A Witches’-Broom Disease of Cultivated Strawberry Associated with ‘Candidatus Phytoplasma Rubi’-Related Strains in Southern Italy
Abstract
1. Introduction
2. Results
2.1. Field Observations
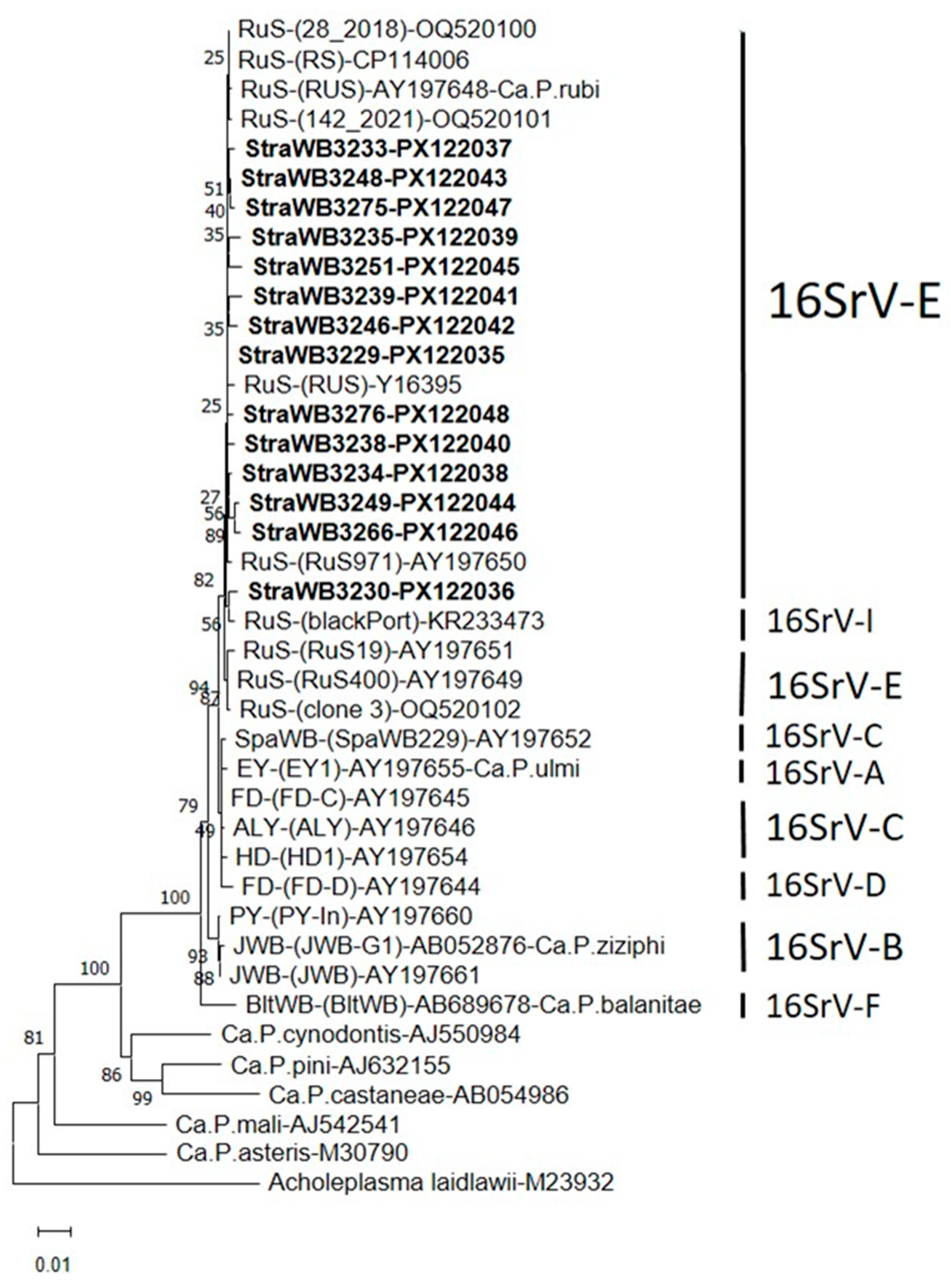
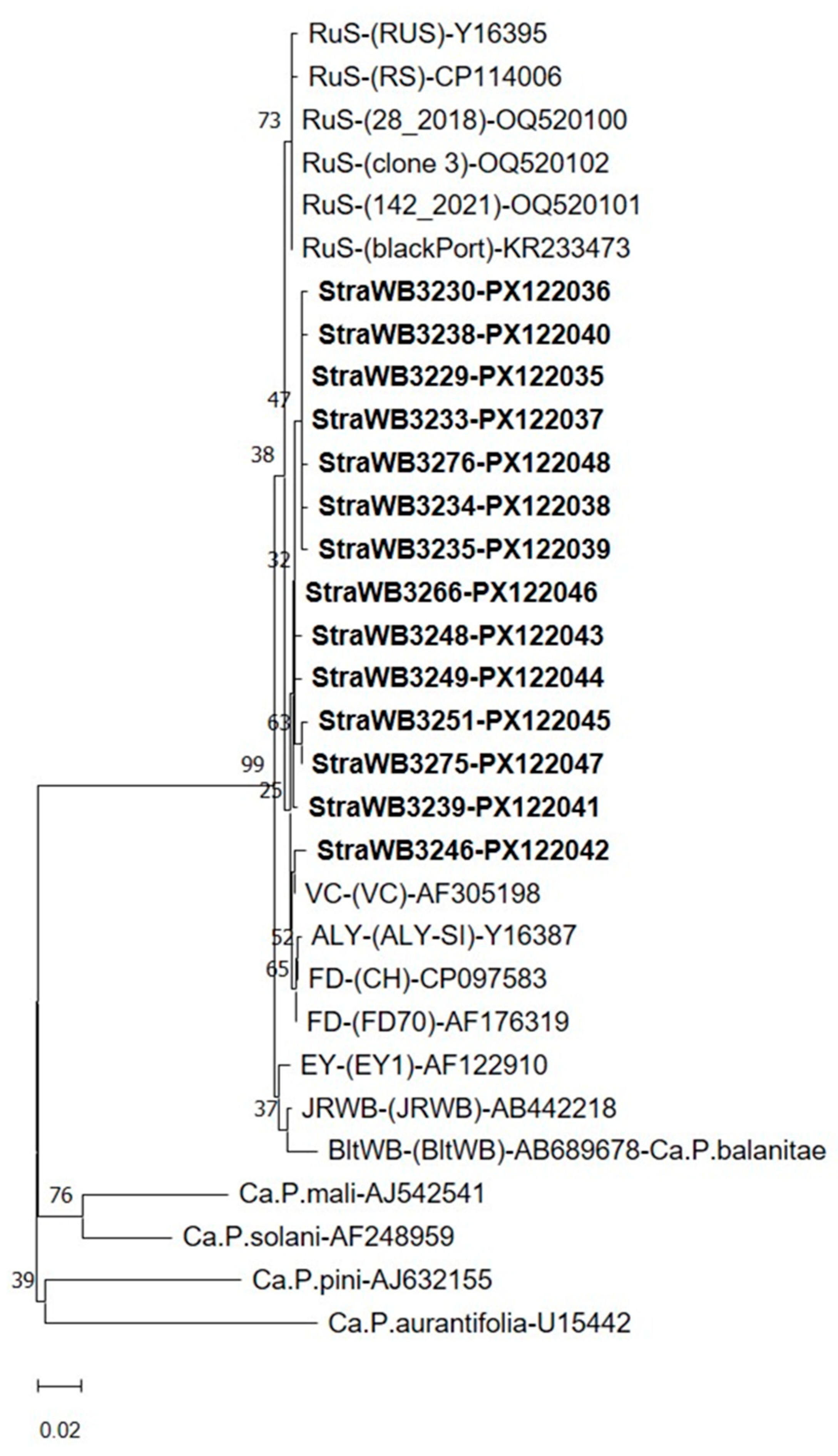
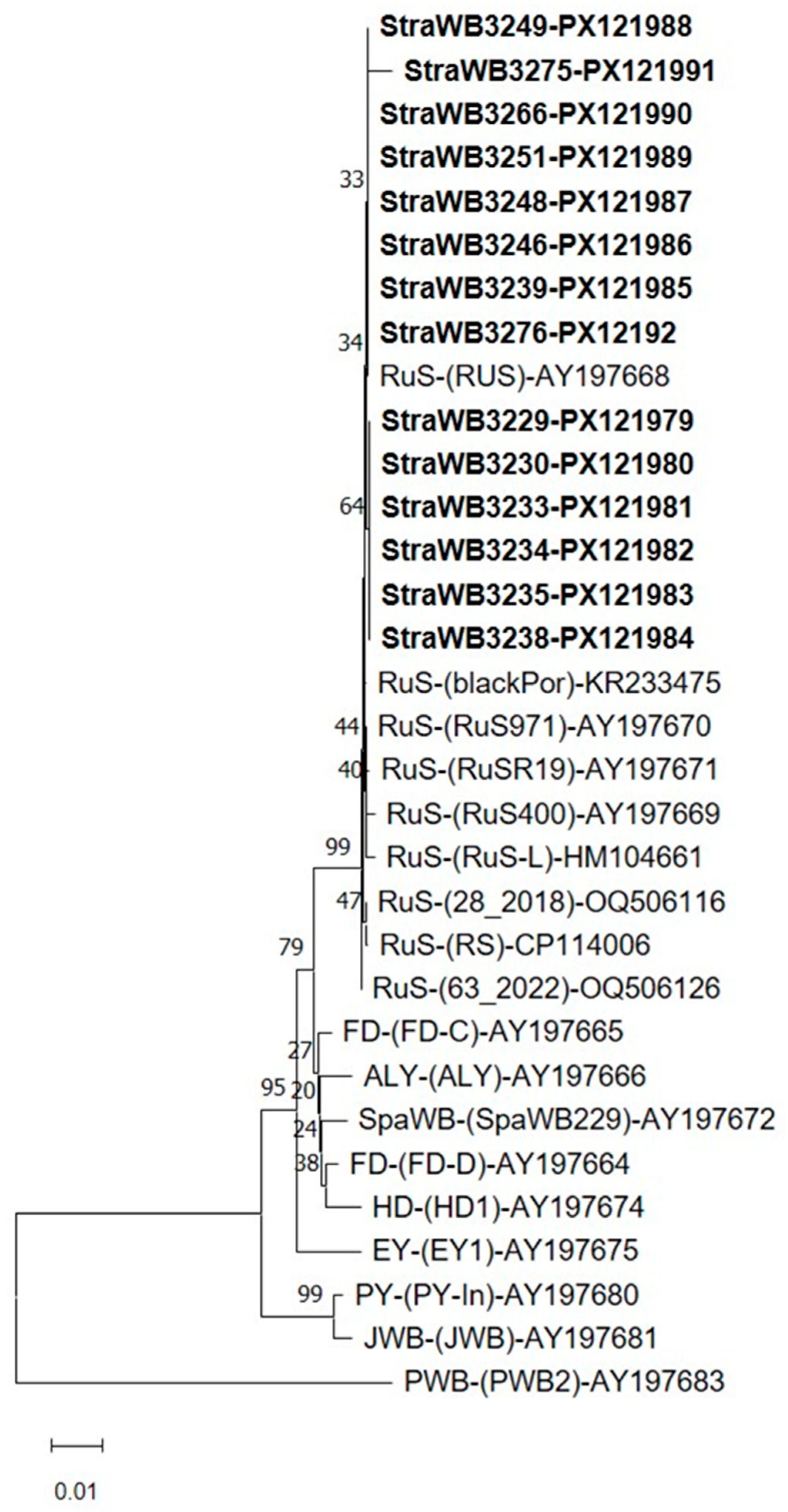
2.2. Phytoplasma Detection and Identification
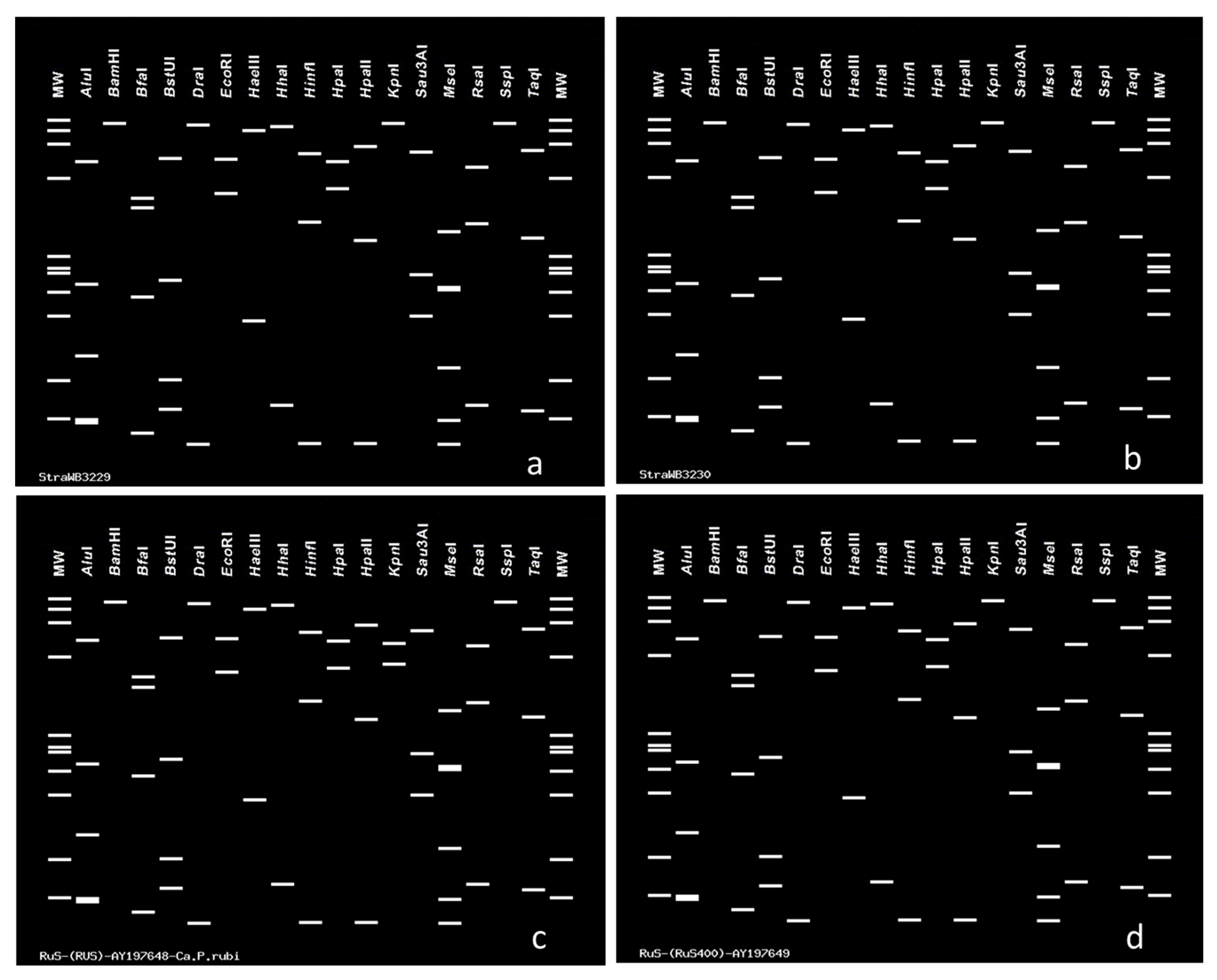
2.3. Virtual RFLP Analysis
3. Discussion
4. Materials and Methods
4.1. Sampling, DNA Isolation, Primers and PCR Amplification
4.2. Sequencing, Phylogenetic and Virtual RFLP Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chiykowski, L.N. Leafhopper-borne diseases. Aster yellows in strawberry. In Virus Diseases of Small Fruits; Converse, R.H., Ed.; United States Department of Agriculture: Washington, DC, USA, 1987; Agriculture Handbook No. 631; pp. 31–34. [Google Scholar]
- Posnette, A.F.; Chiykowski, L.N. Leafhopper-borne diseases. Strawberry green petal and similar diseases. In Virus Diseases of Small Fruits; Converse, R.H., Ed.; United States Department of Agriculture: Washington, DC, USA, 1987; Agriculture Handbook No. 631; pp. 34–38. [Google Scholar]
- Converse, R.H. Leafhopper-borne diseases. Strawberry witches’-broom and multiplier diseases. In Virus Diseases of Small Fruits; Converse, R.H., Ed.; United States Department of Agriculture: Washington, DC, USA, 1987; Agriculture Handbook No. 631; pp. 66–68. [Google Scholar]
- Converse, R.H.; Adams, A.N.; Barbara, D.J.; Clark, M.F.; Casper, R.; Hepp, R.F.; Martin, R.R.; Morris, T.J.; Spiegel, S.; Yoshikawa, N. Laboratory detection of viruses and mycoplasmalike organisms in strawberry. Plant Dis. 1988, 72, 744–749. [Google Scholar] [CrossRef]
- Maas, J.L. Compendium of Strawberry Diseases, 2nd ed.; The American Phytopathological Society (APS Press): St. Paul, MN, USA, 1998; 98p. [Google Scholar]
- Valiunas, D.; Staniulis, J.; Davis, R.E. ‘Candidatus Phytoplasma fragariae’, a novel phytoplasma taxon discovered in yellows diseased strawberry, Fragaria × ananassa. Int. J. Syst. Evol. Microbiol. 2006, 56, 277–281. [Google Scholar] [CrossRef]
- Fernández, F.D.; Meneguzzi, N.G.; Guzmán, F.A.; Kirschbaum, D.S.; Conci, V.C.; Nome, C.F.; Conci, L.R. Detection and identification of a novel 16SrXIII subgroup phytoplasma associated with strawberry red leaf disease in Argentina Int. J. Syst. Evol. Microbiol. 2015, 65, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Brochu, A.-S.; Rodríguez-Martínez, D.; Goulet, C.; Pérez-López, E. Strawberry green petal disease: A diagnostic guide. Plant Health Prog. 2021, 22, 591–595. [Google Scholar] [CrossRef]
- Bertaccini, A. Plants and phytoplasmas: When bacteria modify plants. Plants 2022, 11, 1425. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, P.G.; Beanland, L. Insect vectors of phytoplasmas. Ann. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef]
- Lee, I.-M.; Davis, R.E.; Gundersen-Rindal, D.E. Phytoplasma: Phytopathogenic mollicutes. Annu. Rev. Microbiol. 2000, 54, 221–255. [Google Scholar] [CrossRef]
- Dickinson, M.; Tuffen, M.; Hodgetts, J. The phytoplasmas: An introduction. In Phytoplasma: Methods and Protocols, Methods in Molecular Biology; Dickinson, M., Hodgetts, J., Eds.; Springer Science+Business Media, LLC: New York, NY, USA, 2013; Volume 938, pp. 1–14. [Google Scholar]
- Wei, W.; Shao, J.; Zhao, Y.; Inaba, J.; Ivanauskas, A.; Bottner-Parker, K.D.; Costanzo, S.; Kim, B.M.; Flowers, K.; Escobar, J. iPhyDSDB: Phytoplasma disease and symptom database. Biology 2024, 13, 657. [Google Scholar] [CrossRef]
- IRPCM Phytoplasma/Spiroplasma Working Team—Phytoplasma taxonomy group. ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. Int. J. Syst. Evol. Microbiol. 2004, 54, 1243–1255. [Google Scholar] [CrossRef]
- Bertaccini, A.; Arocha-Rosete, Y.; Contaldo, N.; Duduk, B.; Fiore, N.; Guglielmi Montano, H.; Kube, M.; Kuo, C.-H.; Martini, M.; Oshima, K.; et al. Revision of the ‘Candidatus Phytoplasma’ species description guidelines. Int. J. Syst. Evol. Microbiol. 2022, 72, 005353. [Google Scholar] [CrossRef]
- Wei, W.; Zhao, Y. Phytoplasma Taxonomy: Nomenclature, Classification, and Identification. Biology 2022, 11, 1119. [Google Scholar] [CrossRef]
- Rodrigues Jardim, B.; Tran-Nguyen, L.T.T.; Gambley, C.; Al-Sadi, A.M.; Al-Subhi, A.M.; Foissac, X.; Salar, P.; Cai, H.; Yang, J.-Y.; Davis, R.; et al. The observation of taxonomic boundaries for the 16SrII and 16SrXXV phytoplasmas using genome-based delimitation. Int. J. Syst. Evol. Microbiol. 2023, 73, 005977. [Google Scholar] [CrossRef]
- Rodrigues Jardim, B.; Tran-Nguyen, L.T.T.; Gambley, C.; Webster, C.; Kehoe, M.; Bond, S.; Rodoni, B.; Constable, F.E. ‘Candidatus Phytoplasma vignae’, assigning a species description to a long-known phytoplasma occurring in northern Australia. Int. J. Syst. Evol. Microbiol. 2024, 74, 006502. [Google Scholar] [CrossRef]
- Clark, M.F. Strawberry aster yellows. In Compendium of Strawberry Diseases, 2nd ed.; Maas, J.L., Ed.; The American Phytopathological Society (APS Press): St. Paul, MN, USA, 1998; p. 73. [Google Scholar]
- Clark, M.F. Green petal. In Compendium of Strawberry Diseases, 2nd ed.; Maas, J.L., Ed.; The American Phytopathological Society (APS Press): St. Paul, MN, USA, 1998; pp. 73–74. [Google Scholar]
- Clark, M.F.; Barbara, D.J.; Davies, D.L. Production and characteristics of antisera to Spiroplasma citri and clover phyllody-associated antigens derived from plants. Ann. Appl. Biol. 1983, 103, 251–259. [Google Scholar] [CrossRef]
- Fránova Honetšlegrová, J.; Vibio, M.; Bertaccini, A. Electron microscopy and molecular identification of phytoplasmas associated with strawberry green petals in the Czech Republic. Eur. J. Plant Pathol. 1996, 102, 831–835. [Google Scholar] [CrossRef]
- Harrison, N.A.; Legard, D.E.; Dibonito, R.; Richardson, P.A. Detection and differentiation of phytoplasmas associated with diseases of strawberry in Florida. Plant Dis. 1997, 81, 230. [Google Scholar] [CrossRef] [PubMed]
- Jomantiene, R.; Davis, R.E.; Maas, J.; Dally, E.L. Classification of new phytoplasmas associated with diseases of strawberry in Florida, based on analysis of 16S rRNA and ribosomal protein gene operon sequences. Int. J. Syst. Bacteriol. 1998, 48, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Padovan, A.; Gibb, K.; Persley, D. Association of ‘Candidatus Phytoplasma australiense’ with green petal and lethal yellows diseases in strawberry. Plant Pathol. 2000, 49, 362–369. [Google Scholar] [CrossRef]
- Lee, I.-M.; Gundersen-Rindal, D.E.; Davis, R.E.; Bottner, K.D.; Marcone, C.; Seemüller, E. ‘Candidatus Phytoplasma asteris’, a novel phytoplasma taxon associated with aster yellows and related diseases. Int. J. Syst. Evol. Microbiol. 2004, 54, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, N.; Mejia, J.F.; Paltrinieri, S.; Calari, A.; Bertaccini, A. Identification and GroEl gene characterization of green petal phytoplasma infecting strawberry in Italy. Phytopathog. Mollicutes 2012, 2, 59–62. [Google Scholar] [CrossRef]
- Fernández, F.D.; Conci, V.C.; Kirschbaum, D.S.; Conci, L.R. Molecular characterization of a phytoplasma of the ash yellows group occurring in strawberry (Fragaria × ananassa Duch.) plants in Argentina. Eur. J. Plant Pathol. 2013, 135, 1–4. [Google Scholar] [CrossRef]
- Pérez-López, E.; Dumonceaux, T.J. Detection and identification of the heterogeneous novel subgroup 16SrXIII-(A/I)I phytoplasma associated with strawberry green petal disease and Mexican periwinkle virescence. Int. J. Syst. Evol. Microbiol. 2016, 66, 4406–4415. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Benequen, M.; Silva-Rojas, H.V.; Marbán-Mendoza, N.; Rebollar-Alviter, A. Mexican periwinkle virescence phytoplasma associated with phyllody and virescence in strawberry (Fragaria × ananassa Duch.) in Michoacan, Mexico. Eur. J. Plant Pathol. 2017, 147, 451–454. [Google Scholar] [CrossRef]
- Cui, W.; Quiroga, N.; Curkovic, T.; Zamorano, A.; Fiore, N. Detection and identification of 16SrXIII-F and a novel 16SrXIII phytoplasma subgroups associated with strawberry phyllody in Chile. Eur. J. Plant Pathol. 2019, 155, 1039–1046. [Google Scholar] [CrossRef]
- Plante, N.; Brochu, A.-S.; Goulet, C.; Thibault, P.; Fournier, V.; Pérez-López, E. First evidence of the occurrence of a putative new subgroup of ‘Candidatus Phytoplasma asteris’ (16SrI) associated with strawberry green petal disease in Quebec, Canada. New Dis Rep. 2021, 44, e12038. [Google Scholar] [CrossRef]
- Greber, R.S.; Gowanlock, D.H. Rickettsia-like and mycoplasma-like organisms associated with two yellows-type diseases of strawberry in Queensland. Aust. J. Agric. Res. 1979, 30, 1101–1109. [Google Scholar] [CrossRef]
- Greber, R.S. Strawberry rickettsia yellows and mycoplasma yellows. In Virus Diseases of Small Fruits; Converse, R.H., Ed.; United States Department of Agriculture: Washington, DC, USA, 1987; Agriculture Handbook No. 631; pp. 41–45. [Google Scholar]
- Andersen, M.T.; Longmore, J.; Liefting, L.W.; Wood, G.A.; Sutherland, P.W.; Beck, D.L.; Forster, R.L.S. Phormium yellow leaf phytoplasma is associated with strawberry lethal yellows disease in New Zealand. Plant Dis. 1988, 82, 606–609. [Google Scholar] [CrossRef]
- Streten, C.; Gibb, K.S. Genetic variation in Candidatus Phytoplasma australiense. Plant Pathol. 2005, 54, 8–14. [Google Scholar] [CrossRef]
- Rodrigues Jardim, B.; Gambley, C.; Tran-Nguyen, L.T.T.; Webster, C.; Kehoe, M.; Kinoti, W.M.; Bond, S.; Davis, R.; Jones, L.; Pathania, N.; et al. A metagenomic investigation of phytoplasma diversity in Australian vegetable growing regions. Microb. Genom. 2024, 10, 001213. [Google Scholar] [CrossRef]
- Schwartze, C.D.; Frazier, N.W.; Converse, R.H. Strawberry lethal decline. In Virus Diseases of Small Fruits; Converse, R.H., Ed.; United States Department of Agriculture: Washington, DC, USA, 1987; Agriculture Handbook No. 631; pp. 38–41. [Google Scholar]
- Jomantiene, R.; Maas, J.L.; Dally, E.L.; Davis, R.E. First report of clover yellow edge and STRAWB2 phytoplasmas in strawberry in Maryland. Plant Dis. 1999, 83, 1072. [Google Scholar] [CrossRef]
- Jomantiene, R.; Maas, J.L.; Davis, R.E.; Dally, E.L. Molecular identification and classification of a phytoplasma associated with phyllody of strawberry fruit in Maryland. Plant Dis. 2001, 85, 335. [Google Scholar] [CrossRef]
- Jomantiene, R.; Maas, J.L.; Dally, E.L.; Davis, R.E. Phytoplasmas in strawberry with fruit phyllody. Acta Hortic. 2002, 567, 635–638. [Google Scholar] [CrossRef]
- Jomantiene, R.R.; Maas, J.L.; Takeda, F.; Davis, R.E. Molecular identification and classification of strawberry phylloid fruit phytoplasma in group 16SrI, new subgroup. Plant Dis. 2002, 86, 920. [Google Scholar] [CrossRef]
- Melo, L.; Ventura, J.A.; Costa, H.; Kitajima, E.W.; Ferreira, J.; Bedendo, I.P. Delineation of a novel subgroup 16SrXIII-J phytoplasma, a ‘Candidatus Phytoplasma hispanicum’-related strain, based on computer-simulated RFLP and phylogenetic analysis Int. J. Syst. Evol. Microbiol. 2018, 68, 962–966. [Google Scholar] [CrossRef]
- Zeller, S.M. Preliminary studies on witches’-broom of strawberry. Phytopathology 1927, 18, 329–335. [Google Scholar]
- Clark, M.F. Minor phytoplasma diseases. In Compendium of Strawberry Diseases, 2nd ed.; Maas, J.L., Ed.; The American Phytopathological Society (APS Press): St. Paul, MN, USA, 1998; p. 75. [Google Scholar]
- Boone, D.M. Witches’-broom and multiplier diseases in strawberry. In Virus Diseases of Small Fruits and Grapevines; Frazier, N.W., Ed.; University of California, Division of Agricultural Sciences: Berkeley, CA, USA, 1970; pp. 25–26. [Google Scholar]
- Sehgal, O.P.; Boone, D.M. Multiplier disease of strawberry in Wisconsis. Plant Disease Reptr. 1963, 47, 46–48. [Google Scholar]
- Davis, R.E.; Jomantiene, R.; Maas, J.L.; Legard, D.E.; Postman, J.D. Possible link between the rare plant taxon “Fragaria multicipita” in Canada and a disease problem in strawberry in Florida. Acta Hortic. 1998, 471, 25–30. [Google Scholar] [CrossRef]
- Jomantiene, R.; Davis, R.E.; Dally, E.L.; Maas, J.L. The distinctive morphology of ‘Fragaria multicipita’ is due to phytoplasma. HortScience 1998, 33, 1069–1072. [Google Scholar] [CrossRef]
- Fernández, F.D.; Meneguzzi, N.G.; Conci, L.R. Identification of three novel subgroups within the X-disease group phytoplasma associated with strawberry redness disease. Int. J. Syst. Evol. Microbiol. 2017, 67, 753–758. [Google Scholar] [CrossRef]
- Jomantiene, R.R.; Maas, J.L.; Dally, E.L.; Davis, R.E.; Postman, J.D. First report of clover proliferation phytoplasma in strawberry. Plant Dis. 1999, 83, 967. [Google Scholar] [CrossRef]
- Ferriol-Marchena, X.; Hernández-Rodríguez, L.; Luis-Pantoja, M.; García-García, G.; Banguela-Castillo, A.; Pérez, J.M. First report of a ‘Candidatus Phytoplasma asteris’ isolate affecting strawberry in Cuba. New Dis. Rep. 2013, 28, 19. [Google Scholar] [CrossRef]
- Terlizzi, F.; Babini, A.R.; Credi, R. First report of stolbur phytoplasma (16SrXII-A) on strawberry in northern Italy. Plant Dis. 2006, 90, 831. [Google Scholar] [CrossRef] [PubMed]
- Nourrisseau, J.G.; Lansac, M.; Garnier, M. Marginal chlorosis, a new disease of strawberry associated with a bacteriumlike organism. Plant Dis. 1993, 77, 1055–1059. [Google Scholar] [CrossRef]
- Fos, A.; Danet, J.L.; Zreik, L.; Garnier, M.; Bové, J.M. Use of a monoclonal antibody to detect the stolbur mycoplasmalike organism in plants and insects and to identify a vector in France. Plant Dis. 1992, 76, 1092–1096. [Google Scholar] [CrossRef]
- Danet, J.-L.; Foissac, X.; Zreik, L.; Salar, P.; Verdin, E.; Nourrisseau, J.-G.; Garnier, M. “Candidatus Phlomobacter fragariae” is the prevalent agent of marginal chlorosis of strawberry in French production fields and is transmitted by the planthopper Cixius wagneri (China). Phytopathology 2003, 93, 644–649. [Google Scholar] [CrossRef]
- Oviedo, N.N.; Delgado, J.O.; Corcelles, J.I. A new method for the identification of phytoplasmas in strawberries (Fragaria fragaria). Biosaia 2017, 6, 1. [Google Scholar]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetics Analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Malembic-Maher, S.; Salar, P.; Filippin, L.; Carle, P.; Angelini, E.; Foissac, X. Genetic diversity of European phytoplasmas of the 16SrV taxonomic group and proposal of ‘Candidatus Phytoplasma rubi’. Int. J. Syst. Evol. Microbiol. 2011, 61, 2129–2134. [Google Scholar] [CrossRef]
- Davis, R.E.; Dally, E.L. Revised subgroup classification of group 16SrV phytoplasmas and placement of Flavescence dorée-associated phytoplasmas in two distinct subgroups. Plant Dis. 2001, 85, 790–797. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, W.; Lee, I.-M.; Shao, J.; Suo, X.; Davis, R.E. Construction of an interactive online phytoplasma classification tool, iPhyClassifier, and its application in analysis of the peach X-disease phytoplasma group (16SrIII). Int. J. Syst. Evol. Microbiol. 2009, 59, 2582–2593. [Google Scholar] [CrossRef]
- Lee, I.-M.; Martini, M.; Marcone, C.; Zhu, S.F. Classification of phytoplasma strains in the elm yellows group (16SrV) and proposal of ‘Candidatus Phytoplasma ulmi’ for the phytoplasma associated with elm yellows. Int. J. Syst. Evol. Microbiol. 2004, 54, 337–347. [Google Scholar] [CrossRef]
- Kronenberg, H.E. Report on the lecture before the Netherlands Society for the study of plant diseases November 28, 1942, at Amsterdam. Virus diseases in strawberry. Tijdschr. Over Plantenziekten 1943, 49, 74–76. (In Dutch) [Google Scholar]
- Mäurer, M.; Seemüller, E. Nature and genetic relatedness of the mycoplasma-like organism causing rubus stunt in Europe. Plant Pathol. 1994, 44, 244–249. [Google Scholar] [CrossRef]
- De Jonghe, K.; Goedefroit, T. First report of ‘Candidatus Phytoplasma rubi’ on blackberry in Belgium. New Dis. Rep. 2019, 39, 19. [Google Scholar] [CrossRef]
- Linck, H.; Reineke, A. Rubus stunt: A review of an important phytoplasma disease in Rubus spp. J. Plant Dis. Prot. 2019, 126, 393–399. [Google Scholar] [CrossRef]
- Fránová, J.; Přibylová, J.; Zemek, R.; Tan, J.L.; Hamborg, Z.; Blystad, D.-R.; Lenz, O.; Koloniuk, I. First report of ‘Candidatus Phytoplasma rubi’ associated with rubus stunt disease of raspberry and blackberry in the Czech Republic. Plant Dis. 2023, 107, 2510. [Google Scholar] [CrossRef]
- Fránová, J.; de Sousa, E.; Koloniuk, I.; Mimoso, C.; Matos, J.; Cardoso, F.; Contaldo, N.; Paltrinieri, S.; Bertaccini, A. Multigene characterization of a new ‘Candidatus phytoplasma rubi’-related strain associated with blackberry witches’ broom. Int. J. Syst. Evol. Microbiol. 2016, 66, 1438–1446. [Google Scholar] [CrossRef]
- Jarausch, W.; Jarausch-Wehrheim, B.; Danet, J.L.; Broquaire, J.M.; Dosba, F.; Saillard, C.; Garnier, M. Detection and identification of European stone fruit yellows and other phytoplasmas in wild plants in the surroundings of apricot chlorotic leaf roll-affected orchards in southern France. Eur. J. Plant Pathol. 2001, 107, 209–217. [Google Scholar] [CrossRef]
- Marcone, C.; Hergenhahn, F.; Ragozzino, A.; Seemüller, A. Dodder transmission of pear decline, European stone fruit yellows, rubus stunt, picris echioides yellows and cotton phyllody phytoplasmas to periwinkle. J. Phytopath. 1999, 147, 187–192. [Google Scholar] [CrossRef]
- van der, M. Leafhopper-borne diseases. Rubus stunt. In Virus Diseases of Small Fruits; Converse, R.H., Ed.; United States Department of Agriculture: Washington, DC, USA, 1987; Agriculture Handbook No. 631; pp. 197–203. [Google Scholar]
- Converse, R.H. Leafhopper-transmitted diseases. Rubus stunt. In Compendium of Raspberry and Blackberry Diseases and Insects; Ellis, A., Converse, R.H., Williams, R.N., Williamson, B., Eds.; The American Phytopathological Society (APS Press): St. Paul, MN, USA, 1991; pp. 46–47. [Google Scholar]
- de Fluiter, H.J.; van der Meer, F.A. Rubus stunt, a leafhopper-borne virus disease. Tijdschr. Plantenziekten 1953, 59, 195–197. [Google Scholar]
- de Fluiter, H.J.; van der Meer, F.A. The biology and control of Macropsis fuscula Zett., the vector of Rubus stunt virus. In Proceedings of the Tenth International Congress of Entomology, Montreal, QC, Canada, 17–25 August 1958; Volume 3, pp. 341–346. [Google Scholar]
- Reitzel, J. The leafhopper Macropsis fuscula Zett. In raspberry plantings. Tidsskr. Planteavl 1971, 75, 577–580. [Google Scholar]
- Lehman, W. Euscelis plebejus (Fallén) und Macrosteles cristatus Ribaut als Überträger von Pflanzenkrankheiten von vermuteter Mykoplasma-Ätiologie. Arch. Phytopathol. Pfl. 1973, 9, 363–370. [Google Scholar]
- Jenser, G.; Hegab, A.M.; Dezsery, M. Philaenus spumarius Linn as a vector of the causative pathogen of rubus stunt disease. Acta Phytopathol. Acad. Sci. Hung. 1981, 16, 233–237. [Google Scholar]
- Bertaccini, A.; Vibio, M.; Pastore, M.; Recupero, S.; Guerrini, S.; Grimaldi, D. Nested-PCR assays for detection of phytoplasmas in strawberry. Acta Hortic. 1997, 439, 787–790. [Google Scholar] [CrossRef]
- Pastore, M.; Cubicciotti, G.; Bertaccini, A.; Graziano, V. Phytoplasma infection in strawberry cultivation in southern Italy. Acta Hortic. 2002, 567, 639–642. [Google Scholar] [CrossRef]
- Pastore, M.; Paltrinieri, S.; Bertaccini, A.; Priore, R.; Graziano, V. Phytoplasma sub-groups infecting insects collected in damaged strawberry fields. Acta Hortic. 2006, 708, 161–166. [Google Scholar] [CrossRef]
- Marcone, C.; Ragozzino, A.; Firrao, G.; Locci, R. Detection of a Rubus stunt isolate and characterization by RFLP analysis. Riv. Pat. Veg. 1994, 4, 47–58. [Google Scholar]
- Marcone, C.; Ragozzino, A.; Seemüller, E. Identification and characterization of the phytoplasma associated with elm yellows in southern Italy and its relatedness to other phytoplasmas of the elm yellows group. Eur. J. For. Pathol. 1997, 27, 45–54. [Google Scholar] [CrossRef]
- Marcone, C.; Ragozzino, A.; Schneider, B.; Lauer, U.; Smart, C.D.; Seemüller, E. Genetic characterization and classification of two phytoplasmas associated with spartium witches’-broom disease. Plant Dis. 1996, 80, 365–371. [Google Scholar] [CrossRef]
- Marcone, C.; Pierro, R.; Palmieri, C. Occurrence, impact, and multilocus sequence analysis of alder yellows phytoplasma infecting common alder and Italian alder in southern Italy. Microorganisms 2024, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, U.; Seemüller, E. Detection of DNA of plant pathogenic mycoplasmalike organisms by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology 1992, 82, 828–832. [Google Scholar] [CrossRef]
- Schneider, B.; Seemüller, E.; Smart, C.D.; Kirkpatrick, B.C. Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasmas. In Molecular and Diagnostic Procedures in Mycoplasmology; Razin, S., Tully, J.G., Eds.; Academic Press: San Diego, CA, USA, 1995; Volume I, pp. 369–380. [Google Scholar]
- Gundersen, D.E.; Lee, I.-M. Ultrasensitive detection of phytoplasmas by nested-PCR assays using universal primer pairs. Phytopathol. Mediterr. 1996, 35, 144–151. [Google Scholar]
- Smart, C.D.; Schneider, B.; Blomquist, C.L.; Guerra, L.J.; Harrison, N.A.; Ahrens, U.; Lorenz, K.-H.; Seemüller, E.; Kirkpatrick, B.C. Phytoplasma-specific PCR primers based on sequences of the 16S/23S rRNA spacer region. Appl. Environ. Microbiol. 1996, 62, 2988–2993. [Google Scholar] [CrossRef]
- Maixner, M.; Ahrens, U.; Seemüller, E. Detection of the German grapevine yellows (Vergilbungskrankhet) MLO in grapevine, alternative hosts and a vector by a specific PCR procedure. Eur. J. Plant Pathol. 1995, 101, 241–250. [Google Scholar] [CrossRef]
- Berges, R.; Cousin, M.T.; Roux, J.; Seemüller, E. Detection of phytoplasma infections in declining Populus nigra ‘Italica’ trees and molecular differentiation of the aster yellows phytoplasmas identified in various Populus species. Eur. J. For. Pathol. 1997, 27, 33–43. [Google Scholar] [CrossRef]
- Lee, I.-M.; Gundersen, D.E.; Hammond, R.W.; Davis, R.E. Use of mycoplasmalike organism (MLO) group-specific oligonucleotide primers for nested-PCR assays to detect mixed-MLO infections in a single host plant. Phytopathology 1994, 84, 559–566. [Google Scholar] [CrossRef]
- Arnaud, G.; Malembic-Maher, S.; Salar, P.; Bonnet, P.; Maixner, M.; Marcone, C.; Boudon-Padieu, E.; Foissac, X. Multilocus sequence typing confirms the close genetic interrelatedness of three distinct flavescence dorée phytoplasma strain clusters and group 16SrV phytoplasmas infecting grapevine and alder in Europe. Appl. Environ. Microbiol. 2007, 73, 4001–4010. [Google Scholar] [CrossRef]
- Schneider, B.; Hüttel, B.; Zübert, C.; Kube, M. Genetic variation, phylogenetic relationship and spatial distribution of ‘Candidatus Phytoplasma ulmi’ strains in Germany. Sci. Rep. 2020, 10, 21864. [Google Scholar] [CrossRef]
- Lorenz, K.-H.; Schneider, B.; Ahrens, U.; Seemüller, E. Detection of the apple proliferation and pear decline phytoplasmas by PCR amplification of ribosomal and nonribosomal DNA. Phytopathology 1995, 85, 771–776. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Bottner-Parker, K.D.; Lee, I.-M. PCR-based sequence analysis on multiple genes other than 16S rRNA gene for differentiation of phytoplasmas. In Phytoplasmas: Methods and Protocols, Methods in Molecular Biology; Musetti, R., Pagliari, L., Eds.; Springer Science + Business Media, LLC: New York, NY, USA, 2019; Volume 1875, pp. 97–115. [Google Scholar]
- Seemüller, E.; Marcone, C.; Lauer, U.; Ragozzino, A.; Göschl, M. Current status of molecular classification of the phytoplasmas. J. Plant Pathol. 1998, 80, 3–26. [Google Scholar]
- Duckeck, D.; Zübert, C.; Böhm, J.W.; Carminati, G.; Schneider, B.; Kube, K. Complete Genome of “Candidatus Phytoplasma rubi” RS, a phytopathogenic bacterium associated with rubus stunt disease. Microbiol. Resour. Announc. 2023, 12, e01303-22. [Google Scholar] [CrossRef]
- Griffiths, H.M.; Sinclair, W.A.; Boudon-Padieu, E.; Daire, X.; Lee, I.-M.; Sfalanga, A.; Bertaccini, A. Phytoplasmas associated with elm yellows: Molecular variability and differentiation from related organisms. Plant Dis. 1999, 83, 1101–1104. [Google Scholar] [CrossRef]
- Debonneville, C.; Mandelli, L.; Brodard, J.; Groux, R.; Roquis, D.; Schumpp, O. The complete genome of the “Flavescence dorée” phytoplasma reveals characteristics of low genome plasticity. Biology 2022, 11, 953. [Google Scholar] [CrossRef]
- Jung, H.-Y.; Sawayanagi, T.; Kakizawa, S.; Nishigawa, H.; Wei, W.; Oshima, K.; Miyata, S.-i.; Ugaki, M.; Hibi, T.; Namba, S. ‘Candidatus Phytoplasma ziziphi’, a novel phytoplasma taxon associated with jujube witches’-broom disease. Int. J. Syst. Evol. Microbiol. 2003, 53, 1037–1041. [Google Scholar] [CrossRef]
- Win, N.K.K.; Lee, S.-Y.; Bertaccini, A.; Namba, S.; Jung, H.-Y. ‘Candidatus Phytoplasma balanitae’ associated with witches’ broom disease of Balanites triflora. Int. J. Syst. Evol. Microbiol. 2013, 63, 636–640. [Google Scholar] [CrossRef]
- Kamala-Kannan, S.; Han, S.-S.; Lee, K.-J.; Velmurugan, P.; Lee, Y.H.; Chae, J.-C.; Lee, Y.-S.; Lee, J.-Y.; Oh, B.-T. Association of elm yellows subgroup 16SrV-B phytoplasma with a disease of Hovenia dulcis. J. Phytopathol. 2011, 159, 171–174. [Google Scholar] [CrossRef]
- Marcone, C.; Palmieri, c.; Cuomo, A. Detection and multigene characterization of ‘Candidatus Phytoplasma ulmi’ strains infecting Ulmus spp. in southern Italy. Forests 2024, 15, 2067. [Google Scholar] [CrossRef]
- Trivellone, V.; Ripamonti, M.; Angelini, E.; Filippin, L.; Rossi, M.; Marzachì, C.; Galetto, L. Evidence suggesting interactions between immunodominant membrane protein Imp of Flavescence dorée phytoplasma and protein extracts from distantly related insect species. J. Appl. Microbiol. 2019, 127, 1801–1813. [Google Scholar] [CrossRef]
- Rashidi, M.; Galetto, L.; Bosco, D.; Bulgarelli, A.; Vallino, M.; Veratti, F.; Marzachì, C. Role of the major antigenic membrane protein in phytoplasma transmission by two insect vector species. BMC Microbiol. 2015, 15, 193. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, L.; Jiao, Q.; Yang, S.; Gao, R.; Lu, X.; Zhou, G. Comparative genome analysis of jujube witches’-broom phytoplasma, an obligate pathogen that causes jujube witches’-broom disease. BMC Genom. 2018, 19, 689. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, Y.; Li, H.; Liu, Z.; Gao, W.; Liu, M.; Wang, H.; Liu, P.; Zhao, J. The genome of Candidatus phytoplasma ziziphi provides insights into their biological characteristics. BMC Plant Biol. 2023, 23, 251. [Google Scholar] [CrossRef]
- Martini, M.; Lee, I.-M.; Bottner, K.D.; Zhao, Y.; Botti, S.; Bertaccini, A.; Harrison, N.A.; Carraro, L.; Marcone, C.; Khan, A.J.; et al. Ribosomal protein gene-based phylogeny for finer differentiation and classification of phytoplasmas. Int. J. Syst. Evol. Microbiol. 2007, 57, 2037–2051. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcone, C.; Palmieri, C.; Sellitto, A. A Witches’-Broom Disease of Cultivated Strawberry Associated with ‘Candidatus Phytoplasma Rubi’-Related Strains in Southern Italy. Plants 2025, 14, 2914. https://doi.org/10.3390/plants14182914
Marcone C, Palmieri C, Sellitto A. A Witches’-Broom Disease of Cultivated Strawberry Associated with ‘Candidatus Phytoplasma Rubi’-Related Strains in Southern Italy. Plants. 2025; 14(18):2914. https://doi.org/10.3390/plants14182914
Chicago/Turabian StyleMarcone, Carmine, Carmine Palmieri, and Alberto Sellitto. 2025. "A Witches’-Broom Disease of Cultivated Strawberry Associated with ‘Candidatus Phytoplasma Rubi’-Related Strains in Southern Italy" Plants 14, no. 18: 2914. https://doi.org/10.3390/plants14182914
APA StyleMarcone, C., Palmieri, C., & Sellitto, A. (2025). A Witches’-Broom Disease of Cultivated Strawberry Associated with ‘Candidatus Phytoplasma Rubi’-Related Strains in Southern Italy. Plants, 14(18), 2914. https://doi.org/10.3390/plants14182914






