Effects of Electromagnetically Treated Water (EMTW) on the Properties of Water and Photosynthetic Performance of Spinacia oleracea L.
Abstract
1. Introduction
2. Results
2.1. Nuclear Magnetic Resonance (NMR)
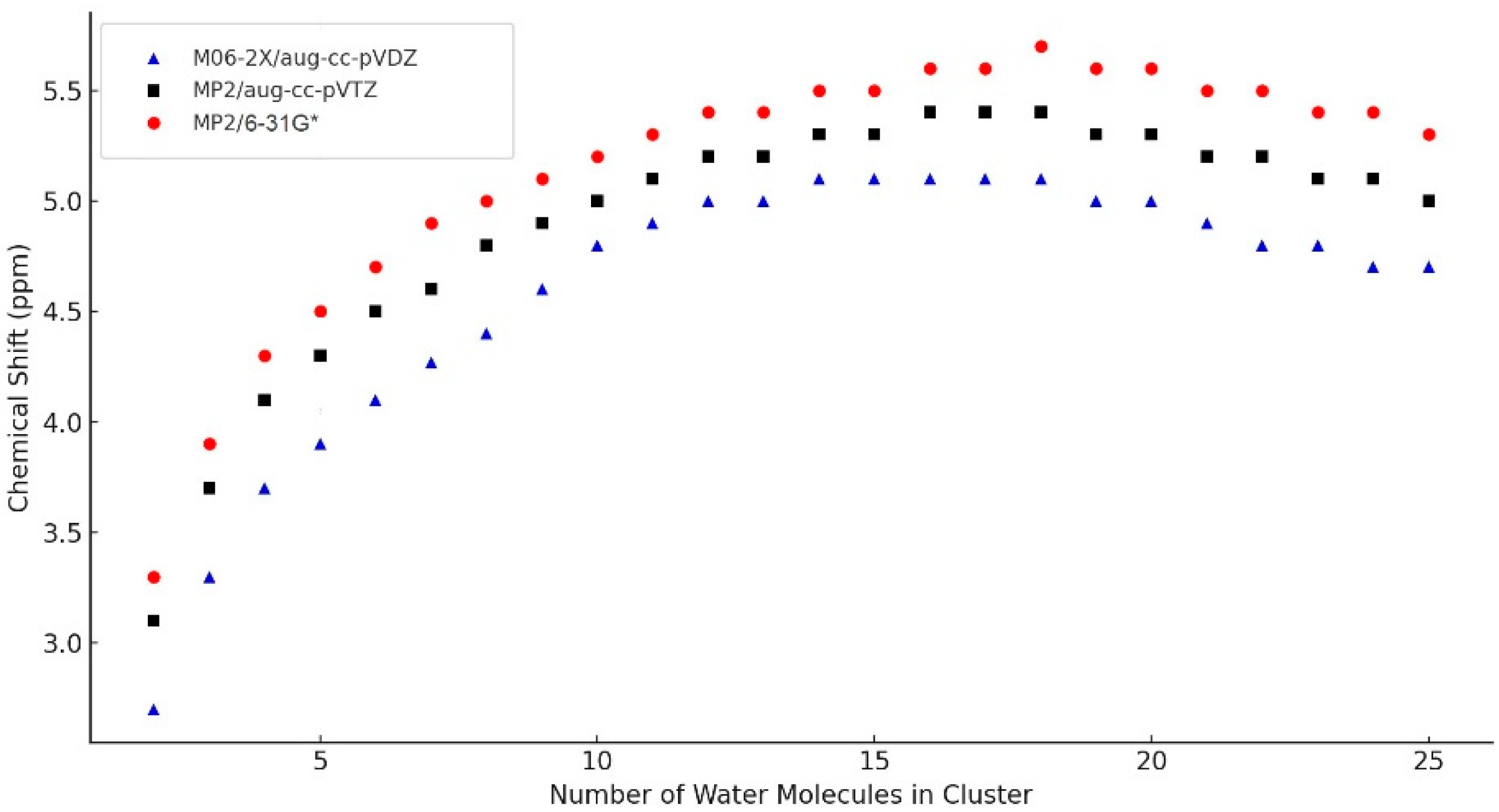
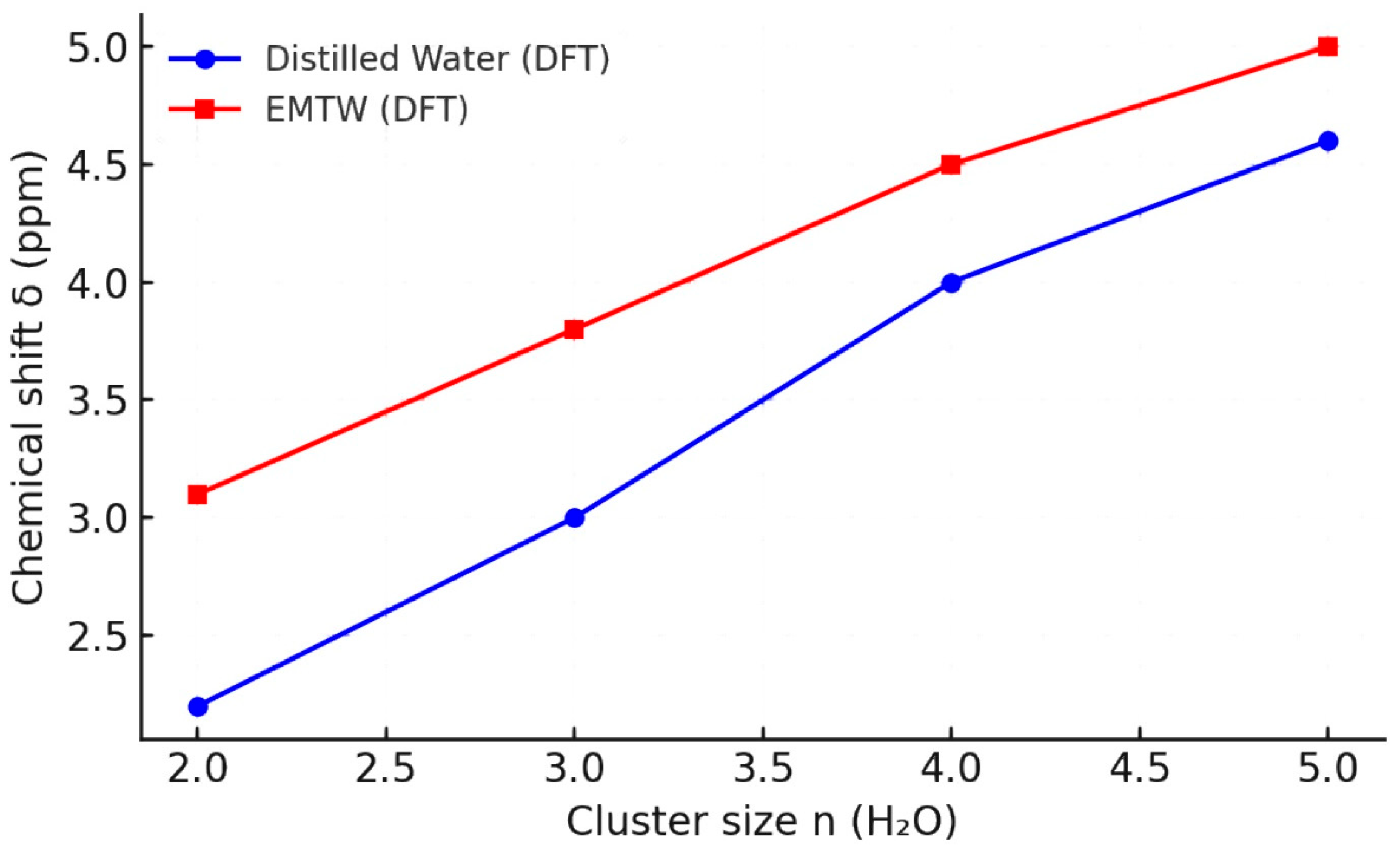
2.2. DFT-Computed Averaged NMR Chemical Shifts on the Size of the Modeled Clusters and Hydrogen Bonds
2.3. Physiological and Biochemical Indicators of Spinacia oleracea L.
3. Discussion
4. Materials and Methods
4.1. Standardized Irrigation Water
4.2. Method for Electromagnetically Treated Water (EMTW)
4.3. Nuclear Magnetic Resonance (NMR)
4.4. Theoretical Calculations for Water Clusters and Hydrogen Bonds
4.5. Plant Material
4.6. Three-Dimensional Analysis of Net Photosynthesis (Anet), Stomatal Conductance (gs), Transpiration (E), and Intercellular CO2 Concentration (Ci)
4.7. Analysis of Total Phenols and Flavonoid Content
4.7.1. Plant Extraction Procedure
4.7.2. Total Phenol Content Analysis
4.7.3. Total Flavonoid Content Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMTW | electromagnetically treated water |
| EM | electromagnetic |
| MTW | magnetically treated water |
| NMR | nuclear magnetic resonance |
| DFT | density functional theory |
| FG | field-grown |
| LG | laboratory-grown |
| Anet | net photosynthesis |
| E | transpiration |
| gs | stomatal conductance |
| Ci | intercellular concentration of CO2 |
References
- Pang, X.; Deng, B. Investigation of changes in properties of water under the action of a magnetic field. Sci. China Ser. G Phys. Mech. Astron. 2008, 51, 1621–1632. [Google Scholar] [CrossRef]
- Moussa, M.; Zarai, B.; Hachicha, M. Magnetic water treatment: Theory and effects on treated water—A systematic review. Euro-Mediterr. J. Environ. Integr. 2025, 10, 2323–2342. [Google Scholar]
- Chibowski, E.; Szcześ, A.; Hołysz, L. Influence of magnetic field on evaporation rate and surface tension of water. Colloids Interfaces 2018, 2, 68. [Google Scholar] [CrossRef]
- Ignatov, I.; Stankov, I.K. Parameters and effects of magnetic field and potassium carbonate in water, Applications. Ukr. J. Phys. 2024, 69, 321–328. [Google Scholar] [CrossRef]
- Ignatov, I.; Stankov, I.K. Electrochemical effects of magnetic field and potassium carbonate in water. Port. Electrochim. Acta 2026, 44, 409–418. [Google Scholar]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic Field (MF) applications in plants: An Overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Aladjadjiyan, A. Study of the influence of magnetic field on some biological characteristics of Zea mays. J. Cent. Eur. Agric. 2002, 3, 89–94. [Google Scholar]
- Ospina-Salazar, D.I.; Cortez-Hernández, G.L.; Benavides-Bolaños, A.J.; Zúñiga-Escobar, O. Fruit yield of tabasco pepper under water deficit with magnetically treated water. Rev. Cienc. Y Tecnol. Agropecu. 2022, 23, 247–266. [Google Scholar]
- Radhakrishnan, R. Magnetic field regulates plant functions, growth and enhances tolerance against environmental stresses. Physiol. Mol. Biol. Plants 2019, 25, 1107–1119. [Google Scholar] [CrossRef]
- Putti, F.F.; Vicente, E.F.; Chaves, P.P.N.; Mantoan, L.P.B.; Cremasco, C.P.; Arruda, B.; Forti, J.C.; Junior, J.F.S.; Campos, M.; Reis, A.R.; et al. Effect of magnetic water treatment on the growth, nutritional status, and yield of lettuce plants with irrigation rate. Horticulturae 2023, 9, 504. [Google Scholar] [CrossRef]
- Răcuciu, M.; Creangă, D.; Olteana, Z. Water based magnetic fluid impact on young plants growing. Rom. Rep. Phys. 2009, 61, 259–268. [Google Scholar]
- Abu-Saied, M.A.; El Desouky, E.A.; Abou Kamer, M.E.; Hafez, M.; Rashad, M. Influence of magnetic field on the physicochemical properties of water molecule under growing of cucumber plant in an arid region. J. King Saud. Univ. Sci. 2023, 35, 102890. [Google Scholar] [CrossRef]
- Aladjadjiyan, A.; Zahariev, A. Influence of a stationary magnetic field on the absorption spectra od some energy plants. J. Environ. Prot. Ecol. 2009, 10, 1032–1036. [Google Scholar]
- Chaplin, M.F. Water Structure and Science, Chapter: Water Activity; London South Bank University: London, UK, 2022. [Google Scholar]
- Hachicha, M.; Kahlaoui, B.; Khamassi, N.; Misle, E.; Jouzdan, O. Effect of electromagnetic treatment of saline water on soil and crops. J. Saudi Soc. Agric. Sci. 2018, 17, 154–162. [Google Scholar] [CrossRef]
- Akrimi, R.; Hajlaoui, H.; Batelli, G.; Ruggiero, A.; Badri, M.; Grillo, S.; Mhamdi, M. Electromagnetic water enhanced metabolism and agro-physiological responses of potato (Solanum tuberosum L) under saline conditions. J. Agron. Crop Sci. 2020, 207, 44–58. [Google Scholar]
- Shafiei, M.; Ojaghlou, N.; Zamfir, S.G.; Bratko, D.; Luzar, A. Modulation of structure and dynamics of water under alternating electric field and the role of hydrogen bonding. Mol. Phys. 2019, 117, 3282–3296. [Google Scholar] [CrossRef]
- Dai, C.; Liao, M.; Li, X.; Chen, S.; Gao, P.; Sheng, P. Electric field-induced giant columns of polarized water molecules. Phys. Rev. Res. 2022, 4, 033164. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, C.; Wang, C.; Wang, Q.; Liu, Y.; Wang, J. Responses of water and fertilizer utilization efficiency and yield of cotton to foliar biostimulant under irrigation with magnetic–electric-activated water. Agronomy 2024, 14, 2117. [Google Scholar]
- Putti, F.F.; de Queiroz Barcelos, J.P.; Goes, B.C.; Alves, R.F.; Neto, M.M.; da Silva, A.O.; Filho, L.R.A.G.; Zanetti, W.A.L.; de Souza, A.V. Effects of water deficit on growth and productivity in tomato crops irrigated with water treated with very low-frequency electromagnetic resonance fields. Plants 2023, 12, 3721. [Google Scholar] [CrossRef]
- Ignatov, I.; Huether, F.; Neshev, N.; Kiselova-Kaneva, Y.; Popova, T.P.; Bankova, R.; Valcheva, N.; Ignatov, A.I.; Angelcheva, M.; Angushev, I.; et al. Research of water molecules cluster structuring during Haberlea rhodopensis Friv. hydration. Plants 2022, 11, 2655. [Google Scholar] [CrossRef]
- Kuroki, S.; Tsenkova, R.; Moyankova, D.; Munkan, J.; Morita, H.; Atanassova, S.; Djilianov, D. Water molecular structure underpins extreme desiccation tolerance of the resurrection plant Haberlea rhodopensis. Sci. Rep. 2019, 9, 3049. [Google Scholar] [CrossRef]
- Ignatov, I.; Huether, F.; Popova, T.P.; Ignatov, A.I.; Iliev, M.T.; Stoyanov, C. Effects of electromagnetic waves on parameters, hydration and in vitro antimicrobial activity of the Brassica oleracea L. var. italica Plenck. and water. Plant Sci. Today 2024, 11, 553–561. [Google Scholar] [CrossRef]
- Bulgarian Ordinance No. 18 of 27 May 2009 on the Quality of Water for Irrigation of Agricultural Crops. The Minister of Environment and Water and the Minister of Agriculture and Food. Available online: https://lex.bg/laws/ldoc/2135636168 (accessed on 4 January 2023).
- Tsanov, E.; Valchev, D.; Ribarova, I.; Dimova, G. Discussion on the need for harvested rainwater quality standards tailored to the reuse purpose. Processes 2023, 11, 665. [Google Scholar] [CrossRef]
- Vassilev, N.; Ignatov, I.; Popova, T.P.; Huether, F.; Ignatov, A.I.; Iliev, M.T.; Marinov, Y. Nuclear Magnetic Resonance (NMR) and Density Functional Theory (DFT) study of water clusters of Hydrogen-rich water (HRW). Water 2024, 16, 3261. [Google Scholar] [CrossRef]
- Ignatov, I.; Marinov, Y.G.; Vassileva, P.; Gluhchev, G.; Pesotskaya, L.A.; Jordanov, I.P.; Iliev, M.T. Nonlinear hydrogen bond network in small water clusters: Combining NMR, DFT, FT-IR, and EIS research. Symmetry 2025, 17, 1062. [Google Scholar] [CrossRef]
- Ignatov, I.; Popova, T.P.; Vassileva, P.; Marinov, Y.G.; Iliev, M.T. Hot Mineral Water as a Medium for Molecular Hydrogen Reactions in the Primordial Hydrosphere for the Origin of Life. Hydrogen 2025, 6, 48. [Google Scholar] [CrossRef]
- Ignatov, I.; Marinov, Y.G.; Vassileva, P.; Popova, T.P.; Gluhchev, G.; Iliev, M.T.; Huether, F.; Dimitrov, Z.; Gotova, I. Formation of ice Ih clusters in solid-phase glacial water with low concentrations of Ca2+ and Mg2+ ions. Crystals 2025, 15, 254. [Google Scholar] [CrossRef]
- Xie, F.; Tikhonov, D.S.; Schell, M. Electric nuclear quadrupole coupling reveals dissociation of HCl with a few water molecules. Science 2024, 384, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Rani, D.; Kobtrakul, K.; De-Eknamkul, W.; Vimolmangkang, S. Magnetized water: A way to enhance isoflavonoids in cultured Pueraria candollei var. mirifica cells. Ind. Crops Prod. 2022, 180, 114779. [Google Scholar] [CrossRef]
- Zareei, E.; Zaare-Nahandi, F.; Oustan, S.; Hajilou, J. Effects of magnetic solutions on some biochemical properties and production of some phenolic compounds in grapevine (Vitis vinifera L.). Sci. Hortic. 2019, 253, 217–226. [Google Scholar] [CrossRef]
- Michalak, A.; Wdowikowska, A.; Janicka, M. Plant plasma Membrane proton pump: One protein with multiple Functions. Cells 2022, 11, 4052. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Lokshin, V.; Mark Sigalov, M.; Larina, N.; Khodorkovsky, V. Dipole moments of conjugated donor–acceptor substituted systems: Calculations vs. experiments. RSC Adv. 2021, 11, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, B.B.; Vicente, E.F.; Nunes Chaves, P.P.; Zanetti, W.A.L.; Ono, E.O.; da Silva, G.F.; dos Reis, A.R.; Putti, F.F. Sugar metabolism and photosynthesis of tomatoes irrigated with water treated with low-frequency electromagnetic resonance fields in different fertigation doses. Horticulturae 2022, 8, 868. [Google Scholar] [CrossRef]
- Javed, N.; Ashraff, M.; Akram, N.A.; Al-Quraity, F. Alleviation of adverse effects of drought stress on growth and some potential physiological attributes in maize (Zea mays L.) by Seed Electromagnetic Treatment. Photochem. Photobiol. 2011, 87, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, S.; Dede, O.; Koseouglu, G. Electromagnetic Water Treatment and Water Quality Effect on Germination, Rooting and Plant Growth on Flower. Asian J. Water Environ. Pollut. 2005, 2, 9–13. [Google Scholar] [CrossRef]
- Ghanati, F.; Mohamadalikhani, S.; Soleimani, M.; Afzalzadeh, R.; Hajnorouzi, A. Change of growth pattern, metabolism, and quality and quantity of maize plants after irrigation with magnetically treated water. Electromagn. Biol. Med. 2015, 34, 211–215. [Google Scholar] [CrossRef]
- Boix, Y.F.; Dubois, E.D.; Hendrix, S.; Luna, L.M.G.; Beenaerts, N.; Manrique, C.E.M.; Victório, C.P.V.; Cuypers, A. Assessment of the antioxidative potential of Rosmarinus officinalis L. (Lamiaceae) Irrigated with static magnetic field-treated Water. Braz. Arch. Biol. Technol. 2020, 63, e20190142. [Google Scholar] [CrossRef]
- Zare, H.; Mohsenzadeh, S. The Effect of Electromagnetic waves on photosynthetic pigments and antioxidant enzymes in Zea mays L. Curr. World Env. 2015, 1, 88. [Google Scholar] [CrossRef]
- Sappington, E.N.; Rifai, H.S. Low-frequency electromagnetic treatment of oilfield produced water for reuse in agriculture: Effect on water quality, germination, and plant growth. Env. Sci. Pollut. Res. 2018, 25, 34380–34391. [Google Scholar] [CrossRef]
- Khaskhoussy, K.; Mohamed Bouhlel, M.; Dahmouni, M.; Hachicha, M. Performance of different magnetic and electromagnetic water treatment devices on soil and two tomato cultivars. Sci. Hortic. 2023, 322, 112447. [Google Scholar] [CrossRef]
- Bouhlel, M.; Khaskhoussy, K.; Hachicha, M. Improvement of salt leaching efficiency and water content of soil through irrigation with electro-magnetized saline water. Water 2024, 16, 3010. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Xu, X.; Xu, P. A critical review of the application of electromagnetic fields for scaling control in water systems: Mechanisms, characterization, and operation. npj Clean Water 2020, 3, 25. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Wang, Q.; Wang, C. Responses of the growth characteristics of spinach to different moisture contents in soil under irrigation with magnetoelectric water. Agronomy 2023, 13, 657. [Google Scholar] [CrossRef]
- Kamble, S.; Agrawal, S.; Cherumukki, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting Zeta potential, the key feature of interfacial phenomena, with applications and recent advancements. ChemistrySelect 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Mateus-Vargas, R.H.; Kemper, N.; Volkmann, N.; Kietzmann, M.; Meissner, J.; Schulz, J. Low-frequency electromagnetic fields as an alternative to sanitize water of drinking systems in poultry production? PLoS ONE 2019, 14, e0220302. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Shields, R.M.; Temelso, B.; Archer, K.A.; Morrell, T.E.; Shields, G.C. Accurate Predictions of water cluster formation, (H2O)n=2–10. J. Phys. Chem. A 2010, 114, 11725–11737. [Google Scholar] [CrossRef]
- Sun, N.; Li, Z.; Qiu, N.; Yu, X.; Zhang, X.; Li, Y.; Yang, L.; Luo, K.; Huang, Q.; Du, S. Ab initio studies on the clathrate hydrates of some nitrogen- and sulfur-containing gases. J. Phys. Chem. A 2017, 121, 2620–2626. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Duong, C.H.; Kelleher, P.J.; Johnson, M.A. Capturing intrinsic site-dependent spectral signatures and lifetimes of isolated OH oscillators in extended water networks. Nat. Chem. 2020, 12, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, A.; Bandyopadhyay, P.; Heindel, J.P.; Xantheas, S.S. Atlas of putative minima and low-lying energy networks of water clusters n = 3–25. J. Chem. Phys. 2019, 151, 214307. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Klein, R.A. Ab initio conformational studies on diols and binary diol-water systems using DFT methods. Intramolecular hydrogen bonding and 1:1 complex formation with water. J. Comput. Chem. 2002, 23, 585–599. [Google Scholar] [CrossRef]
- Qian, P.; Song, W.; Lu, L.; Yang, Z. Ab initio investigation of water clusters (H2O)n (n = 2–34). Int. J. Quantum Chem. 2010, 110, 1923–1937. [Google Scholar] [CrossRef]
- Klein, R.A. Hydrogen bonding in diols and binary diol–water systems investigated using DFT methods. II. Calculated infrared OH-stretch frequencies, force constants, and NMR chemical shifts correlate with hydrogen bond geometry and electron density topology. A re-evaluation of geometrical criteria for hydrogen bonding. J. Comput. Chem. 2003, 24, 1120–1131. [Google Scholar] [PubMed]
- Michiu, D.; Socaciu, M.-I.; Fogarasi, M.; Jimborean, A.M.; Ranga, F.; Mureşan, V.; Semeniuc, C.A. Implementation of an analytical method for spectrophotometric evaluation of total phenolic content in essential oils. Molecules 2022, 27, 1345. [Google Scholar] [CrossRef] [PubMed]
- Koleva-Valkova, L.; Piperkova, N.; Petrov, V.; Vassilev, A. Biochemical responses of peach leaves infected with Taphrina deformans Berk/Tul. Acta Univ. Agric. Silvic. Mendel. Brun. 2017, 65, 871–878. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, Y.; Zhu, W.; Yin, Y. Assessment of the fruit chemical characteristics and antioxidant activity of different mulberry cultivars (Morus spp.) in semi-arid, Sandy Regions of China. Foods 2023, 12, 3495. [Google Scholar] [CrossRef]




| Sample | δ, ppm | Δν1/2, Hz | Comment |
|---|---|---|---|
| 1 | 4.192 | 7.09 | Control sample irrigation water |
| 2 | 4.253 | 7.68 | Electromagnetically treated irrigation water |
| Plant Growth Regime | Treatments | Anet | E | gs | Ci | Chlorophyll |
|---|---|---|---|---|---|---|
| LG | A | 6.28 | 0.57 | 0.07 | 258 | 147 |
| B | 11.41 | 0.95 | 0.16 | 284 | 161 | |
| p < 0.001 | p < 0.001 | p < 0.01 | p < 0.01 | p < 0.01 | ||
| r = 0.744 | r = 0.640 | r = 0.510 | r = 0.810 | r = 0.712 | ||
| FG | A | 6.22 | 0.49 | 0.09 | 197 | 139 |
| B | 11.20 | 0.94 | 0.16 | 280 | 152 | |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.01 | ||
| r = 0.612 | r = 0.389 | r = 0.481 | r = 0.612 | r = 0.602 |
| r | |
|---|---|
| A (photosynthesis) ↔ E (transpiration) | 0.992 |
| A (photosynthesis) ↔ gs (stomatal conductance) | 0.983 |
| E (transpiration) ↔ gs (stomatal conductance) | 0.952 |
| Plant Growth Regime | Treatments | mgGAE·g−1 FW | mgQ·g−1 FW |
|---|---|---|---|
| FG | A | 2.68 | 1.03 |
| B | 2.88 | 1.11 | |
| p < 0.001 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koleva-Valkova, L.; Ignatov, I.; Huether, F.; Bojinov, B.; Marinkov, K.; Popova, T.P.; Ignatov, A.I.; Marinov, Y.G.; Iliev, M.T. Effects of Electromagnetically Treated Water (EMTW) on the Properties of Water and Photosynthetic Performance of Spinacia oleracea L. Plants 2025, 14, 2972. https://doi.org/10.3390/plants14192972
Koleva-Valkova L, Ignatov I, Huether F, Bojinov B, Marinkov K, Popova TP, Ignatov AI, Marinov YG, Iliev MT. Effects of Electromagnetically Treated Water (EMTW) on the Properties of Water and Photosynthetic Performance of Spinacia oleracea L. Plants. 2025; 14(19):2972. https://doi.org/10.3390/plants14192972
Chicago/Turabian StyleKoleva-Valkova, Lyubka, Ignat Ignatov, Fabio Huether, Bojin Bojinov, Kiril Marinkov, Teodora P. Popova, Alexander I. Ignatov, Yordan G. Marinov, and Mario T. Iliev. 2025. "Effects of Electromagnetically Treated Water (EMTW) on the Properties of Water and Photosynthetic Performance of Spinacia oleracea L." Plants 14, no. 19: 2972. https://doi.org/10.3390/plants14192972
APA StyleKoleva-Valkova, L., Ignatov, I., Huether, F., Bojinov, B., Marinkov, K., Popova, T. P., Ignatov, A. I., Marinov, Y. G., & Iliev, M. T. (2025). Effects of Electromagnetically Treated Water (EMTW) on the Properties of Water and Photosynthetic Performance of Spinacia oleracea L. Plants, 14(19), 2972. https://doi.org/10.3390/plants14192972







