Identification of High- and Low-Cadmium (Cd)-Accumulating Rice Cultivars Using Combined Molecular Markers
Abstract
1. Introduction
2. Results
2.1. Total Cadmium Concentration in the Soils
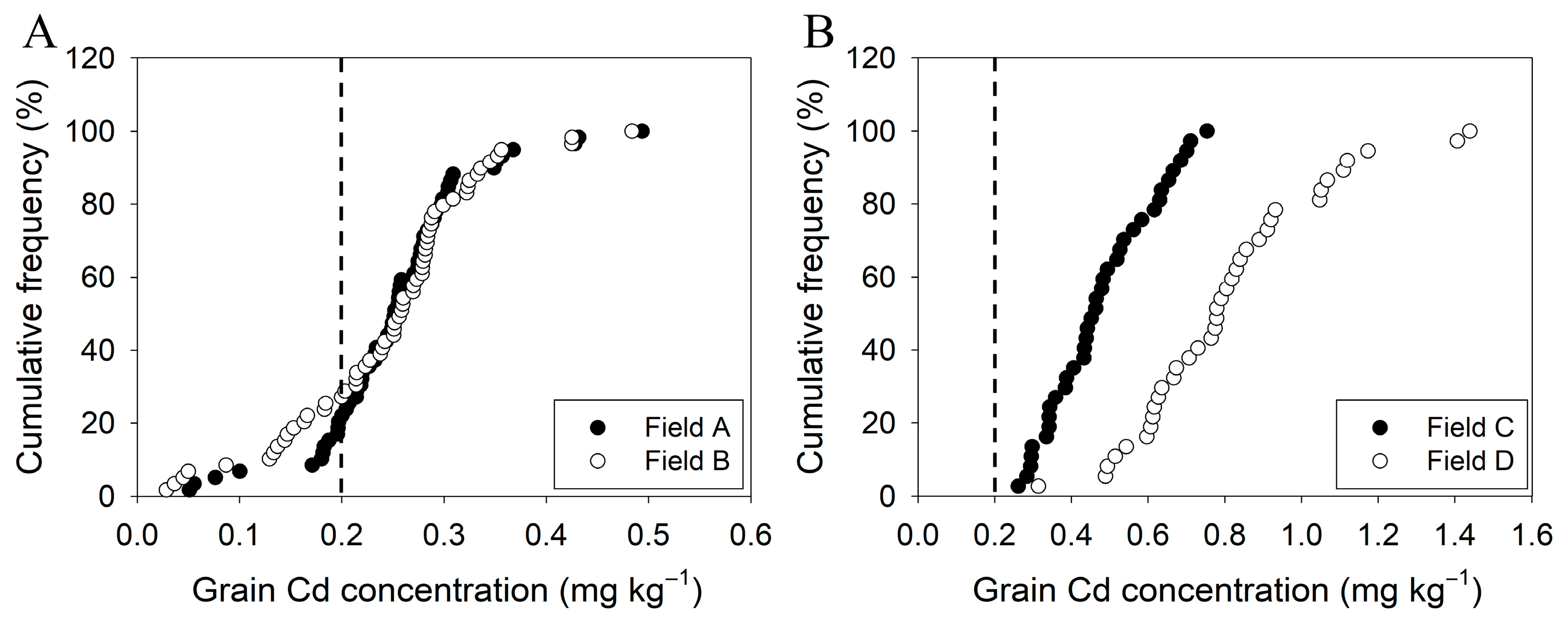
2.2. Cadmium Concentrations in Rice Grain
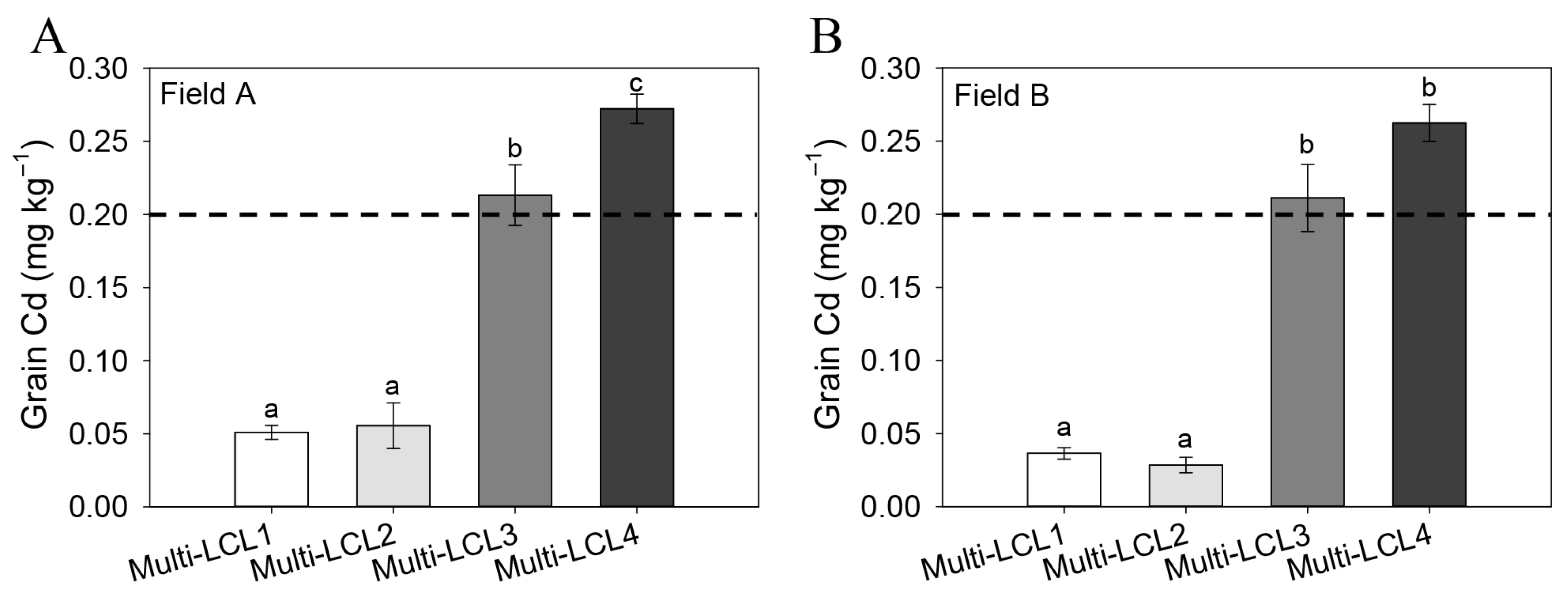
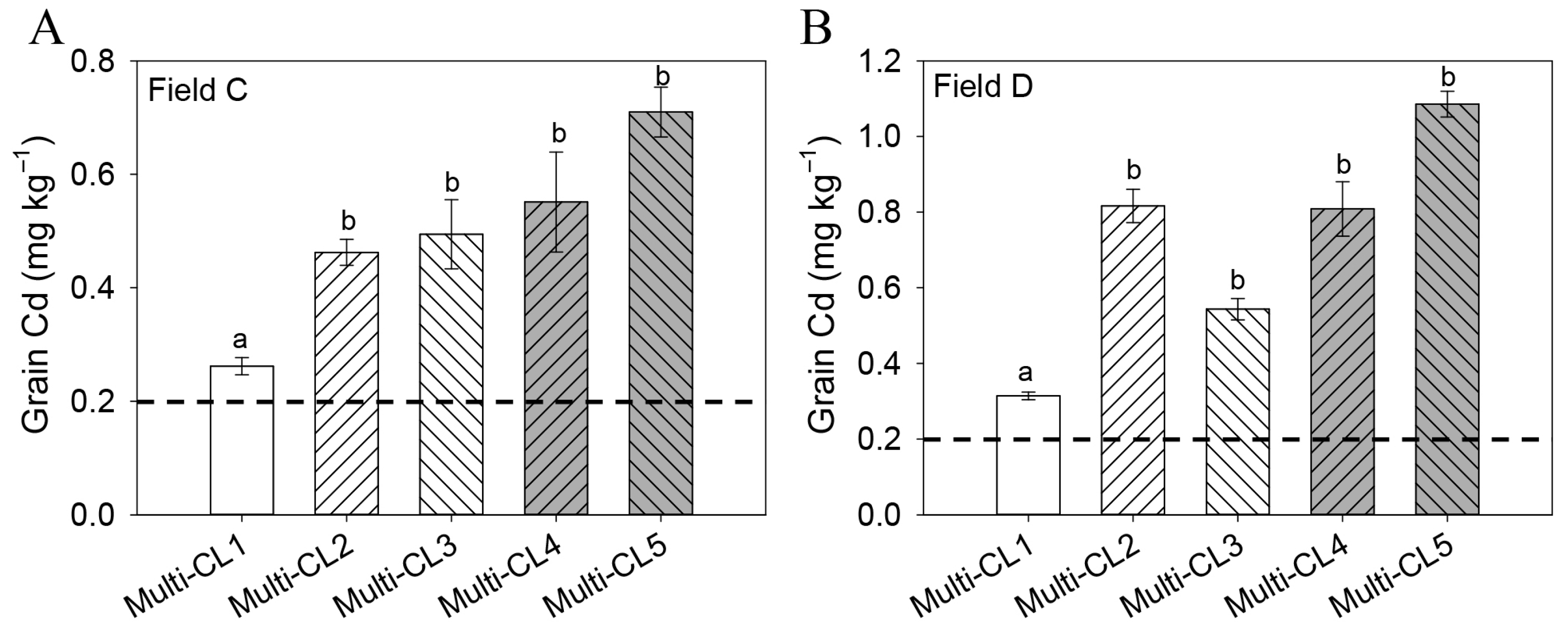
2.3. Screening of Rice Genotypes for High- and Low-Cd-Accumulating Cultivars Using Molecular Markers
2.4. Efficacy of Combined Molecular Markers in Classifying High- and Low-Cd-Accumulating Rice Cultivars
3. Discussion
4. Materials and Methods
4.1. Rice Cultivars
4.2. Field Sites
4.3. Rice Cultivation
4.4. Genomic DNA Isolation and Genotyping
4.5. Rice Grain and Soil Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC. Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 58, 1–415. [Google Scholar]
- NTP. National Toxicology Program, Tenth Report on Carcinogens; Department of Health and Human Services, Research Triangle Park: Durham, NC, USA, 2000; pp. III-42–III-44.
- McLaughlin, M.J.; Smolders, E.; Zhao, F.J.; Grant, C.; Montalvo, D. Managing cadmium in agricultural systems. In Advances in Agronomy, Vol 166; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; 129p. [Google Scholar]
- Hou, D.Y.; Jia, X.Y.; Wang, L.W.; McGrath, S.P.; Zhu, Y.G.; Hu, Q.; Zhao, F.J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef]
- The Ministry of Environmental Protection (MEP). The Ministry of Land and Resources Report on the National Soil Contamination Survey. Available online: https://www.gov.cn/foot/2014-04/17/content_2661768.htm (accessed on 17 April 2014).
- Zhao, F.J.; Ma, Y.B.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Shao, D.W.; Zhan, Y.; Zhou, W.J.; Zhu, L.Z. Current status and temporal trend of heavy metals in farmland soil of the Yangtze River Delta Region: Field survey and meta-analysis. Environ. Pollut. 2016, 219, 329–336. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.P.; Kopittke, P.M.; Zhao, F.J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef]
- Meharg, A.A.; Norton, G.; Deacon, C.; Williams, P.; Adomako, E.E.; Price, A.; Zhu, Y.G.; Li, G.; Zhao, F.J.; McGrath, S.; et al. Variation in Rice Cadmium Related to Human Exposure. Environ. Sci. Technol. 2013, 47, 5613–5618. [Google Scholar] [CrossRef]
- Zhao, F.J.; Wang, P. Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 2020, 446, 1–21. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, P.; Zhao, F.-J. Toxic metals and metalloids in food: Current status, health risks, and mitigation strategies. Curr. Environ. Health Rep. 2024, 11, 468–483. [Google Scholar] [CrossRef]
- Tsuchiya, K. Epidemiological studies on cadmium in the environment in Japan: Etiology of itai-itai disease. Fed. Proc. 1976, 35, 2412–2418. [Google Scholar] [PubMed]
- Satarug, S.; Moore, M.R. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ. Health Perspect. 2004, 112, 1099–1103. [Google Scholar] [CrossRef]
- Authority, E.F.S. Scientific opinion on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar]
- Song, Y.; Wang, Y.B.N.; Mao, W.F.; Sui, H.X.; Yong, L.; Yang, D.J.; Jiang, D.G.; Zhang, L.; Gong, Y.Y. Dietary cadmium exposure assessment among the Chinese population. PLoS ONE 2017, 12, e0177978. [Google Scholar] [CrossRef]
- Wang, P.; Kopittke, P.M.; McGrath, S.P.; Zhao, F.-J.; Grp, I.W. Cadmium Transfer from Soil to Plants and its Potential Risk to Human Health; Catena Soil Sciences: Stuttgart, Germany, 2017; pp. 138–147. [Google Scholar]
- Chen, H.P.; Yang, X.P.; Wang, P.; Wang, Z.X.; Li, M.; Zhao, F.J. Dietary cadmium intake from rice and vegetables and potential health risk: A case study in Xiangtan, southern China. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef]
- Huang, N.; Wang, B.; Liu, S.; Wang, K.; Wang, R.; Liu, F.; Chen, C. Cadmium exposure in infants and children: Toxicity, health effects, dietary risk assessment and mitigation strategies. Crit. Rev. Food Sci. Nutr. 2024, 65, 5085–5107. [Google Scholar] [CrossRef]
- Wu, D.Z.; Yamaji, N.; Yamane, M.; Kashino-Fujii, M.; Sato, K.; Ma, J.F. The HvNramp5 Transporter Mediates Uptake of Cadmium and Manganese, But Not Iron. Plant Physiol. 2016, 172, 1899–1910. [Google Scholar] [CrossRef]
- Sui, F.Q.; Chang, J.D.; Tang, Z.; Liu, W.J.; Huang, X.Y.; Zhao, F.J. Nramp5 expression and functionality likely explain higher cadmium uptake in rice than in wheat and maize. Plant Soil 2018, 433, 377–389. [Google Scholar] [CrossRef]
- Hu, Y.N.; Cheng, H.F.; Tao, S. The Challenges and Solutions for Cadmium-contaminated Rice in China: A Critical Review. Environ. Int. 2016, 92–93, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.H.; Chen, C.; Xu, C.; Zhu, Q.H.; Huang, D.Y. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef]
- Chaney, R.L.; Kim, W.I.; Yang, J.E.; Ok, Y.S. Integrated management strategies for arsenic and cadmium in rice paddy environments Preface. Geoderma 2016, 270, 1–2. [Google Scholar] [CrossRef]
- Zhao, F.J.; Tang, Z.; Song, J.J.; Huang, X.Y.; Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol. Plant 2022, 15, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Noor, I.; Sohail, H.; Akhtar, M.T.; Cui, J.; Lu, Z.; Mostafa, S.; Hasanuzzaman, M.; Hussain, S.; Guo, N.; Jin, B. From stress to resilience: Unraveling the molecular mechanisms of cadmium toxicity, detoxification and tolerance in plants. Sci. Total Environ. 2024, 954, 176462. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of Water Management on Cadmium and Arsenic Accumulation and Dimethylarsinic Acid Concentrations in Japanese Rice. Environ. Sci. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal Soil Eh, pH, and Water Management for Simultaneously Minimizing Arsenic and Cadmium Concentrations in Rice Grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef]
- Huang, F.Y.; Li, Z.M.; Yang, X.; Liu, H.J.; Chen, L.; Chang, N.; He, H.R.; Zeng, Y.; Qiu, T.Y.; Fang, L.C. Silicon reduces toxicity and accumulation of arsenic and cadmium in cereal crops: A meta-analysis, mechanism, and perspective study. Sci. Total Environ. 2024, 918, 170663. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Wang, H.; Li, C.; Zhang, Y.; Dong, Z.; Fu, C.; Ye, Y.; Wang, F.; Chen, X. Effects of different phosphorus fertilizers on cadmium absorption and accumulation in rice under low-phosphorus and rich-cadmium soil. Environ. Sci. Pollut. Res. 2024, 31, 11898–11911. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Chen, F.X.; Zhang, Y.H.; Chen, X.J. Predicting heavy metal concentration in crop grain using automated machine learning models. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2025, 36, 1889–1897. [Google Scholar]
- Duan, G.L.; Shao, G.S.; Tang, Z.; Chen, H.P.; Wang, B.X.; Tang, Z.; Yang, Y.P.; Liu, Y.C.; Zhao, F.J. Genotypic and Environmental Variations in Grain Cadmium and Arsenic Concentrations Among a Panel of High Yielding Rice Cultivars. Rice 2017, 10, 9. [Google Scholar] [CrossRef]
- Tang, Z.; You, T.T.; Li, Y.F.; Tang, Z.X.; Bao, M.Q.; Dong, G.; Xu, Z.R.; Wang, P.; Zhao, F.J. Rapid identification of high and low cadmium (Cd) accumulating rice cultivars using machine learning models with molecular markers and soil Cd levels as input data. Environ. Pollut. 2023, 326, 121501. [Google Scholar] [CrossRef]
- Pinson, S.R.M.; Tarpley, L.; Yan, W.G.; Yeater, K.; Lahner, B.; Yakubova, E.; Huang, X.Y.; Zhang, M.; Guerinot, M.L.; Salt, D.E. Worldwide Genetic Diversity for Mineral Element Concentrations in Rice Grain. Crop Sci. 2015, 55, 294–311. [Google Scholar] [CrossRef]
- Zhang, M.; Pinson, S.R.M.; Tarpley, L.; Huang, X.Y.; Lahner, B.; Yakubova, E.; Baxter, I.; Guerinot, M.L.; Salt, D.E. Mapping and validation of quantitative trait loci associated with concentrations of 16 elements in unmilled rice grain. Theor. Appl. Genet. 2014, 127, 137–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lu, K.; Zhao, F.J.; Xie, W.B.; Ramakrishna, P.; Wang, G.Y.; Du, Q.Q.; Liang, L.M.; Sun, C.J.; Zhao, H.; et al. Genome-Wide Association Studies Reveal the Genetic Basis of Ionomic Variation in Rice. Plant Cell 2018, 30, 2720–2740. [Google Scholar] [CrossRef]
- Palali Delen, S.; Lee, J.; Yang, J. Improving the metal composition of plants for reduced Cd and increased Zn content: Molecular mechanisms and genetic regulations. Cereal Res. Commun. 2024, 52, 901–918. [Google Scholar] [CrossRef]
- Sun, L.; Wang, R.G.; Tang, W.B.; Chen, Y.C.; Zhou, J.Q.; Ma, H.R.; Li, S.; Deng, H.B.; Han, L.; Chen, Y.B.; et al. Robust identification of low-Cd rice varieties by boosting the genotypic effect of grain Cd accumulation in combination with marker-assisted selection. J. Hazard. Mater. 2022, 424, 127703. [Google Scholar] [CrossRef]
- Norton, G.J.; Deacon, C.M.; Xiong, L.Z.; Huang, S.Y.; Meharg, A.A.; Price, A.H. Genetic mapping of the rice ionome in leaves and grain: Identification of QTLs for 17 elements including arsenic, cadmium, iron and selenium. Plant Soil 2010, 329, 139–153. [Google Scholar] [CrossRef]
- Abe, T.; Nonoue, Y.; Ono, N.; Omoteno, M.; Kuramata, M.; Fukuoka, S.; Yamamoto, T.; Yano, M.; Ishikawa, S. Detection of QTLs to reduce cadmium content in rice grains using LAC23/Koshihikari chromosome segment substitution lines. Breed. Sci. 2013, 63, 284–291. [Google Scholar] [CrossRef] [PubMed]
- State Council of the People’s Republic of China. Action Plan for Soil Pollution Prevention. 2016. Available online: http://www.gov.cn/zhengce/content/2016-05/31/content_5078377.htm (accessed on 10 July 2025).
- Ueno, D.; Kono, I.; Yokosho, K.; Ando, T.; Yano, M.; Ma, J.F. A major quantitative trait locus controlling cadmium translocation in rice (Oryza sativa). New Phytol. 2009, 182, 644–653. [Google Scholar] [CrossRef]
- Du, J.; Zeng, D.L.; Wang, B.; Qian, Q.; Zheng, S.S.; Ling, H.Q. Environmental effects on mineral accumulation in rice grains and identification of ecological specific QTLs. Environ. Geochem. Health 2013, 35, 161–170. [Google Scholar] [CrossRef]
- Yan, H.L.; Xu, W.X.; Xie, J.Y.; Gao, Y.W.; Wu, L.L.; Sun, L.; Feng, L.; Chen, X.; Zhang, T.; Dai, C.H.; et al. Variation of a major facilitator superfamily gene contributes to differential cadmium accumulation between rice subspecies. Nat. Commun. 2019, 10, 2562. [Google Scholar] [CrossRef]
- Wang, C.C.; Tang, Z.; Zhuang, J.Y.; Tang, Z.; Huang, X.Y.; Zhao, F.J. Genetic mapping of ionomic quantitative trait loci in rice grain and straw reveals OsMOT1;1 as the putative causal gene for a molybdenum QTL qMo8. Mol. Genet. Genom. 2020, 295, 391–407. [Google Scholar] [CrossRef]
- Ishikawa, S.; Ishimaru, Y.; Igura, M.; Kuramata, M.; Abe, T.; Senoura, T.; Hase, Y.; Arao, T.; Nishizawa, N.K.; Nakanishi, H. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc. Natl. Acad. Sci. USA 2012, 109, 19166–19171. [Google Scholar] [CrossRef]
- Chen, J.G.; Zou, W.L.; Meng, L.J.; Fan, X.R.; Xu, G.H.; Ye, G.Y. Advances in the Uptake and Transport Mechanisms and QTLs Mapping of Cadmium in Rice. Int. J. Mol. Sci. 2019, 20, 3417. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Hu, M.; Jiang, Y.; Yan, H.; Xu, W.; Yu, Y.; He, Z. Development and utilization of KASP marker LCd-38 for cadmium accumulation in rice grain. China Agric. Sci. Technol. Her. 2022, 24, 40–47. [Google Scholar]
- Luo, J.S.; Huang, J.; Zeng, D.L.; Peng, J.S.; Zhang, G.B.; Ma, H.L.; Guan, Y.; Yi, H.Y.; Fu, Y.L.; Han, B.; et al. A defensin-like protein drives cadmium efflux and allocation in rice. Nat. Commun. 2018, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.Y.; Qi, Z.A.; Wang, Y.T.; Chen, S.Y.; Yan, J.; Qiu, H.P.; Yu, Y.X.; Fang, Z.J.; Wang, J.M.; Gong, J.M. An endophytic fungus interacts with the defensinlike protein OsCAL1 to regulate cadmium allocation in rice. Mol. Plant 2024, 17, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Zhou, J.Q.; Wang, J.R.; Sun, L. The Genetic Architecture for Phenotypic Plasticity of the Rice Grain Ionome. Front. Plant Sci. 2020, 11, 12. [Google Scholar] [CrossRef]
- Gong, W.-q.; Li, L.-q.; Pan, G.-x. Cd uptake and accumulation in grains by hybrid rice in two paddy soils: Interactive effect of soil type and cultivars. Huanjing Kexue 2006, 27, 1647–1653. [Google Scholar]
- Sun, L.; Xu, X.X.; Jiang, Y.R.; Zhu, Q.H.; Yang, F.; Zhou, J.Q.; Yang, Y.Z.; Huang, Z.Y.; Li, A.H.; Chen, L.H.; et al. Genetic Diversity, Rather than Cultivar Type, Determines Relative Grain Cd Accumulation in Hybrid Rice. Front. Plant Sci. 2016, 7, 1407. [Google Scholar] [CrossRef]
- He, Z.Y.; Ma, M.; Xu, W.X.; Yan, H.L. Application of SNP Molecular Marker of Cadmium Content Related Gene LCd-31 in Rice Grain. Patent China CN105624319B, 25 December 2018. [Google Scholar]
- He, Z.Y.; Ma, M.; Xu, W.X.; Yan, H.L. Application of SNP Molecular Marker of Cadmium Content Related Gene LCd-38 in Rice Grain. Patent China CN105543397B, 25 December 2018. [Google Scholar]
- Gong, J.M.; Luo, J.X. A Short Peptide Reducing Cytoplasmic Cadmium Accumulation, Its Encoding Gene and Application. Patent China CN108250280B, 1 June 2021. [Google Scholar]
- He, Z.Y.; Ma, M.; Xu, W.X.; Yan, H.L. Application of SNP Molecular Marker of Cadmium Content Related Gene LCd-41 in Rice Grain. Patent China CN105671164B, 8 March 2019. [Google Scholar]
- Neff, M.M.; Turk, E.; Kalishman, M. Web-based primer design for single nucleotide polymorphism analysis. TRENDS Genet. 2002, 18, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–13. [Google Scholar]
- McGrath, S.P.; Cunliffe, C.H. A simplified method for the extraction of the metals Fe, Zn, Cu, Ni, Cd, Pb, Cr, Co and Mn from soils and sewage sludges. J. Sci. Food Agric. 1985, 36, 794–798. [Google Scholar] [CrossRef]
| Site | Cd (mg kg−1) |
|---|---|
| Field A | 0.48 |
| Field B | 0.67 |
| Field C | 0.78 |
| Field D | 0.69 |
| Genotype NO. | Field A | Field B | Mean Accuracy (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Marker | High-Cd Genotype | Low-Cd Genotype | High-Cd Accuracy (%) | Low-Cd Accuracy (%) | Overall Accuracy (%) | High-Cd Accuracy (%) | Low-Cd Accuracy (%) | Overall Accuracy (%) | |
| LCd-41 | 57 | 2 | 80.7 | 100.0 | 81.4 | 75.4 | 100.0 | 76.3 | 78.8 |
| CAL1 | 58 | 1 | 79.3 | 100.0 | 79.7 | 74.1 | 100.0 | 74.6 | 77.1 |
| LCd-38 | 46 | 13 | 87.0 | 53.8 | 79.7 | 76.1 | 38.5 | 67.8 | 73.7 |
| LCd-41-CAL1-LCd-38 | 57 | 2 | 80.7 | 100.0 | 81.4 | 75.4 | 100.0 | 76.3 | 78.8 |
| Genotype NO. | Field C | Field D | Mean Accuracy (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Marker | High-Cd Genotype | Low-Cd Genotype | High-Cd Accuracy (%) | Low-Cd Accuracy (%) | Overall Accuracy (%) | High-Cd Accuracy (%) | Low-Cd Accuracy (%) | Overall Accuracy (%) | |
| CAL1 | 36 | 1 | 100.0 | 0.0 | 97.2 | 100.0 | 0.0 | 97.2 | 97.2 |
| LCd-31 | 3 | 34 | 100.0 | 0.0 | 8.1 | 100.0 | 0.0 | 8.1 | 8.1 |
| CAL1-LCd-31 | 36 | 1 | 100.0 | 0.0 | 97.20 | 100.0 | 0.0 | 97.2 | 97.2 |
| Variation | SS | df | MS | F | p-Value | SSE/SST (%) a | |
|---|---|---|---|---|---|---|---|
| Early rice cultivars | Markers | 0.593 | 1 | 0.593 | 25.858 | <0.001 | 13 |
| Field | 21.41 | 2 | 10.705 | 466.425 | <0.001 | 84.4 | |
| Marker × Field | 22.003 | 3 | 7.334 | 319.569 | <0.001 | 84.7 | |
| Error | 3.971 | 173 | 0.023 | ||||
| Late rice cultivars | Markers | 0.264 | 1 | 0.264 | 7.143 | 0.009 | 9.1 |
| Field | 2.038 | 1 | 2.038 | 55.177 | <0.001 | 43.7 | |
| Marker × Field | 2.302 | 2 | 1.151 | 31.16 | <0.001 | 46.7 | |
| Error | 2.622 | 71 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Ding, F.; Rong, Q.; Lu, Z.; Fu, J.; Zhou, C. Identification of High- and Low-Cadmium (Cd)-Accumulating Rice Cultivars Using Combined Molecular Markers. Plants 2025, 14, 2931. https://doi.org/10.3390/plants14182931
Wang C, Ding F, Rong Q, Lu Z, Fu J, Zhou C. Identification of High- and Low-Cadmium (Cd)-Accumulating Rice Cultivars Using Combined Molecular Markers. Plants. 2025; 14(18):2931. https://doi.org/10.3390/plants14182931
Chicago/Turabian StyleWang, Chengcheng, Fangfang Ding, Qinlei Rong, Zhihong Lu, Junru Fu, and Chunhuo Zhou. 2025. "Identification of High- and Low-Cadmium (Cd)-Accumulating Rice Cultivars Using Combined Molecular Markers" Plants 14, no. 18: 2931. https://doi.org/10.3390/plants14182931
APA StyleWang, C., Ding, F., Rong, Q., Lu, Z., Fu, J., & Zhou, C. (2025). Identification of High- and Low-Cadmium (Cd)-Accumulating Rice Cultivars Using Combined Molecular Markers. Plants, 14(18), 2931. https://doi.org/10.3390/plants14182931







