High Dose of Nickel Unbalances Carbon Metabolism and Nitrogen Assimilation in Barley (Hordeum vulgare L.)
Abstract
1. Introduction
2. Results
2.1. Effects of Ni2+ on Barley Growth
2.2. Genotoxic Damage Induced by Ni2+ Exposure
2.3. Changes in Main Photosynthetic Parameters
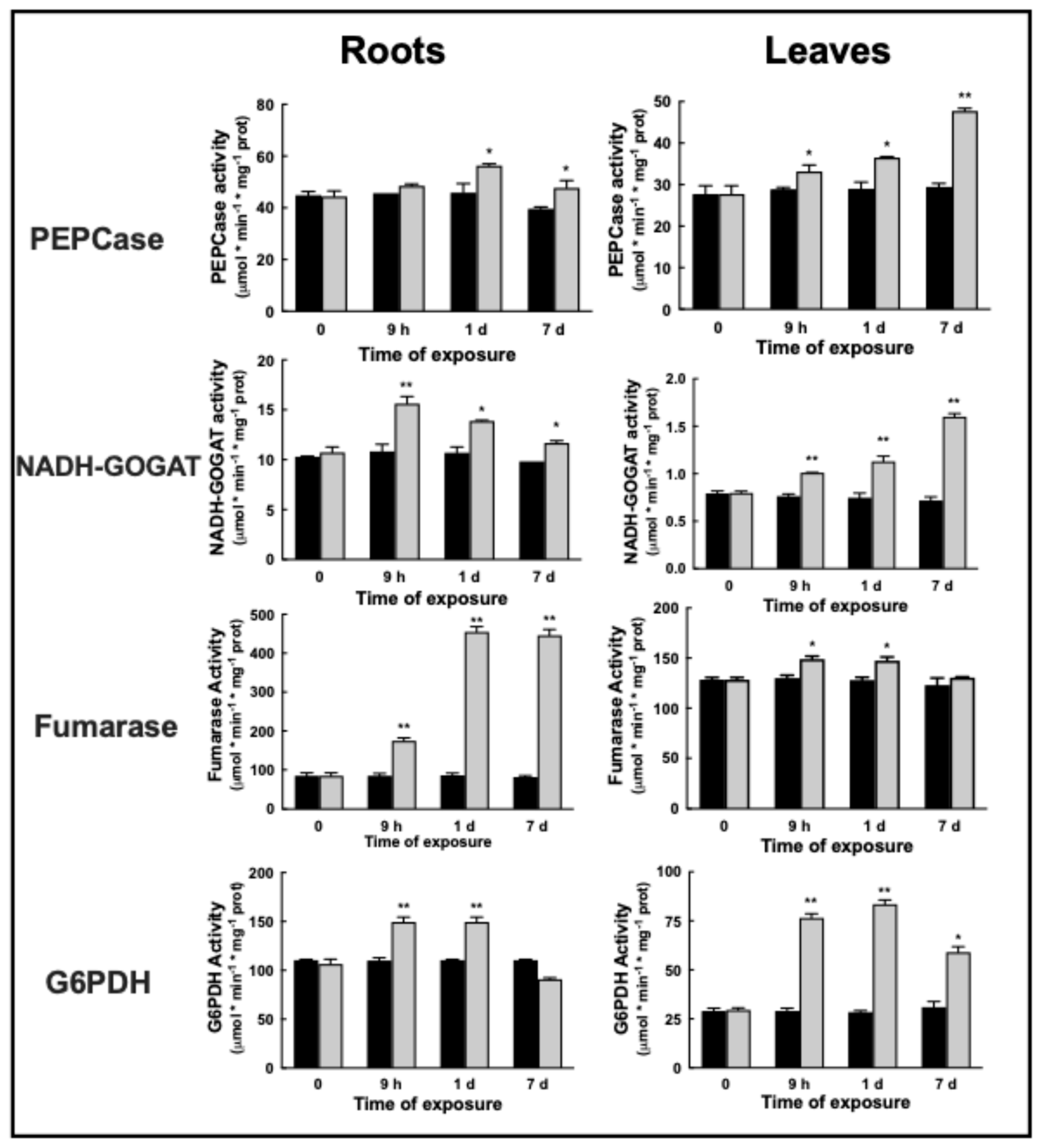
2.4. Enzymatic Activities and Changes in Proteins upon Ni2+ Exposure
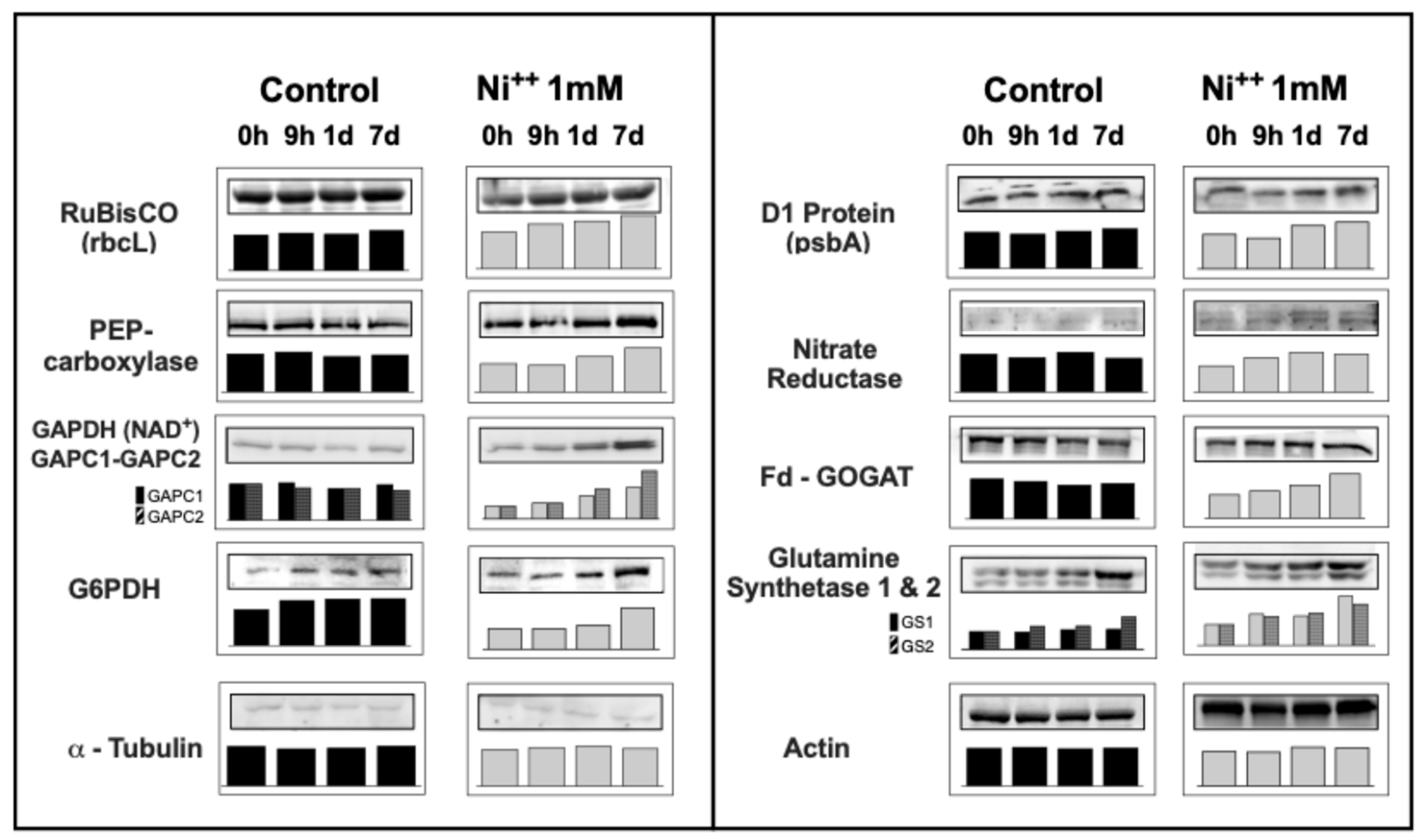
2.5. Changes in Presence of Proteins and Enzymes upon Ni2+ Stress in Barley Leaves
2.5.1. Proteins and Enzymes Involved in Photosynthesis and Carbon Metabolism
2.5.2. Enzymes Involved in Inorganic Nitrogen Assimilation and Metabolism
2.6. Changes in Gene Expression upon Ni2+ Stress in Barley Leaves
3. Discussion
3.1. Cell Damage and Growth
3.2. Photosynthetic Efficiency
3.3. Carbon Metabolism
3.4. Nitrogen Assimilation
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Growth Variation in Barley Plants
4.3. Photosynthesis Measurements
4.4. Comet Assays
4.5. Preparation of Protein Extracts
4.6. Extraction and Assays of Enzymes
4.7. Gel Electrophoresis (SDS-PAGE) and Immunoblotting Analyses
4.8. RNA Isolation, cDNA Production and Real-Time PCR Analyses
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Fd-GOGAT | Ferredoxin-dependent glutamine oxoglutarate aminotransferase (glutamate synthase)—EC 1.4.7.1 |
| G6PDH | Glucose-6P dehydrogenase—EC 1.1.1.49 |
| GS1/GS2 | Glutamine synthetase 1(cytosolic)/glutamine synthetase 2 (chloroplast)—EC 6.3.1.2 |
| NADH- GOGAT | NADH-dependent glutamine oxoglutarate aminotransferase (glutamate synthase)—EC 1.4.1.14 |
| NR | Nitrate reductase—EC 1.7.1.1 |
| PEPCase | Phosphoenolpyruvate carboxylase—EC 4.1.1.31 |
| PMSF | Phenyl-methyl-sulfonyl-fluoride |
| RuBisCO | Ribulose-1,5-bisphosphate carboxylase/oxygenase—EC 4.1.1.39 |
References
- Costa Rodrigues, W.P.; Breno, P.; Lisboa, A.; Esrom Lima, J.; Klein Ricachenevsky, F.; Del-Bem, L.-E. Ferrous iron uptake via IRT1/ZIP evolved at least twice in green plants. New Phytol. 2023, 237, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Palmgren, M.G.; Kraemer, U. A long way ahead: Understanding and engineering plant metal accumulation. Trends Plant Sci. 2002, 7, 309–315. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Goyal, D.; Yadav, A.; Prasad, M.; Singh, T.B.; Shrivastav, P.; Ali, A.; Dantu, P.K.; Mishra, S. Effect of Heavy Metals on Plant Growth: An Overview. In Contaminants in Agriculture, 1st ed.; Naeem, M., Ansari, A., Gill, S., Eds.; Springer: Cham, Switzerland, 2020; pp. 79–101. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Conte, B.; Golia, B.; Montanari, S.; Cobianchi, R.C.; Esposito, S. Antioxidant activity in extracts from Leptodictyum riparium (Bryophyta), stressed by heavy metals, heat shock, and salinity. Plant Biosyst. 2011, 145, 77–80. [Google Scholar] [CrossRef]
- Esposito, S.; Sorbo, S.; Conte, B.; Basile, A. Effects of heavy metals on ultrastructure and HSP70s induction in the aquatic moss Leptodictyum riparium Hedw. Int. J. Phytorem. 2012, 14, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.; Dubey, N.; Chauhan, D. Influence of High and Low Levels of Plant-Beneficial Heavy Metal Ions on Plant Growth and Development. Front. Environ. Sci. 2016, 4, 69. [Google Scholar] [CrossRef]
- dos Reis, D.A.; da Fonseca Santiago, A.; Nascimento, L.P.; Roeser, H.M.P. Influence of environmental and anthropogenic factors at the bottom sediments in a Doce River tributary in Brazil. Environ. Sci. Pollut. Res. 2017, 24, 7456. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Jasiewicz, C.; Koncewicz-Baran, M.; Sendor, R. Nickel bioaccumulation by the chosen plant species. Acta Physiol. Plant. 2016, 38, 40. [Google Scholar] [CrossRef]
- Rooney, C.P.; Zhao, F.-J.; McGrath, S.P. Phytotoxicity of nickel in a range of European soils: Influence of soil properties, Ni solubility and speciation. Environ. Pollut. 2007, 145, 596–605. [Google Scholar] [CrossRef]
- Wang, P.; Kopittke, P.M.; De Schamphelaere, K.A.C.; Zhao, F.-J.; Zhou, D.-M.; Lock, K.; Ma, Y.-B.; Peijnenburg, W.J.G.M.; McGrath, S.P. Evaluation of an electrostatic toxicity model for predicting Ni2+ toxicity to barley root elongation in hydroponic cultures and in soils. New Phytol. 2011, 192, 414–427. [Google Scholar] [CrossRef]
- Baccouch, S.; Chaoui, A.; El Ferjani, E. Nickel toxicity: Effects on growth and metabolism of maize. J. Plant Nutr. 1998, 21, 577–588. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.R.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]
- Qin, C.; Shen, J.; Ahanger, M.A. Supplementation of nitric oxide and spermidine alleviates the nickel stress-induced damage to growth, chlorophyll metabolism, and photosynthesis by upregulating ascorbate–glutathione and glyoxalase cycle functioning in tomato. Front. Plant Sci. 2022, 13, 1039480. [Google Scholar] [CrossRef] [PubMed]
- Roccotiello, E.; Nicosia, E.; Pierdonà, L.; Marescotti, P.; Ciardiello, M.A.; Giangrieco, I.; Mari, A.; Zennaro, D.; Dozza, D.; Brancucci, M.; et al. Tomato (Solanum lycopersicum L.) accumulation and allergenicity in response to nickel stress. Sci. Rep. 2022, 12, 5432. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Kozhevnikova, A.D. Physiological Role of Nickel and Its Toxic Effects on Higher Plants. Russ. J. Plant Physiol. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- Kovácik, J.; Klejdus, B.; Stork, F.; Hedbavny, J. Physiological responses of Tillandsia albida (Bromeliaceae) to long-term foliar metal application. J. Hazard. Mater. 2012, 239–240, 175–182. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, C.; Lv, K.; Li, Y.; Cheng, J.; Chen, X.; Fang, X.; Yu, X. Genotypic Variation in Nickel Accumulation and Translocation and Its Relationships with Silicon, Phosphorus, Iron, and Manganese among 72 Major Rice Cultivars from Jiangsu Province, China. Int. J. Environ. Res. Public Health 2019, 16, 3281. [Google Scholar] [CrossRef]
- Rizwan, M.; Imtiaz, M.; Dai, Z.; Mehmood, S.; Adeel, M.; Liu, J.; Tu, S. Nickel stressed response of rice in Ni subcellular distribution, antioxidant production and osmolyte accumulation. Environ. Sci. Pollut. Res. 2017, 24, 20587–20598. [Google Scholar] [CrossRef]
- Gupta, V.; Jatav, P.K.; Verma, R.; Kothari, S.L.; Kachlwaha, S. Nickel accumulation and its effect on growth, physiological and biochemical parameters in millets and oats. Environ. Sci. Pollut. Res. 2017, 24, 23915–23925. [Google Scholar] [CrossRef]
- Uruç Parlak, K. Effect of nickel on growth and biochemical characteristics of wheat (Triticum aestivum L.) seedlings. NJAS Wagening. J. Life Sci. 2016, 76, 1–5. [Google Scholar] [CrossRef]
- Molas, J.; Baran, S. Relationship between the chemical form of nickel applied to the soil and its uptake and toxicity to barley plants (Hordeum vulgare L.). Geoderma 2004, 122, 247–255. [Google Scholar] [CrossRef]
- Luo, Z.; Bani Mfarrej, M.F.; Saleem, M.H.; Ma, J.; Saleh, I.A.; Abdel-Maksoud, M.A.; Zakri, A.M.; Chen, F.; Gómez Oliván, L.M. Individual and combinatorial application of nanosilica and carbon nanoparticles alleviate nickel stress in barley (Hordeum vulgare L.): Impacts on gene expression, AsA − GSH cycle, cellular fractionation, and proline metabolism. Sci. Total Environ. 2024, 954, 176304. [Google Scholar] [CrossRef]
- Kovácik, J.; Vydra, M. The impact of nickel on plant growth and oxidative balance. Physiol. Plant. 2024, 176, e14595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, S.; Xu, Y.; Zhou, Y.; Ran, S.; Zhao, H.; Huang, W.; Xu, R.; Zhong, F. Effect of Nickel Ions on the Physiological and Transcriptional Responses to Carbon and Nitrogen Metabolism in Tomato Roots under Low Nitrogen Levels. Int. J. Mol. Sci. 2022, 23, 11398. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.U.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities-a review. Environ. Sci. Pollut. Res. Int. 2019, 26, 12673–12688. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Stoleru, V.; Gavrilescu, M. Analysis of Heavy Metal Impacts on Cereal Crop Growth and Development in Contaminated Soils. Agriculture 2023, 13, 1983. [Google Scholar] [CrossRef]
- Fargasovà, A. Plants as models for chromium and nickel risk assessment. Ecotoxicology 2012, 21, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Tamás, L.; Dudíková, J.; Ďurčeková, K.; Huttová, J.; Mistrík, I.; Zelinová, V. The impact of heavy metals on the activity of some enzymes along the barley root. Environ. Exp. Bot. 2008, 62, 86–91. [Google Scholar] [CrossRef]
- Lentini, M.; De Lillo, A.; Paradisone, V.; Liberti, D.; Landi, S.; Esposito, S. Early responses to cadmium exposure in barley plants: Effects on biometric and physiological parameters. Acta Physiol. Plant. 2018, 40, 178. [Google Scholar] [CrossRef]
- Feigl, G.; Kumar, D.; Lehotai, N.; Tugyi, N.; Molnár, A.; Ordög, A.; Szepesi, A.; Gémes, K.; Laskay, G.; Erdei, L.; et al. Physiological and morphological responses of the root system of Indian mustard (Brassica juncea L. Czern.) and rapeseed (Brassica napus L.) to copper stress. Ecotoxicol. Environ. Saf. 2013, 94, 179–189. [Google Scholar] [CrossRef]
- Barrameda-Medina, Y.; Montesinos-Pereira, D.; Romero, L.; Ruiz, J.M.; Blasco, B. Comparative study of the toxic effect of Zn in Lactuca sativa and Brassica oleracea plants: I. Growth, distribution, and accumulation of Zn, and metabolism of carboxylates. Environ. Exp. Bot. 2014, 107, 98–104. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M. Nickel-induced changes in nitrogen metabolism in wheat shoots. J. Plant Physiol. 2009, 166, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Mukherjee, A. Sensitivity of Allium cepa and Vicia faba towards cadmium toxicity. J. Soil Sci. Plant Nutr. 2014, 14, 447–458. [Google Scholar] [CrossRef]
- Erturk, F.A.; Ay, H.; Nardemir, G.; Agar, G. Molecular determination of genotoxic effects of cobalt and nickel on maize (Zea mays L.) by RAPD and protein analyses. Toxicol. Ind. Health 2013, 29, 662–671. [Google Scholar] [CrossRef]
- Santos, C.L.V.; Pourrut, B.; Ferreira de Oliveira, J.M.P. The use of comet assay in plant toxicology: Recent advances. Front. Genet. 2015, 6, 216. [Google Scholar] [CrossRef]
- Ackova, D.G.; Kadifkova-Panovska, T.; Bačeva Andonovska, K.; Stafilov, T. Evaluation of genotoxic variations in plant model systems in a case of metal stressors. J. Environ. Sci. Health B 2016, 51, 340–349. [Google Scholar] [CrossRef]
- Drążkiewicz, M.; Baszyński, T. Interference of nickel with the photosynthetic apparatus of Zea mays. Ecotoxicol. Environ. Saf. 2010, 73, 982–986. [Google Scholar] [CrossRef]
- Gajewska, E.; Niewiadomska, E.; Tokarz, K.; Słabad, M.; Skłodowska, M. Nickel-induced changes in carbon metabolism in wheat shoots. J. Plant Physiol. 2013, 170, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.M.; Dwivedi, R.; Zeeshan, M. Growth, photosynthetic electron transport, and antioxidant responses of young soybean seedlings to simultaneous exposure of nickel and UV-B stress. Photosynthetica 2005, 43, 177. [Google Scholar] [CrossRef]
- Monreal, J.A.; Arias-Baldrich, C.; Tossi, V.; Feria Ana, B.; Rubio-Casal, A.; García-Mata, C.; Lamattina, L.; García-Mauriño, S. Nitric oxide regulation of leaf phosphoenolpyruvate carboxylase-kinase activity: Implication in sorghum responses to salinity. Planta 2013, 238, 859–869. [Google Scholar] [CrossRef]
- Blasco, B.; Graham, N.S.; Broadley, M.R. Antioxidant response and carboxylate metabolism in Brassica rapa exposed to different external Zn, Ca, and Mg supply. J. Plant Physiol. 2015, 176, 16–24. [Google Scholar] [CrossRef]
- Abbas, S.; Basit, F.; Tanwir, K.; Zhu, X.; Hu, J.; Guan, Y.; Hu, W.; Sheteiwy, M.S.; Yang, H.; El-Keblawy, A.; et al. Exogenously applied sodium nitroprusside alleviates nickel toxicity in maize by regulating antioxidant activities and defense-related gene expression. Physiol. Plant. 2023, 175, e13985. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Nunes-Nesi, A.; Nikoloski, Z.; Sweetlove, L.J.; Fernie, A.R. Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ. 2012, 35, 1–21. [Google Scholar] [CrossRef]
- Jallouli, S.; Ayadi, S.; Landi, S.; Capasso, G.; Santini, G.; Chamekh, Z.; Zouari, I.; Ben Azaiez, F.E.; Trifa, Y.; Esposito, S. Physiological and Molecular Osmotic Stress Responses in Three Durum Wheat (Triticum turgidum ssp. durum) Genotypes. Agronomy 2019, 9, 550. [Google Scholar] [CrossRef]
- Esposito, S.; Massaro, G.; Vona, V.; Di Martino Rigano, V.; Carfagna, S.; Rigano, C. Ammonium induction of a novel isoform of glucose-6P dehydrogenase in barley roots. Physiol. Plant. 2001, 113, 469–476. [Google Scholar] [CrossRef]
- Esposito, S. Nitrogen Assimilation, Abiotic Stress and Glucose 6-Phosphate Dehydrogenase: The Full Circle of Reductants. Plants 2016, 5, 24. [Google Scholar] [CrossRef]
- Landi, S.; Vitale, E.; Lanzilli, M.; Arena, C.; D’Ippolito, G.; Fontana, A.; Esposito, S. Lack of Arabidopsis chloroplastic glucose-6-phosphate dehydrogenase 1 (G6PD1) affects lipid synthesis during cold stress response. Plant Sci. 2024, 349, 112260. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J.; von Schaewen, A. The oxidative pentose phosphate pathway: Structure and organisation. Curr. Opin. Plant Biol. 2003, 6, 236–246. [Google Scholar] [CrossRef]
- Fortunato, S.; Nigro, D.; Lasorella, C.; Laria Marcotuli, K.I.; Gadaleta, A.; de Pinto, M.C. The Role of Glutamine Synthetase (GS) and Glutamate Synthase (GOGAT) in the Improvement of Nitrogen Use Efficiency in Cereals. Biomolecules 2023, 13, 1771. [Google Scholar] [CrossRef]
- Navarro-León, E.; Barrameda-Medina, Y.; Lentini, M.; Esposito, S.; Juan, M.; Ruiz, J.M.; Blasco, B. Comparative study of Zn deficiency in L. sativa and B. oleracea plants: NH4+ assimilation and nitrogen derived protective compounds. Plant Sci. 2016, 248, 8–16. [Google Scholar] [CrossRef]
- Esposito, S.; Guerriero, G.; Vona, V.; Di Martino Rigano, V.; Carfagna, S.; Rigano, C. Glutamate synthase activities and protein changes in relation to nitrogen nutrition in barley: The dependence on different plastidic glucose-6P dehydrogenase isoforms. J. Exp. Bot. 2005, 56, 55–64. [Google Scholar] [CrossRef]
- Goel, P.; Singh, A.K. Abiotic stresses downregulate key genes involved in nitrogen uptake and assimilation in Brassica juncea L. PLoS ONE 2015, 10, e0143645. [Google Scholar] [CrossRef]
- Cardi, M.; Chibani, K.; Cafasso, D.; Rouhier, N.; Jacquot, J.-P.; Esposito, S. Abscisic acid effects on activity and expression of barley (Hordeum vulgare) plastidic glucose-6-phosphate-dehydrogenase. J. Exp. Bot. 2011, 62, 4013–4023. [Google Scholar] [CrossRef]
- Cardi, M.; Castiglia, D.; Ferrara, M.; Guerriero, G.; Chiurazzi, M.; Esposito, S. The effects of salt stress cause a diversion of basal metabolism in barley roots: Possible different roles for glucose-6-phosphate dehydrogenase isoforms. Plant Physiol. Bioch. 2015, 86, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Massaro, G.; Vona, V.; Di Martino Rigano, V.; Carfagna, S. Glutamate synthesis in barley roots: The role of the plastidic glucose-6-phosphate dehydrogenase. Planta 2003, 216, 639–647. [Google Scholar] [CrossRef]
- Rigano, C.; Di Martino Rigano, V.; Vona, V.; Carfagna, S.; Carillo, P.; Esposito, S. Ammonium assimilation by young plants of Hordeum vulgare in light and darkness: Effects on respiratory oxygen consumption by roots. New Phytol. 1996, 132, 375–383. [Google Scholar] [CrossRef]
- Arena, C.; Conti, S.; Francesca, S.; Melchionna, G.; Hájek, J.; Barták, M.; Barone, A.; Rigano, M.M. Eco-Physiological Screening of Different Tomato Genotypes in Response to High Temperatures: A Combined Field-to-Laboratory Approach. Plants 2020, 9, 508. [Google Scholar] [CrossRef]
- Esposito, S.; Carillo, P.; Carfagna, S. Ammonium metabolism stimulation of glucose-6P dehydrogenase and phosphoenolpyruvate carboxylase in young barley roots. J. Plant Physiol. 1998, 153, 61–66. [Google Scholar] [CrossRef]
- Cardi, M.; Chibani, K.; Castiglia, D.; Cafasso, D.; Pizzo, E.; Rouhier, N.; Jacquot, J.-P.; Esposito, S. Overexpression, purification and enzymatic characterization of a recombinant plastidial glucose-6-phosphate dehydrogenase from barley (Hordeum vulgare cv. Nure) roots. Plant Physiol. Biochem. 2013, 73, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ben Azaiez, F.E.; Ayadi, S.; Capasso, G.; Landi, S.; Paradisone, V.; Jallouli, S.; Hammami, Z.; Chamekh, Z.; Zouari, I.; Trifa, Y.; et al. Salt stress induces differentiated nitrogen uptake and antioxidant responses in two contrasting barley landraces from MENA region. Agronomy 2020, 10, 1426. [Google Scholar] [CrossRef]
- Ayadi, S.; Karmous, C.; Chamekh, Z.; Hammami, Z.; Baraket, M.; Esposito, S.; Rezgui, S.; Trifa, Y. Effects of nitrogen rates on grain yield and nitrogen agronomic efficiency of durum wheat genotypes under different environments. Ann. Appl. Biol. 2016, 168, 264–273. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lillo, A.; De Rosa, I.; Capasso, G.; Santini, G.; Di Napoli, C.; Russo, N.; Vitale, E.; Grillo, S.; Esposito, S.; Landi, S. High Dose of Nickel Unbalances Carbon Metabolism and Nitrogen Assimilation in Barley (Hordeum vulgare L.). Plants 2025, 14, 2927. https://doi.org/10.3390/plants14182927
De Lillo A, De Rosa I, Capasso G, Santini G, Di Napoli C, Russo N, Vitale E, Grillo S, Esposito S, Landi S. High Dose of Nickel Unbalances Carbon Metabolism and Nitrogen Assimilation in Barley (Hordeum vulgare L.). Plants. 2025; 14(18):2927. https://doi.org/10.3390/plants14182927
Chicago/Turabian StyleDe Lillo, Alessia, Ivana De Rosa, Giorgia Capasso, Giorgia Santini, Concetta Di Napoli, Noemi Russo, Ermenegilda Vitale, Stefania Grillo, Sergio Esposito, and Simone Landi. 2025. "High Dose of Nickel Unbalances Carbon Metabolism and Nitrogen Assimilation in Barley (Hordeum vulgare L.)" Plants 14, no. 18: 2927. https://doi.org/10.3390/plants14182927
APA StyleDe Lillo, A., De Rosa, I., Capasso, G., Santini, G., Di Napoli, C., Russo, N., Vitale, E., Grillo, S., Esposito, S., & Landi, S. (2025). High Dose of Nickel Unbalances Carbon Metabolism and Nitrogen Assimilation in Barley (Hordeum vulgare L.). Plants, 14(18), 2927. https://doi.org/10.3390/plants14182927










