Research on the Physiological Mechanisms of Nitrogen in Alleviating Plant Drought Tolerance
Abstract
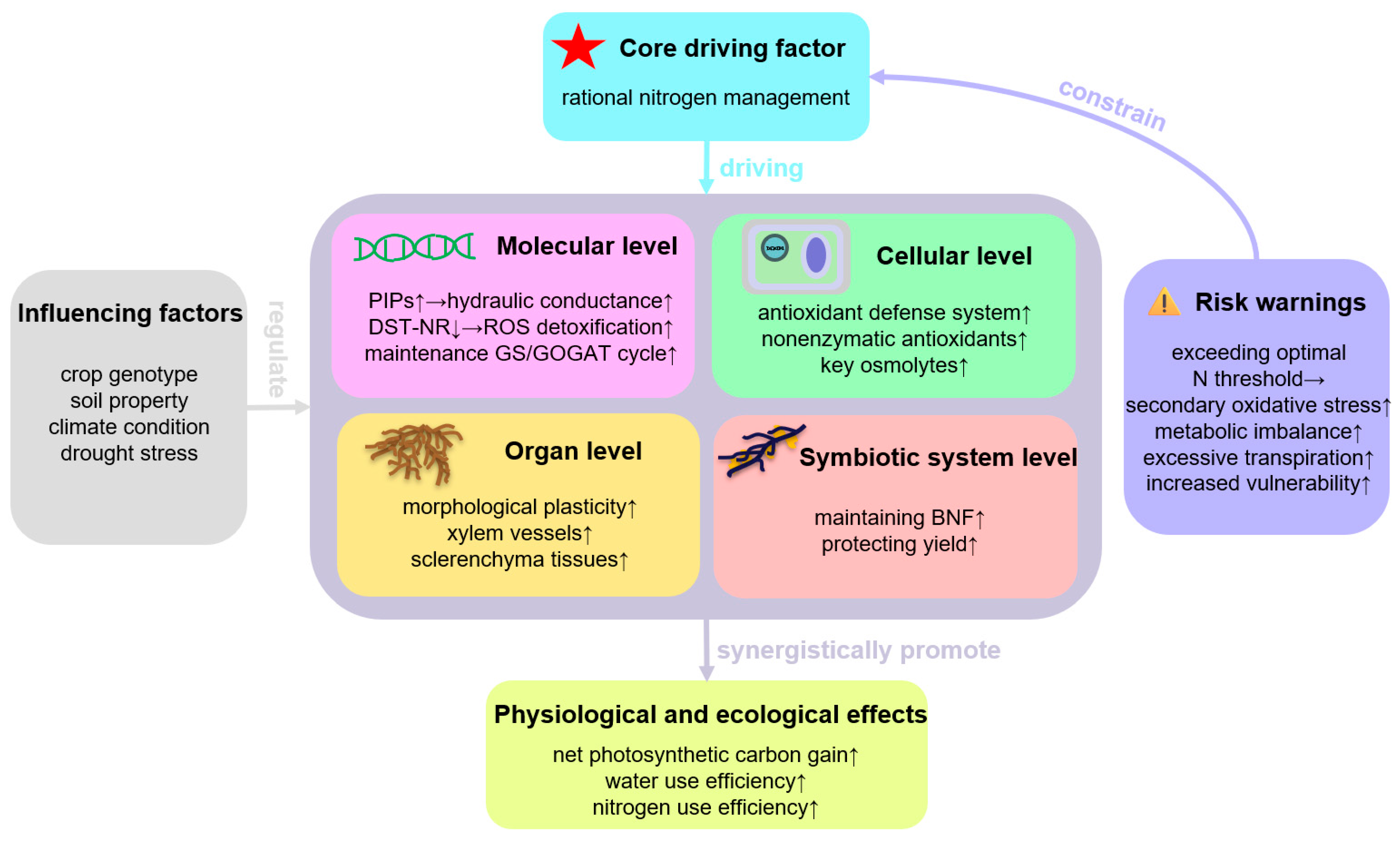
1. Introduction
2. Physiological Mechanisms of N in Alleviating Plant Drought Stress
2.1. Regulation of Water Use and Photosynthesis by N Under Drought Stress
| Crop Species | N Treatment | Key Physiological Changes | Reference |
|---|---|---|---|
| Maize (Zea mays) | 7.5 mM N supply | Photosynthetic parameters (Pn, Fv/Fm) recovered to 80–95% of controls; H2O2 and O2− levels reduced by over 40% | [29] |
| Winter Wheat (Triticum aestivum) | Moderate N supply (≈7 mM total N) | Activities of glutamine synthetase (GS1) and glutamate synthase (Fd-GOGAT) maintained at 80–90% of controls | [34] |
| Catalpa bungei | 1 mM N + V-PRD | WUEi maximized; xylem vessel radial expansion enhanced hydraulic conductivity | [28] |
| Leymus chinensis | 5 mM NO3− supplementation | Fv/Fm increased by 12% compared to N-deficient groups | [27] |
| Winter wheat (Triticum aestivum) | High N treatment (≈30 g N m−2) | Stomatal conductance decreased; transpiration loss increased; yield reduced due to rapid soil moisture depletion | [35] |
| Malus prunifolia | 1 mM NH4NO3 | Net NH4+ influx increased; AMT1;2 and AMT4;2 up-regulated; biomass and photosynthesis higher than low-N drought group | [31] |
2.2. Regulatory Mechanisms of N Signaling and N Assimilation Metabolism on Drought Stress
2.3. Antioxidant and Osmotic Regulation Mechanisms Mediated by N Under Drought
2.4. N-Mediated Root Morphology and Optimized Resource Acquisition Under Drought Stress
2.5. Drought Adaptation Mechanisms of BNF Systems
| Regulation Strategy | Technical Details | Effects Under Drought Conditions | Reference |
|---|---|---|---|
| Inoculation with drought-tolerant rhizobia and PGPR | Co-inoculation with osmotic stress-tolerant rhizobia (e.g., Bradyrhizobium elkanii) and PGPR (e.g., Azospirillum brasilense) | Stabilized nifH gene expression and Nase activity; soybean yield increased by 15–25% compared to single rhizobia inoculation | [96] |
| Water-retentive soil management | No-till combined with organic amendments to enhance soil organic matter (SOM) and water-holding capacity | Reduced nodule inactivation by 30–40%; sustained carbon supply to nodules; alleviated BNF inhibition | [104] |
| Use of slow-wilting genotypes | Selection of deep-rooted or water-conservative cultivars; delayed canopy wilting under water deficit | Mitigated drought impacts on nodule water potential; maintained BNF function until grain filling | [96] |
| Targeted N supplementation | Application of 40–60 kg N ha−1 NH4NO3 during critical drought stages (e.g., soybean pod filling); avoidance of excessive N to prevent BNF suppression | Temporarily compensated for reduced BNF; yield increased by 15–25% vs. sole reliance on BNF; high N (>80 kg N ha−1) ineffective or harmful | [105] |
2.6. N Regulation Strategies Under Multiple Stress Scenarios
2.7. Molecular Regulatory Mechanisms and Breeding Targets
3. Conclusions
4. Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, R.X.; Sun, H.Q.; Xing, L.S.; Li, R.Z.; Li, M.; Zhang, Q. Effects of anthropogenic climate change on the drought characteristics in China: From frequency, duration, intensity, and affected area. J. Hydrol. 2023, 617, 10. [Google Scholar] [CrossRef]
- Chiang, F.; Mazdiyasni, O.; AghaKouchak, A. Evidence of anthropogenic impacts on global drought frequency, duration, and intensity. Nat. Commun. 2021, 12, 2754. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of soil water deficit on growth and development of plants: A review. In Soil Water Deficit and Physiological Issues in Plants; Springer: Singapore, 2021; pp. 393–488. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G. Hormones and nitrate: A two-way connection. Plant Mol. Biol. 2016, 91, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.A.; Alvarez, J.M.; Araus, V.; Riveras, E.; Brooks, M.D.; Krouk, G.; Ruffel, S.; Lejay, L.; Crawford, N.M.; Coruzzi, G.M.; et al. Nitrate in 2020: Thirty Years from Transport to Signaling Networks. Plant Cell 2020, 32, 2094–2119. [Google Scholar] [CrossRef]
- Patel, J.; Khatri, K.; Khandwal, D.; Gupta, N.K.; Choudhary, B.; Hapani, D.; Koshiya, J.; Syed, S.N.; Phillips, D.W.; Jones, H.D.; et al. Modulation of physio-biochemical and photosynthesis parameters by overexpressing SbPIP2 gene improved abiotic stress tolerance of transgenic tobacco. Physiol. Plant. 2024, 176, e14384. [Google Scholar] [CrossRef]
- Zhu, G.; Gu, L.; Shi, Y.; Chen, H.; Liu, Y.; Lu, F.; Ren, Z.; Wang, Y.; Lu, H.; Tabassum, A.; et al. Plant hydraulic conductivity determines photosynthesis in rice under PEG-induced drought stress. Pak. J. Bot. 2021, 53, 409–417. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 251, 84. [Google Scholar] [CrossRef]
- Serraj, R.; Sinclair, T.R.; Purcell, L.C. Symbiotic N2 fixation response to drought. J. Exp. Bot. 1999, 50, 143–155. [Google Scholar] [CrossRef]
- King, C.A.; Purcell, L.C. Soybean nodule size and relationship to nitrogen fixation response to water deficit. Crop Sci. 2001, 41, 1099–1107. [Google Scholar] [CrossRef]
- Balkos, K.D.; Britto, D.T.; Kronzucker, H.J. Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant Cell Environ. 2010, 33, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.S.; Miao, L.F.; Yang, F. Drought and Nitrogen Application Modulate the Morphological and Physiological Responses of Dalbergia odorifera to Different Niche Neighbors. Front. Plant Sci. 2021, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Wei, Z.H.; Wan, H.; Zhang, J.R.; Liu, J.; Liu, F.L. CO2 Elevation and Nitrogen Supply Alter the Growth and Physiological Responses of Tomato and Barley Plants to Drought Stress. Agronomy 2022, 12, 1821. [Google Scholar] [CrossRef]
- Cerda, A.; Alvarez, J.M. Insights into molecular links and transcription networks integrating drought stress and nitrogen signaling. New Phytol. 2024, 241, 560–566. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef]
- Evans, J.R.; Clarke, V.C. The nitrogen cost of photosynthesis. J. Exp. Bot. 2019, 70, 7–15. [Google Scholar] [CrossRef]
- Wang, M.; Ding, L.; Gao, L.M.; Li, Y.R.; Shen, Q.R.; Guo, S.W. The Interactions of Aquaporins and Mineral Nutrients in Higher Plants. Int. J. Mol. Sci. 2016, 17, 1229. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Carvajal, M.; Henzler, T.; Waterhouse, R.N.; Smyth, A.J.; Cooke, D.T.; Steudle, E. Root hydraulic conductance: Diurnal aquaporin expression and the effects of nutrient stress. J. Exp. Bot. 2000, 51, 61–70. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Douthe, C.; Dreyerc, E.; Ferrio, J.P.; Gago, J.; et al. Corrigendum to ‘Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 196, 31. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Plett, D.C.; Ranathunge, K.; Melino, V.J.; Kuya, N.; Uga, Y.; Kronzucker, H.J. The intersection of nitrogen nutrition and water use in plants: New paths toward improved crop productivity. J. Exp. Bot. 2020, 71, 4452–4468. [Google Scholar] [CrossRef]
- Wei, X.W.; Han, L.; Xu, N.; Sun, M.Y.; Yang, X.C. Nitrate nitrogen enhances the efficiency of photoprotection in Leymus chinensis under drought stress. Front. Plant Sci. 2024, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, X.; Xu, S.; Zhao, M.; Shu, P.; Xu, F.; Ma, J.; Sun, Y.; Dong, H.; Guo, Z.; et al. Nitrogen availability affects stem development and response to differential root-zone drought stress in Catalpa bungei. Environ. Exp. Bot. 2021, 186, 12. [Google Scholar] [CrossRef]
- Song, Y.; Li, J.; Liu, M.; Meng, Z.; Liu, K.; Sui, N. Nitrogen increases drought tolerance in maize seedlings. Funct. Plant Biol. 2019, 46, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.L.; Li, Y.K. Effect of Drought Stress and Drought Tolerance Heredity on Nitrogen Efficiency of Winter Wheat; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 62–63. [Google Scholar] [CrossRef]
- Huang, L.L.; Li, M.J.; Zhou, K.; Sun, T.T.; Hu, L.Y.; Li, C.Y.; Ma, F.W. Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol. Biochem. 2018, 127, 185–193. [Google Scholar] [CrossRef]
- Hao, D.-L.; Zhou, J.-Y.; Yang, S.-Y.; Qi, W.; Yang, K.-J.; Su, Y.-H. Function and regulation of ammonium transporters in plants. Int. J. Mol. Sci. 2020, 21, 3557. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, H.; Liu, H.; Yang, L.; Mi, G.; Wang, P. Enhancing Crop Nitrogen Efficiency: The Role of Mixed Nitrate and Ammonium Supply in Plant Growth and Development. Biology 2025, 14, 546. [Google Scholar] [CrossRef]
- Gayatri; Jayaraman, K.; Sinha, S.K.; Roy, P.; Mandal, P.K. Comparative Analysis of GS2 and Fd-GOGAT Genes in Cultivated Wheat and Their Progenitors Under N Stress. Plant Mol. Biol. Rep. 2021, 39, 520–545. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.T.; Wang, W.E.; Yan, H. Impact of nitrogen on photosynthesis, remobilization, yield, and efficiency in winter wheat under heat and drought stress. Agric. Water Manag. 2024, 302, 15. [Google Scholar] [CrossRef]
- Jozefowicz, A.M.; Bienert, M.D.; Garibay, A.; Giehl, R.; Matros, A.; Schum, A.; Bienert, G.P.; Mock, H.-P. Plasma membrane intrinsic proteins PIP1; 1 and PIP1; 3 contribute to the tolerance to nitrogen deficiency in potato. Authorea Prepr. 2020. [Google Scholar] [CrossRef]
- Schley, T.R.; Zhu, T.; Geist, B.; Crabos, A.; Dietrich, D.; Alandes, R.A.; Bennett, M.; Nacry, P.; Schäffner, A.R. The Arabidopsis PIP1;1 Aquaporin Represses Lateral Root Development and Nitrate Uptake Under Low Nitrate Availability. Plant Cell Environ. 2025, 48, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Q.; Zhang, Z.M.; Wang, H.Y.; Chen, Y.L.; Zhang, M.X. Soil Water Deficit Reduced Root Hydraulic Conductivity of Common Reed (Phragmites australis). Plants 2023, 12, 3543. [Google Scholar] [CrossRef]
- Mu, Z.; Zhang, S.; Zhang, L.; Liang, A.; Liang, Z. Hydraulic conductivity of whole root system is better than hydraulic conductivity of single root in correlation with the leaf water status of maize. Bot. Stud. 2006, 47, 145–151. [Google Scholar]
- Zahedi, S.M.; Karimi, M.; Venditti, A.; Zahra, N.; Siddique, K.H.M.; Farooq, M. Plant Adaptation to Drought Stress: The Role of Anatomical and Morphological Characteristics in Maintaining the Water Status. J. Soil Sci. Plant Nutr. 2025, 25, 409–427. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, C.; Hu, S.; Zuo, K. Arabidopsis calcium-dependent protein kinase CPK6 regulates drought tolerance under high nitrogen by the phosphorylation of NRT1.1. J. Exp. Bot. 2023, 74, 5682–5693. [Google Scholar] [CrossRef]
- Ho, C.-H.; Lin, S.-H.; Hu, H.-C.; Tsay, Y.-F. CHL1 functions as a nitrate sensor in plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef]
- Liu, K.-H.; Niu, Y.; Konishi, M.; Wu, Y.; Du, H.; Chung, H.S.; Li, L.; Boudsocq, M.; McCormack, M.; Maekawa, S.; et al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 2017, 545, 311–316. [Google Scholar] [CrossRef]
- Han, M.-L.; Lv, Q.-Y.; Zhang, J.; Wang, T.; Zhang, C.-X.; Tan, R.-J.; Wang, Y.-L.; Zhong, L.-Y.; Gao, Y.-Q.; Chao, Z.-F.; et al. Decreasing nitrogen assimilation under drought stress by suppressing DST-mediated activation of Nitrate Reductase 1.2 in rice. Mol. Plant 2022, 15, 167–178. [Google Scholar] [CrossRef]
- Fortunato, S.; Nigro, D.; Lasorella, C.; Marcotuli, I.; Gadaleta, A.; de Pinto, M.C. The Role of Glutamine Synthetase (GS) and Glutamate Synthase (GOGAT) in the Improvement of Nitrogen Use Efficiency in Cereals. Biomolecules 2023, 13, 1771. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef] [PubMed]
- Sulpice, R.; Pyl, E.-T.; Ishihara, H.; Trenkamp, S.; Steinfath, M.; Witucka-Wall, H.; Gibon, Y.; Usadel, B.; Poree, F.; Piques, M.C.; et al. Starch as a major integrator in the regulation of plant growth. Proc. Natl. Acad. Sci. USA 2009, 106, 10348–10353. [Google Scholar] [CrossRef] [PubMed]
- Dikilitas, M.; Simsek, E.; Roychoudhury, A. Role of proline and glycine betaine in overcoming abiotic stresses. Biochem. Mol. Perspect. 2020. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. Oxidative stress and antioxidative systems: Recipes for successful data collection and interpretation. Plant Cell Environ. 2016, 39, 1140–1160. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I. Reactive Oxygen Species in Plants; Springer: Berlin/Heidelberg, Germany, 2023; Available online: https://link.springer.com/series/7651 (accessed on 14 September 2025).
- Farhan, M.; Sathish, M.; Kiran, R.; Mushtaq, A.; Baazeem, A.; Hasnain, A.; Hakim, F.; Naqvi, S.A.H.; Mubeen, M.; Iftikhar, Y.; et al. Plant Nitrogen Metabolism: Balancing Resilience to Nutritional Stress and Abiotic Challenges. Phyton Int. J. Exp. Bot. 2024, 93, 581–609. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef]
- Ahmad, R.; Waraich, E.A.; Ashraf, M.Y.; Ahmad, S.; Aziz, T. Does nitrogen fertilization enhance drought tolerance in sunflower? A review. J. Plant Nutr. 2014, 37, 942–963. [Google Scholar] [CrossRef]
- Iqbal, A.; Dong, Q.; Wang, X.; Gui, H.; Zhang, H.; Zhang, X.; Song, M. High Nitrogen Enhance Drought Tolerance in Cotton through Antioxidant Enzymatic Activities, Nitrogen Metabolism and Osmotic Adjustment. Plants 2020, 9, 178. [Google Scholar] [CrossRef]
- Ahmadi, A.; Emam, Y.; Pessarakli, M. Biochemical changes in maize seedlings exposed to drought stress conditions at different nitrogen levels. J. Plant Nutr. 2010, 33, 541–556. [Google Scholar] [CrossRef]
- Agami, R.A.; Alamri, S.A.M.; Abd El-Mageed, T.A.; Abousekken, M.S.M.; Hashem, M. Role of exogenous nitrogen supply in alleviating the deficit irrigation stress in wheat plants. Agric. Water Manag. 2018, 210, 261–270. [Google Scholar] [CrossRef]
- Römheld, V.; Kirkby, E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, S.X. Effects of application of N and K fertilizers on nitrogen metabolism of two genetype varieties of maize under water-stressed condition. J. Plant Nutr. Fertil. 2007, 13, 554–560. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Bolong, M.; Weiyi, M.; Qingzi, L.; Zhuoliang, H.; Jiaxuan, G.; Yixuan, C. The effect mechanism of drought and frost damage on plant water transport xylem embolism and cavitation fatigue. Tree Health 2024, 1, 9–17. [Google Scholar]
- Zimmermann, M.H. Xylem Structure and the Ascent of Sap; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Available online: https://link.springer.com/series/760 (accessed on 14 September 2025).
- Maqbool, S.; Hassan, M.A.; Xia, X.; York, L.M.; Rasheed, A.; He, Z. Root system architecture in cereals: Progress, challenges and perspective. Plant J. Cell Mol. Biol. 2022, 110, 23–42. [Google Scholar] [CrossRef]
- Lombardi, M.; De Gara, L.; Loreto, F. Determinants of root system architecture for future-ready, stress-resilient crops. Physiol. Plant. 2021, 172, 2090–2097. [Google Scholar] [CrossRef]
- Sun, X.; Wang, P.; Mi, G. Genotypic Differences in Maize Root Morphology in Response to Low-Nitrogen Stress. Agronomy 2025, 15, 332. [Google Scholar] [CrossRef]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014, 166, 590–602. [Google Scholar] [CrossRef]
- Lynch, J.P. Harnessing root architecture to address global challenges. Plant J. Cell Mol. Biol. 2022, 109, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Chen, J.; Chen, A.; Wang, L.; Guo, X.; Niu, Y.; Liu, S.; Mi, G.; Gao, Q. Reducing basal nitrogen rate to improve maize seedling growth, water and nitrogen use efficiencies under drought stress by optimizing root morphology and distribution. Agric. Water Manag. 2019, 212, 328–337. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; Gruber, B.D.; von Wirén, N. Its time to make changes: Modulation of root system architecture by nutrient signals. J. Exp. Bot. 2014, 65, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Baena-González, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007, 448, 938–942. [Google Scholar] [CrossRef]
- Eghball, B.; Maranville, J.W. Root development and nitrogen influx of corn genotypes grown under combined drought and nitrogen stresses. Agron. J. 1993, 85, 147–152. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Cortasa, M.S.; Loges, R. Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant Soil 2009, 322, 101–114. [Google Scholar] [CrossRef]
- Adu, M.O. Variations in Root System Architecture and Root Growth Dynamics of Brassica Rapa Genotypes Using a New Scanner-Based Phenotyping System. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2014. Available online: https://eprints.nottingham.ac.uk/id/eprint/14259 (accessed on 14 September 2025).
- Jia, Z. Exploiting Natural Variation to Uncover Genes Modulating Root Foraging Responses to Low Nitrogen in Arabidopsis Thaliana. Ph.D. Thesis, Martin-Luther-Universität Halle-Wittenberg, Halle (Saale), Germany, 2019. [Google Scholar] [CrossRef]
- Ullah, A.; Tian, Z.W.; Xu, L.B.; Abid, M.; Lei, K.Q.; Khanzada, A.; Zeeshan, M.; Sun, C.J.; Yu, J.H.; Dai, T.B. Improving the effects of drought priming against post-anthesis drought stress in wheat (Triticum aestivum L.) using nitrogen. Front. Plant Sci. 2022, 13, 965996. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Q.; Gao, J.; Liu, Z. The Physiological Mechanisms and Hurdles of Efficient Water-Nitrogen Utilization in Maize Production: A Review. Plants 2025, 14, 1899. [Google Scholar] [CrossRef]
- Shi, H.; Ma, W.; Song, J.; Lu, M.; Rahman, S.U.; Bui, T.T.X.; Vu, D.D.; Zheng, H.; Wang, J.; Zhang, Y. Physiological and transcriptional responses of Catalpa bungei to drought stress under sufficient- and deficient-nitrogen conditions. Tree Physiol. 2017, 37, 1457–1468. [Google Scholar] [CrossRef]
- Pierret, A.; Maeght, J.L.; Clément, C.; Montoroi, J.P.; Hartmann, C.; Gonkhamdee, S. Understanding deep roots and their functions in ecosystems: An advocacy for more unconventional research. Ann. Bot. 2016, 118, 621–635. [Google Scholar] [CrossRef]
- Hofer, D.; Suter, M.; Buchmann, N.; Lüscher, A. Nitrogen status of functionally different forage species explains resistance to severe drought and post-drought overcompensation. Agric. Ecosyst. Environ. 2017, 236, 312–322. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; McElroy, M.S.; Chapagain, T.; Papadopoulos, Y.A.; Raizada, M.N. Belowground nitrogen transfer from legumes to non-legumes under managed herbaceous cropping systems. A review. Agron. Sustain. Dev. 2016, 36, 16. [Google Scholar] [CrossRef] [PubMed]
- Concha, C.; Doerner, P. The impact of the rhizobia-legume symbiosis on host root system architecture. J. Exp. Bot. 2020, 71, 3902–3921. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lang, G.; Zhang, J.; Liu, G.; Li, J. Deficit irrigation during the vegetative stage improves tomato growth and yield by optimizing root architecture and delaying root senescence. Sci. Hortic. 2025, 349, 114218. [Google Scholar] [CrossRef]
- Wakrim, R.; Wahbi, S.; Tahi, H.; Aganchich, B.; Serraj, R. Comparative effects of partial root drying (PRD) and regulated deficit irrigation (RDI) on water relations and water use efficiency in common bean (Phaseolus vulgaris L.). Agric. Ecosyst. Environ. 2005, 106, 275–287. [Google Scholar] [CrossRef]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-Regulated Auxin Transport by NRT1.1 Defines a Mechanism for Nutrient Sensing in Plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef]
- Bouguyon, E.; Perrine-Walker, F.; Pervent, M.; Rochette, J.; Cuesta, C.; Benkova, E.; Martinière, A.; Bach, L.; Krouk, G.; Gojon, A.; et al. Nitrate Controls Root Development through Posttranscriptional Regulation of the NRT1.1/NPF6.3 Transporter/Sensor. Plant Physiol. 2016, 172, 1237–1248. [Google Scholar] [CrossRef]
- Bienert, M.D.; Diehn, T.A.; Richet, N.; Chaumont, F.; Bienert, G.P. Heterotetramerization of Plant PIP1 and PIP2 Aquaporins Is an Evolutionary Ancient Feature to Guide PIP1 Plasma Membrane Localization and Function. Front. Plant Sci. 2018, 9, 382. [Google Scholar] [CrossRef]
- Kotur, Z.; Mackenzie, N.; Ramesh, S.; Tyerman, S.D.; Kaiser, B.N.; Glass, A.D.M. Nitrate transport capacity of the Arabidopsis thaliana NRT2 family members and their interactions with AtNAR2.1. New Phytol. 2012, 194, 724–731. [Google Scholar] [CrossRef]
- Frungillo, L.; Skelly, M.J.; Loake, G.J.; Spoel, S.H.; Salgado, I. S-nitrosothiols regulate nitric oxide production and storage in plants through the nitrogen assimilation pathway. Nat. Commun. 2014, 5, 5401. [Google Scholar] [CrossRef] [PubMed]
- Cossani, C.M.; Sadras, V.O. Water–nitrogen colimitation in grain crops. Adv. Agron. 2018, 150, 231–274. [Google Scholar] [CrossRef]
- Quemada, M.; Gabriel, J.L. Approaches for increasing nitrogen and water use efficiency simultaneously. Glob. Food Secur. Agric. Policy 2016, 9, 29–35. [Google Scholar] [CrossRef]
- Arrese-Igor, C.; González, E.M.; Marino, D.; Ladrera, R.; Larrainzar, E.; Gil-Quintana, E.J. Physiological responses of legume nodules to drought. Plant Stress 2011, 5, 24–31. Available online: https://academica-e.unavarra.es/handle/2454/23973 (accessed on 14 September 2025).
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Verma, D.K.; Kaur, B.; Pandey, A.K.; Asthir, B. Nitrogenase: A Key Enzyme in Microbial Nitrogen Fixation for Soil Health. In Microbiology for Sustainable Agriculture, Soil Health, and Environmental Protection; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 61–294. [Google Scholar] [CrossRef]
- Khatun, M.; Sarkar, S.; Era, F.M.; Islam, A.K.M.M.; Anwar, P.; Fahad, S.; Datta, R.; Islam, A.K.M.A. Drought stress in grain legumes: Effects, tolerance mechanisms and management. Agronomy 2021, 11, 2374. [Google Scholar] [CrossRef]
- Marino, D.; Frendo, P.; Ladrera, R.; Zabalza, A.; Puppo, A.; Arrese-Igor, C.; González, E.M. Nitrogen fixation control under drought stress. Localized or systemic? Plant Physiol. 2007, 143, 1968–1974. [Google Scholar] [CrossRef]
- de Freitas, V.F.; Cerezini, P.; Hungria, M.; Nogueira, M.A. Strategies to deal with drought-stress in biological nitrogen fixation in soybean. Appl. Soil Ecol. 2022, 172, 10. [Google Scholar] [CrossRef]
- Cassán, F.; Diaz-Zorita, M. Azospirillum sp. in current agriculture: From the laboratory to the field. Soil Biol. Biochem. 2016, 103, 117–130. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Burity, H.A.; Martínez, C.R.; Chanway, C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008, 40, 182–188. [Google Scholar] [CrossRef]
- de Almeida Leite, R.; Martins, L.C.; Ferreira, L.V.d.S.F.; Barbosa, E.S.; Alves, B.J.R.; Zilli, J.E.; Araújo, A.P.; Jesus, E.D.C. Co-inoculation of Rhizobium and Bradyrhizobium promotes growth and yield of common beans. Appl. Soil Ecol. 2022, 172, 104356. [Google Scholar] [CrossRef]
- Cely, M.V.T.; Siviero, M.A.; Emiliano, J.; Spago, F.R.; Freitas, V.F.; Barazetti, A.R.; Goya, E.T.; Lamberti, G.d.S.; dos Santos, I.M.O.; De Oliveira, A.G.; et al. Inoculation of Schizolobium parahyba with mycorrhizal fungi and plant growth-promoting rhizobacteria increases wood yield under field conditions. Front. Plant Sci. 2016, 7, 1708. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Ijaz, M.; Ali, Q.; Ul-Allah, S.; Sattar, A.; Ahmad, S. Biological nitrogen fixation in nutrient management. In Agronomic Crops: Volume 2: Management Practices; Springer: Singapore, 2019; pp. 127–147. Available online: https://link.springer.com/chapter/10.1007/978-981-32-9783-8_8#citeas (accessed on 14 September 2025).
- Blanco-Canqui, H.; Lal, R. No-tillage and soil-profile carbon sequestration: An on-farm assessment. Soil Sci. Soc. Am. J. 2008, 72, 693–701. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production; Food & Agriculture Org.: Rome, Italy, 2005; p. 80. Available online: https://www.oregondigital.org/concern/documents/df70rp71x (accessed on 14 September 2025).
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Co-inoculation of soybeans and common beans with rhizobia and azospirilla: Strategies to improve sustainability. Biol. Fertil. Soils 2013, 49, 791–801. [Google Scholar] [CrossRef]
- Purcell, L.C.; Serraj, R.; Sinclair, T.R.; De, A. Soybean N2 fixation estimates, ureide concentration, and yield responses to drought. Crop Sci. 2004, 44, 484–492. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Marrou, H.; Soltani, A.; Vadez, V.; Chandolu, K.C. Soybean production potential in Africa. Glob. Food Secur. 2014, 3, 31–40. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef]
- Gao, H.; Yang, D.; Yang, L.; Han, S.; Liu, G.; Tang, L.; Chen, J.; Wang, D.; Guo, C. Co-inoculation with Sinorhizobium meliloti and Enterobacter ludwigii improves the yield, nodulation, and quality of alfalfa (Medicago sativa L.) under saline-alkali environments. Ind. Crops Prod. 2023, 199, 116818. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Yue, A.; Guo, S.; Wang, P.; Wang, L.; Yang, T.; Zhang, H.; Zhang, Y.; Gao, C.; et al. Effects of nitrogen nutrition and rhizobium inoculation on nodulation and nitrogen fixation and growth of soybean. Acta Agric. Boreali-Sin. 2022, 37, 95–102. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Plant responses to climate change: Metabolic changes under combined abiotic stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Chang. 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, H.; Wu, B.; Wang, C. Technology. Combined effects of drought stress and different forms of nitrogen deposition as response mechanism to environmental change. Int. J. Environ. Sci. Technol. 2023, 20, 6697–6712. Available online: https://link.springer.com/article/10.1007/s13762-022-04408-0#citeas (accessed on 14 September 2025). [CrossRef]
- Barmeier, G.; Hu, Y.; Schmidhalter, U. Partitioning and Translocation of Dry Matter and Nitrogen During Grain Filling in Spring Barley Varieties and Their Roles in Determining Malting Quality. Front. Plant Sci. 2021, 12, 722871. [Google Scholar] [CrossRef]
- Gaju, O.; Allard, V.; Martre, P.; Le Gouis, J.; Moreau, D.; Bogard, M.; Hubbart, S.; Foulkes, M.J. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars. Field Crops Res. 2014, 155, 213–223. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Fan, B.L.; Chen, L.H.; Chen, L.L.; Guo, H. Integrative Multi-Omics Approaches for Identifying and Characterizing Biological Elements in Crop Traits: Current Progress and Future Prospects. Int. J. Mol. Sci. 2025, 26, 1466. [Google Scholar] [CrossRef]
- Saleem, M.H.; Noreen, S.; Ishaq, I.; Saleem, A.; Khan, K.A.; Ercisli, S.; Anas, M.; Khalid, A.; Ahmed, T.; Hassan, A.; et al. Omics technologies: Unraveling abiotic stress tolerance mechanisms for sustainable crop improvement. J. Plant Growth Regul. 2025, 44, 4165–4187. Available online: https://link.springer.com/article/10.1007/s00344-025-11674-y#citeas (accessed on 14 September 2025). [CrossRef]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M.J.H. Reactive oxygen species generation, hazards, and defense mechanisms in plants under environmental (abiotic and biotic) stress conditions. In Handbook of Plant and Crop Physiology; Hindawi Publishing Corporation: Cairo, Egypt, 2021; pp. 617–658. [Google Scholar] [CrossRef]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: A review on molecular and biochemical aspects. Physiol. Plant. 2020, 168, 318–344. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Veselov, D.; Ye, Q.; Reinhardt, H.; Knipfer, T.; Fricke, W.; Chaumont, F. Short-term control of maize cell and root water permeability through plasma membrane aquaporin isoforms. Plant Cell Environ. 2012, 35, 185–198. [Google Scholar] [CrossRef]
- Yang, S.; Vanderbeld, B.; Wan, J.; Huang, Y. Narrowing down the targets: Towards successful genetic engineering of drought-tolerant crops. Mol. Plant 2010, 3, 469–490. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.Z.; Luo, P.; Xian, Y.N.; Xiao, R.L.; Wu, J.S. Glutamine synthetase plays an important role in ammonium tolerance of Myriophyllum aquaticum. Sci. Total Environ. 2022, 848, 12. [Google Scholar] [CrossRef]
- Santos, T.d.O.; Junior, A.T.D.A.; Moulin, M.M. Maize breeding for low nitrogen inputs in agriculture: Mechanisms underlying the tolerance to the abiotic stress. Stresses 2023, 3, 136–152. [Google Scholar] [CrossRef]
- Habash, D.; Massiah, A.; Rong, H.; Wallsgrove, R.; Leigh, R.J. The role of cytosolic glutamine synthetase in wheat. Ann. Appl. Biol. 2001, 138, 83–89. [Google Scholar] [CrossRef]
- Obara, M.; Kajiura, M.; Fukuta, Y.; Yano, M.; Hayashi, M.; Yamaya, T.; Sato, T. Mapping of QTLs associated with cytosolic glutamine synthetase and NADH-glutamate synthase in rice (Oryza sativa L.). J. Exp. Bot. 2001, 52, 1209–1217. [Google Scholar] [CrossRef][Green Version]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Lang, Z.; Botella, J.R.; Zhu, J.-K. Genome editing—Principles and applications for functional genomics research and crop improvement. Crit. Rev. Plant Sci. 2017, 36, 291–309. Available online: https://www.x-mol.com/paperRedirect/548454 (accessed on 14 September 2025). [CrossRef]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.G.; Rodgers-Melnick, E.; Buckler, E.S. On the Road to Breeding 4.0: Unraveling the Good, the Bad, and the Boring of Crop Quantitative Genomics. Annu. Rev. Genet. 2018, 52, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Marzaki, I.; Supriyadi, A.A.; Arief, S. Leveraging drone technology for advancements in photogrammetry, remote sensing, and military intelligence: A review. Remote Sens. Technol. Def. Environ. 2024, 1, 1–9. [Google Scholar] [CrossRef]
- La, W.J.; Sudduth, K.A.; Kim, H.J.; Chung, S.O. Fusion of spectral and electrochemical sensor data for estimating soil macronutrients. Trans. ASABE 2016, 59, 787–794. [Google Scholar] [CrossRef]
- Cohen, A.R.; Chen, G.; Berger, E.M.; Warrier, S.; Lan, G.; Grubert, E.; Dellaert, F.; Chen, Y. Engineering, T. Dynamically controlled environment agriculture: Integrating machine learning and mechanistic and physiological models for sustainable food cultivation. ACS EST Eng. 2021, 2, 3–19. [Google Scholar] [CrossRef]
- Skobelev, P.; Mayorov, I.; Simonova, E.; Goryanin, O.; Zhilyaev, A.; Tabachinskiy, A.; Yalovenko, V. Development of models and methods for creating a digital twin of plant within the cyber-physical system for precision farming management. J. Phys. Conf. Ser. 2020, 1703, 012022. [Google Scholar] [CrossRef]
- Dubey, A. Climate changes in soil microorganism–plant interactions. In Climate Change and the Microbiome: Sustenance of the Ecosphere; Springer: Cham, Switzerland, 2021; pp. 187–198. Available online: https://link.springer.com/chapter/10.1007/978-3-030-76863-8_9#citeas (accessed on 14 September 2025).
- de Sousa, R.N.; Grichar, W.J. Strategic Tillage and Soil Management: New Perspectives; BoD–Books on Demand; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.; Schnable, P.S. TWAS results are complementary to and less affected by linkage disequilibrium than GWAS. Plant Physiol. 2021, 186, 1800–1811. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Tian, Y.; Lyv, X.; Zhang, H.; Hong, H.; Gao, H.; Li, Y.-F.; Zhao, C.; Wang, J.; et al. TWAS facilitates gene-scale trait genetic dissection through gene expression, structural variations, and alternative splicing in soybean. Plant Commun. 2024, 5, 101010. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Zou, C.; Lu, Y.; Xie, C.; Zhang, X.; Prasanna, B.M.; Olsen, M.S. Enhancing genetic gain in the era of molecular breeding. J. Exp. Bot. 2017, 68, 2641–2666. [Google Scholar] [CrossRef]
| Regulation Type | Key Substance/Enzyme | Effects of Moderate N Treatment | Potential Risks of Excessive N | Reference |
|---|---|---|---|---|
| Antioxidant enzyme system | SOD | Activity significantly enhanced in sunflower under 80–120 kg N ha−1; O2− scavenging efficiency improved | Induced secondary oxidative burst; exacerbated membrane damage | [54] |
| CAT | Activity enhanced in cotton under 5 mM N; H2O2 content reduced to 55% of low-N drought groups | Carbon-N metabolic imbalance; increased respiratory load | [55] | |
| APX | Synergized with GR to maintain ascorbate–glutathione cycle stability | Accumulation of photorespiratory H2O2 | ||
| Osmotic Adjustment | Proline | Synthesis increased by 24% in maize under 180 kg N ha−1; activated via P5CS pathway | Metabolic disorder due to overaccumulation | [56] |
| Soluble sugars (sucrose, fructans) | Content increased by 30% in wheat under 0.6 g N kg−1 soil; enhanced cellular osmolarity and membrane stability | Uncoupling from N assimilation; toxic soluble sugar accumulation | [57] | |
| Structural reinforcement | Xylem vessels and sclerenchyma | Xylem vessel diameter increased by 18% and sclerenchyma thickness by 25% in wheat; reduced drought-induced cavitation | Promoted succulent growth; increased water demand | [57] |
| N Level | Root Structural Responses | Implications for Drought Adaptation | Representative Crop | Reference |
|---|---|---|---|---|
| Moderate N | Increased deep root proportion (30–50% higher root length density in >40 cm soil layers); proliferation of fine roots (<0.5 mm diameter) | Enhanced deep soil water uptake; improved hydraulic conductance; increased drought survival rate | Maize (Zea mays) | [68] |
| Radial expansion of xylem vessels; synergized with fine root proliferation to optimize water transport efficiency | Improved root-to-canopy water transport; maintained transpiration-photosynthesis balance | Catalpa bungei | [28] | |
| N deficiency | Increased shallow fine root density; prioritized carbon allocation to roots for expanded absorption area | Enhanced surface soil resource capture, but insufficient deep water acquisition | Maize (Zea mays) | [66] |
| High N supply | Shallow root distribution; reduced R/S; decreased total root biomass | Rapid depletion of surface soil moisture; increased hydraulic vulnerability under drought | Winter wheat (Triticum aestivum) | [30] |
| N form difference | Preferential NH4+ uptake in Malus prunifolia under drought; upregulated AMT1;2/4;2 transporter expression | Conserved energy consumption; maintained nodule vitality under N-limited drought stress | Apple rootstock (Malus prunifolia) | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Miao, Q.; Gu, Y.; Yang, L.; Wang, P. Research on the Physiological Mechanisms of Nitrogen in Alleviating Plant Drought Tolerance. Plants 2025, 14, 2928. https://doi.org/10.3390/plants14182928
Sun X, Miao Q, Gu Y, Yang L, Wang P. Research on the Physiological Mechanisms of Nitrogen in Alleviating Plant Drought Tolerance. Plants. 2025; 14(18):2928. https://doi.org/10.3390/plants14182928
Chicago/Turabian StyleSun, Xichao, Qi Miao, Yingchen Gu, Lan Yang, and Peng Wang. 2025. "Research on the Physiological Mechanisms of Nitrogen in Alleviating Plant Drought Tolerance" Plants 14, no. 18: 2928. https://doi.org/10.3390/plants14182928
APA StyleSun, X., Miao, Q., Gu, Y., Yang, L., & Wang, P. (2025). Research on the Physiological Mechanisms of Nitrogen in Alleviating Plant Drought Tolerance. Plants, 14(18), 2928. https://doi.org/10.3390/plants14182928








