed]
- Puente-Garza, C.A.; Meza-Miranda, C.; Ochoa-Martínez, D.; García-Lara, S. Effect of in vitro drought stress on phenolic acids, flavonols, saponins, and antioxidant activity in Agave salmiana. Plant Physiol. Biochem. 2017, 115, 400–407. [Google Scholar] [CrossRef]
- Zhao, L.; Tao, X.; Qi, Y.; Xu, L.; Yin, L.; Peng, J. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biol. 2018, 16, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Aditya, M.; Dey, N.; Banik, N.; Bhattacharjee, S. Antioxidant Signaling and Redox Regulation in Drought- and Salinity-Stressed Plants. In Drought Stress Tolerance in Plants; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.S.P., Eds.; Springer: Cham, Switzerland, 2016; pp. 489–512. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Chen, J.; Chen, L.; Wan, X. Chloride and amino acids are associated with K+-alleviated drought stress in tea (Camellia sinesis). Funct. Plant Biol. 2020, 47, 398–408. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Shi, Y.; Li, R.; Fan, F.; Huang, Y.; Li, W.; Chen, N.; Huang, L.; Dai, Z.; et al. Elucidation of the complete biosynthetic pathway of the main triterpene glycosylation products of Panax notoginseng using a synthetic biology platform. Metab. Eng. 2020, 61, 131–140. [Google Scholar] [CrossRef]
- Bick, J.A.; Lange, B.M. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: Unidirectional transport of intermediates across the chloroplast envelope membrane. Arch. Biochem. Biophys. 2003, 415, 146–154. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Fan, Y.; Dong, J.; Gao, P.; Jiang, W.; Yang, T.; Che, D. RNA sequencing analysis reveals PgbHLH28 as the key regulator in response to methyl jasmonate-induced saponin accumulation in Platycodon grandiflorus. Hortic. Res. 2024, 11, uhae058. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Huang, Y.; Yang, X.; Wang, R.; Zhang, M. Increasing the Proportion of Broadleaf Species in Mixed Conifer-Broadleaf Forests Improves Understory Plant Composition and Promotes Soil Carbon Fixation. Plants 2025, 14, 1392. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Zhang, M.; Bai, T.; Zhang, B.; Yang, L.; Dang, S.; Yang, X.; Gao, R. Dendroclimatic response of Pinus tabuliformis Carr. along an altitudinal gradient in the warm temperate region of China. Front. Plant Sci. 2023, 14, 1147229. [Google Scholar] [CrossRef]
- LY/T 1228-2015; Nitrogen Determination Methods of Forest Soils. Forestry Industry Standards of the People’s Republic of China: Beijing, China, 2015.
- LY/T 1237-1999; Determination of Organic Matter in Forest Soil and Calculation Carbon-Nitrogen Ratio. Forestry Industry Standards of the People’s Republic of China: Beijing, China, 1999.
- LY/T 1232-2015; Phosphorus Determination Methods of Forest Soils. Forestry Industry Standards of the People’s Republic of China: Beijing, China, 2015.
- Zhang, M.; Liu, Z.; Yang, Z.; Shen, H.; Wang, J.; Wu, X. Altitudinal Variation in Species Diversity, Distribution, and Regeneration Status of a Secondary Picea Forest in Guandi Mountain, Northern China. Forests 2024, 15, 771. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Orczyk, M.; Gutberlet, T.; Geue, T. Complexation of phospholipids and cholesterol by triterpenic saponins in bulk and in monolayers. Biochim. Biophys. Acta (BBA)—Biomembr. 2016, 1858, 363–373. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Sun, Y.; Chen, Y. Does stand density affect understory vegetation and soil properties of differently aged Robinia pseudoacacia plantations? For. Ecol. Manag. 2023, 548, 121444. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. [136] Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified Thiobarbituric Acid Assay for Measuring Lipid Oxidation in Sugar-Rich Plant Tissue Extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Lai, J.; Zhu, W.; Cui, D.; Mao, L.; Liao, J. Extension of theglmm.hppackage to zero-inflated generalized linear mixed models and multiple regression. J. Plant Ecol. 2023, 16, rtad038. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L.; Zhang, W.-H. glmm.hp: An R package for computing individual effect of predictors in generalized linear mixed models. J. Plant Ecol. 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Hu, X.; Liu, Y.; Daryanto, S.; Cherubini, F. Trading-off ecosystem services for better ecological restoration: A case study in the Loess Plateau of China. J. Clean. Prod. 2020, 257, 120469. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Kim, N.; Lee, T.; Quagliato, L. Extreme gradient boosting-inspired process optimization algorithm for manufacturing engineering applications. Mater. Des. 2023, 226, 111625. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, G.I. Model multifactor analysis of soil heavy metal pollution on plant germination in Southeast Chengdu, China: Based on redundancy analysis, factor detector, and XGBoost-SHAP. Sci. Total Environ. 2024, 954, 176605. [Google Scholar] [CrossRef] [PubMed]
- Vayssières, M.P.; Plant, R.E.; Allen-Diaz, B.H. Classification trees: An alternative non-parametric approach for predicting species distributions. J. Veg. Sci. 2000, 11, 679–694. [Google Scholar] [CrossRef]
- Lombard, M.A.; Bryan, M.S.; Jones, D.K.; Bulka, C.; Bradley, P.M.; Backer, L.C.; Focazio, M.J.; Silverman, D.T.; Toccalino, P.; Argos, M.; et al. Machine Learning Models of Arsenic in Private Wells Throughout the Conterminous United States As a Tool for Exposure Assessment in Human Health Studies. Environ. Sci. Technol. 2021, 55, 5012–5023. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ding, Y.; Cheng, J.C.P.; Tan, Y.; Gan, V.J.L.; Zhang, J. Analyzing the Leading Causes of Traffic Fatalities Using XGBoost and Grid-Based Analysis: A City Management Perspective. IEEE Access 2019, 7, 148059–148072. [Google Scholar] [CrossRef]
- Bhagat, S.K.; Tiyasha, T.; Awadh, S.M.; Tung, T.M.; Jawad, A.H.; Yaseen, Z.M. Prediction of sediment heavy metal at the Australian Bays using newly developed hybrid artificial intelligence models. Environ. Pollut. 2021, 268, 115663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, Y.; Du, Y.; Li, Z.; Wang, T. Causality analysis and prediction of soil saturated hydraulic conductivity by combining empirical modeling and machine learning techniques. J. Hydrol. 2024, 644, 132104. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Zhao, Q.; Liu, C.; Jim, C.Y.; Johnson, V.C.; Tan, M.L. Determining the main contributing factors to nutrient concentration in rivers in arid northwest China using partial least squares structural equation modeling. J. Environ. Manag. 2023, 343, 118249. [Google Scholar] [CrossRef] [PubMed]

| Drought Treatment | SR | PR | Height (cm) | Diameter (cm) |
|---|---|---|---|---|
| CK | 0.81 ± 0.03 b | 0.24 ± 0.02 b | 10.53 ± 1.07 a | 0.61 ± 0.11 ab |
| MID | 0.92 ± 0.07 a | 0.18 ± 0.03 c | 12.30 ± 1.14 a | 0.78 ± 0.10 a |
| MD | 0.65 ± 0.05 c | 0.28 ± 0.01 b | 11.27 ± 1.41 a | 0.52 ± 0.10 b |
| SD | 0.54 ± 0.02 d | 0.33 ± 0.02 a | 11.06 ± 1.03 a | 0.51 ± 0.12 b |
| p-value | p < 0.001 *** | p < 0.001 *** | 0.3688 | 0.05745 |
| Drought Treatment | CK | MID | MD | HD | p-Value |
|---|---|---|---|---|---|
| SOD (U·g−1FW) | 85.98 ± 3.17 d | 178.20 ± 13.97 a | 152.37 ± 2.34 b | 114.48 ± 1.59 c | p < 0.001 *** |
| CAT (U·g−1FW) | 1.33 ± 0.01 d | 1.44 ± 0.03 c | 1.67 ± 0.01 b | 1.98 ± 0.01 a | p < 0.001 *** |
| MDA (μmol·g−1FW) | 4.35 ± 0.23 c | 5.18 ± 0.80 bc | 7.61 ± 2.57 b | 12.27 ± 0.71 a | p < 0.001 *** |
| Pro (μg·mL−1FW) | 53.44 ± 4.89 d | 76.37 ± 0.21 c | 95.58 ± 2.56 b | 118.51 ± 3.08 a | p < 0.001 *** |
| SS (%DW) | 0.29 ± 0.02 c | 0.45 ± 0.01 a | 0.45 ± 0.04 a | 0.36 ± 0.01 b | p < 0.001 *** |
| SP (mg·g−1FW) | 7.90 ± 0.18 b | 8.49 ± 0.12 a | 7.60 ± 0.07 b | 6.50 ± 0.41 c | p < 0.001 *** |
| Chl (mg·g−1FW) | 3.67. ± 0.28 c | 4.46 ± 0.01 b | 4.77 ± 0.01 a | 4.65 ± 0.01 ab | p < 0.001 *** |
| Pn (μmol·m−2·s−1) | 4.28 ± 0.59 a | 3.18 ± 0.41 b | 1.76 ± 0.30 c | 0.70 ± 0.30 c | p < 0.001 *** |
| Tr (mmol·m−2·s−1) | 0.77 ± 0.1 a | 0.64 ± 0.03 a | 0.43 ± 0.05 b | 0.28 ± 0.11 b | p < 0.001 *** |
| Gs (μm) | 3.74 ± 0.55 a | 2.53 ± 0.43 b | 1.69 ± 0.37 bc | 1.03 ± 0.44 c | p < 0.001 *** |
| Ci (μmol·mol−1) | 99.33 ± 2.08 d | 154.67 ± 5.03 c | 218.67 ± 12.10 b | 295.00 ± 13.75 a | p < 0.001 *** |
| WUE (μmol·mmol−1) | 3.74 ± 0.46 ab | 4.49 ± 0.75 a | 3.07 ± 0.54 bc | 2.48 ± 0.28 c | p < 0.01 ** |
| Drought Treatment | DW (g) | Moisture (%) | Dio (mg·g−1) |
|---|---|---|---|
| CK | 0.58 ± 0.16 c | 8.02 ± 0.35 b | 3.2 ± 0.3 b |
| MID | 0.79 ± 0.15 a | 8.50 ± 0.34 a | 6.5 ± 0.1 a |
| MD | 0.66 ± 0.14 b | 7.91 ± 0.56 c | 2.3 ± 0.3 c |
| SD | 0.62 ± 0.14 bc | 7.68 ± 0.58 d | 1.1 ± 0.2 d |
| p-value | p < 0.001 *** | p < 0.001 *** | p < 0.001 *** |
| Drought Treatment | CK | MID | MD | SD |
|---|---|---|---|---|
| The interval of watering/d | 3 | 5 | 8 | 10 |
| The relative soil water content/% | 70–80 | 50–60 | 35–50 | 20–35 |
| Drought degree | No drought | Mild drought | Moderate drought | Severe drought |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Xue, Z.; Li, Z.; Cao, H.; Wang, J.; He, R.; Gao, H.; Gao, R. Mild Drought Promotes Biomass Accumulation and Increases Diosgenin Content in Rhizomes of Dioscorea nipponica. Plants 2025, 14, 2998. https://doi.org/10.3390/plants14192998
Wang R, Xue Z, Li Z, Cao H, Wang J, He R, Gao H, Gao R. Mild Drought Promotes Biomass Accumulation and Increases Diosgenin Content in Rhizomes of Dioscorea nipponica. Plants. 2025; 14(19):2998. https://doi.org/10.3390/plants14192998
Chicago/Turabian StyleWang, Ran, Zhigang Xue, Zixing Li, Huan Cao, Jiayu Wang, Runze He, Haoyuan Gao, and Runmei Gao. 2025. "Mild Drought Promotes Biomass Accumulation and Increases Diosgenin Content in Rhizomes of Dioscorea nipponica" Plants 14, no. 19: 2998. https://doi.org/10.3390/plants14192998
APA StyleWang, R., Xue, Z., Li, Z., Cao, H., Wang, J., He, R., Gao, H., & Gao, R. (2025). Mild Drought Promotes Biomass Accumulation and Increases Diosgenin Content in Rhizomes of Dioscorea nipponica. Plants, 14(19), 2998. https://doi.org/10.3390/plants14192998






