Advancing the Chemical Characterization of Eperua oleifera Duke Oleoresin: A UHPLC-HRMS-Based Approach
Abstract
1. Introduction
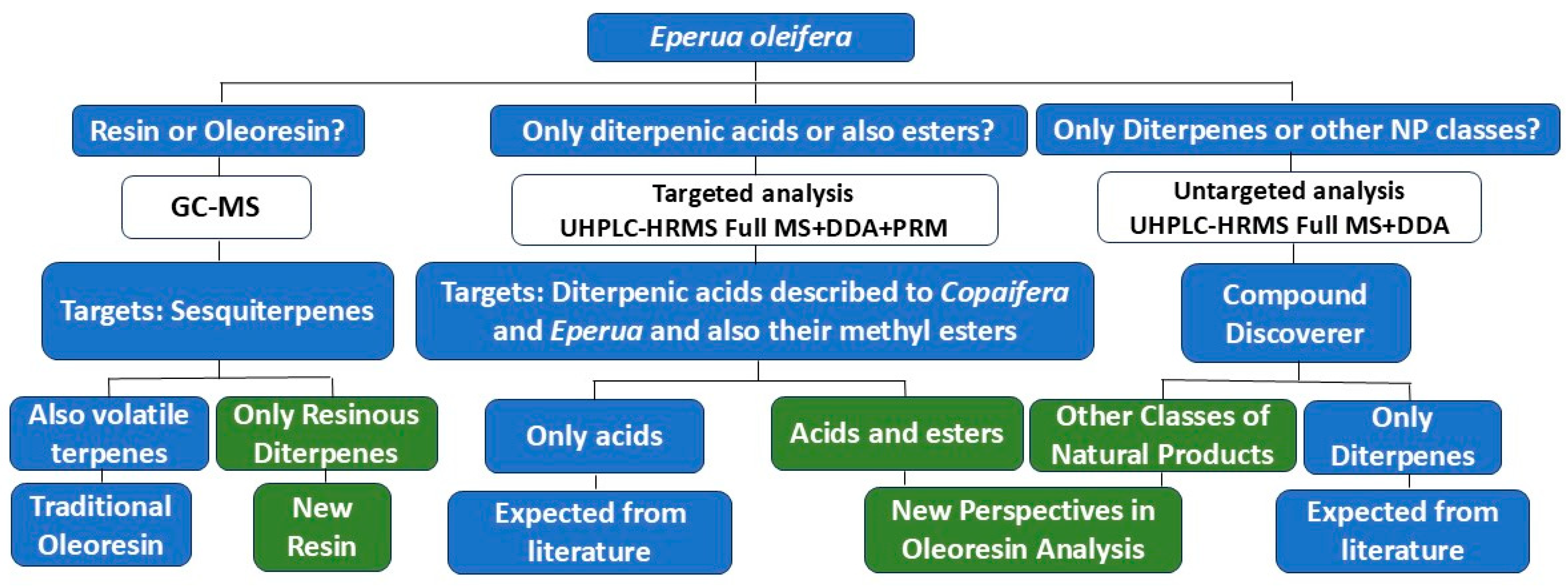
2. Results and Discussion
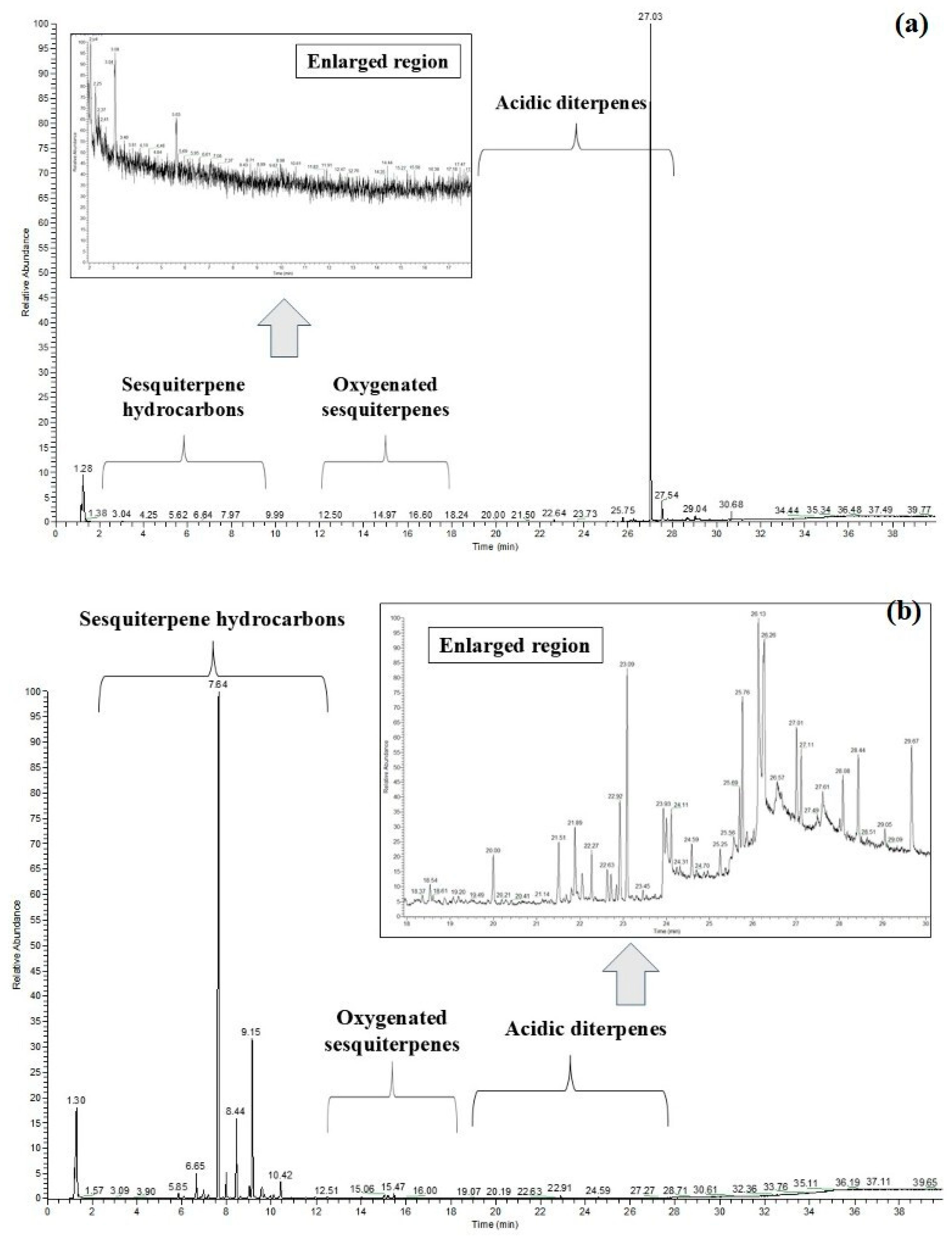
2.1. Evaluation of Sesquiterpene Presence Using GC-MS
2.2. Chemical Characterization of Diterpenes (Targeted) by UHPLC-HRMS
2.2.1. Evaluation of the Kinetics of Diterpenoate Methyl Ester Formation
Oleoresin Dissolved in Methanol Containing 0.1% Formic Acid
Oleoresin Dissolved in Acetonitrile
2.3. Putative Identification of Other Substances in E. oleifera Resin Using the UHPLC-HRMS Approach
3. Materials and Methods
3.1. Plant Material
3.2. GC-MS Analysis and Instrument Conditions
3.3. UHPLC-HRMS Analysis and Instrument Conditions
3.3.1. Evaluation of the Kinetics of Methyl Ester Formation by UHPLC-HRMS
3.3.2. Data Processing and Data Analysis Workflow
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jardim Botânico do Rio de Janeiro. Available online: https://jabot.jbrj.gov.br/ (accessed on 7 September 2025).
- Gomes, F.T.A.; de Araújo Boleti, A.P.; Leandro, L.M.; Squinello, D.; Aranha, E.S.P.; Vasconcelos, M.C.; Cos, P.; Veiga-Junior, V.F.; Lima, E.S. Biological Activities and Cytotoxicity of Eperua oleifera Ducke Oil-Resin. Pharmacogn. Mag. 2017, 13, 542. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.M.; Veiga-Junior, V.F. O Gênero Eperua Aublet: Uma Revisão. Sci. Amazon. 2012, 1, 14–22. [Google Scholar]
- Leandro, L.M.; da Veiga-Junior, V.F.; Sales, A.P.B.; do O Pessoa, C. Composição Química e Atividade Citotóxica Dos Óleos Essenciais Das Folhas e Talos de Eperua duckeana Cowan. Bol. Latinoam. Caribe Plantas Med. Aromat. 2015, 14, 42–47. [Google Scholar]
- de Sousa, J.P.B.; Brancalion, A.P.S.; Junior, M.G.; Bastos, J.K. A Validated Chromatographic Method for the Determination of Flavonoids in Copaifera Langsdorffii by HPLC. Nat. Prod. Commun. 2012, 7, 1934578X1200700110. [Google Scholar] [CrossRef]
- Xavier Junior, F.H.; Gueutin, C.; do Vale Morais, A.R.; do Nascimento Alencar, E.; do Egito, E.S.T.; Vauthier, C. HPLC Method for the Dosage of Paclitaxel in Copaiba Oil: Development, Validation, Application to the Determination of the Solubility and Partition Coefficients. Chromatographia 2016, 79, 405–412. [Google Scholar] [CrossRef]
- Souza, A.B.; Moreira, M.R.; Borges, C.H.G.; Simão, M.R.; Bastos, J.K.; de Sousa, J.P.B.; Ambrosio, S.R.; Veneziani, R.C.S. Development and Validation of a Rapid RP-HPLC Method for Analysis of (−)-copalic Acid in Copaíba Oleoresin. Biomed. Chromatogr. 2013, 27, 280–283. [Google Scholar] [CrossRef]
- Bertolucci, S.K.V.; Pereira, A.B.D.; Pinto, J.E.B.P.; de Aquino Ribeiro, J.A.; de Oliveira, A.B.; Braga, F.C. Development and Validation of an RP-HPLC Method for Quantification of Cinnamic Acid Derivatives and Kaurane-Type Diterpenes in Mikania Laevigata and Mikania Glomerata. Planta Med. 2009, 75, 280–285. [Google Scholar] [CrossRef]
- Al-Sulaiti, H.; Almaliti, J.; Naman, C.B.; Al Thani, A.A.; Yassine, H.M. Metabolomics Approaches for the Diagnosis, Treatment, and Better Disease Management of Viral Infections. Metabolites 2023, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Lu, H.; Lee, Y.H. Challenges and Emergent Solutions for LC-MS/MS Based Untargeted Metabolomics in Diseases. Mass. Spectrom. Rev. 2018, 37, 772–792. [Google Scholar] [CrossRef]
- Couttas, T.A.; Jieu, B.; Rohleder, C.; Leweke, F.M. Current State of Fluid Lipid Biomarkers for Personalized Diagnostics and Therapeutics in Schizophrenia Spectrum Disorders and Related Psychoses: A Narrative Review. Front. Psychiatry 2022, 13, 885904. [Google Scholar] [CrossRef]
- Lazofsky, A.; Brinker, A.; Rivera-Núñez, Z.; Buckley, B. A Comparison of Four Liquid Chromatography–Mass Spectrometry Platforms for the Analysis of Zeranols in Urine. Anal. Bioanal. Chem. 2023, 415, 4885–4899. [Google Scholar] [CrossRef]
- Li, C.; Chu, S.; Tan, S.; Yin, X.; Jiang, Y.; Dai, X.; Gong, X.; Fang, X.; Tian, D. Towards Higher Sensitivity of Mass Spectrometry: A Perspective from the Mass Analyzers. Front. Chem. 2021, 9, 813359. [Google Scholar] [CrossRef] [PubMed]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Ebbels, T.M.D.; van der Hooft, J.J.J.; Chatelaine, H.; Broeckling, C.; Zamboni, N.; Hassoun, S.; Mathé, E.A. Recent Advances in Mass Spectrometry-Based Computational Metabolomics. Curr. Opin. Chem. Biol. 2023, 74, 102288. [Google Scholar] [CrossRef]
- Kontou, E.E.; Walter, A.; Alka, O.; Pfeuffer, J.; Sachsenberg, T.; Mohite, O.S.; Nuhamunada, M.; Kohlbacher, O.; Weber, T. UmetaFlow: An Untargeted Metabolomics Workflow for High-Throughput Data Processing and Analysis. J. Cheminform. 2023, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B. New Software Tools, Databases, and Resources in Metabolomics: Updates from 2020. Metabolomics 2021, 17, 49. [Google Scholar] [CrossRef]
- Souza, A.L.; Patti, G.J. A Protocol for Untargeted Metabolomic Analysis: From Sample Preparation to Data Processing. In Mitochondrial Medicine: Volume 2: Assessing Mitochondria; Springer: New York, NY, USA, 2021; pp. 357–382. [Google Scholar]
- Rivera-Pérez, A.; Garrido Frenich, A. Comparison of Data Processing Strategies Using Commercial vs. Open-Source Software in GC-Orbitrap-HRMS Untargeted Metabolomics Analysis for Food Authentication: Thyme Geographical Differentiation and Marker Identification as a Case Study. Anal. Bioanal. Chem. 2024, 416, 4039–4055. [Google Scholar] [CrossRef]
- Shahzadi, I.; Nadeem, R.; Hanif, M.A.; Mumtaz, S.; Jilani, M.I.; Nisar, S. Chemistry and Biosynthesis Pathways of Plant Oleoresins: Important Drug Sources. Int. J. Chem. Biochem. Sci. 2017, 12, 18–52. [Google Scholar]
- Patitucci, M.L.; Veiga Jr, V.F.; Pinto, A.C.; Zoghbi, M.G.B.; Silva, J.R.A. Utilização de Cromatografia Gasosa de Alta Resolução Na Detecção de Classe de Terpenos Em Extratos Brutos Vegetais. Quim. Nova 1995, 18, 262–265. [Google Scholar]
- da Silva Antonio, A.; Oliveira, D.S.; Dos Santos, G.R.C.; Pereira, H.M.G.; Wiedemann, L.S.M.; da Veiga-Junior, V.F. UHPLC-HRMS/MS on Untargeted Metabolomics: A Case Study with Copaifera (Fabaceae). RSC Adv. 2021, 11, 25096–25103. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Cole, R.B.; Harrata, A.K. Solvent Effect on Analyte Charge State, Signal Intensity, and Stability in Negative Ion Electrospray Mass Spectrometry; Implications for the Mechanism of Negative Ion Formation. J. Am. Soc. Mass. Spectrom. 1993, 4, 546–556. [Google Scholar] [CrossRef]
- Arruda, C.; Aldana Mejía, J.A.; Ribeiro, V.P.; Gambeta Borges, C.H.; Martins, C.H.G.; Sola Veneziani, R.C.; Ambrósio, S.R.; Bastos, J.K. Occurrence, Chemical Composition, Biological Activities and Analytical Methods on Copaifera Genus—A Review. Biomed. Pharmacother. 2019, 109, 1–20. [Google Scholar] [CrossRef]
- Barbosa, K.d.S.; Yoshida, M.; Scudeller, V. V Detection of Adulterated Copaiba (Copaifera multijuga Hayne) Oil-Resins by Refractive Index and Thin Layer Chromatography. Rev. Bras. Farmacogn. 2009, 19, 57–60. [Google Scholar] [CrossRef]
- do Nascimento, M.E.; Zoghbi, M.G.B.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Chemical Variability of the Volatiles of Copaifera Langsdorffii Growing Wild in the Southeastern Part of Brazil. Biochem. Syst. Ecol. 2012, 43, 1–6. [Google Scholar] [CrossRef]
- Silva, W.G.D.; Cortesi, N.; Fusari, P. Copaiba Oleoresin: Evaluation of the Presence of Polycyclic Aromatic Hydrocarbons (PAHs). Braz. J. Pharm. Sci. 2010, 46, 597–602. [Google Scholar] [CrossRef]
- Cavalcanti, B.C.; Costa-Lotufo, L.V.; Moraes, M.O.; Burbano, R.R.; Silveira, E.R.; Cunha, K.M.A.; Rao, V.S.N.; Moura, D.J.; Rosa, R.M.; Henriques, J.A.P. Genotoxicity Evaluation of Kaurenoic Acid, a Bioactive Diterpenoid Present in Copaiba Oil. Food Chem. Toxicol. 2006, 44, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, A.; Yan, L.T.; Ito, S.; Edatsugi, H.; Iwata, D.; Komoda, Y. The Isolation and in Vivo Potent Antitumor Activity of Clerodane Diterpenoid from the Oleoresin of the Brazilian Medicinal Plant, Copaifera Langsdorfi Desfon. Bioorg. Med. Chem. Lett. 1994, 4, 2889–2892. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, K. Accelerator of Collagen Production. US Patent App. 10/870,495, 2005/0004217 A1, 6 January 2005. [Google Scholar]
- Símaro, G.V.; Lemos, M.; da Silva, J.J.M.; Ribeiro, V.P.; Arruda, C.; Schneider, A.H.; de Souza Wanderley, C.W.; Carneiro, L.J.; Mariano, R.L.; Ambrósio, S.R. Antinociceptive and Anti-Inflammatory Activities of Copaifera Pubiflora Benth Oleoresin and Its Major Metabolite Ent-Hardwickiic Acid. J. Ethnopharmacol. 2021, 271, 113883. [Google Scholar] [CrossRef]
- Bandara, B.M.R.; Wimalasiri, W.R.; Bandara, K.A.N.P. Isolation and Insecticidal Activity of (-)-Hardwickiic Acid from Croton Aromaticus. Planta Med. 1987, 53, 575. [Google Scholar] [CrossRef]
- Royer, M.; Stien, D.; Beauchêne, J.; Herbette, G.; McLean, J.P.; Thibaut, A.; Thibaut, B. Extractives of the Tropical Wood Wallaba (Eperua Falcata Aubl.) as Natural Anti-Swelling Agents. Holzforschung 2010, 64, 211–215. [Google Scholar] [CrossRef]
- Braz Filho, R.; Gottlieb, O.R.; Pinho, S.L.V.; Monte, F.J.Q.; Da Rocha, A.I. Flavonoids from Amazonian Leguminosae. Phytochemistry 1973, 12, 1184–1186. [Google Scholar] [CrossRef]
- Jiang, C.; Gates, P.J. Systematic Characterisation of the Fragmentation of Flavonoids Using High-Resolution Accurate Mass Electrospray Tandem Mass Spectrometry. Molecules 2024, 29, 5246. [Google Scholar] [CrossRef]
- Parailloux, M.; Godin, S.; Lobinski, R. Nontargeted Screening for Flavonoids in Salicornia Plant by Reversed-Phase Liquid Chromatography–Electrospray Orbitrap Data-Dependent MS2/MS3. Molecules 2023, 28, 3022. [Google Scholar] [CrossRef]
- Śliwka-Kaszyńska, M.; Anusiewicz, I.; Skurski, P. The Mechanism of a Retro-Diels–Alder Fragmentation of Luteolin: Theoretical Studies Supported by Electrospray Ionization Tandem Mass Spectrometry Results. Molecules 2022, 27, 1032. [Google Scholar] [CrossRef] [PubMed]
- NIST, Mass Spectrometry Data Center. Tandem Mass Spectral Library; National Institute of Standards and Technology (NIST)—U.S. Department of Commerce: Gaithersburg, MD, USA, 2017. Available online: https://www.nist.gov/programs-projects/tandem-mass-spectral-library (accessed on 7 September 2025).
| Compound | Molecular Formula [M] | Retention Time (min) | [M–H]−/[M+H]+ | (N)CE (%) | Fragment Ions/Intensity (%) |
|---|---|---|---|---|---|
| Hardwickiic acid | C20H28O3 | 12.95 | 315.1966 | 40 | 301.1820 (14%)/285.18582 (11%)/273.2223 (26%)/257.1909 (16%) |
| Patagonic acid | C20H28O4 | 11.29 | 331.1915 | 40 | 287.2019 (100%)/259.2066 (34%)/243.1752 (28%) |
| Copalic acid | C20H32O2 | 14.00 | 303.2330 | 40 | 285.1861 (5%)/259.2069 (10%) |
| Agathic acid | C20H30O4 | 11.95 | 333.2071 | 40 | 301.1809 (66%)/289.2177 (28%) |
| Dihydroagathic (pinifolic) acid | C20H32O4 | 12.22 | 335.2227 | 40 | 303.1963 (40%)/285.1845 (100%)/245.1908 (88%) |
| Eperuic acid | C20H34O2 | 14.06 | 305.2486 | 40 | 287.2377 (10%) |
| Kolavenic acid | C20H32O2 | 14.02 | 303.2329 | 40 | 285.1861 (5%)/243.1754 (5%) |
| Clerod-3-en-15,18-dioic acid | C20H32O4 | 11.49 | 335.2227 | 40 | 303.1963 (40%)/285.1858 (100%)/275.1908 (46%) |
| 14,15,16-trinor-hardwikiic acid ** | C17H26O4 | 10.90/14.43 | 293.1758 | 40 | 249.1853 (20%) |
| 2-oxokolavenic acid | C20H30O3 | 11.84 | 317.2122 | 40 | 273.2226 (20%)/285.1858 (12%) |
| Methyl hardwickiate | C21H30O3 | 13.90 | 329.2122 | 40 | 301.1802 (2%)/285.1855 (20%)/257.1905 (100%) |
| Methyl copalate | C21H34O2 | 14.90 | 317.2486 | 40 | 285.1862 (48%)/257.1913 (10%) |
| Methyl 3β-hydroxy copalate | C21H34O3 | 12.81 | 333.2435 | 40 | 301.1807 (10%)/273.2227 (20%)/257.1912 (25%) |
| Methyl 3β-acetoxy copalate | C23H36O4 | 13.88 | 375.2541 | 40 | 317.2121 (12%)/301.1804 (5%)/287.1655 (25%) |
| Methyl patagonate | C21H32O4 | 13.89 | 345.2071 | 40 | 301.2173 (10%)/243.1753 (32%)/255.1749 (40%) |
| Methyl agathate | C21H32O4 | 12.29 | 347.2227 | 40 | 319.1899 (7%)/303.1966 (100%)/287.1635 (10%)/243.1754 (39%) |
| Methyl eperuate | C21H36O2 | 14.95 | 319.2642 | 40 | 301.2170 (5%)/289.2173 (10%) |
| Cativic acid * | C20H34O2 | 14.10 | 305.2486 | 40 | 261.18573 (2%) |
| 8,17-dihydroxy-13-labden-16,15-olid-19-oate * | C21H32O6 | 12.21 | 439.2340 [M-H-60]− | 30 | 357.2052 (15%) |
| Effusanin A * | C20H28O5 | 10.84 | 347.1865 | 30 | 319. 1899 (3%)/303.1969 (37%)/259.2072 (11%) |
| 18-hydroxy-clerod-3-en-15-oic acid * | C20H34O3 | 13.13 | 321.2437 | 30 | 277.2171 (60%)/247.2069 (14%) |
| Craterellin A * | C22H34O4 | 13.16 | 380.2792 [M+NH4]+ | 30 | 336.2792 (32%)/296.2474 (18%) |
| 14-deoxy-11,12-didehydroandrographolide * | C20H28O4 | 11.94 | 315.1953 [M+H]-18 | 30 | 297.1845 (45%)/283.1699 (32%) |
| 12-hydroxy-7-carboxy- abiet-8(13)-en-18-oic acid * | C20H30O4 | 12.53 | 335.2216 | 30 | 317.2111 (60%)/303.1960 (90%) |
| Aphidicolin * | C20H34O4 | 12.08 | 339.2529 | 30 | 295.2529 (60%)/277.2530 (32%) |
| 7-keto, 12-hydroxy, abiet-8-14-en-18-oic acid | C20H30O4 | 12.71 | 333.2071 | 30 | 301.1803 (10%)/289.2176 (8%) |
| (-)-7β-hydroxycleroda-8(17),13E-diene-15-oic acid * | C20H32O3 | 13.47 | 319.2278 | 30 | 275.2381 (60%) |
| 16-oxo-13,14H-hardwikiic acid * | C20H28O4 | 11.26 | 331.1914 | 30 | 287.2016 (100%)/243.1748 (10%) |
| Nor-hardwikiic acid * | C17H26O4 | 12.16 | 293.1758 | 30 | 249.1853 (100%) |
| 7-oxo-labda-8-ene-15-oic acid * | C20H30O3 | 11.86 | 317.2122 | 30 | 285.1861 (5%)/273.2218 (100%) |
| (-)-cleroda-7,13E-diene-15-oic acid * | C20H32O2 | 14.62 | 303.2329 | 30 | 285.18536 (74%)/259.16934 (39%) |
| 6β,7β-dihydroxykaurenoic acid * | C20H30O4 | 11.48 | 333.2071 | 30 | 301.18033 (70%)/289.21765 (32%) |
| 8-hydroxyoctadeca-9,12-dienoic acid * | C18H32O3 | 13.86 | 295.2278 | 30 | 251.23836 (5%)/155.14264 (5%) |
| Ent-16β,17-dihydroxy-19-kaurenoic acid * | C20H32O4 | 13.05 | 335.2227 | 30 | 291.23301 (40%)/273.2227 (32%) |
| Experiment Dates | Target Analytes or Target Substances | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hardwickiic Acid | CV% | Methyl Hardwickiate | CV% | Copalic Acid | CV% | Methyl Copalate | CV% | Patagonic Acid | CV% | Methyl Patagonate | CV% | Agathic Acid | CV% | Methyl Ester of Agathic Acid | CV% | |
| 15 May 2024 | 1,975,121,726 | 8.0 | 1,512,316 | 5.1 | 1,203,063,795 | 7.7 | 921,009 | 4.4 | 74,788,718 | 7.7 | 891,007 | 5.2 | 399,396,558 | 7.5 | 509,843 | 6.2 |
| 20 May 2024 | 1,926,711,350 | 1,341,619 | 1,114,681,593 | 870,510 | 64,791,889 | 862,986 | 406,791,632 | 498,032 | ||||||||
| 25 May 2024 | 1,711,628,436 | 1,470,515 | 122,3025,777 | 941,617 | 74,937,278 | 789,675 | 453,361,272 | 569,872 | ||||||||
| 4 June 2024 | 2,075,347,716 | 1,421,008 | 1,098,075,485 | 858,979 | 77,429,128 | 871,585 | 381,595,128 | 543,929 | ||||||||
| Experiment Dates | Target Analytes or Target Substances | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hardwickiic Acid | CV% | Methyl Hardwickiate | CV% | Copalic Acid | CV% | Methyl Copalate | CV% | Patagonic Acid | CV% | Methyl Patagonate | CV% | Agathic Acid | CV% | Methyl Ester of Agathic Acid | CV% | |
| 15 May 2024 | 177,534,771 | 6.0 | 122,541 | 6.1 | 109,567,508 | 5.2 | 76,870 | 8.5 | 7,278,871 | 7.6 | 79,100 | 8.5 | 37,939,253 | 4.9 | 48,209 | 4.1 |
| 20 May 2024 | 169,568,903 | 130,981 | 152,698,547 | 64,191 | 6,479,188 | 75,298 | 39,891,163 | 47,981 | ||||||||
| 25 May 2024 | 162,671,135 | 131,701 | 191,469,162 | 67,078 | 7,093,297 | 75,465 | 40,459,027 | 50,629 | ||||||||
| 4 June 2024 | 186,713,428 | 142,216 | 100,330,879 | 65,132 | 7,798,235 | 89,698 | 36,289,712 | 52,298 | ||||||||
| Class of Natural Products | Substance Detected | Molecular Formula [M] | [M-H]−/ [M+H]+ | Fragment Ions |
|---|---|---|---|---|
| Polyacetylene | (R)-(-)-Falcarinol | C17H24O | 243.1753 | 181.15877 (10%)/155.08554 (8%) |
| Benzoquinone | 5-O-ethyl embelin | C19H30O4 | 321.2073 | 277.2167 (100%)/247.2060 (15%) |
| Embelin | C17H26O4 | 293.1758 | 249.1758 (100%)/219.1758 (10%) | |
| Fatty Acid | Methyl palmitate | C17H34O2 | 288.2893 | 220.9344 (11%)/90.9771 (100%) |
| (13Z)-8-hydroxyoctadecene-9,11-diynoic acid | C18H26O3 | 289.1812 | 256.9605 (10%)/156.9426 (5%) | |
| α-Linolenic acid | C18H30O2 | 277.2174 | 247.2061 (25%)/259.2041 (10%)/123.0077 (15%) | |
| Ricinoleic Acid | C18H34O3 | 297.2438 | 253.21616 (12%)/183.01044 (18%) | |
| Azelaic acid | C9H16O4 | 187.0971 | 169.0856 (2%)/125.09560 (100%) | |
| Amino Acid | L-Tyrosine methyl ester | C10H13NO3 | 194.0818 | 179.0577 (8%)/164.0831 (20%)/150.0548 (8%) |
| Polyene | (9cis)-Retinal | C20H28O | 285.2211 | 267.2105 (17%)/95.0496 (17%) |
| Diterpene | (E,E,E)-3,7,11,15-Tetramethylhexadeca-1,3,6,10,14-pentaene | C20H32 | 273.2575 | 163.1480 (79%)/149.1325 (55%) |
| Triterpene | Betulin | C30H50O2 | 443.3881 | 425.3881 (36%)/385.3881 (24%) |
| Ursolic acid | C30H48O3 | 455.3531 | 409.2443 (74%)/391.2338 (100%)/387.2158 (30%)/319.2263 (30%) | |
| Phenolic | 1-(5-Hexyl-2,4-dihydroxyphenyl)ethenone | C14H20O3 | 254.1748 [M+NH4]+ | 237.1636 (11%)/98.9846 (100%) |
| 1-(2,6-Dihydroxyphenyl)-1,3-dodecanedione | C18H26O4 | 307.1901 | 276.1719 (87%)/261.1484 (47%)/233.1533 (43%)/107.0858 (89%) | |
| p-hydroxy benzoic acid | C7H6O3 | 137.0244 | 109.0279 (4%)/93.0332 (33%) | |
| Gallic acid | C7H6O5 | 171.0288 | 153.0154 (92%)/127.0369 (85%)/109.0265 (64%) | |
| Ellagic acid | C14H6O8 | 300.9989 | 257.0100 (36%)/283.9970 (66%)/229.0150 (45%), 185.0241 (40%) | |
| Flavonoids | 7-Hydroxy-2-methyl-4H-chromen-4-one | C10H8O3 | 177.0546 | 159.0546 (42%)/115.0467 (30%) |
| Catechin | C15H14O6 | 289.0717 | 245.0814 (12%)/203.0709 (54%)/151.0403 (40%)/125.0238 (31%)/109.0290 (100%) | |
| Epicatechin | C15H14O6 | 289.0717 | 245.0814 (15%)/(203.0709 (65%)/151.0403 (56%)/125.0238 (40%)/109.0290 (100%) | |
| Quinic acid | C7H12O6 | 191.0561 | 191.0565 (7%)/173.0455 (5%)/127.0404 (15%)/111.0459 (8%)/93.0356 (61%)/83.0305 (100%) | |
| Quercitrin | C21H20O11 | 447.0933 | 301.0341 (100%)/151.0027 (8%) | |
| Quercetin | C15H10O7 | 301.0354 | 273.0359 (7%)/151.0031 (100%)/121.0294 (25%)/107.0133 (24%) | |
| Luteolin | C15H10O6 | 285.0405 | 217.0507 (10%)/175.0390 (20%)/151.0033 (15%)/133.0291 (100%) | |
| Apigenin | C15H10O5 | 269.0455 | 151.0032 (20%)/117.0341 (100%) | |
| Dihydromyricetin | C15H12O8 | 319.0459 | 193.0135 (%)/165.0170 (10%)/137.02 (30%)/125.0228 (42%)/109.0296 (30%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, R.; Carneiro, G.R.A.; Santos, G.R.C.d.; Gomes, M.V.d.S.; Pereira, H.M.G.; Padilha, M.C.; Veiga-Junior, V.F. Advancing the Chemical Characterization of Eperua oleifera Duke Oleoresin: A UHPLC-HRMS-Based Approach. Plants 2025, 14, 2893. https://doi.org/10.3390/plants14182893
Ribeiro R, Carneiro GRA, Santos GRCd, Gomes MVdS, Pereira HMG, Padilha MC, Veiga-Junior VF. Advancing the Chemical Characterization of Eperua oleifera Duke Oleoresin: A UHPLC-HRMS-Based Approach. Plants. 2025; 14(18):2893. https://doi.org/10.3390/plants14182893
Chicago/Turabian StyleRibeiro, Rayssa, Gabriel Reis Alves Carneiro, Gustavo Ramalho Cardoso dos Santos, Márcio Vinícius da Silva Gomes, Henrique Marcelo Gualberto Pereira, Monica Costa Padilha, and Valdir F. Veiga-Junior. 2025. "Advancing the Chemical Characterization of Eperua oleifera Duke Oleoresin: A UHPLC-HRMS-Based Approach" Plants 14, no. 18: 2893. https://doi.org/10.3390/plants14182893
APA StyleRibeiro, R., Carneiro, G. R. A., Santos, G. R. C. d., Gomes, M. V. d. S., Pereira, H. M. G., Padilha, M. C., & Veiga-Junior, V. F. (2025). Advancing the Chemical Characterization of Eperua oleifera Duke Oleoresin: A UHPLC-HRMS-Based Approach. Plants, 14(18), 2893. https://doi.org/10.3390/plants14182893








