Intraspecific Variation and Environmental Determinants of Leaf Functional Traits in Polyspora chrysandra Across Yunnan, China
Abstract
1. Introduction
- (1)
- What are the patterns of ITV in P. chrysandra across different biogeographical regions of Yunnan Province? Given the high environmental heterogeneity in its distribution area, we hypothesize that under the synergistic effects of environmental factors such as GCs, CFs, SPs, and UVRFs, the ITV exhibits a moderate or strong variation level while also demonstrating a broader range of variability (Hypothesis 1).
- (2)
- Which environmental factors drive the variation in LFTs, and what are their relative importance? Previous studies have indicated that ITV is comprehensively regulated by multiple environmental factors [13,15,16]. Therefore, we hypothesize that GCs, CFs, SPs, and UVRFs collectively drive the ITV; among these, owing to the intense ultraviolet radiation (UV) in Yunnan Province, UVRFs likely exert a pronounced effect on the ITV (Hypothesis 2).
2. Results
2.1. Leaf Functional Traits Characteristics of P. chrysandra
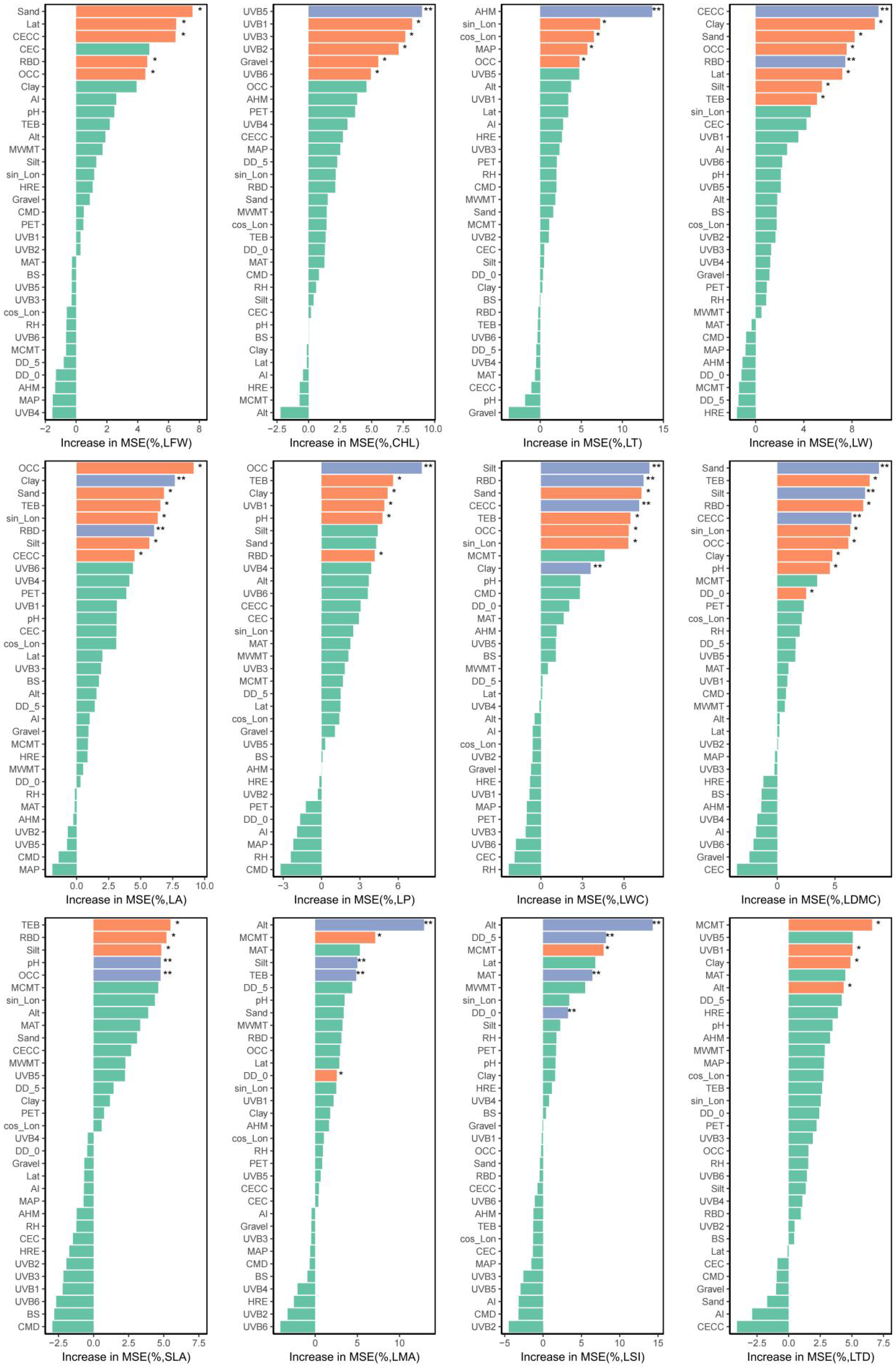
2.2. Variable Screening Results
2.2.1. Screening Results of Response Variables
2.2.2. Screening Results of Explanatory Variables
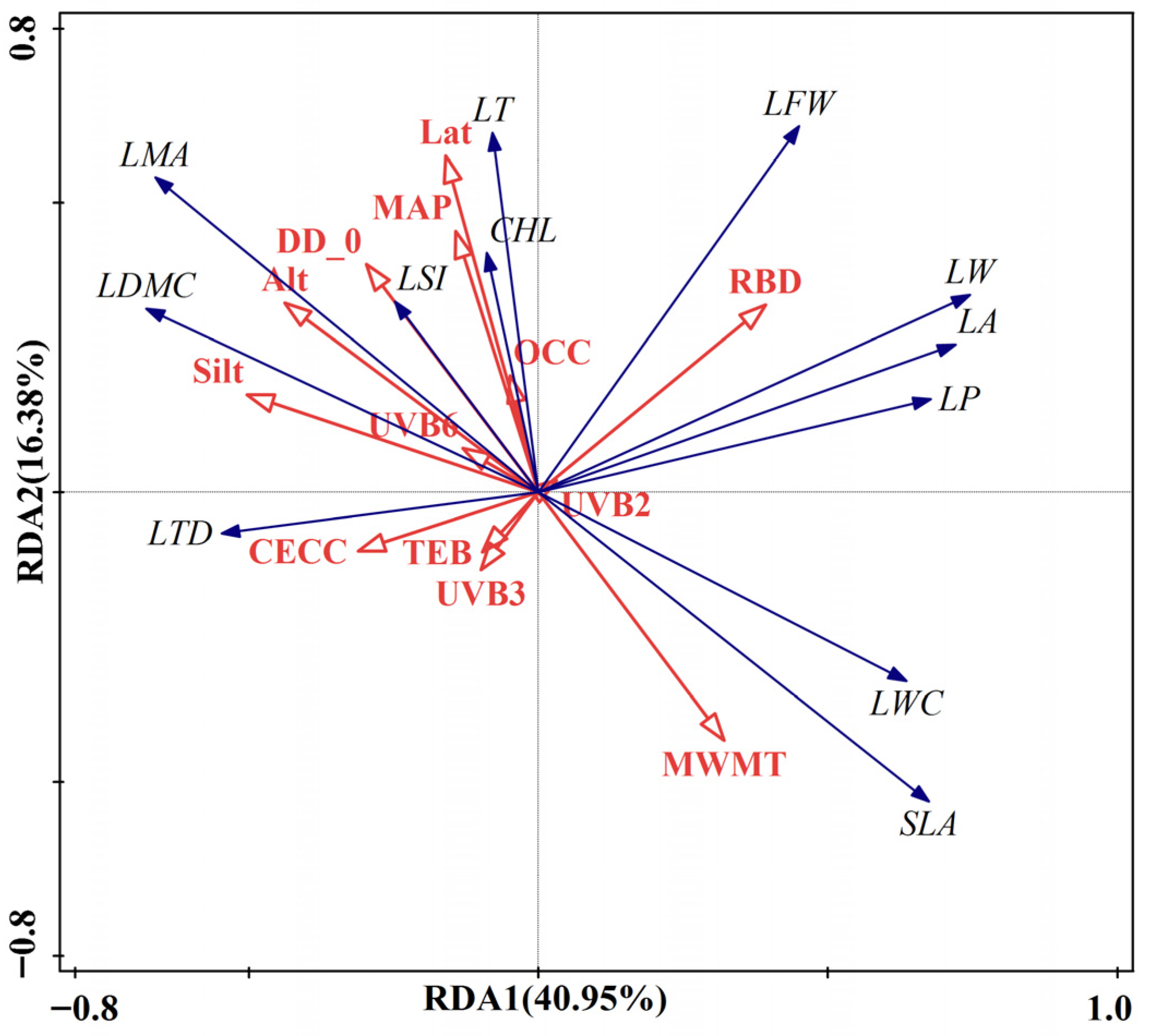
2.2.3. RDA Analysis of Leaf Functional Traits and Environmental Factors and Interpretation of Relative Importance
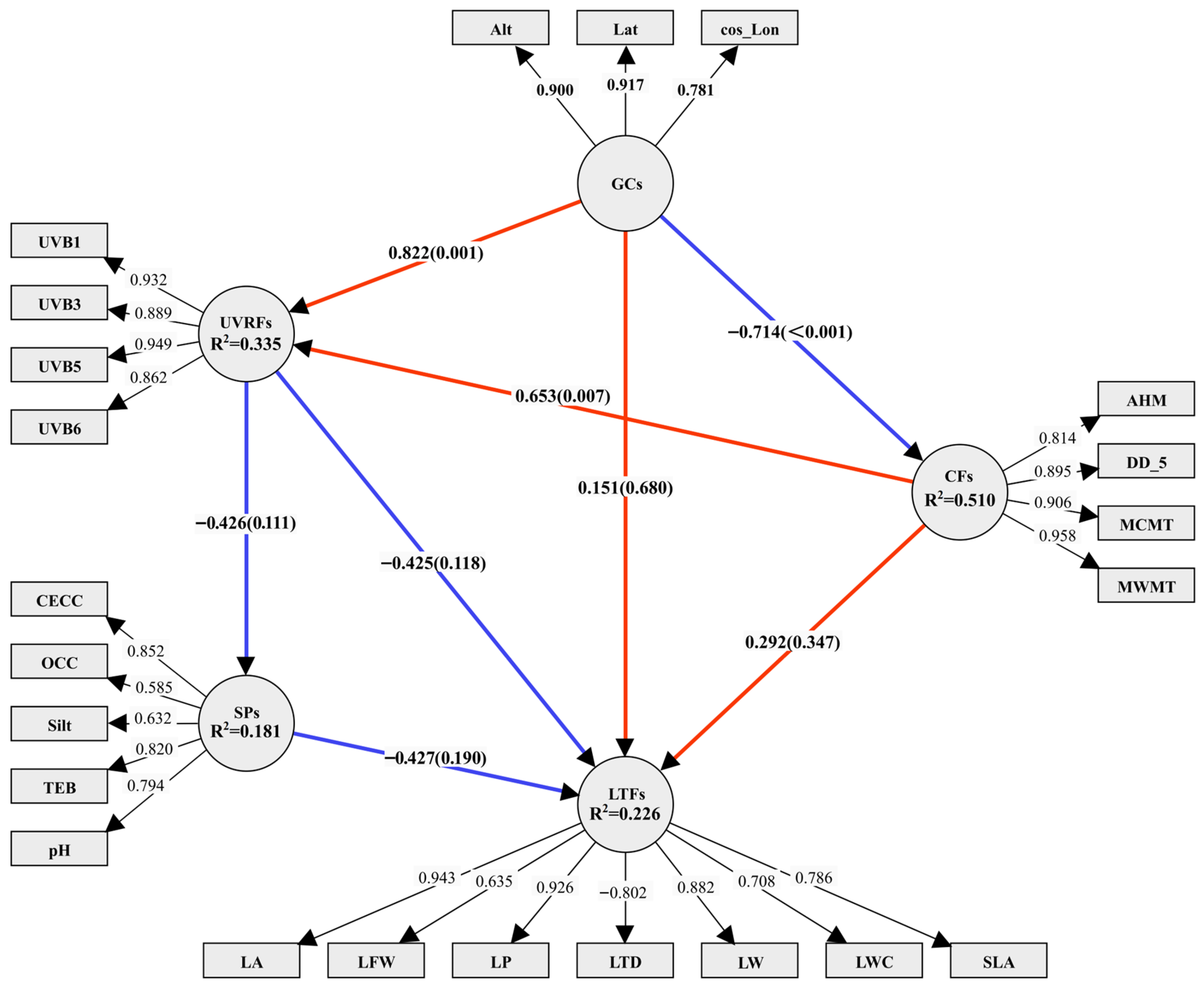
2.3. Results of CCA and the Optimal PLS-SEM Model
2.4. Assessment of Influencing Factors of Leaf Functional Traits
3. Discussion
3.1. Variation Characteristics of Leaf Functional Traits of P. chrysandra
3.2. Effects of Soil Properties on Leaf Functional Traits of P. chrysandra
3.3. Effects of Ultraviolet Radiation Factors on Leaf Functional Traits of P. chrysandra
3.4. Effects of Geographical Conditions on Leaf Functional Traits of P. chrysandra
3.5. Effects of Climate Factors on Leaf Functional Traits of P. chrysandra
3.6. Potential Limitations of the Study
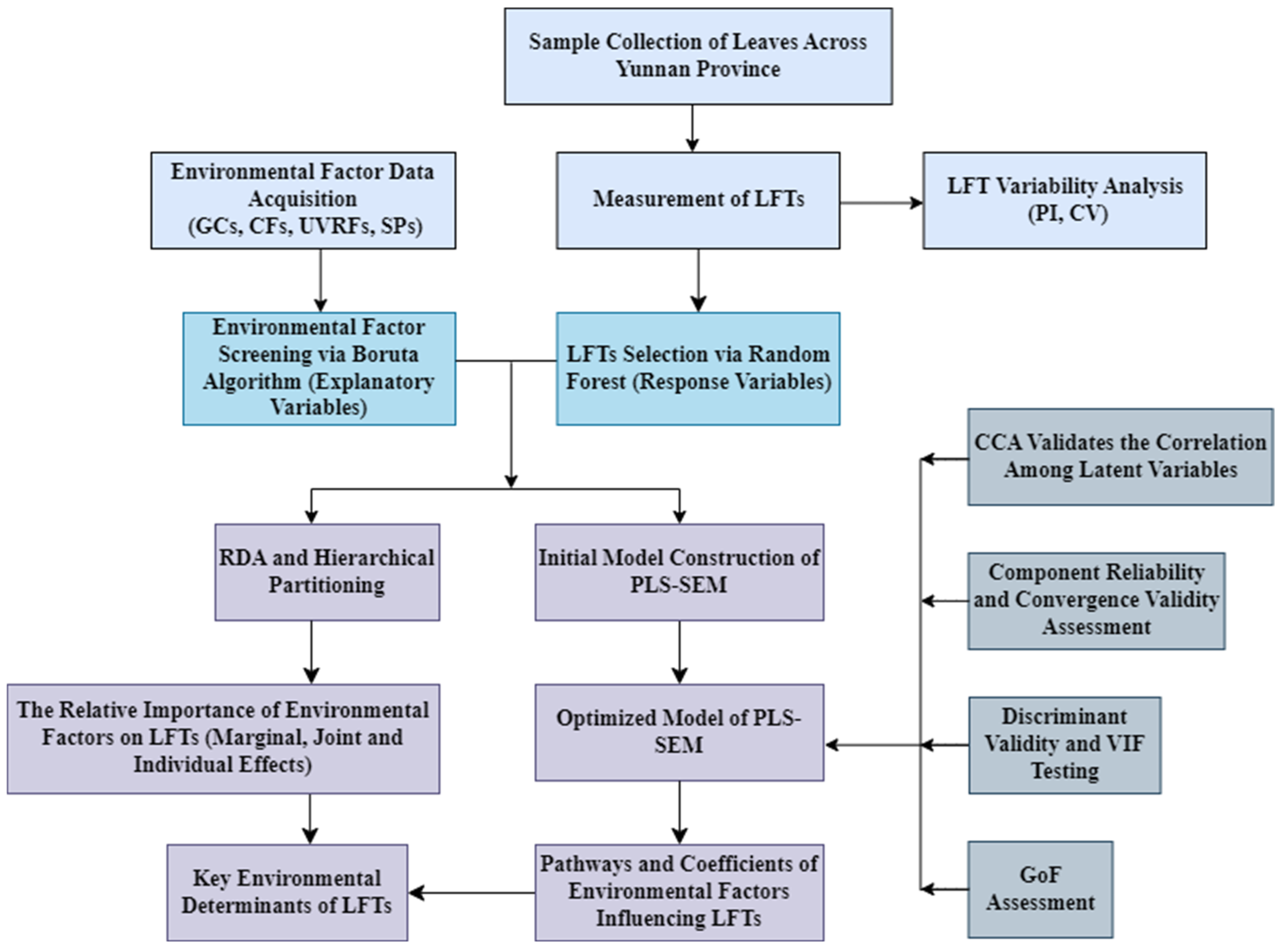
4. Materials and Methods
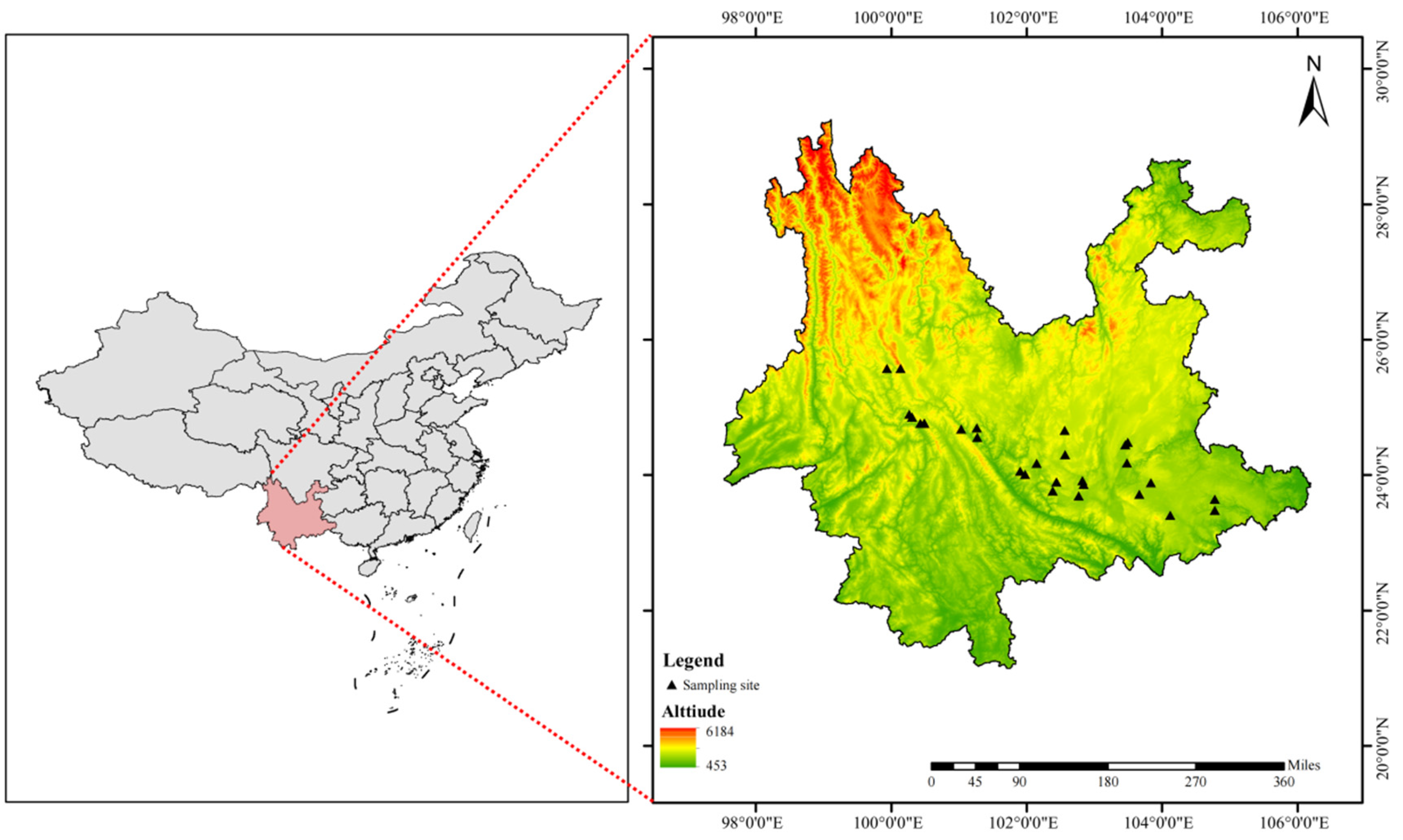
4.1. Study Area
4.2. Sample Collection and Leaf Functional Trait Measurements
4.3. Acquisition of Environmental Factor Data
4.4. Data Analysis and Processing
4.4.1. Data Preprocessing and Variable Screening
4.4.2. Effects of Environmental Factors on Leaf Functional Traits and Their Relative Importance
4.4.3. Construction and Evaluation of the PLS-SEM Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function: Enhanced species-level trait dataset. Sci. Data 2022, 9, 755. [Google Scholar] [CrossRef]
- Ding, X.; Liu, J.; Wang, Y.; Wang, J.; Liu, C.; Qin, M.; Xu, Y.; Ma, Y.; Yang, J.; Xu, Z. Effects of nitrogen addition on leaf functional traits of dominant species in Bayanbulak grassland, Xinjiang, China. Plants 2025, 14, 597. [Google Scholar] [CrossRef] [PubMed]
- Pigliucci, M. Phenotypic integration: Studying the ecology and evolution of complex phenotypes. Ecol. Lett. 2003, 6, 265–272. [Google Scholar] [CrossRef]
- Hu, M.; Chen, H.Y.H.; Chang, S.X.; Leuzinger, S.; Dukes, J.S.; Langley, J.A.; Bader, M.K.F.; Van Sundert, K.; Liao, H.; Ma, Z. Plant functional traits affect biomass responses to global change: A meta-analysis. J. Ecol. 2025, 113, 2046–2065. [Google Scholar] [CrossRef]
- Barabás, G.; Parent, C.; Kraemer, A.; Van de Perre, F.; De Laender, F. The evolution of trait variance creates a tension between species diversity and functional diversity. Nat. Commun. 2022, 13, 2521. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.A.; Wang, X.; Shrestha, N.; Zhang, Y.; Sun, Y.; Yao, S.; Li, J.; Hou, Q.; Hu, W.; Ran, J.; et al. Variations and driving factors of leaf functional traits in the dominant desert plant species along an environmental gradient in the drylands of China. Sci. Total Environ. 2023, 897, 165394. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zheng, H.; Wang, Z.; Wen, Z.; Yang, Y. Effects of plant functional traits on ecosystem services: A review. Chin. J. Plant Ecol. 2021, 45, 1140–1153. [Google Scholar] [CrossRef]
- Carrera, A.L.; Mazzarino, M.J.; Bertiller, M.B.; Del Valle, H.F.; Carretero, E.M. Plant impacts on nitrogen and carbon cycling in the Monte Phytogeographical Province, Argentina. J. Arid Environ. 2009, 73, 192–201. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Z.; Chen, K.; Ma, X.; Zhou, S.; He, X.; Xie, D. Investigating the variation in leaf traits within the Allium prattii C.H. Wright population and its environmental adaptations. Plants 2025, 14, 541. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Rifai, S.W.; Deng, X.; Ter Steege, H.; Thomson, E.; Corral-Rivas, J.J.; Guimaraes, A.F.; Muller, S.; Klipel, J.; Fauset, S.; et al. Canopy functional trait variation across Earth’s tropical forests. Nature 2025, 641, 129–136. [Google Scholar] [CrossRef]
- Slot, M.; Cala, D.; Aranda, J.; Virgo, A.; Michaletz, S.T.; Winter, K. Leaf heat tolerance of 147 tropical forest species varies with elevation and leaf functional traits, but not with phylogeny. Plant Cell Environ. 2021, 44, 2414–2427. [Google Scholar] [CrossRef] [PubMed]
- Koffel, T.; Umemura, K.; Litchman, E.; Klausmeier, C.A. A general framework for species-abundance distributions: Linking traits and dispersal to explain commonness and rarity. Ecol. Lett. 2022, 25, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.; Aakala, T.; Abedi, M.; et al. TRY plant trait database-enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef]
- Serrano-León, H.; Nitschke, R.; Scherer-Lorenzen, M.; Forrester, D.I. Intra-specific leaf trait variability of F. sylvatica, Q. petraea and P. abies in response to inter-specific competition and implications for forest functioning. Tree Physiol. 2022, 42, 253–272. [Google Scholar] [CrossRef]
- Niu, K.; Zhang, S.; Lechowicz, M.J. Harsh environmental regimes increase the functional significance of intraspecific variation in plant communities. Funct. Ecol. 2020, 34, 1666–1677. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; He, X.; Qi, Z.; Luo, P. The influence of three kinds of trees on slope deformation and stability under wind load. Trans. Chin. Soc. Agric. Eng. 2023, 39, 110–119. [Google Scholar]
- Ministry of Ecology and Environment of PRC, Chinese Academy of Sciences. Red List of China’s Biodiversity-Higher Plants; Ministry of Ecology and Environment of PRC, Chinese Academy of Sciences: Beijing, China, 2023; Volume 2020.
- Beech, E.; Barstow, M.; Rivers, M. The Red List of Theaceae; Encyclopædia Britannica Inc.: Richmond, UK, 2017. [Google Scholar]
- Yang, S. Taxonomic Treatment of Chinese Polyspora Sweet (Theaceae). J. Trop. Subtrop. Bot. 2005, 13, 363–365. [Google Scholar]
- Sun, F.; Zhong, Z. Quantitative characters of reproductive adaptation of Gordonia acuminata population in Mt. Jin Yun. Chin. J. Plant Ecol. 1997, 3–6, 9–10. [Google Scholar]
- Zhang, X.; Ren, Q.; Liu, M.; You, Y.; Pan, L.; Fu, H. Chemical constituents from the stems of Gordonia kwangsiensis. J. Chin. Med. Mater. 2020, 43, 80–83. [Google Scholar]
- Mu, B.; Li, M.; Yang, C.; Deng, L.; Pan, D.; Mu, J.; Li, C. Study on Gordonia acuminata community in Xishui National Nature Reserve. Seed 2011, 30, 62–66. [Google Scholar]
- Fan, Z.; Zhou, B.; Ma, C.; Gao, C.; Han, D.; Chai, Y. Impacts of climate change on species distribution patterns of Polyspora sweet in China. Ecol. Evol. 2022, 12, e9516. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gong, M.; Ma, C.; Fan, Z.; Gao, C.; Wang, L.; Deng, L. Characterization of leaf phenotypic traits in natural populations of three Polyspora species. Plant Sci. J. 2025, 43, 21–31. [Google Scholar]
- Escobar-Bravo, R.; Klinkhamer, P.G.; Leiss, K.A. Interactive effects of UV-B light with abiotic factors on plant growth and chemistry, and their consequences for defense against arthropod herbivores. Front. Plant Sci. 2017, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Wang, B.; Chen, S.; Wang, Y.; Suo, A.; Liu, X.; Chen, F. Changes of leaf functional traits of Pinus tabuliformis in burned areas with different fire severities. Chin. J. Appl. Ecol. 2022, 33, 1497–1504. [Google Scholar]
- Wu, T.; Long, C.; Xiong, L.; Liu, Q. Variation and adaptation of functional leaf traits of different plant types in karst forests. Chin. J. Appl. Environ. Biol. 2023, 29, 1043–1049. [Google Scholar]
- Ding, J.; Wu, Q.; Yan, H.; Zhang, S. Effects of topographic variations and soil characteristics on plant functional traits in a subtropical evergreen broad-leaved forest. Biodivers. Sci. 2011, 19, 158–167. [Google Scholar]
- Liu, R.; Huang, Z.; Yu, R.; Bao, J.; Mo, Y. The impact of red tourism on national identity of tourists. J. Nat. Resour. 2021, 36, 1673–1683. [Google Scholar] [CrossRef]
- Wu, X.; Ji, B.; He, J.; Ren, X.; Yu, H.; Wang, Z. Effects of controlling precipitation gradient on leaf functional traits and soil nutrients of dominant plants in desert steppe. Acta Ecol. Sin. 2021, 41, 2719–2727. [Google Scholar]
- Li, X.; Wen, H.; Wang, X.; Yang, J.; Huang, C. Phenotypic plasticity of Distylium chinense leaves in relation to soil environmental factors in heterogeneous habitats in the Three Gorges Reservoir Region. Acta Ecol. Sin. 2018, 38, 3581–3591. [Google Scholar] [CrossRef]
- Lin, W.; Yu, Z.; Luo, Y.; He, W.; Yan, G.; Peng, C. Photoprotection differences between dominant tree species at mid-and late-successional stages in subtropical forests in different seasonal environments. Int. J. Mol. Sci. 2022, 23, 5417. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of drought stress on Chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities in lettuce seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Ni, Y.; Xia, R.; Li, J. Changes of epicuticular wax induced by enhanced UV-B radiation impact on gas exchange in Brassica napus. Acta Physiol. Plant. 2014, 36, 2481–2490. [Google Scholar] [CrossRef]
- He, X.; Xu, L.; Pan, C.; Gong, C.; Wang, Y.; Liu, X.; Yu, Y. Drought resistance of Camellia oleifera under drought stress: Changes in physiology and growth characteristics. PLoS ONE 2020, 15, e0235795. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y.; Zhao, Y.; Liu, B.; Chen, J. Effect of drought stress on physiology of Camellia nitidissima saplings. Fujian J. Agric. Sci. 2018, 33, 614–620. [Google Scholar]
- Liu, K.; He, N.; Hou, J. Spatial patterns and influencing factors of specific leaf area in typical temperate forests. Acta Ecol. Sin. 2022, 42, 872–883. [Google Scholar] [CrossRef]
- Yang, H.; Wei, H.; Sang, M.; Shang, Z.; Mao, Y.; Wang, X.; Liu, F.; Gu, W. Phenotypic plasticity of Schisandra sphenanthera leaf and the effect of environmental factors on leaf phenotype. Chin. Bull. Bot. 2016, 51, 322–334. [Google Scholar]
- Auld, J.R.; Agrawal, A.A.; Relyea, R.A. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B Biol. Sci. 2010, 277, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Pélabon, C.; Hilde, C.H.; Einum, S.; Gamelon, M. On the use of the coefficient of variation to quantify and compare trait variation. Evol. Lett. 2020, 4, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Bao, W.; Wu, N. Morphological and physiological responses of current Sophora davidii seedlings to drought stress. Acta Ecol. Sin. 2009, 29, 5406–5416. [Google Scholar]
- Luo, T.; Yu, F.; Lian, J.; Wang, J.; Shen, J.; Wu, Z.; Ye, W. Impact of canopy vertical height on leaf functional traits in a lower subtropical evergreen broad-leaved forest of Dinghushan. Biodivers. Sci. 2022, 30, 21414. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Xiong, L.; Long, C.; Liang, S.; Wu, T.; Liu, Q.; Liao, Q.; Xue, F. Response of leaf functional traits of woody plants to soil characteristics in Karst forests. J. Trop. Subtrop. Bot. 2024, 32, 310–318. [Google Scholar]
- Wang, L.; Ali, A. Functional identity regulates aboveground biomass better than trait diversity along abiotic conditions in global forest metacommunities. Ecography 2022, 2022, e05854. [Google Scholar] [CrossRef]
- Rosas, T.; Mencuccini, M.; Barba, J.; Cochard, H.; Saura-Mas, S.; Martínez-Vilalta, J. Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytol. 2019, 223, 632–646. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Su, Q.; Chen, S.; Jiang, Y.; Luo, M.; Liang, S. Exploring community assembly of Guilin karst hills based on functional traits and phylogeny. J. Guangxi Norm. Univ. (Nat. Sci.) 2023, 41, 171–181. [Google Scholar]
- Abalos, D.; van Groenigen, J.W.; Philippot, L.; Lubbers, I.M.; De Deyn, G.B. Plant trait-based approaches to improve nitrogen cycling in agroecosystems. J. Appl. Ecol. 2019, 56, 2454–2466. [Google Scholar] [CrossRef]
- Weemstra, M.; Roumet, C.; Cruz-Maldonado, N.; Anthelme, F.; Stokes, A.; Freschet, G.T. Environmental variation drives the decoupling of leaf and root traits within species along an elevation gradient. Ann. Bot. 2022, 130, 419–430. [Google Scholar] [CrossRef]
- Lucas Erickson, R.; Kessler, M.; Carneiro, I. Factors driving trait-convergence linked to leaf economic spectrum in tropical ferns. Bot. Lett. 2023, 170, 518–531. [Google Scholar] [CrossRef]
- Wu, T.; Long, C.; Xiong, L.; Li, J.; Liu, Q. Relationship between plant leaf functional traits and soil factors at different succession stages in karst forest of Maolan. Guihaia 2023, 43, 463–472. [Google Scholar]
- Zhou, Y.; Yang, G.; Qin, Z.; Yang, Y.; Jiang, Y.; Huang, L. Functional traits of fine roots and leaves of coastal herbaceous plants and their relationship with soil factors. Guihaia 2023, 43, 1975–1985. [Google Scholar]
- Song, X.; Nie, J.; Yang, M.; Yu, M.; Tao, L.; Feng, H.; Pan, J. Photosynthetic characteristics and reproductive strategy of Polygonum viviparum at different altitudes in Qilian Mountains. Chin. J. Appl. Environ. Biol. 2022, 28, 1527–1533. [Google Scholar]
- Sun, P.; Wei, X.; Ye, W.; Shen, H. Differences in leaf functional trait responses to heterogeneous habitats between dominant canopy and understory tree species in a south subtropical evergreen broad-leaved forest. Guihaia 2022, 42, 510–519. [Google Scholar]
- Tong, R.; Cao, Y.; Zhu, Z.; Lou, C.; Zhou, B.; Wu, T. Solar radiation effects on leaf nitrogen and phosphorus stoichiometry of Chinese fir across subtropical China. For. Ecosyst. 2021, 8, 62. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Liu, J.; Guo, Y.; Shen, G.; Gu, S. The structures and ecological adaptability of plant leaves of three compositae species on the Qinghai-Tibet plateau at different altitude. Acta Ecol. Sin. 2016, 36, 1559–1570. [Google Scholar] [CrossRef]
- Ou, Z.; Zheng, W.; Pang, S.; He, F.; Shen, W.; Tan, Y.; Chen, S. Altitude variation in covariation of the leaf functional traits of dominant woody plants in Mao’er Mountain of Guangxi. Ecol. Sci. 2024, 43, 95–101. [Google Scholar]
- Duan, G.; Wen, Z.; Xue, W.; Bu, Y.; Lu, J.; Wen, B.; Wang, B.; Chen, S. Agents affecting the plant functional traits in National Soil and Water Conservation Demonstration Park (China). Plants 2022, 11, 2891. [Google Scholar] [CrossRef]
- Xu, K.; Liu, X.; Zhao, C.; Pan, Q.; Chen, X.; Jiang, N.; Du, C.; Xu, Y.; Shao, M.; Qu, B. Nitrogen deposition further increases Ambrosia trifida root exudate invasiveness under global warming. Environ. Monit. Assess. 2023, 195, 759. [Google Scholar] [CrossRef]
- Collado, C.E.; Hwang, S.J.; Hernández, R. Supplemental greenhouse lighting increased the water use efficiency, crop growth, and cutting production in Cannabis sativa. Front. Plant Sci. 2024, 15, 1371702. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wu, H.; Lü, L.; Xiao, Z.; Yang, T.; Shi, H.; Wei, X. Geographic patterns of leaf functional traits and environmental drivers of national key protected wild plant Davidia involucrata Baillon. Plant Sci. J. 2024, 42, 160–169. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, J.; Cai, Z.; Wu, H.; Wang, S.; Xiao, Z.; Jiang, M.; Wei, X. Responses of leaf functional traits of endangered plant Celastrus orbiculatus to environmental factors. Acta Ecol. Sin. 2023, 43, 7252–7262. [Google Scholar]
- Pan, Y.; Chen, X.; Jiang, Y.; Liang, S.; Lu, Z.; Huang, Y.; Ni, M.; Qin, C.; Liu, R. Changes in leaf functional traits and soil environmental factors in response to slope gradient in Karst hills of Guilin. Acta Ecol. Sin. 2018, 38, 1581–1589. [Google Scholar] [CrossRef]
- Zhu, H.; Tan, Y. Flora and vegetation of Yunnan, Southwestern China: Diversity, origin and evolution. Diversity 2022, 14, 340. [Google Scholar] [CrossRef]
- Ai, Z.; Xu, T.; Zhou, Z.; Ma, F. Leaf morphological trait variations in natural populations of Caragana microphylla. Acta Bot. Boreali-Occident. Sin. 2020, 40, 1595–1604. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H.T.; Morgan, H.D.; Heijden, M.G.A.V.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167. [Google Scholar] [CrossRef]
- Meng, J.; Wang, G.; Cao, G.; Hu, N.; Zhao, M.; Zhao, Y.; Xue, Z.; Liu, B.; Piao, W.; Jiang, M. Patterns and drivers of plant species richness in Phragmites australis marshes in China. Biodivers. Sci. 2024, 32, 45–57. [Google Scholar] [CrossRef]
- Lyu, Z.; Li, W.; Huang, X.; Zhang, Z. Predicting suitable distribution area of three dominant tree species under climate change scenarios in Hebei Province. Sci. Silvae Sin. 2019, 55, 13–21. [Google Scholar]
- Li, X.; Duan, A.; Zhang, J. Site index for Chinese fir plantations varies with climatic and soil factors in southern China. J. For. Res. 2022, 33, 1765–1780. [Google Scholar] [CrossRef]
- Xu, Q.; Lei, X.; Zang, H.; Zeng, W. Climate change effects on height–diameter allometric relationship vary with tree species and size for larch plantations in Northern and Northeastern China. Forests 2022, 13, 468. [Google Scholar] [CrossRef]
- Wang, T.; Wang, G.; Innes, J.L.; Seely, B.; Chen, B. ClimateAP: An application for dynamic local downscaling of historical and future climate data in Asia Pacific. Front. Agric. Sci. Eng. 2017, 4, 448. [Google Scholar] [CrossRef]
- Lin, M.; Huang, Y.; Li, Y.; Sun, J. Geographical distribution characteristics and influencing factors of plant survival strategies in an alpine grassland. Chin. J. Plant Ecol. 2023, 47, 41. [Google Scholar] [CrossRef]
- Li, L.; Tao, C.; Lin, J.; Li, M.; Ma, X.; Wu, P. Response of needle anatomical structure of different Chinese fir clones to atmospheric warming. Acta Ecol. Sin. 2022, 42, 8385–8397. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Li, K.; Guo, Y. Estimation of soil organic carbon content in Bohu Lake Basin based on composite image and multivariate. Chin. J. Environ. Sci. 2025, 46, 4428–4440. [Google Scholar] [CrossRef]
- Chen, J.; Shen, C.; Fan, Z.; Zhang, X.; Huang, Z. Influencing factors of soil organic carbon in citrus orchard in the three Gorges Reservoir Area. Resour. Environ. Yangtze Val. 2024, 33, 2440–2450. [Google Scholar]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, R.; Zhang, X.; Tan, X.; Liu, T.; Wei, A. Identification of national priority protected areas of medicinal plant species based on random forest model. J. Southwest Univ. Nat. Sci. Ed. 2022, 44, 67–76. [Google Scholar]
- Zhang, L.; Wang, L.; Zhang, X.; Liu, S.; Sun, P.; Wang, T. The basic principle of random forest and its applications in ecology: A case study of Pinus yunnanensis. Acta Ecol. Sin. 2014, 34, 650–659. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres Neto, P.R. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Yu, Y.; Hu, W.; Lai, J. Application of “rdacca.hp” R. package in ecological data analysis: Case and progress. Chin. J. Plant Ecol. 2023, 47, 134. [Google Scholar] [CrossRef]
- Fornell, C.; Bookstein, F.L. Two structural equation models: LISREL and PLS applied to consumer exit-voice theory. J. Mark. Res. 1982, 19, 440–452. [Google Scholar] [CrossRef]
- Cao, W.; Liu, T.; Wang, G.; Luo, Y.; Fu, Q. Direct and indirect effects of habitat conditions on plant community in Horqin Sandy Land based on SEM. Chin. J. Ecol. 2019, 38, 1221–1229. [Google Scholar]
- Sarstedt, M.; Hair, J.F.; Ringle, C.M.; Thiele, K.O.; Gudergan, S.P. Estimation issues with PLS and CBSEM: Where the bias lies! J. Bus. Res. 2016, 69, 3998–4010. [Google Scholar] [CrossRef]
- Hu, Q.; Lin, Q.; Lin, Q. The influence mechanism of restorative perception on tourists’ environmental responsibility behavior in forest parks. Issues For. Econ. 2023, 43, 539–550. [Google Scholar]
- Teh, P.L.; Sander, T. SmartPLS for the human resources field to evaluate a model. In Proceedings of the New Challenges of Economic and Business Development 2014, Riga, Latvia, 8–10 May 2014. [Google Scholar]
- Fornell, C.; Larcker, D.F. Evaluating structural equation models with unobservable variables and measurement error. J. Mark. Res. 1981, 18, 39–50. [Google Scholar] [CrossRef]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 2nd ed.; Guiford Press: New York, NY, USA, 1998. [Google Scholar]
- Henseler, J.; Ringle, C.M.; Sarstedt, M. A new criterion for assessing discriminant validity in variance-based structural equation modeling. J. Acad. Mark. Sci. 2015, 43, 115–135. [Google Scholar] [CrossRef]
- Hair, J.F.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M. A Primer on Partial Least Squares Structural Equation Modeling (PLS-SEM); Sage Publications: New York, NY, USA, 2022. [Google Scholar]
- Kock, N.; Lynn, G. Lateral collinearity and misleading results in variance-based SEM: An illustration and recommendations. J. Assoc. Inf. Syst. 2012, 13, 546–580. [Google Scholar] [CrossRef]
- Darity, W. International Encyclopedia of the Social Sciences, 2nd ed.; The Gale Group: Detroit, MI, USA, 2008. [Google Scholar]
- Batista-Foguet, J.M.; Esteve, M.; van Witteloostuijn, A. Measuring leadership an assessment of the Multifactor Leadership Questionnaire. PLoS ONE 2021, 16, e0254329. [Google Scholar] [CrossRef]
- Shmueli, G.; Sarstedt, M.; Hair, J.F.; Cheah, J.; Ting, H.; Vaithilingam, S.; Ringle, C.M. Predictive model assessment in PLS-SEM: Guidelines for using PLSpredict. Eur. J. Mark. 2019, 53, 2322–2347. [Google Scholar] [CrossRef]
- Wong, K.K. Partial least squares structural equation modeling (PLS-SEM) techniques using SmartPLS. Mark. Bull. 2013, 24, 1. [Google Scholar]
- Henseler, J.; Sarstedt, M. Goodness-of-fit indices for partial least squares path modeling. Comput. Stat. 2013, 28, 565–580. [Google Scholar] [CrossRef]


| LFTs | Max | Min | Mean ± SD | PI | CV (%) | Q1 | Q3 | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|---|
| PL | 9.724 | 5.489 | 7.352 ± 1.243 | 0.436 | 16.906 | 6.472 | 7.981 | 0.668 | −0.557 |
| PD | 2.835 | 1.824 | 2.248 ± 0.257 | 0.357 | 11.452 | 2.020 | 2.457 | 0.342 | −0.678 |
| LFW | 1.975 | 0.816 | 1.261 ± 0.284 | 0.587 | 22.528 | 1.081 | 1.370 | 0.952 | 0.651 |
| LDW | 0.922 | 0.346 | 0.532 ± 0.125 | 0.625 | 23.544 | 0.453 | 0.589 | 1.335 | 2.365 |
| CHL | 72.771 | 44.281 | 59.895 ± 6.846 | 0.392 | 11.432 | 57.453 | 62.778 | −0.279 | 0.137 |
| LT | 1.085 | 0.493 | 0.758 ± 0.136 | 0.546 | 17.995 | 0.650 | 0.859 | 0.149 | −0.165 |
| LSN | 56.100 | 25.400 | 38.343 ± 7.567 | 0.547 | 19.734 | 32.725 | 43.425 | 0.584 | −0.084 |
| LSD | 1.731 | 0.986 | 1.334 ± 0.204 | 0.430 | 15.272 | 1.223 | 1.477 | 0.100 | −0.648 |
| LL | 18.453 | 9.075 | 12.530 ± 1.998 | 0.508 | 15.944 | 11.454 | 13.746 | 0.927 | 1.582 |
| LW | 5.435 | 3.305 | 4.300 ± 0.467 | 0.392 | 10.866 | 4.013 | 4.528 | 0.534 | 0.465 |
| LA | 65.600 | 19.052 | 36.928 ± 9.472 | 0.710 | 25.650 | 29.499 | 42.136 | 0.986 | 1.945 |
| LP | 42.149 | 21.723 | 29.129 ± 4.453 | 0.485 | 15.294 | 26.438 | 31.853 | 0.885 | 1.390 |
| LWR | 3.474 | 2.534 | 2.936 ± 0.280 | 0.271 | 9.522 | 2.691 | 3.178 | 0.215 | −1.127 |
| LSF | 0.615 | 0.462 | 0.538 ± 0.042 | 0.249 | 7.861 | 0.512 | 0.576 | 0.239 | −0.921 |
| LWC | 67.371 | 49.673 | 57.049 ± 4.034 | 0.263 | 7.071 | 54.506 | 59.119 | 0.581 | 0.688 |
| LDMC | 50.327 | 32.629 | 43.069 ± 3.986 | 0.352 | 9.255 | 41.047 | 45.494 | −0.677 | 0.955 |
| SLA | 117.573 | 45.608 | 72.168 ± 16.086 | 0.612 | 22.292 | 59.925 | 78.523 | 1.129 | 1.514 |
| LMA | 0.022 | 0.009 | 0.015 ± 0.003 | 0.591 | 19.978 | 0.013 | 0.017 | 0.108 | 0.121 |
| LSI | 0.029 | 0.015 | 0.021 ± 0.003 | 0.483 | 16.098 | 0.019 | 0.023 | 0.343 | 0.115 |
| LTD | 0.303 | 0.119 | 0.207 ± 0.045 | 0.607 | 21.941 | 0.179 | 0.235 | 0.199 | −0.130 |
| Latent Variables Group | Canonical Correlation Coefficients (Rc) | Eigenvalue | p-Value | Wilk’s | DF |
|---|---|---|---|---|---|
| GCs-CFs | 0.932 | 6.608 | 0.000 *** | 0.046 | 54.225 |
| GCs-SPs | 0.627 | 0.646 | 0.259 | 0.446 | 55.613 |
| CFs-SPs | 0.676 | 0.844 | 0.529 | 0.250 | 70.000 |
| GCs-LFTs | 0.853 | 2.669 | 0.001 ** | 0.066 | 43.371 |
| CFs-LFTs | 0.905 | 4.528 | 0.054 | 0.020 | 70.881 |
| SPs-LFTs | 0.878 | 3.380 | 0.004 ** | 0.019 | 65.728 |
| GCs-UVRFs | 0.963 | 12.820 | 0.000 *** | 0.011 | 64.005 |
| CFs-UVRFs | 0.990 | 49.445 | 0.000 *** | 0.000 | 60.210 |
| UVRFs-SPs | 0.977 | 20.686 | 0.000 *** | 0.000 | 64.315 |
| UVRFs-LFTs | 0.999 | 647.935 | 0.193 | 0.000 | 19.022 |
| Cronbach’s α | CR | AVE | R2 | Q2 | |
|---|---|---|---|---|---|
| CFs | 0.954 | 0.989 | 0.881 | 0.510 | 0.198 |
| GCs | 0.845 | 0.933 | 0.754 | — | 0.452 |
| LFTs | 0.731 | 0.930 | 0.670 | 0.226 | 0.223 |
| SPs | 0.796 | 0.790 | 0.554 | 0.181 | 0.147 |
| UVRFs | 0.933 | 0.934 | 0.833 | 0.335 | 0.409 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Ma, C.; Zhou, L.; Gui, Q.; Gong, M.; Yang, H.; Liu, J.; Chai, Y.; Sun, Y.; Wu, X. Intraspecific Variation and Environmental Determinants of Leaf Functional Traits in Polyspora chrysandra Across Yunnan, China. Plants 2025, 14, 2953. https://doi.org/10.3390/plants14192953
Yang J, Ma C, Zhou L, Gui Q, Gong M, Yang H, Liu J, Chai Y, Sun Y, Wu X. Intraspecific Variation and Environmental Determinants of Leaf Functional Traits in Polyspora chrysandra Across Yunnan, China. Plants. 2025; 14(19):2953. https://doi.org/10.3390/plants14192953
Chicago/Turabian StyleYang, Jianxin, Changle Ma, Longfei Zhou, Qing Gui, Maiyu Gong, Hengyi Yang, Jia Liu, Yong Chai, Yongyu Sun, and Xingbo Wu. 2025. "Intraspecific Variation and Environmental Determinants of Leaf Functional Traits in Polyspora chrysandra Across Yunnan, China" Plants 14, no. 19: 2953. https://doi.org/10.3390/plants14192953
APA StyleYang, J., Ma, C., Zhou, L., Gui, Q., Gong, M., Yang, H., Liu, J., Chai, Y., Sun, Y., & Wu, X. (2025). Intraspecific Variation and Environmental Determinants of Leaf Functional Traits in Polyspora chrysandra Across Yunnan, China. Plants, 14(19), 2953. https://doi.org/10.3390/plants14192953






