Effects of Alternative Food Sources and Different Substrates on the Mass Rearing of Amblyseius andersoni
Abstract
1. Introduction
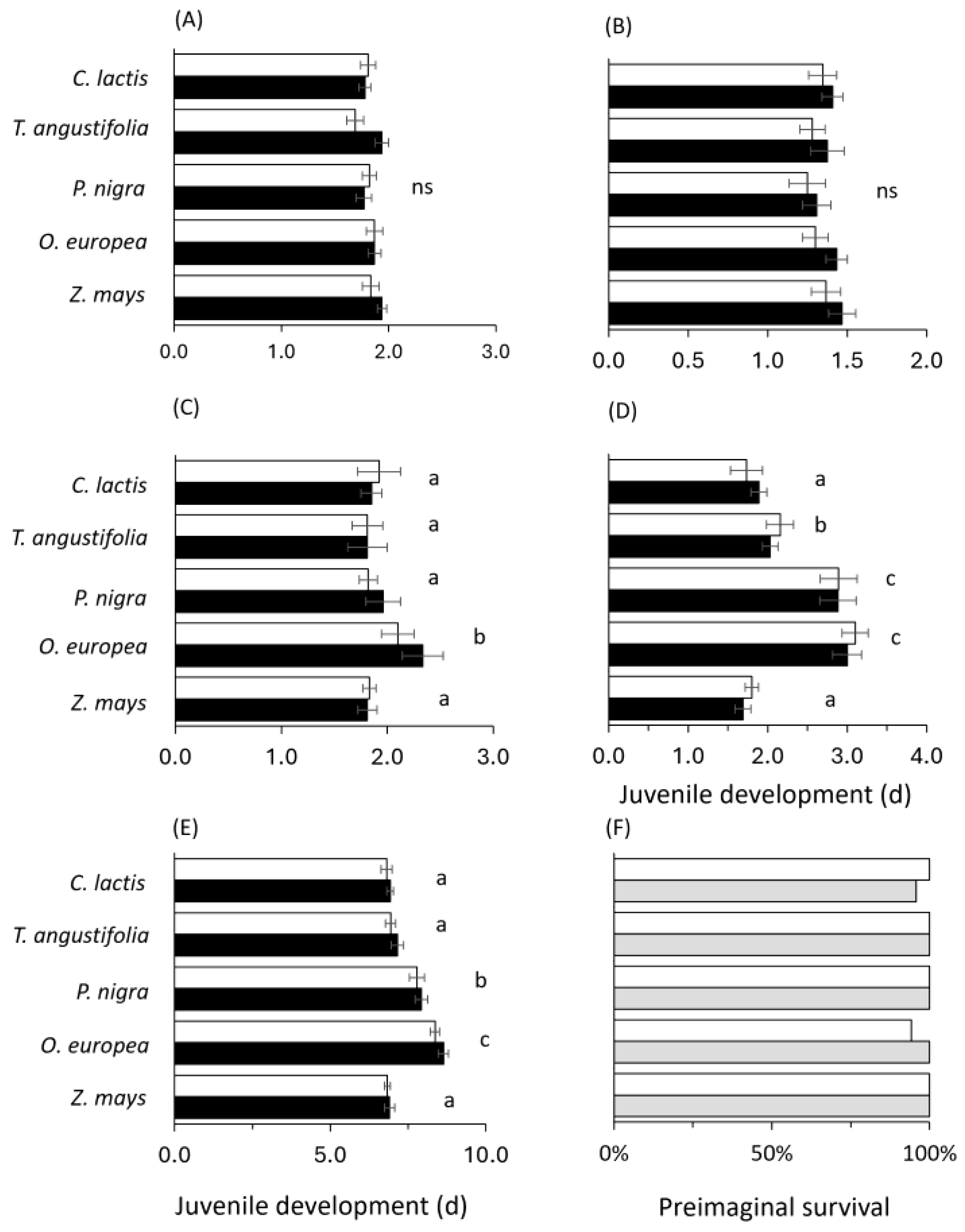
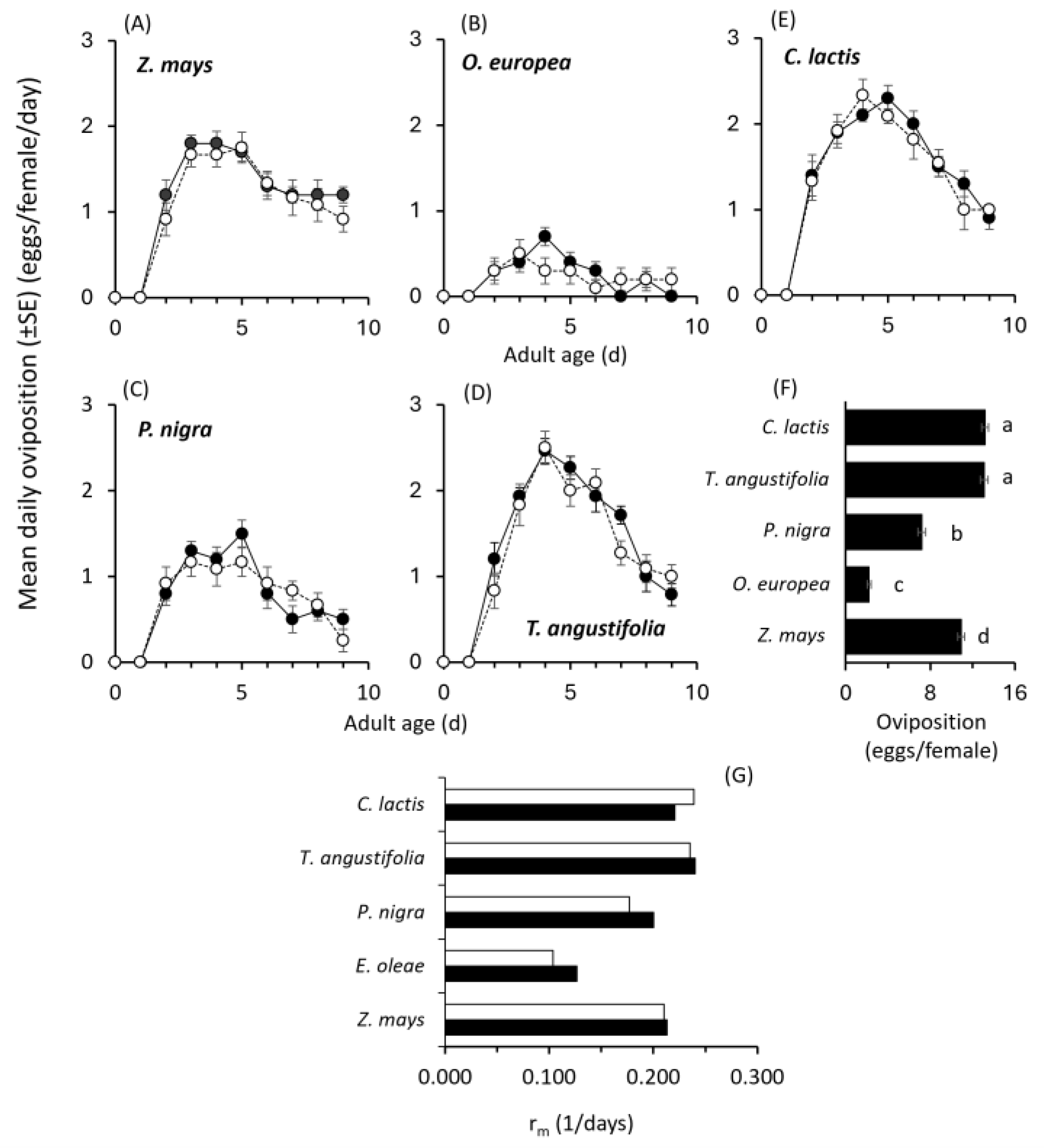
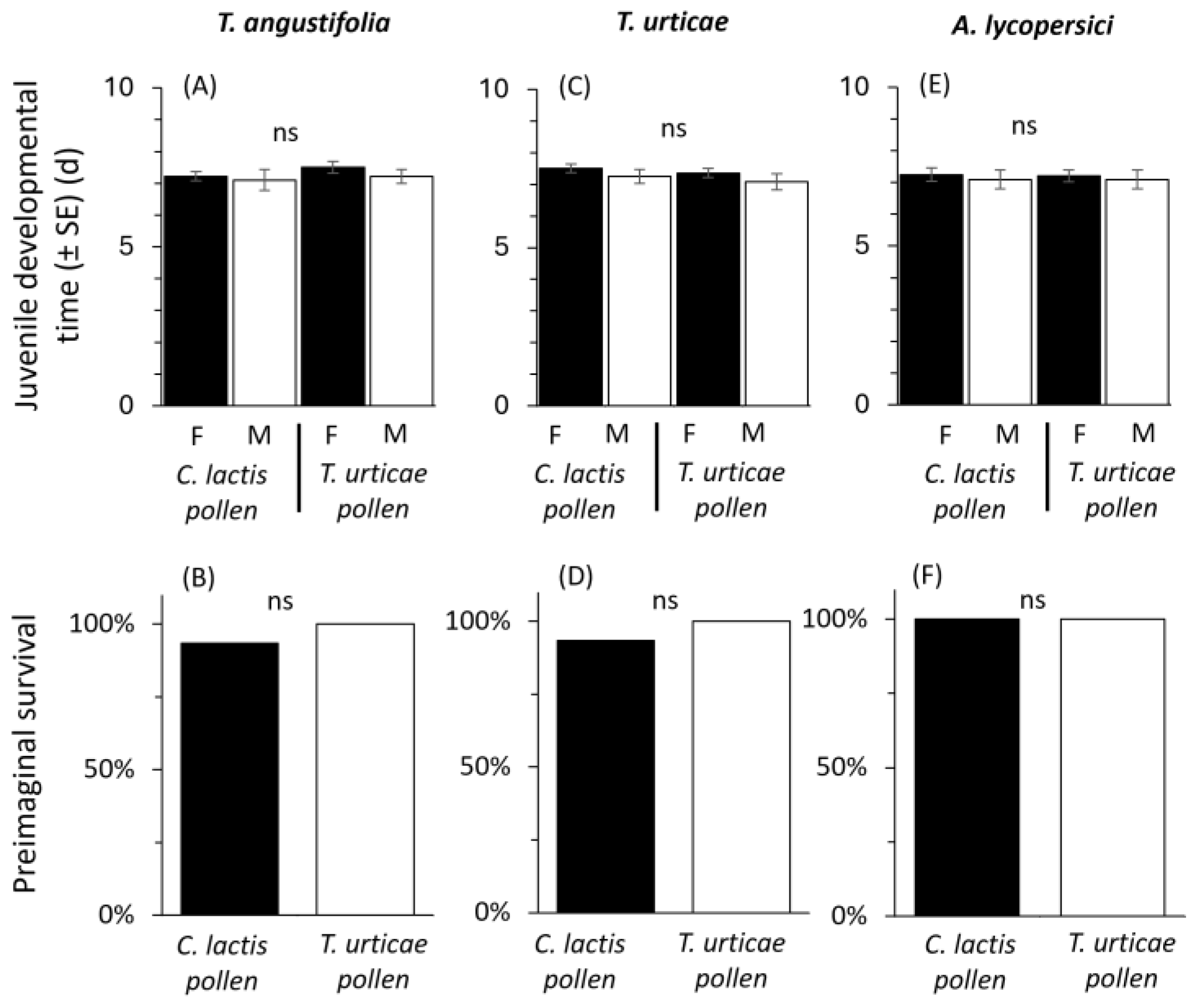
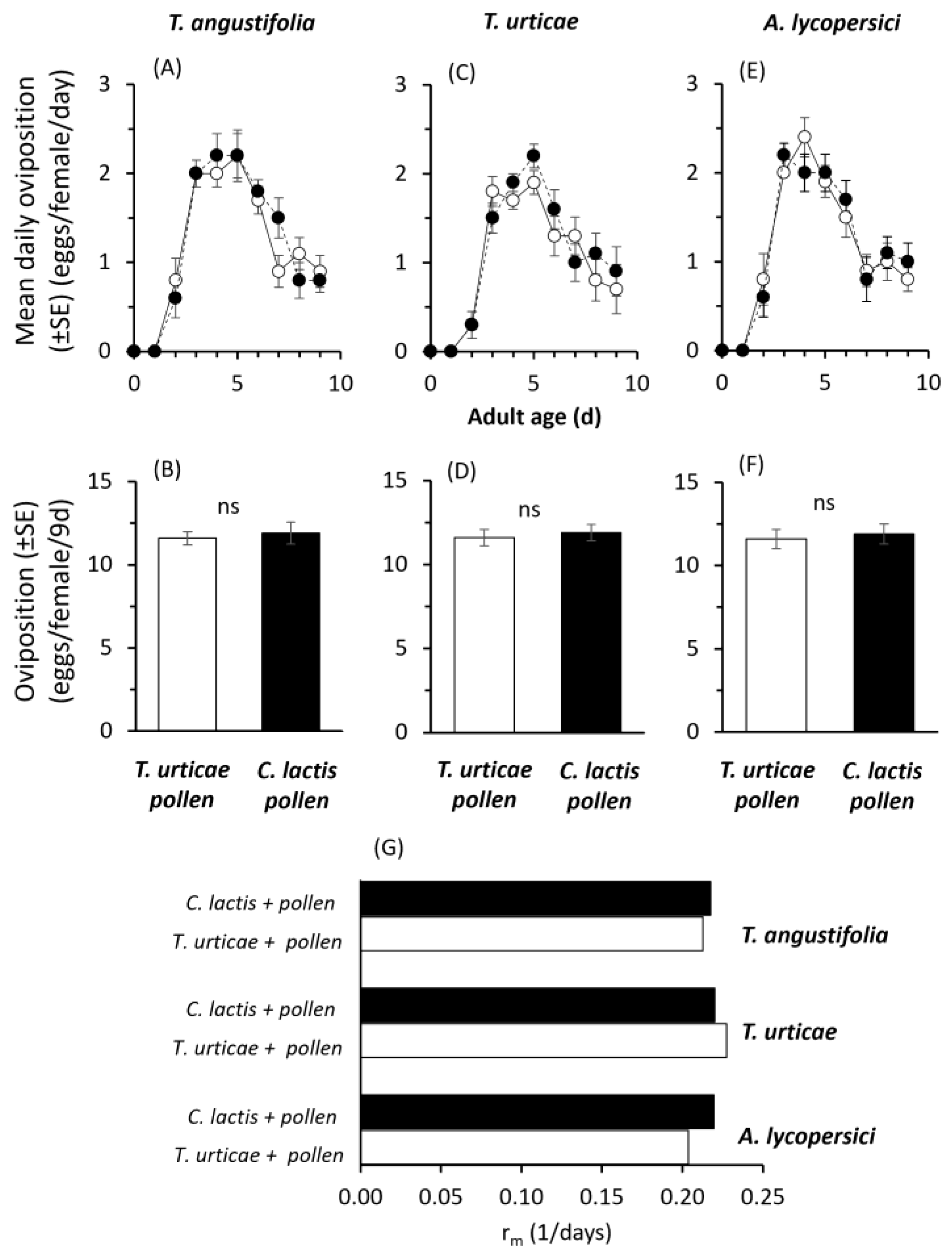
2. Results
2.1. Effects of Different Substrates and Food Sources on Predator Performance
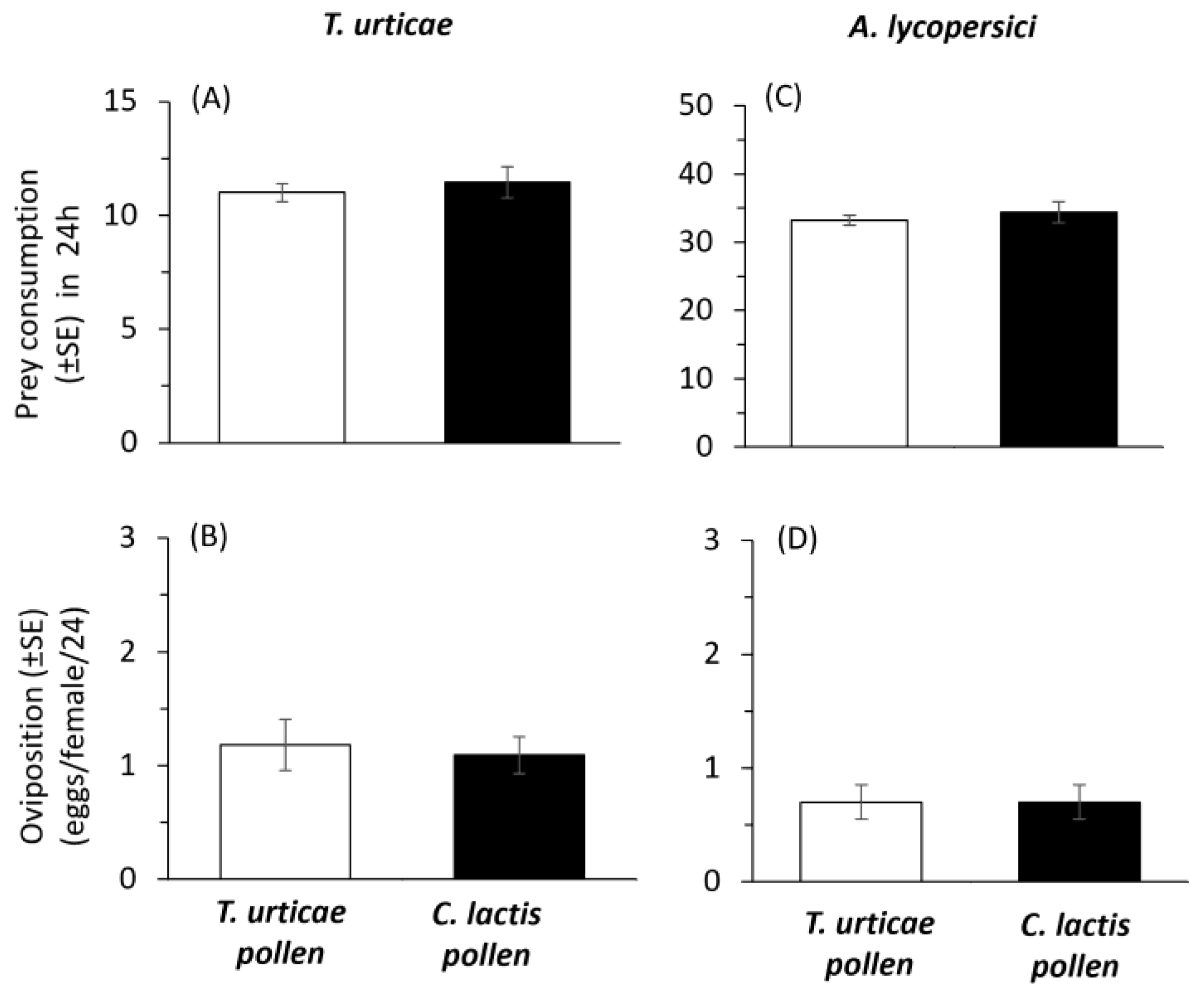
2.2. Mass-Rearing Effects on Predator Performance
3. Discussion
4. Materials and Methods
4.1. Predator Rearing
4.2. Plants
4.3. Experimental Arenas
4.4. Effects of Different Substrates and Food Sources on Predator Performance
4.4.1. Food Sources
4.4.2. Juvenile Development, Survival, and Oviposition of A. andersoni on Different Diets
4.5. Mass-Rearing Effects on Predator Performance
Predator Fitness
4.6. Intrinsic Rates of Population Increase (rm)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kogan, M. Integrated Pest Management: Historical Perspectives and Contemporary Developments. Annu. Rev. Entomol. 1998, 43, 243–270. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Alomar, O.; Ravensberg, W.J.; Urbaneja, A. Biological Control Agents for Control of Pests in Greenhouses. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 409–439. [Google Scholar]
- Galli, M.; Feldmann, F.; Vogler, U.K.; Kogel, K.-H. Can biocontrol be the game-changer in integrated pest management? A review of definitions, methods and strategies. J. Plant Dis. Prot. 2024, 131, 265–291. [Google Scholar] [CrossRef]
- Han, P.; Rodriguez-Saona, C.; Zalucki, M.P.; Liu, S.-s.; Desneux, N. A theoretical framework to improve the adoption of green Integrated Pest Management tactics. Commun. Biol. 2024, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Castañé, C.; Alomar, O.; Rocha, A.; Vila, E.; Riudavets, J. Control of Aculops lycopersici with the Predatory Mite Transeius montdorensis. Insects 2022, 13, 1116. [Google Scholar] [CrossRef]
- Gallego, J.R.; Solano-Rojas, Y.; Tiseyra, B.; Gamez, M.; Cabello, T. Population dynamics of mites in slow-release sachets used in biological control: A new study methodology. Exp. Appl. Acarol. 2022, 87, 325–335. [Google Scholar] [CrossRef]
- Lopez, L. Meet Amblyseius swirskii (Acari: Phytoseiidae): A commonly used predatory mite in vegetable crops. J. Integr. Pest Manag. 2023, 14, 20. [Google Scholar] [CrossRef]
- Palevsky, E.; Walzer, A.; Gal, S.; Schausberger, P. Evaluation of dry-adapted strains of the predatory mite Neoseiulus californicus for spider mite control on cucumber, strawberry and pepper. Exp. Appl. Acarol. 2008, 45, 15–27. [Google Scholar] [CrossRef]
- Gerson, U.; Smiley, R.L.; Ochoa, R. Mites (Acari) for Pest Control; Wiley: Hoboken, NJ, USA, 2007; pp. 1–550. [Google Scholar]
- Knapp, M.; Houten, Y.; Baal, E.; Groot, T. Use of predatory mites in commercial biocontrol: Current status and future prospects. Acarologia 2018, 58, 72–82. [Google Scholar] [CrossRef]
- Novljan, M.; Bohinc, T.; Kreiter, S.; Döker, I.; Trdan, S. The indigenous species of predatory mites (Acari: Phytoseiidae) as biological control agents of plant pests in Slovenia. Acarologia 2023, 63, 1048–1061. [Google Scholar] [CrossRef]
- Messing, R.H.; Croft, B.A. Biosystematics of Amblyseius andersoni and A. potentillae (Acarina: Phytoseiidae): Implications for biological control. Exp. Appl. Acarol. 1991, 10, 267–278. [Google Scholar] [CrossRef]
- Duso, C.; Camporese, P. Developmental times and oviposition rates of predatory mites Typhlodromus pyri and Amblyseius andersoni (Acari: Phytoseiidae) reared on different foods. Exp. Appl. Acarol. 1991, 13, 117–128. [Google Scholar] [CrossRef]
- Puchalska, E.; Zagrodzki, S.K.; Kozak, M.; Rector, B.G.; Mauer, A. A Preliminary Assessment of Amblyseius andersoni (Chant) as a Potential Biocontrol Agent against Phytophagous Mites Occurring on Coniferous Plants. Insects 2021, 12, 664. [Google Scholar] [CrossRef]
- Tixier, M.S.; Douin, M.; Kreiter, S. Phytoseiidae (Acari: Mesostigmata) on plants of the family Solanaceae: Results of a survey in the south of France and a review of world biodiversity. Exp. Appl. Acarol. 2020, 81, 357–388. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, J.A.; De Moraes, G.J.; Sourassou, N.F. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 2013, 18, 297–320. [Google Scholar] [CrossRef]
- McMurtry, J.A.; Croft, B.A. Life-styles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol. 1997, 42, 291–321. [Google Scholar] [CrossRef]
- Duso, C. Role of Amblyseius aberrans (Oud.), Typhlodromus pyri Scheuten and Amblyseius andersoni (Chant) (Acari, Phytoseiidae) in vineyards. J. Appl. Entomol. 1992, 114, 455–462. [Google Scholar] [CrossRef]
- Shaw, B.; Nagy, C.; Fountain, M.T. Organic control strategies for use in ipm of invertebrate pests in apple and pear orchards. Insects 2021, 12, 1106. [Google Scholar] [CrossRef]
- Duso, C.; Pozzebon, A.; Capuzzo, C.; Bisol, P.M.; Otto, S. Grape downy mildew spread and mite seasonal abundance in vineyards: Evidence for the predatory mites Amblyseius andersoni and Typhlodromus pyri. Biol. Control 2003, 27, 229–241. [Google Scholar] [CrossRef]
- Pozzebon, A.; Duso, C. Grape downy mildew Plasmopara viticola, an alternative food for generalist predatory mites occurring in vineyards. Biol. Control 2008, 45, 441–449. [Google Scholar] [CrossRef]
- Pozzebon, A.; Loeb, G.M.; Duso, C. Grape powdery mildew as a food source for generalist predatory mites occurring in vineyards: Effects on life-history traits. Ann. Appl. Biol. 2009, 155, 81–89. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Vangansbeke, D.; De Clercq, P. Performance of four species of phytoseiid mites on artificial and natural diets. Biol. Control 2015, 80, 56–62. [Google Scholar] [CrossRef]
- Malagnini, V.; Pozzebon, A.; Facchin, P.; Paganelli, A.; Duso, C. Airborne pollen can affect the abundance of predatory mites in vineyards: Implications for conservation biological control strategies. Pest Manag. Sci. 2022, 78, 1963–1975. [Google Scholar] [CrossRef]
- Pijnakker, J.; Hürriyet, A.; Petit, C.; Vangansbeke, D.; Duarte, M.V.A.; Arijs, Y.; Moerkens, R.; Sutter, L.; Maret, D.; Wäckers, F. Evaluation of Phytoseiid and Iolinid Mites for Biological Control of the Tomato Russet Mite Aculops lycopersici (Acari: Eriophyidae). Insects 2022, 13, 1146. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bennison, J.; Alomar, O.; Ingegno, B.L.; Tavella, L.; Shipp, L.; Palevsky, E.; Wäckers, F.L. Approaches to conserving natural enemy populations in greenhouse crops: Current methods and future prospects. BioControl 2014, 59, 377–393. [Google Scholar] [CrossRef]
- Broufas, G.D.; Koveos, D.S. Effect of different pollens on development, survivorship and reproduction of Euseius finlandicus (Acari: Phytoseiidae). Environ. Entomol. 2000, 29, 743–749. [Google Scholar] [CrossRef]
- Broufas, G.D.; Pappas, M.L.; Koveos, D.S. Development, survival, and reproduction of the predatory mite Kampimodromus aberrans (Acari: Phytoseiidae) at different constant temperatures. Environ. Entomol. 2007, 36, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Goleva, I.; Zebitz, C.P.W. Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii (Acari, Phytoseiidae). Exp. Appl. Acarol. 2013, 61, 259–283. [Google Scholar] [CrossRef]
- Pappas, M.L.; Xanthis, C.; Samaras, K.; Koveos, D.S.; Broufas, G.D. Potential of the predatory mite Phytoseius finitimus (Acari: Phytoseiidae) to feed and reproduce on greenhouse pests. Exp. Appl. Acarol. 2013, 61, 387–401. [Google Scholar] [CrossRef]
- Samaras, K.; Pappas, M.L.; Fytas, E.; Broufas, G.D. Pollen suitability for the development and reproduction of Amblydromalus limonicus (Acari: Phytoseiidae). BioControl 2015, 60, 773–782. [Google Scholar] [CrossRef]
- Samaras, K.; Pappas, M.L.; Fytas, E.; Broufas, G.D. Pollen provisioning enhances the performance of Amblydromalus limonicus on an unsuitable prey. Front. Ecol. Evol. 2019, 7, 122. [Google Scholar] [CrossRef]
- Van Rijn, P.C.J.; Tanigoshi, L.K. Pollen as food for the predatory mites Iphiseius degenerans and Neoseiulus cucumeris (Acari: Phytoseiidae): Dietary range and life history. Exp. Appl. Acarol. 1999, 23, 785–802. [Google Scholar] [CrossRef]
- Vangansbeke, D.; Nguyen, D.T.; Audenaert, J.; Verhoeven, R.; Gobin, B.; Tirry, L.; De Clercq, P. Food supplementation affects interactions between a phytoseiid predator and its omnivorous prey. Biol. Control 2014, 76, 95–100. [Google Scholar] [CrossRef]
- Beltrà, A.; Calabuig, A.; Navarro-Campos, C.; José Ramírez-Soria, M.; Soto, A.; Garcia-Marí, F.; Wäckers, F.L.; Pekas, A. Provisioning of food supplements enhances the conservation of phytoseiid mites in citrus. Biol. Control 2017, 115, 18–22. [Google Scholar] [CrossRef]
- Riahi, E.; Fathipour, Y.; Talebi, A.A.; Mehrabadi, M. Natural diets versus factitious prey: Comparative effects on development, fecundity and life table of Amblyseius swirskii (Acari: Phytoseiidae). Syst. Appl. Acarol. 2017, 22, 711–723. [Google Scholar] [CrossRef]
- Bolckmans, K.; van Houten, Y. Mite Composition, Use Thereof, Method for Rearing the Phytoseiid Predatory Mite Amblyseius swirskii, Rearing System for Rearing Said Phytoseiid Mite and Methods for Biological Pest Control on a Crop. World Intellectual Property Organisation. WO Patent WO/2006/057552 A1, 1 June 2006. [Google Scholar]
- Nguyen, D.T.; Vangansbeke, D.; Lü, X.; De Clercq, P. Development and reproduction of the predatory mite Amblyseius swirskii on artificial diets. BioControl 2013, 58, 369–377. [Google Scholar] [CrossRef]
- Midthassel, A.; Leather, S.R.; Wright, D.J.; Baxter, I.H. The functional and numerical response of Typhlodromips swirskii (Acari: Phytoseiidae) to the factitious prey Suidasia medanensis (Acari: Suidasidae) in the context of a breeding sachet. Biocontrol Sci. Technol. 2014, 24, 361–374. [Google Scholar] [CrossRef]
- Simoni, S.; Nannelli, R.; Goggioli, D.; Guidi, S.; Castagnoli, M. Biological and demographic parameters of Neoseiulus californicus (McGregors) (Acari Phytoseiidae) reared on two astigmatid mites. Redia 2006, 89, 59–63. [Google Scholar]
- Pirayeshfar, F.; Safavi, S.A.; Sarraf Moayeri, H.R.; Messelink, G.J. The potential of highly nutritious frozen stages of Tyrophagus putrescentiae as a supplemental food source for the predatory mite Amblyseius swirskii. Biocontrol Sci. Technol. 2020, 30, 403–417. [Google Scholar] [CrossRef]
- Pirayeshfar, F.; Safavi, S.A.; Moayeri, H.R.S.; Messelink, G.J. Provision of astigmatid mites as supplementary food increases the density of the predatory mite Amblyseius swirskii in greenhouse crops, but does not support the omnivorous pest, western flower thrips. BioControl 2021, 66, 511–522. [Google Scholar] [CrossRef]
- Buitenhuis, R.; Glemser, E.; Brommit, A. Practical placement improves the performance of slow release sachets of Neoseiulus cucumeris. Biocontrol Sci. Technol. 2014, 24, 1153–1166. [Google Scholar] [CrossRef]
- Pochubay, E.; Tourtois, J.; Himmelein, J.; Grieshop, M. Slow-release sachets of Neoseiulus cucumeris predatory mites reduce intraguild predation by dalotia coriaria in greenhouse biological control systems. Insects 2015, 6, 489–507. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Q.; Zhang, Z.Q. Fresh and frozen dried fruit mites (Carpoglyphus lactis) supported the rearing of a predatory mite Phytoseius leaki (Acari: Phytoseiidae) with specialised niche requirements. J. Stored Prod. Res. 2025, 112, 102651. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Y.X.; Lin, J.Z.; Chen, X.; Sun, L.; Saito, Y. Life histories of three predatory mites feeding upon Carpoglyphus lactis (Acari, Phytoseiidae; Carpoglyphidae). Syst. Appl. Acarol. 2015, 20, 491–496. [Google Scholar] [CrossRef]
- Solano-Rojas, Y.; Gallego, J.R.; Gamez, M.; Lopez, I.; Castillo, P.; Cabello, T. Effect of Relative Humidity on the Population Dynamics of the Predator Amblyseius swirskii and Its Prey Carpoglyphus lactis in the Context of Slow-Release Sachets for Use in Biological Control in Greenhouses. Plants 2022, 11, 2493. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Li, L.; Zhang, Z.-Q. Development and reproduction of four predatory mites (Parasitiformes: Phytoseiidae) feeding on the spider mites Tetranychus evansi and T. urticae (Trombidiformes: Tetranychidae) and the dried fruit mite Carpoglyphus lactis (Sarcoptiformes: Carpoglyphidae). Syst. Appl. Acarol. 2024, 29, 269–284. [Google Scholar] [CrossRef]
- Nguyen, H.; Nguyen, B.; Mainali, B.; Maselko, M. Diet optimization for rearing Transeius montdorensis predatory mites under laboratory conditions. Scientific Reports 2025, 15, 9761. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, B.; Li, Z.; Wang, E.; Wei, G.-S.; Xu, X. Phenotypic plasticity of predatory mite Amblyseius orientalis in response to diet switch. Syst. Appl. Acarol. 2022, 27, 1098–1108. [Google Scholar] [CrossRef]
- Khanamani, M.; Basij, M.; Fathipour, Y. Effectiveness of factitious foods and artificial substrate in mass rearing and conservation of Neoseiulus californicus (Acari: Phytoseiidae). Int. J. Acarol. 2021, 47, 273–280. [Google Scholar] [CrossRef]
- Lee, M.H.; Zhang, Z.-Q. Assessing the augmentation of Amblydromalus limonicus with the supplementation of pollen, thread, and substrates to combat greenhouse whitefly populations. Sci. Rep. 2018, 8, 12189. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, S.; Fathipour, Y.; Riahi, E.; Zalucki, M.P. Mass Production of Neoseiulus cucumeris (Acari: Phytoseiidae): An Assessment of 50 Generations Reared on Almond Pollen. J. Econ. Entomol. 2021, 114, 2255–2263, 2259. [Google Scholar] [CrossRef]
- Yazdanpanah, S.; Fathipour, Y. How mixture of plant and prey diets affects long-term rearing of predatory mite Neoseiulus cucumeris (Acari: Phytoseiidae). Ann. Entomol. Soc. Am. 2023, 116, 185–194. [Google Scholar] [CrossRef]
- vantornhout, I.; Minnaert, H.; Tirry, L.; de Clercq, P. Effect of pollen, natural prey and factitious prey on the development of Iphiseius degenerans. BioControl 2004, 49, 627–644. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Z.-Q. The dried fruit mite Carpoglyphus lactis (Acari: Carpoglyphidae) is a suitable alternative prey for Amblyseius herbicolus (Acari: Phytoseiidae). Syst. Appl. Acarol. 2021, 26, 2167–2176, 2110. [Google Scholar] [CrossRef]
- Wäckers, F.L. Suitability of (extra-)floral nectar, pollen, and honeydew as insect food sources. In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and its Applications; Wäckers, F.L., van Rijn, P.C.J., Bruin, J., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 17–74. [Google Scholar]
- Goleva, I.; Rubio Cadena, E.C.; Ranabhat, N.B.; Beckereit, C.; Zebitz, C.P.W. Dietary effects on body weight of predatory mites (Acari, Phytoseiidae). Exp. Appl. Acarol. 2015, 66, 541–553. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khanamani, M.; Fathipour, Y.; Talebi, A.A.; Mehrabadi, M. Linking pollen quality and performance of Neoseiulus californicus (Acari: Phytoseiidae) in two-spotted spider mite management programmes. Pest Manag. Sci. 2017, 73, 452–461. [Google Scholar] [CrossRef]
- Khanamani, M.; Fathipour, Y.; Talebi, A.A.; Mehrabadi, M. Quantitative Analysis of Long-Term Mass Rearing of Neoseiulus californicus (Acari: Phytoseiidae) on Almond Pollen. J. Econ. Entomol. 2017, 110, 1442–1450. [Google Scholar] [CrossRef]
- Asgari, F.; Moayeri, H.R.S.; Kavousi, A.; Enkegaard, A.; Chi, H. Demography and Mass Rearing of Amblyseius swirskii (Acari: Phytoseiidae) Fed on Two Species of Stored-Product Mites and Their Mixture. J. Econ. Entomol. 2020, 113, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Nemati, A.; Riahi, E. Does feeding on pollen grains affect the performance of Amblyseius swirskii (Acari: Phytoseiidae) during subsequent generations? Bull. Entomol. Res. 2020, 110, 449–456. [Google Scholar] [CrossRef]
- De Clercq, P.; Arijs, Y.; Van Meir, T.; Van Stappen, G.; Sorgeloos, P.; Dewettinck, K.; Rey, M.; Grenier, S.; Febvay, G. Nutritional value of brine shrimp cysts as a factitious food for Orius laevigatus (Heteroptera: Anthocoridae). Biocontrol Sci. Technol. 2005, 15, 467–479. [Google Scholar] [CrossRef]
- Yazdanpanah, S.; Fathipour, Y.; Riahi, E.; Zalucki, M.P. Effects of diet switching from almond pollen to natural prey on predation capacity of Neoseiulus cucumeris. J. Appl. Entomol. 2023, 147, 72–84. [Google Scholar] [CrossRef]
- Li, G.-Y.; Pattison, N.; Zhang, Z.-Q. Immature development and survival of Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) on eggs of Tyrophagus curvipenis (Fain & Fauvel) (Acari: Acaridae). Acarologia 2021, 61, 84–93. [Google Scholar] [CrossRef]
- Puchalska, E.K.; Kozak, M. Typhlodromus pyri and Euseius finlandicus (Acari: Phytoseiidae) as potential biocontrol agents against spider mites (Acari: Tetranychidae) inhabiting willows: Laboratory studies on predator development and reproduction on four diets. Exp. Appl. Acarol. 2016, 68, 39–53. [Google Scholar] [CrossRef]
- Janssen, A.; Sabelis, M.W. Phytoseiid life-histories, local predator-prey dynamics, and strategies for control of tetranychid mites. Exp. Appl. Acarol. 1992, 14, 233–250. [Google Scholar] [CrossRef]
- Nomikou, M.; Janssen, A.; Schraag, R.; Sabelis, M.W. Phytoseiid predators as potential biological control agents for Bemisia tabaci. Exp. Appl. Acarol. 2001, 25, 271–291. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bechtsoudis, A.; Pappas, M.L.; Samaras, K.; Broufas, G.D. Effects of Alternative Food Sources and Different Substrates on the Mass Rearing of Amblyseius andersoni. Plants 2025, 14, 2912. https://doi.org/10.3390/plants14182912
Bechtsoudis A, Pappas ML, Samaras K, Broufas GD. Effects of Alternative Food Sources and Different Substrates on the Mass Rearing of Amblyseius andersoni. Plants. 2025; 14(18):2912. https://doi.org/10.3390/plants14182912
Chicago/Turabian StyleBechtsoudis, Angelos, Maria L. Pappas, Konstantinos Samaras, and George D. Broufas. 2025. "Effects of Alternative Food Sources and Different Substrates on the Mass Rearing of Amblyseius andersoni" Plants 14, no. 18: 2912. https://doi.org/10.3390/plants14182912
APA StyleBechtsoudis, A., Pappas, M. L., Samaras, K., & Broufas, G. D. (2025). Effects of Alternative Food Sources and Different Substrates on the Mass Rearing of Amblyseius andersoni. Plants, 14(18), 2912. https://doi.org/10.3390/plants14182912







