Substrates in Organic Mint Cultivation: Growth, Phytochemistry and Biological Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Soil and Substrates
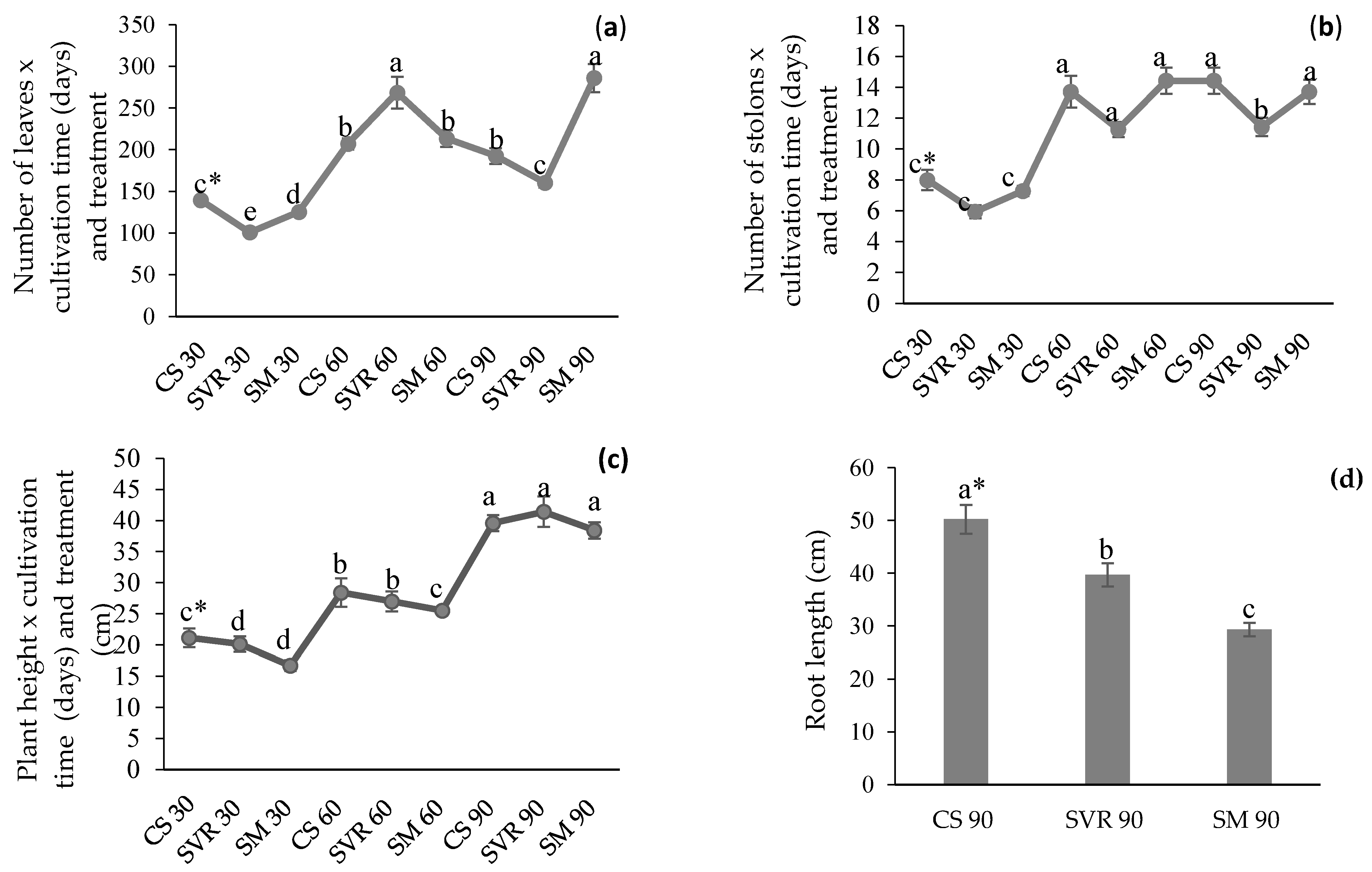
2.2. Agronomic Characteristics of the Plant
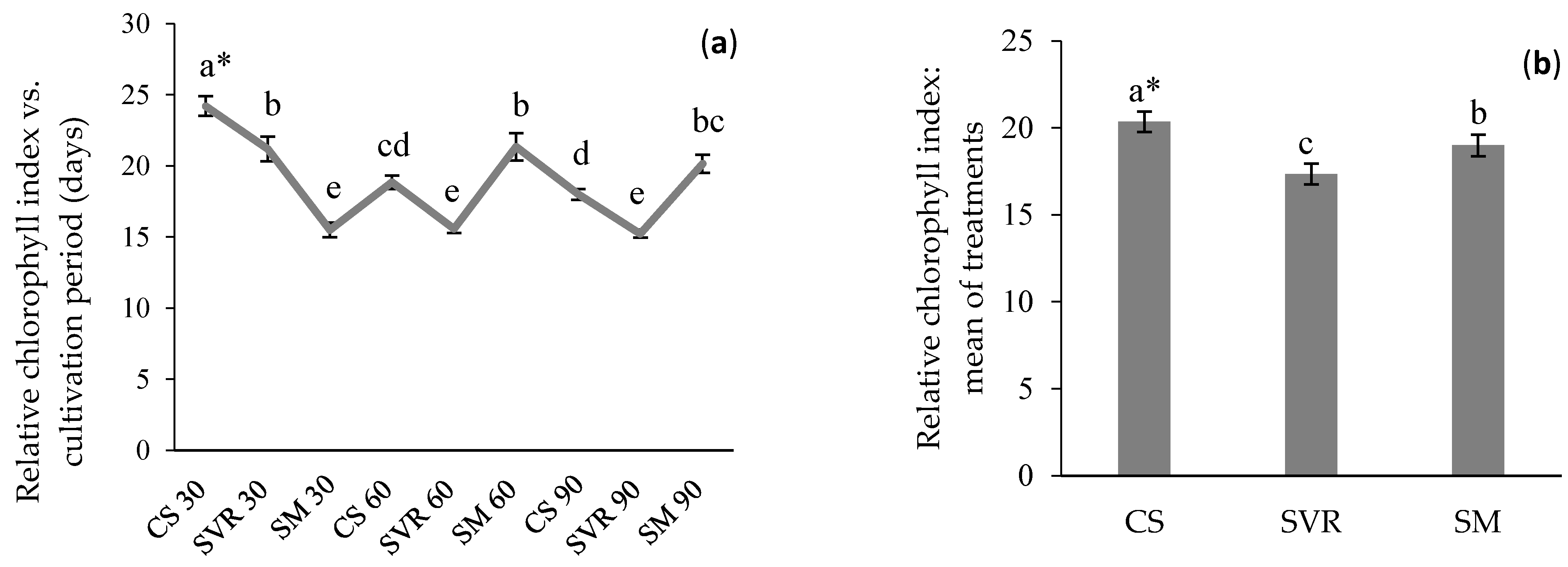
2.3. Relative Chlorophyll Index
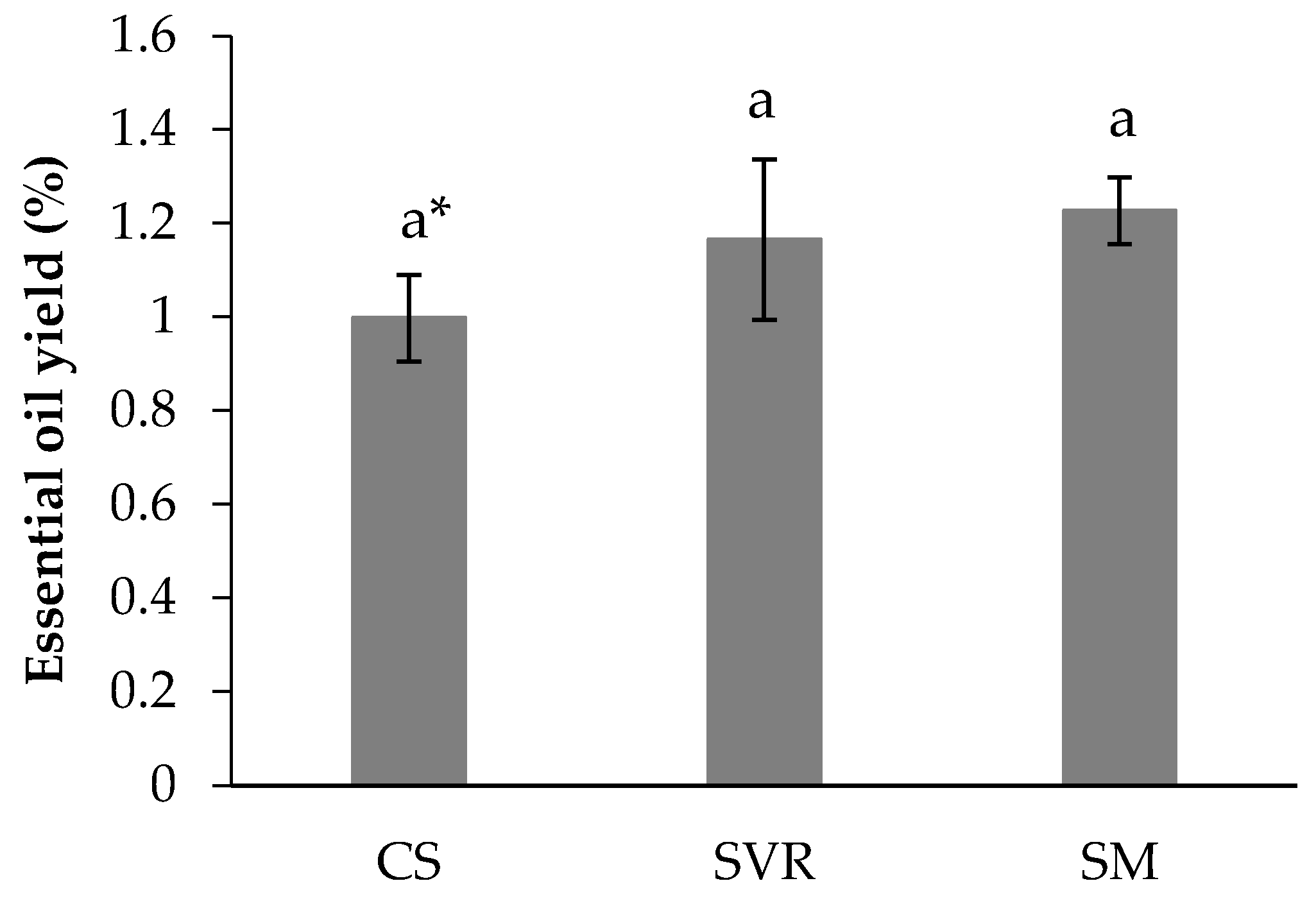
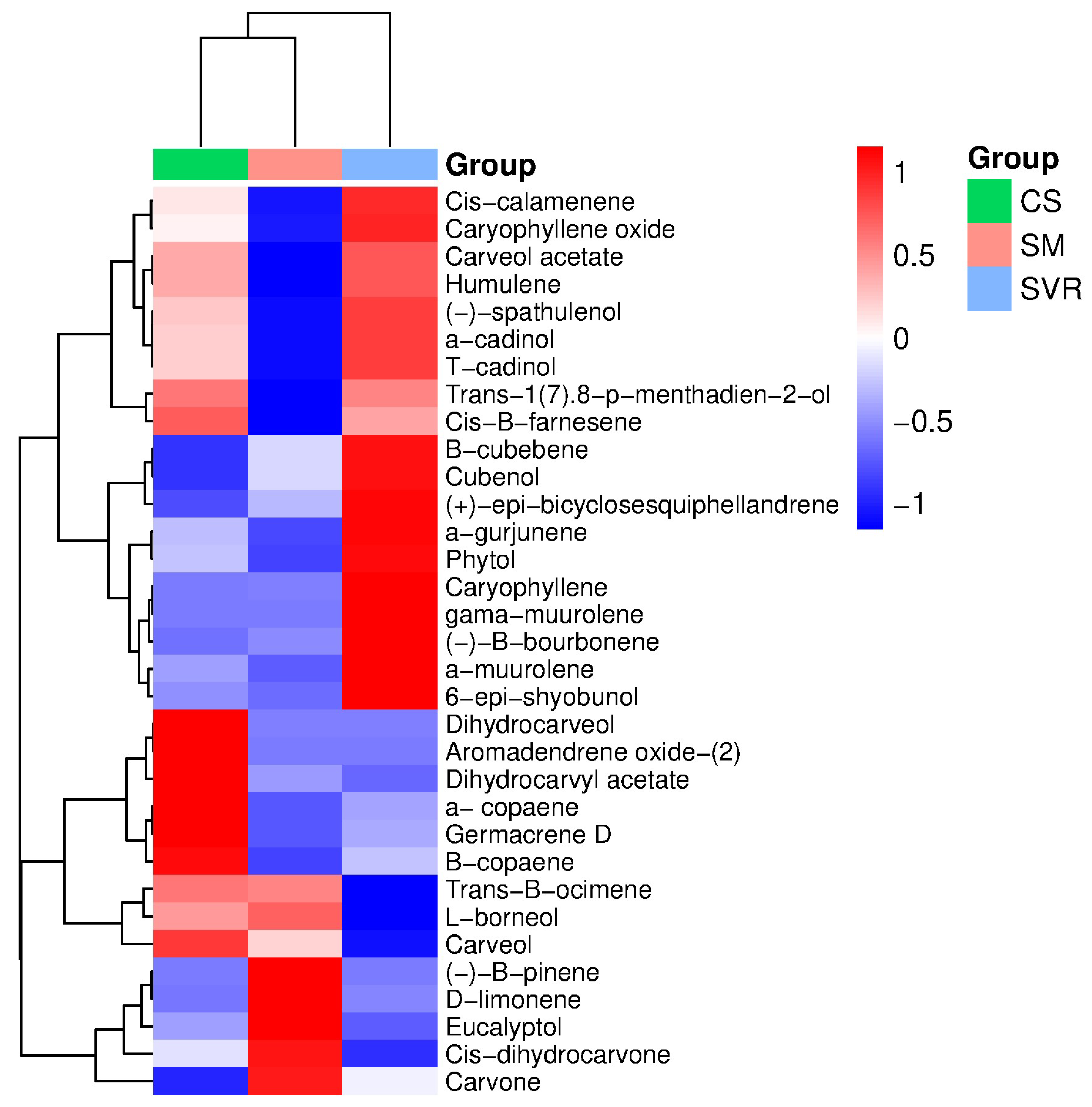
2.4. Phytochemical and Biological Aspects of the Plant
3. Materials and Methods
3.1. Substrates and Plant Cultivation
3.2. Pest Control
3.3. Enzymatic Analysis of Substrates
3.4. Agronomic Characteristics of the Plant
3.5. Plant Physiological Indices
3.6. Phytochemical and Biological Aspects of the Plant
3.6.1. Essential Oil Extraction and Chemical Characterization
3.6.2. Determination of Antioxidant Activity, Flavonoids, and Anthocyanins in the Extract
3.6.3. Determination of Phenylalanine Ammonia-Lyase (PAL) Enzyme Activity in Leaves
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Celenk, S.; Tarimcilar, G.; Bicakci, A.; Kaynak, G.; Malyer, H. A palynological study of the genus Mentha L. (Lamiaceae). Bot. J. Linn. Soc. 2008, 157, 141–154. [Google Scholar] [CrossRef]
- Rahimi, Y.; Taleei, A.; Ranjbar, M. Long-term water deficit modulates antioxidant capacity of peppermint (Mentha piperita L.). Sci. Hortic. 2018, 237, 36–43. [Google Scholar] [CrossRef]
- Beiki, M.; Zhaveh, S.; Khalili, S.T.; Rahmani-Cherati, T.; Abollah, A.; Bayat, M.; Tabatabae, M.; Mohsenifar, A. Encapsulation of Mentha piperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crops Prod. 2014, 54, 310–319. [Google Scholar] [CrossRef]
- Yi, W.; Chen, R.; Xie, F.; Xu, C.; Tian, W. Anti-inflammatory and immunomodulatory properties of Mentha piperita green-formulated gold nanoparticles and its effect on ovalbumin-induced asthma and lung pathological changes in rats. J. Exp. Nanosci. 2022, 17, 163–172. [Google Scholar] [CrossRef]
- Batool, I.; Nisar, S.; Hamrouni, L.; Jilani, M.I. Extraction, production and analysis techniques for menthol: A review. Int. J. Chem. Biochem. Sci. 2018, 14, 71–76. [Google Scholar]
- Jahdkaran, E.; Hosseini, S.E.; Nafchi, A.M.; Nouri, L. The effects of methylcellulose coating containing carvacrol or menthol on the physicochemical, mechanical, and antimicrobial activity of polyethylene films. Food Sci. Nutr. 2021, 9, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Ito, M. Sedative effects of l-menthol, d-camphor, phenylethyl alcohol, and geraniol. J. Nat. Med. 2021, 75, 319–325. [Google Scholar] [CrossRef]
- Yoshida, E.; Kojima, M.; Suzuki, M.; Matsuda, F.; Shimbo, K.; Onuki, A.; Nishio, Y.; Usuda, Y.; Kondo, A.; Ishii, J. Increased carvone production in Escherichia coli by balancing limonene conversion enzyme expression via targeted quantification concatamer proteome analysis. Sci. Rep. 2021, 11, 22126. [Google Scholar] [CrossRef]
- Gonçalves, R.K.S.; Freitas, H.R.; Nascimento-Junior, B.J.; Almeida, L.R.S.; Leite, I.O.; Moura, A.S. Agricultura urbana no semiárido: Produção de plantas medicinais no sertão baiano. Cad. Agroecol. 2020, 15, 01–05. [Google Scholar]
- Ye, L.; Zhao, X.; Bao, E.; Li, J. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef]
- Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA. Estratégias para Reduzir a Dependência de Fertilizantes Importados. Available online: https://www.embrapa.br/busca-de-noticias/-/noticia/70879925/mesa-redonda-aborda-as-estrategias-para-reduzir-a-dependencia-de-fertilizantes-importados (accessed on 20 June 2024).
- Rashad, A.M. Vermiculite as a construction material—A short guide for Civil Engineer. Constr. Build. Mater. 2016, 125, 53–62. [Google Scholar] [CrossRef]
- Pan, J.; Shang, Y.; Zhang, W.J.; Che, X.; Cui, Z. Improving soil quality for higher grain yields in Chinese wheat and maize production. Land Degrad. Dev. 2019, 31, 1125–1137. [Google Scholar] [CrossRef]
- Pisa, C.; Parwada, C.; Chiripanyanga, S.; Dunjana, N. Evaluation of vermiculite application rates on growth and yield of Brassica napus (Rape). Herit. Sci. 2020, 4, 46–50. [Google Scholar] [CrossRef]
- Lim, S.L.; Wu, T.Y.; Lim, P.N.; Shak, K.P.Y. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Figueiroa, J.C.R.; Mendoza, M.I.N.R.; Castorena, M.C.G.; Escudero, J.S. Vermicompost as a substrate in the production of peppermint (Mentha piperita L.). Rev. Mex. Cienc. Agric. 2013, 4, 889–899. [Google Scholar]
- Loureiro, F.E.L.; Melamed, R.; Figueiredo Neto, E.J. Fertilizantes Agroindústria & Sustentabilidade; CETEM/MCT: Rio de Janeiro, Brazil, 2009; 645p. [Google Scholar]
- Lajús, C.R.; Luz, G.L.; Silva, C.G.; Dalcanton, F.; Barichello, R.; Sauer, A.V.; Piaia, T.A.; Piva, A.J.D. Aspectos qualitativos e quantitativos de variedades de alface submetidas a concentrações de pó de rocha em cultivo orgânico. Braz. J. Dev. 2021, 7, 49489–49512. [Google Scholar]
- Mousavinik, S.M.; Asgharipour, M.R.; Sardashti, S. Manure and light intensity affect growth characteristics and essential oil of peppermint (Mentha piperita L.). J. Essent. Oil Bear. Plants 2016, 19, 2029–2036. [Google Scholar] [CrossRef]
- Shushupti, O.; Orpa, R.S.; Tarannum, T.; Chitra, N.N.; Suchi, S.J.H.; Rahman, M.K. Influence of various commercially available organic manures on growth, yield and nutrient accumulation in mint plants (Mentha sp.). J. Biodivers. Conserv. Bioresour. Manag. 2022, 7, 73–84. [Google Scholar] [CrossRef]
- Amorim, E.L.; Silva, F.; Neto, M.T.C.; Alves, L.S.; Oliveira, M.E.F.; Pacheco, J.L.F. Avaliação de diferentes substratos orgânicos na produção de biomassa da hortelã (Mentha piperita L.): Evaluation of different organic substrates in the biomass production of mint (Mentha piperita L.). Lat. Am. J. Dev. 2021, 3, 3313–3319. [Google Scholar] [CrossRef]
- Vaz, A.P.A.; Jorge, M.H.A. Série Plantas Medicinais, Condimentares e Aromáticas: Hortelã, 1st ed.; EMBRAPA: Corumbá, Brazil, 2006. [Google Scholar]
- Durán-Lara, E.F.; Valderrama, A.; Adolfo Marican, A. Natural organic compounds for application in organic farming. Agriculture 2020, 10, 41. [Google Scholar] [CrossRef]
- Keshavarz-Mirzamohammadi, H.; Tohidi-Moghadam, H.R.; Hosseini, S.J. Is there any relationship between agronomic traits. soil properties and essential oil profile of peppermint (Mentha piperita L.) treated by fertilizer treatments and irrigation regimes? Ann. Appl. Biol. 2021, 179, 331–344. [Google Scholar] [CrossRef]
- Ramos, C.; Querol, X.; Oliveira, M.; Pires, K.; Kautzmann, R.; Oliveira, L. A preliminary evaluation of volcanic rock powder for application in agriculture as soil a remineralizer. Sci. Total Environ. 2015, 512, 371–380. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal: Nutrientes Essenciais, Deficiências e Distúrbios Vegetais, 6th ed.; Artmed Editora: São Paulo, Brazil, 2017; 888p. [Google Scholar]
- Lal, R. Soil health and carbon management. Food Energy Secur. 2016, 5, 212–222. [Google Scholar] [CrossRef]
- Cardoso, E.J.B.N.; Andreote, F.D. Microbiologia do Solo, 2nd ed.; Universidade de São Paulo: São Paulo, Brazil, 2016; 225p. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Matsuoka, M.; Mendes, I.C.; Loureiro, M.F. Biomassa microbiana e atividade enzimática em solos sob vegetação nativa e sistemas agrícolas anuais e perenes na região de Primavera do Leste (MT). Rev. Bras. Cienc. Solo. 2003, 27, 425–433. [Google Scholar] [CrossRef][Green Version]
- Acosta-Martinez, V.; Cano, A.; Johnson, J. Simultaneous determination of multiple soil enzyme activities for soil health-biogeochemical indices. Appl. Soil Ecol. 2018, 126, 121–128. [Google Scholar] [CrossRef]
- Hřebečková, T.; Wiesnerová, L.; Hanč, A. Changes of enzymatic activity during a large-scale vermicomposting process with continuous feeding. J. Clean. Prod. 2019, 239, 118127. [Google Scholar] [CrossRef]
- Soltanbeigi, A.; Özgüven, M.; Hassanpouraghdam, M.B. Planting-date and cutting-time affect the growth and essential oil composition of Mentha piperita and Mentha arvensis. Ind. Crops Prod. 2021, 170, 113790. [Google Scholar] [CrossRef]
- Desai, S.; Pushpa, T.N.; Srikantaprasad, D.; Kantharaju, V.; Biradar, I.B.; Shalini, R.M.; Asha, M.R. Effect of dates of planting on growth. yield and quality of menthol mint (Mentha arvensis L.) cultivars planted during Rabi season. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 625–633. [Google Scholar] [CrossRef]
- Al-Zyadi, Q.A. Effect of planting and harvesting dates on the growth and essential oil content of peppermint (Mentha piperita L.). Plant Arch. 2019, 19, 319–322. [Google Scholar]
- Verma, E.K.; Verma, R.S.; Rahman, L.; Kalra, A.; Patra, D.D. Integrated nutrient management on biomass, oil yields and essential oil composition of peppermint (Mentha piperita L.) and residual fertility in a Hilly soil. J. Essent. Oil Bear. Plants 2016, 19, 582–591. [Google Scholar] [CrossRef]
- Asadi, M.; Nasiri, Y.; Maggi, F.; Rasouli, F.; Morshedloo, M.R. Biomass yield and essential oil chemical composition of Mentha × piperita as affected by amino acids and different fertilizer resources. J. Soil Sci. Plant. Nutr. 2023, 23, 668–682. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Ogura, T.; Goeschl, C.; Filiault, D.; Mirea, M.; Slovak, R.; Wolhrab, B.; Satbhai, S.B.; Busch, W. Root system depth in arabidopsis is shaped by EXOCYST70A3 via the dynamic modulation of auxin transport. Cell 2019, 178, 400–412. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Mur, L.A.J.; Shen, Q.; Guo, S. Unravelling the roles of nitrogen nutrition in plant disease defences. Int. J. Mol. Sci. 2020, 21, 572. [Google Scholar] [CrossRef]
- Silva, D.A.S.; Silva Júnior, M.L.; Viégas, I.J.M.; Lobato, A.K.S.; Melo, V.S.; Botelho, S.M.A.; Silva, G.R.; Freitas, J.M.N.; Oliveira Neto, C.F.; Cunha, M.L.A.; et al. Growth and visual symptoms of nutrient deficiencies in young Mentha piperita plants. J. Food Agric. Environ. 2014, 12, 292–296. [Google Scholar]
- Lothe, N.B.; Mazeed, A.; Pandey, J.; Patairiya, V.; Verma, K.; Semwal, M.; Verma, R.S.; Verma, R.K. Maximizing yields and economics by supplementing additional nutrients for commercially grown menthol mint (Mentha arvensis L.) cultivars. Ind. Crops Prod. 2021, 160, 113110. [Google Scholar] [CrossRef]
- Vatansever, R.; Ibrahim, O.; Ertugrul, F. Essential and beneficial trace elements in plants, and their transport in roots: A review. Appl. Biochem. Biotechnol. 2017, 181, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Dabrowski, P.; Cetner, M.D.; Samborska, I.A.; Lukasik, I.; Brestic, M.; Ziccak, M.; Tomasz, H.; Mojski, J.; Kociel, H.; et al. A comparison between different chlorophyll content meters under nutrient deficiency conditions. J. Plant Nutr. 2017, 40, 1024–1034. [Google Scholar] [CrossRef]
- Tarasevičienė, Ž.; Velička, A.; Jarienė, E.; Paulauskienė, A.; Kieltyka-Dadasiewicz, A.; Sawicka, B.; Gajewski, M. Comparison of chemical composition and colour parameters of different Mentha genus plants grown under organic conditions. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 92–99. [Google Scholar] [CrossRef]
- Nabi, A.; Naeem, M.; Aftab, T.; Khan, M.M.A. Alterations in photosynthetic pigments, antioxidant machinery, essential oil constituents and growth of menthol mint (Mentha arvensis L.) upon nickel exposure. Braz. J. Bot. 2020, 43, 721–731. [Google Scholar] [CrossRef]
- Nozzi, V.; Graber, A.; Schmautz, Z.; Mathis, A.; Junge, R. Nutrient management in aquaponics: Comparison of three approaches for cultivating lettuce, mint and mushroom herbs. Agronomy 2018, 8, 27. [Google Scholar] [CrossRef]
- Pitarokili, D.; Couladis, M.; Petsikos-Panayotarou, N.; Tzakou, O. Composition and antifungal activity on soil-borne pathogens of the essential oil of Salvia sclarea from Greece. J. Agric. Food Chem. 2002, 50, 6688–6691. [Google Scholar] [CrossRef]
- Lopes, D.; Strobl, H.; Kolodziejczyk, P. 14-Methylpentadecano-15-lactone (Muscolide): A new macrocyclic lactone from the oil of Angelica archangelica L. Chem. Biodivers. 2004, 1, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Juliani, H.R.; Zygadlo, J.A.; Scrivanti, R.; de la Sota, E.; Simon, J.E. The essential oil of Anemia tomentosa (Savigny) Sw. var. anthriscifolia (Schrad.) Mickel. Flavour Fragr. J. 2004, 19, 541–543. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Zekavat, B.; Sereshti, H. Characterization of essential oil components of Iranian geranium oil using gas chromatography-mass spectrometry combined with chemometric resolution techniques. J. Chromatogr. A 2006, 1114, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Yassa, N.; Akhani, H. The Essential Oils Composition in Two Species of the Genus Eriocycla Lindl. (Apiaceae) from Iran. Available online: https://l1nq.com/8lWmX (accessed on 29 August 2025).
- Pino, J.A.; Marbot, R.; Quert, R.; García, H. Study of essential oils of Eucalyptus resinifera Smith, E. tereticornis Smith and Corymbia maculata (Hook.) KD Hill & LAS Johnson, grown in Cuba. Flavour Fragr. J. 2002, 17, 1–4. [Google Scholar] [CrossRef]
- Andriamaharavo, N.R.; NIST Mass Spectrometry Data Center. Retention Data. 2014. Available online: https://webbook.nist.gov/cgi/cbook.cgi?Source=2014AND%2319410M&Units=SI&Mask=2000 (accessed on 31 August 2025).
- Gallori, S.; Flamini, G.; Bilia, A.R.; Morelli, I.; Landini, A.; Vincieri, F.F. Chemical composition of some traditional herbal drug preparations: Essential oil and aromatic water of costmary (Balsamita suaveolens Pers.). J. Agric. Food Chem. 2001, 49, 5907–5910. [Google Scholar] [CrossRef]
- Kim, M.R.; Abd El-Aty, A.M.; Kim, I.S.; Shim, J.H. Determination of volatile flavor components in danggui cultivars by solvent free injection and hydrodistillation followed by gas chromatographic-mass spectrometric analysis. J. Chromatogr. A 2006, 1116, 259–264. [Google Scholar] [CrossRef]
- Salido, S.; Altarejos, J.; Nogueras, M.; Sánchez, A.; Pannecouque, C.; Witvrouw, M.; De Clercq, E. Chemical studies of essential oils of Juniperus oxycedrus ssp. Badia. J. Ethnopharmacol. 2002, 81, 129–134. [Google Scholar] [CrossRef]
- Flamini, G.; Luigi Cioni, P.; Morelli, I. Volatiles from leaves, fruits, and virgin oil from Olea europaea Cv. Olivastra seggianese from Italy. J. Agric. Food Chem. 2003, 51, 1382–1386. [Google Scholar] [CrossRef]
- Shafiee, A.; Javidnia, K.; Tabatabai, M. Volatile constituents and antimicrobial activity of Zataria multiflora, population Iran. J. Chem. Chem. Eng. 1999, 18, 1–5. [Google Scholar]
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the Essential Oils of Thymus and Origanum Species from Algeria and Their Antioxidant and Antimicrobial Activities. J. Agric. Food Chem. 2006, 54, 6314–6321. [Google Scholar] [CrossRef] [PubMed]
- Güllüce, M.; Özer, H.; Baris, Ö.; Daferera, D.; Sahin, F.; Polissiou, M. Chemical Composition of the Essential Oil of Salvia aethiopis L. Turk. J. Biol. 2006, 30, 231–233. [Google Scholar]
- Silva-Brandão, K.L.; Solferini, V.N.; Trigo, J.R. Chemical and phylogenetic relationships among Aristolochia L. (Aristolochiaceae) from southeastern Brazil. Biochem. Syst. Ecol. 2006, 34, 291–302. [Google Scholar] [CrossRef]
- Elias, V.O.; Simoneit, B.R.T.; Cardoso, J.N. Analysis of volatile sesquiterpenoids in environmental and geological samples. J. High Res. Chromatogr. 1997, 20, 305–309. [Google Scholar] [CrossRef]
- Zeng, Y.-X.; Zhao, C.-X.; Liang, Y.-Z.; Yang, H.; Fang, H.-Z.; Yi, L.-Z.; Zeng, Z.-D. Comparative analysis of volatile components from Clematis species growing in China. Anal. Chim. Acta. 2007, 595, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Tzakou, O.; Vagias, C.; Gani, A.; Yannitsaros, A. Volatile constituents of essential oils isolated at different growth stages from three Conyza species growing in Greece. Flavour Fragr. J. 2004, 19, 425–428. [Google Scholar]
- Yu, Y.; Huang, T.; Yang, B.; Liu, X.; Duan, G. Development of gas chromatography-mass spectrometry with microwave distillation and simultaneous solid-phase microextraction for rapid determination of volatile constituents in ginger. J. Pharm. Biomed. Anal. 2007, 43, 24–31. [Google Scholar] [CrossRef]
- Zhao, C.; Zeng, Y.; Wan, M.; Li, R.; Liang, Y.; Li, C.; Zeng, Z.; Chau, F.-T. Comparative analysis of essential oils from eight herbal medicines with pungent flavor and cool nature by GC-MS and chemometric resolution methods. J. Sep. Sci. 2009, 32, 660–670. [Google Scholar] [CrossRef]
- Potzernheim, M.C.L.; Bizzo, H.R.; Vieira, R.F. Analysis of the essential oil of three species of Piper collected in the region of the Distrito Federal (Cerrado-Brazilian Savannah) and comparison with oils of plants from region of Paraty, State of Rio de Janeiro (Atlantic Rain Forest). Rev. Bras. Farmacogn. 2006, 16, 246–251. [Google Scholar]
- Asuming, W.A.; Beauchamp, P.S.; Descalzo, J.T.; Dev, B.C.; Dev, V.; Frost, S.; Ma, C.W. Essential oil composition of four Lomatium Raf. species and their chemotaxonomy. Biochem. Syst. Ecol. 2005, 33, 17–26. [Google Scholar] [CrossRef]
- Sabulal, B.; Dan, M.; Anil, J.J.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef]
- Argyropoulou, C.; Daferera, D.; Tarantilis, P.A.; Fasseas, C.; Polissiou, M. Chemical composition of the essential oil from leaves of Lippia citriodora HBK (Verbenaceae) at two developmental stages. Biochem. Syst. Ecol. 2007, 35, 831–837. [Google Scholar] [CrossRef]
- Roussis, V.; Tsoukatou, M.; Petrakis, P.V.; Chinou, I.; Skoula, M.; Harborne, J.B. Volatile constituents of four Helichrysum species growing in Greece. Biochem. Syst. Ecol. 2000, 28, 163–175. [Google Scholar] [CrossRef]
- Kristiawan, M.; Sobolik, V.; Al-Haddad, M.; Allaf, K. Effect of pressure-drop rate on the isolation of cananga oil using instantaneous controlled pressure-drop process. Chem. Eng. Process. 2008, 47, 66–75. [Google Scholar] [CrossRef]
- Cavalli, J.F.; Tomi, F.; Bernardini, A.F.; Casanova, J. Composition and chemical variability of the bark oil of Cedrelopsis grevei H. Baillon from Madagascar. Flavour Fragr. J. 2003, 18, 532–538. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liang, Y.Z.; Fang, H.Z.; Li, X.N. Temperature-programmed retention indices for gas chromatography-mass spectroscopy analysis of plant essential oils. J. Chromatogr. A 2005, 1096, 76–85. [Google Scholar] [CrossRef]
- Santos, R.A.M.D.; Souza, F.D.O.; Pilau, E.J.; Porto, C.; Gonçalves, J.E.; Oliveira, A.J.B.D.; Gonçalves, R.A.C. Biotransformation of (+)-carvone and (−)-carvone using human skin fungi: A green method of obtaining fragrances and flavours. Biocatal. Biotransform. 2018, 36, 396–400. [Google Scholar] [CrossRef]
- Van Der Werf, M.J.; Boot, A.M. Metabolism of carveol and dihydrocarveol in Rhodococcus erythropolis DCL14. Microbiology 2000, 146, 1129–1141. [Google Scholar] [CrossRef]
- Orio, L.; Cravotto, G.; Binello, A.; Pignata, G.; Nicolab, S.; Chematc, F. Hydrodistillation and in situ microwavegenerated hydrodistillation of fresh and dried mint leaves: A comparison study. J. Sci. Food Agric. 2012, 92, 3085–3090. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Li, Z.; Li, X.; Fan, G. Bioactive properties of the aromatic molecules of spearmint (Mentha spicata L.) essential oil: A review. Food Funct. 2022, 13, 3110–3132. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.C.C.R.; Fonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene? Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Chen, X.; Gao, X.; Wu, Q.; Zhu, D. Synthesis of optically active dihydrocarveol via a stepwise or one-pot enzymatic reduction of (R)- and (S)-carvone. Tetrahedron Asymmetry 2012, 23, 734–738. [Google Scholar] [CrossRef]
- Hendawy, S.F.; El Gendy, A.G.; Omer, E.A.; Pistelli, L.; Pistelli, L. Growth, yield and chemical composition of essential oil of Mentha piperita var. multimentha grown under different agro-ecological locations in Egypt. J. Essent. Oil Bear. Plants 2018, 21, 23–39. [Google Scholar] [CrossRef]
- Costa, A.G.; Bertolucci, S.K.V.; Chagas, J.H.; Ferraz, E.O.; Pinto, J.E.B.P. Biomass production, yield and chemical composition of peppermint essential oil using different organic fertilizer sources. Cienc. Agrotecnologia 2013, 37, 202–210. [Google Scholar] [CrossRef]
- Beigi, M.; Torki-Harchegani, M.; Ghasemi Pirbalouti, A. Quantity and chemical composition of essential oil of peppermint (Mentha × piperita L.) leaves under different drying methods. Int. J. Food Prop. 2018, 21, 267–276. [Google Scholar] [CrossRef]
- Barros, A.S.; Morais, S.M.; Ferreira, P.A.T.; Vieira, I.G.P.; Craveiro, A.A.; Fontenelle, R.O.S.; Menezes, J.E.S.; Silva, F.W.F.; Sousa, H.A.S. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crops Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Alsaraf, S.; Hadi, Z.; Akhtar, M.J.; Khan, S.A. Chemical profiling. cytotoxic and antioxidant activity of volatile oil isolated from the mint (Mentha spicata L.) grown in Oman. Biocatal. Agric. Biotechnol. 2021, 34, 102034. [Google Scholar] [CrossRef]
- Satmi, F.R.S.; Hossain, M.A. In vitro antimicrobial potential of crude extracts and chemical compositions of essential oils of leaves of Mentha piperita L. native to the Sultanate of Oman. Pac. Sci. Rev. 2016, 18, 103–106. [Google Scholar] [CrossRef]
- Rezende, D.A.C.S.; Souza, R.V.; Magalhães, M.L.; Caetano, A.R.S.; Carvalho, M.S.S.; Souza, E.C.; Guimarães, L.G.L.; Nelson, D.L.; Batista, L.R.; Cardoso, M.G. Characterization of the biological potential of the essential oils from five species of medicinal plants. Am. J. Plant Sci. 2017, 8, 154–170. [Google Scholar] [CrossRef]
- Grulova, D.; Martino, L.; Mancini, E.; Salamon, I.; Feo, V.D. Seasonal variability of the main components in essential oil of Mentha × piperita L. J. Sci. Food Agric. 2015, 95, 621–627. [Google Scholar] [CrossRef]
- Abdel-Hameed, R.S.; Alfakeer, M.; Abdallah, M. Inhibiting properties of some heterocyclic amide derivatives as potential nontoxic corrosion inhibitors for carbon steel in 1.0 M sulfuric acid. Surf. Engin. Appl. Electrochem. 2018, 54, 599–606. [Google Scholar] [CrossRef]
- Bouyahya, A.; Mechchate, H.; Benali, T.; Ghchime, R.; Charfi, S.; Balahbib, A.; Burkov, P.; Shariati, M.A.; Lorenzo, J.M.; El Omari, N. Health benefits and pharmacological propertiesofcarvone. Biomolecules 2021, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Wu, L.; Yu, K.; Xu, R.; Wei, Y.; Chinnathambi, A.; Alahmadi, T.A.; Zhou, M. D-Carvone inhibit cerebral ischemia/reperfusion induced inflammatory response TLR4/NLRP3 signaling pathway. Biomed. Pharmacother. 2020, 132, 110870. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Du, J. Anti-inflammatory and protective effects of D-carvone on lipopolysaccharide (LPS)-induced acute lung injury in mice. J. King Saud Univ. Sci. 2020, 32, 1592–1596. [Google Scholar] [CrossRef]
- Brosnan, R.J.; Ramos, K.; Aguiar, A.J.A.; Cenani, A.; Knych, H.K. Anesthetic pharmacology of the mint extracts L-Carvone and methyl salicylate. Pharmacology 2022, 107, 167–178. [Google Scholar] [CrossRef]
- Lange, B.M. Biosynthesis and biotechnology of high-value p-menthane monoterpenes, including menthol, carvone, and limonene. Adv. Biochem. Eng. Biotechnol. 2015, 148, 319–353. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Gershenzon, J. Changes in the levels of plant secondary metabolites under water and nutrient stress. In Phytochemical Adaptations to Stress, 1st ed.; Timmermann, B.N., Steelink, C., Loewus, F.A., Eds.; Springer: Boston, MA, USA, 1984; pp. 273–320. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, F.; Chang, Q.; Li, T.; Li, F. Effects of vermicomposts on tomato yield and quality and soil fertility in greenhouse under different soil water regimes. Agric. Water Manag. 2015, 160, 98–105. [Google Scholar] [CrossRef]
- Keshavarz, H.; Sanavy, S.A.M.M. Yield and oil content of mint under different nitrogen fertilizer treatments. Nat. Sci. Biol. 2018, 10, 92–96. [Google Scholar] [CrossRef][Green Version]
- Paula de Souza, M.E.; Cardoso, I.M.; de Carvalho, A.M.X.; Lopes, A.P.; Jucksch, I.; Janssen, A. Rock powder can improve vermicompost chemical properties and plant nutrition: An on-farm experiment. Commun. Soil Sci. Plant Anal. 2018, 49, 1–12. [Google Scholar] [CrossRef]
- Eckhardt, D.P.; Redin, M.; Santana, N.A.; Conti, L.D.; Dominguez, J.; Jacques, R.J.S.; Antoniolli, Z.I. Cattle manure bioconversion effect on the availability of nitrogen, phosphorus, and potassium in soil. Rev. Bras. Cienc. Solo. 2018, 42, e0170327. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Ma, A.; Wang, M.; Sun, Z. In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef]
- Garcia-Mier, L.; Meneses-Reyes, A.; Jimenez-Garcia, S.N.; Luna, A.M.; Trejo, J.F.G.; Contreras-Medina, L.M.; Feregrino-Perez, A.A. Polyphenol content and antioxidant activity of stevia and peppermint as a result of organic and conventional fertilization. J. Food Qual. 2021, 28, 6620446. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonicb, A.; Katalinicc, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Raeisi, M.; Hashemi, M.; Aminzare, M.; Afshari, A.; Zeinali, T.; Jannat, B. An investigation of the effect of Zataria multiflora Boiss and Mentha piperita essential oils to improve the chemical stability of minced meat. Vet. World 2018, 11, 1656–1662. [Google Scholar] [CrossRef]
- Farnad, N.; Heidari, R.; Aslanipour, B. Phenolic composition and comparison of antioxidant activity of alcoholic extracts of Peppermint (Mentha piperita). J. Food Meas. Charact. 2014, 8, 113–121. [Google Scholar] [CrossRef]
- Calgaro, L.C.; Melisinas, V.A.P.S.; Gonçalves, J.E.; Magalhães, H.M. Biochemical responses and volatile compounds in a peppermint chemotype grown in a controlled environment. Res. Sq. 2023, 1, 1–29. [Google Scholar] [CrossRef]
- Nichols, S.N.; Hofmann, R.W.; Williams, W.M. Physiological drought resistance and accumulation of leaf phenolics in white clover interspecific hybrids. Environ. Exp. Bot. 2015, 119, 40–47. [Google Scholar] [CrossRef]
- Mirzamohammadi, H.K.; Modarres-Sanavy, S.A.M.; Sefidkon, F.; Mokhtassi-Bidgoli, A.; Mirjalili, M.H. Irrigation and fertilizer treatments affecting rosmarinic acid accumulation, total phenolic content, antioxidant potential and correlation between them in peppermint (Mentha piperita L.). Irrig. Sci. 2021, 39, 671–683. [Google Scholar] [CrossRef]
- Ferrari, M.P.S.; Queiroz, M.S.; Andrade, M.M.; Trettel, J.R.; Magalhães, H.M. Growth regulators. sucrose and potassium in the growth and biochemical activity of Curcuma longa L. micropropagated. Sci. Agr. Par. 2020, 19, 18–26. [Google Scholar] [CrossRef]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.C.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef]
- You, X.; Fang, H.; Wang, R.; Wang, G.-L.; Ning, Y. Phenylalanine ammonia lyases mediate broad-spectrum resistance to pathogens and insect pests in plants. Sci. Bull. 2020, 65, 1425–1427. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, N.; Ghassemi-Golezani, K. Physiological changes of Mentha pulegium in response to exogenous salicylic acid under salinity. Sci. Hortic. 2020, 267, 109325. [Google Scholar] [CrossRef]
- Ortega-García, F.; Blanco, S.; Peinado, M.Á.; Peragón, J. Phenylalanine ammonia-lyase and phenolic compounds in leaves and fruits of Olea europaea L. cv. Picual during ripening. J. Sci. Food Agric. 2009, 89, 398–406. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant Phenylalanine/Tyrosine Ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.-J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.G. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; 355p. [Google Scholar]
- Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA. Atlas Climático da Região Sul do Brasil, 2nd ed.; Embrapa: Brasília, Brazil, 2012; 334p. [Google Scholar]
- Instituto de Desenvolvimento Rural do Paraná—IDR. Boletim Agrometeorológico IDR-Paraná. Available online: https://www.idrparana.pr.gov.br/ (accessed on 29 January 2025).
- Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA. Manual de Métodos de Análise de Solo. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1085209/manual-de-metodos-de-analise-de-solo (accessed on 23 November 2023).
- Myiazawa, M.; Barbosa, G.M.C. Effects of mechanical agitation and soil organic matter on soil particle size analysis. Rev. Bras. Eng. Agríc. Ambient. 2011, 15, 680–685. [Google Scholar] [CrossRef]
- Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA. BioAS: Tecnologia de Bioanálise de Solo. Available online: https://www.embrapa.br/busca-de-solucoes-tecnologicas/-/produto-servico/6047/bioas--tecnologia-de-bioanalise-de-solo-- (accessed on 30 January 2025).
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 1st ed.; Potafos: Piracicaba, Brazil, 1997; 319p. [Google Scholar]
- Radünz, L.L.; Melo, E.C.; Barbosa, L.C.A.; Santos, R.H.S. Influência da temperatura do ar de secagem no rendimento do óleo essencial de hortelã-comum (Mentha × villosa Huds). Eng. Agric. 2006, 14, 250–257. [Google Scholar]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-Candida activity of Brazilian medicinal plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Current Protocols in Food Analytical Chemistry: Determination of Total Phenolics; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Fernandes, F.; Alves, R.E.; Brito, E.S. Free radical-scavenging behaviour of some north-east Brazilian fruits in a DPPH system. Food Chem. 2009, 114, 693–695. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas Pelo Método de Redução do Ferro (FRAP); Embrapa: Fortaleza, Brazil, 2006. [Google Scholar]
- Rodríguez-Bonilla, P.; Gandía-Herrero, F.; Matencio, A.; García-Carmona, F.; López-Nicolás, J. Comparative Study of the Antioxidant Capacity of Four Stilbenes Using ORAC. ABTS+, and FRAP Techniques. Food Anal. Methods 2017, 10, 2994–3000. [Google Scholar] [CrossRef]
- Mattos, L.M.; Moretti, C.L.; Muniz, L.B.; Silva, E.Y.Y. Protocolo de Análise Para Determinação da Atividade Antioxidante Total em Hortaliças no Sistema Beta-Caroteno/ÁCido Linoleico, 1st ed.; Embrapa: Brasília, Brazil, 2009. [Google Scholar]
- Francis, F.J. Analysis of anthocyanins. In Anthocyanins as Food Colors; Markakis., P., Ed.; Academic Press: London, UK, 1982; pp. 181–206. [Google Scholar]
- Redman, R.; Freeman, S.; Clifton, D.; Morrel, J.; Brown, G.; Rodriguez, R. Biochemical analysis of plant protection afforded by a nonpathogenic endophytic mutant of Colletotrichum magna. Plant Physiol. 1999, 119, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Umesha, S. Phenylalanine ammonia lyase activity in tomato seedlings and its relationship to bacterial canker disease resistance. Phytoparasitica 2006, 34, 68–71. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
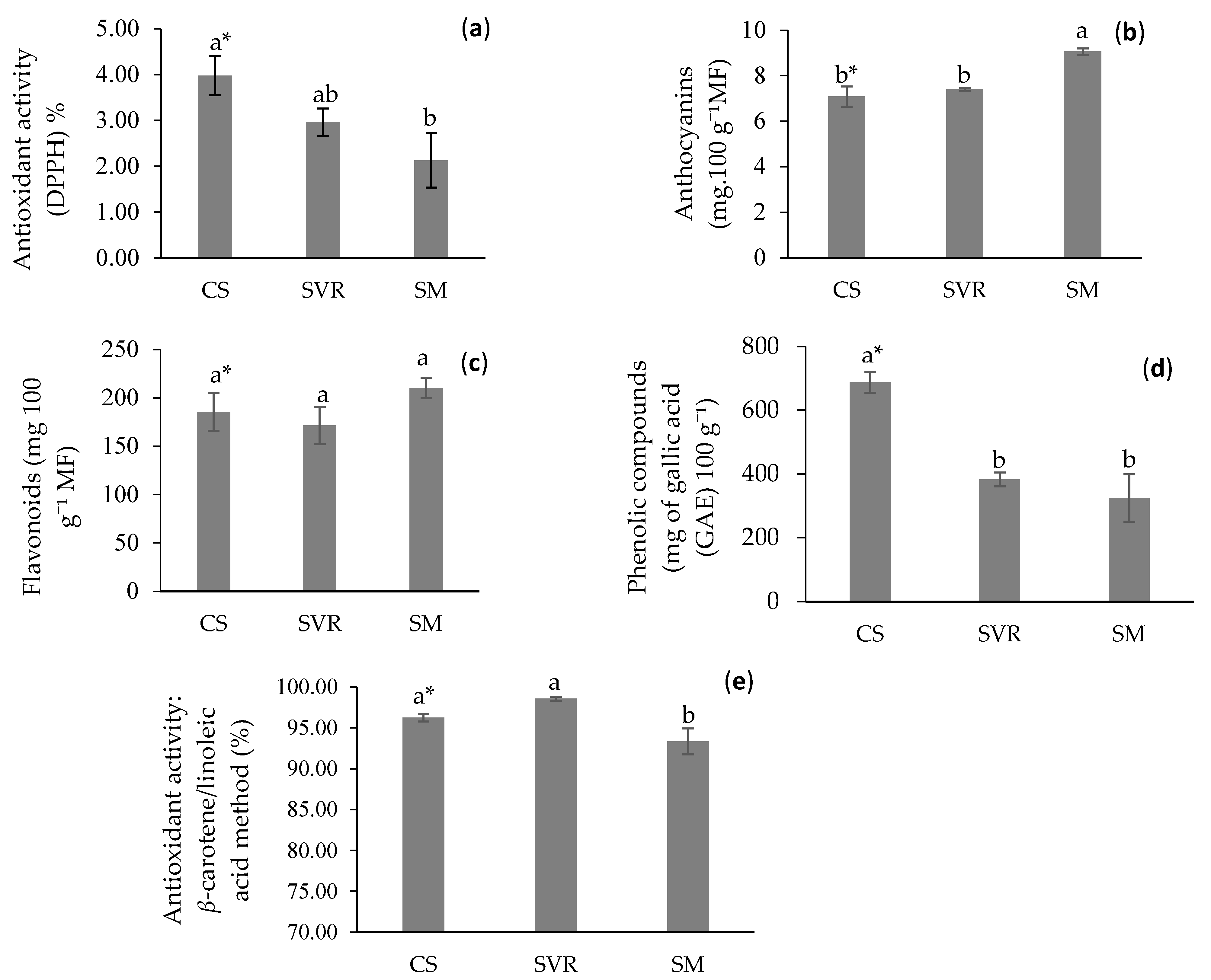
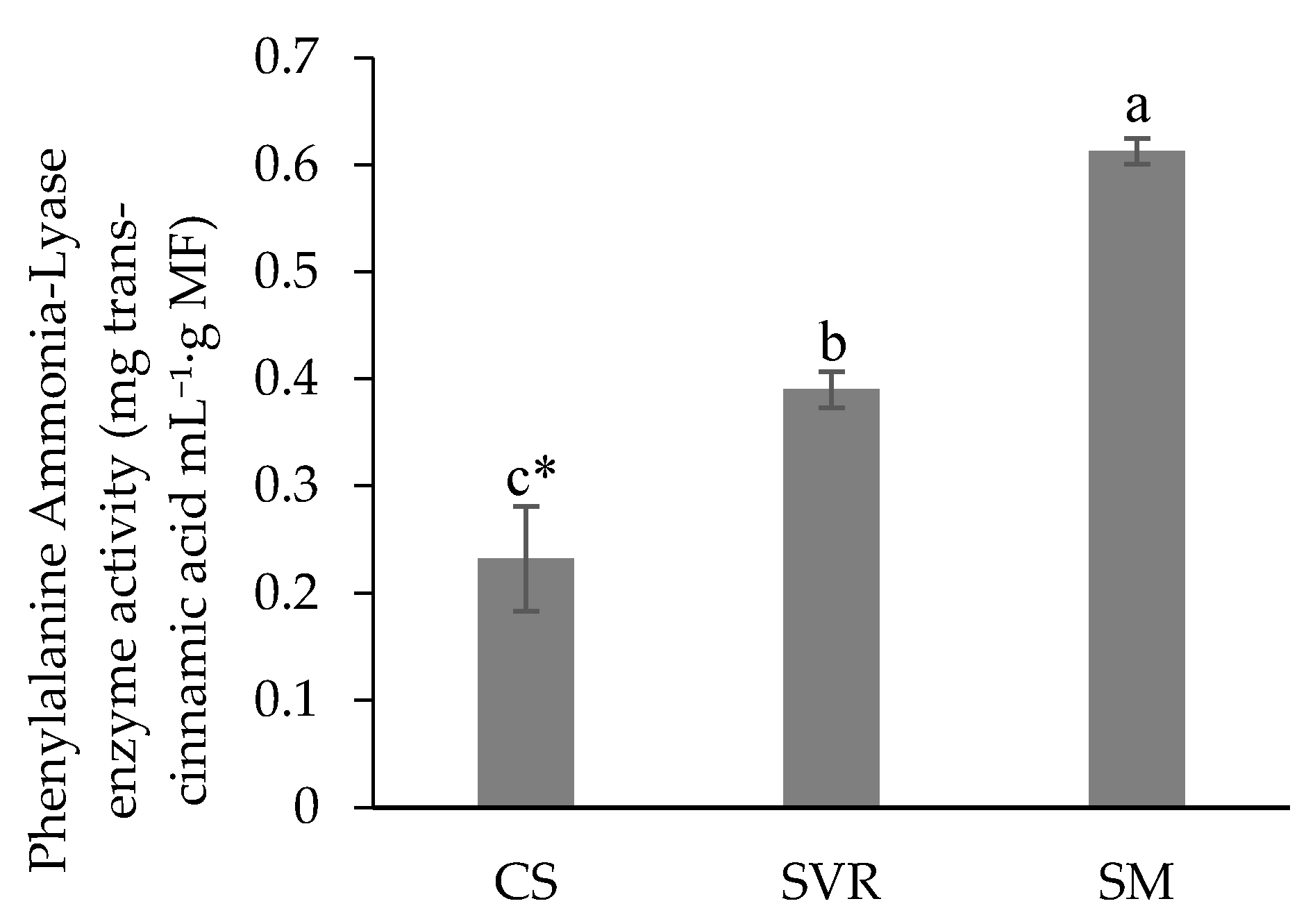
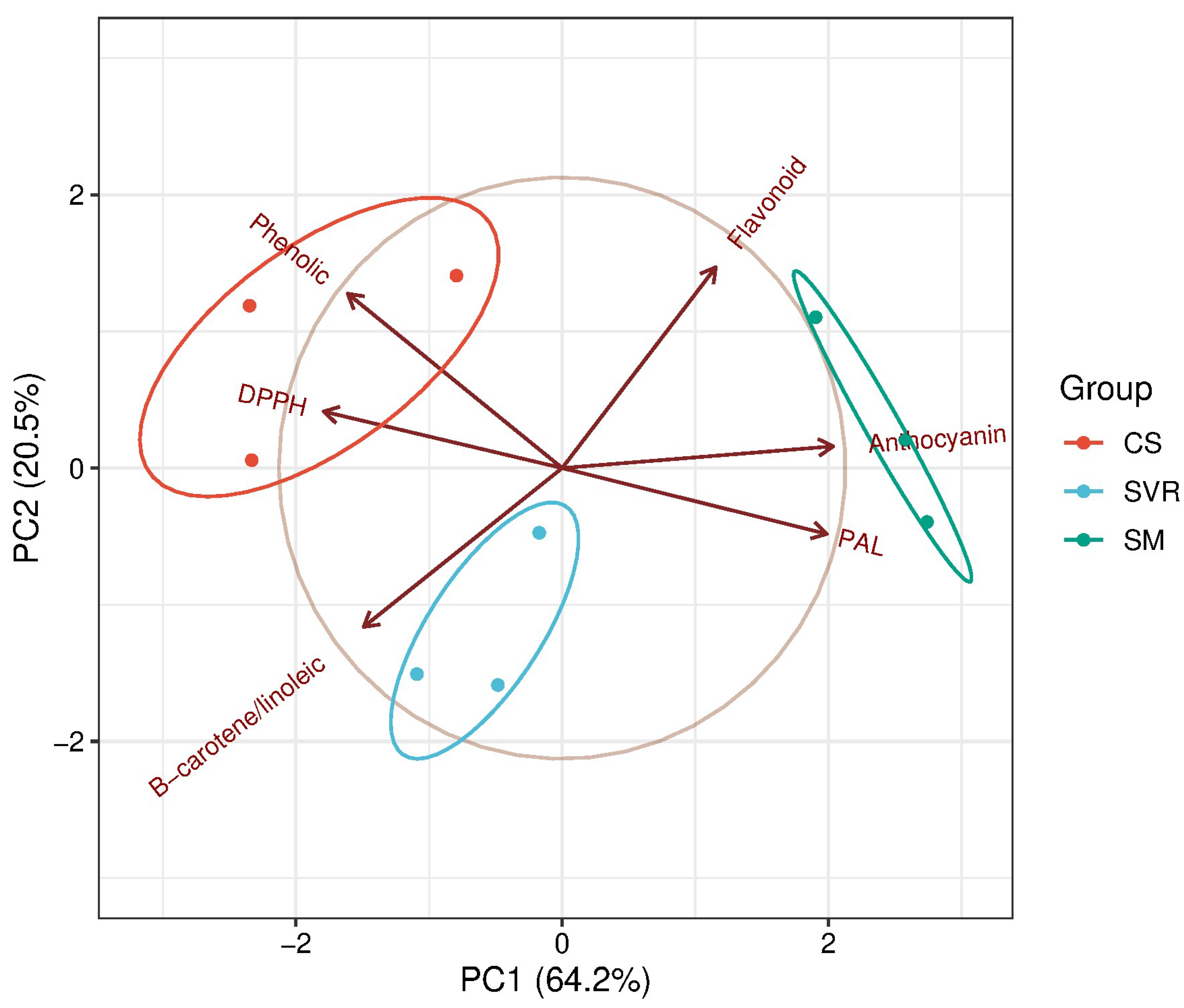
| Substrate | β-glucosidase | Arylsulfatase |
|---|---|---|
| CS | 25.09 ± 2.70 b * | 10.67 ± 0.00 b |
| SVR | 25.49 ± 1.19 b | 52.15 ± 4.24 a |
| SM | 63.89 ± 4.65 a | 39.91 ± 2.29 a |
| Parameter | CS | SVR | SM |
|---|---|---|---|
| Fresh mass aerial parts | 58.50 ± 1.95 b * | 54.67 ± 1.50 b | 92.33 ± 2.33 a |
| Dry mass aerial parts | 18.65 ± 0.62 b | 11.95 ± 0.33 c | 29.93 ± 0.76 a |
| Fresh mass roots | 350.00 ± 10.81 a | 180.25 ± 11.51 b | 335.75 ± 21.97 a |
| Dry mass roots | 48.90 ± 3.75 ab | 39.22 ± 2.25 b | 58.55 ± 4.54 a |
| Total mass | |||
| Fresh mass | 408.50 | 234.92 | 428.08 |
| Dry mas | 67.55 | 51.17 | 88.48 |
| Fresh mass ratio: aerial/root | 0.17 | 0.30 | 0.27 |
| Dry mass ratio: aerial/root | 0.38 | 0.30 | 0.51 |
| Treatment | Total N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|
| CS leaves | 14.05 ± 0.09 b * | 2.82 ± 0.14 a | 13.45 ± 0.43 b | 18.60 ± 0.98 a | 7.30 ± 0.20 a | 1.16 ± 0.09 a |
| SVR leaves | 14.90 ± 0.75 b | 2.46 ± 0.08 a | 26.10 ± 0.75 a | 18.60 ± 0.87 a | 4.45 ± 0.20 c | 1.06 ± 0.03 a |
| SM leaves | 20.73 ± 1.42 a | 2.93 ± 0.17 a | 14.00 ± 0.35 b | 15.45 ± 0.43 b | 5.53 ± 0.24 b | 1.13 ± 0.09 a |
| CS roots | 7.96 ± 0.64 b | 0.51 ± 0.01 b | 5.72 ± 0.53 c | 3.50 ± 0.75 a | 1.40 ± 0.12 b | 0.30 ± 0.00 a |
| SVR roots | 7.53 ± 0.18 b | 0.56 ± 0.04 b | 12.23 ± 0.47 a | 3.45 ± 0.03 a | 2.35 ± 0.14 a | 0.20 ± 0.00 a |
| SM roots | 11.45 ± 0.03 a | 0.85 ± 0.00 a | 8.85 ± 0.26 b | 2.95 ± 0.14 a | 1.30 ± 0.06 b | 0.30 ± 0.00 a |
| Treatment | Cu | Fe | Mn | Zn |
|---|---|---|---|---|
| CS leaves | 8.16 ± 0.58 a * | 291.95 ± 26.18 a | 211.43 ± 6.18 a | 41.66 ± 2.17 a |
| SVR leaves | 8.75 ± 0.26 a | 265.76 ± 23.60 a | 101.83 ± 1.98 c | 47.83 ± 3.30 a |
| SM leaves | 9.00 ± 0.00 a | 179.95 ± 1.01 a | 170.25 ± 4.01 b | 37.36 ± 5.13 a |
| CS roots | 6.50 ± 0.87 b | 1503.90 ± 61.26 b | 103.25 ± 11.63 c | 22.40 ± 1.27 b |
| SVR roots | 16.80 ± 0.17 a | 5172.50 ± 7.22 a | 164.85 ± 7.19 a | 24.65 ± 1.88 ab |
| SM roots | 7.90 ± 0.17 b | 1483.60 ± 56.00 b | 135.15 ± 1.65 b | 29.40 ± 2.31 a |
| Peak | RT | Compound | Ri Calc | Ri Lit | CS | SVR | SM | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.559 | (-)β-pinene | 941 | 949 | - | - | 0.58 | [48] |
| 2 | 8.163 | D-limonene | 1032 | 1032 | 0.70 | 0.86 | 6.35 | [49] |
| 3 | 8.174 | Eucalyptol | 1032 | 1033 | 0.46 | 0.32 | 1.17 | [50] |
| 4 | 8.234 | Trans-β-ocimene | 1034 | 1039 | 0.64 | - | 0.62 | [51] |
| 5 | 13.037 | L-borneol | 1161 | 1166 | 0.40 | - | 0.46 | [52] |
| 6 | 14.343 | Trans-1(7),8-p-menthadien-2-ol | 1165 | 1165 | 1.00 | 0.95 | - | [53] |
| 7 | 14.554 | Cis-dihydrocarvone | 1192 | 1194 | 0.96 | - | 2.36 | [54] |
| 8 | 15.657 | Dihydrocarveol | 1194 | 1195 | 34.45 | - | - | [55] |
| 9 | 16.085 | Carveol | 1222 | 1225 | 2.08 | 1.23 | 1.78 | [56] |
| 10 | 16.708 | Carvone | 1248 | 1242 | 31.98 | 51.33 | 74.18 | [57] |
| 11 | 20.586 | Dihydrocarvyl acetate | 1339 | 1344 | 1.07 | 0.67 | 0.72 | [58] |
| 12 | 21.218 | Carveol acetate | 1355 | 1345 | 1.27 | 1.58 | - | [59] |
| 13 | 22.488 | (-)-β-bourbonene | 1384 | 1384 | 1.84 | 5.36 | 2.09 | [60] |
| 14 | 22.817 | α-copaene | 1391 | 1394 | 1.93 | 0.57 | 0.26 | [51] |
| 15 | 23.547 | β-cubebene | 1417 | 1418 | 0.18 | 10.34 | 3.97 | [61] |
| 16 | 23.884 | α-gurjunene | 1425 | 1425 | 0.14 | 0.50 | - | [62] |
| 17 | 24.620 | β-copaene | 1443 | 1442 | 1.91 | 0.56 | - | [63] |
| 18 | 24.891 | Caryophyllene | 1449 | 1444 | 2.03 | 5.92 | 2.09 | [51] |
| 19 | 24.896 | Humulene | 1449 | 1452 | 0.88 | 1.01 | 0.37 | [62] |
| 20 | 24.980 | Cis-β-farnesene | 1451 | 1457 | 1.99 | 1.75 | 0.55 | [64] |
| 21 | 25.393 | γ-muurolene | 1460 | 1461 | - | 0.54 | - | [65] |
| 22 | 26.162 | Germacrene D | 1477 | 1480 | 5.39 | 1.45 | 0.45 | [62] |
| 23 | 27.296 | (+)-epi-bicyclosesquiphellandrene | 1527 | 1521 | - | 3.15 | 0.81 | [66] |
| 24 | 27.840 | α-muurolene | 1555 | 1540 | 0.15 | 0.94 | - | [67] |
| 25 | 28.452 | Cis-calamenene | 1564 | 1566 | 1.60 | 2.25 | 0.74 | [68] |
| 26 | 29.955 | (-)-spathulenol | 1585 | 1582 | 0.76 | 1.11 | [69] | |
| 27 | 30.148 | Caryophyllene oxide | 1587 | 1589 | 0.53 | 0.76 | 0.26 | [69] |
| 28 | 32.390 | Cubenol | 1664 | 1664 | 0.95 | 1.44 | 1.13 | [70] |
| 29 | 32.449 | T-cadinol | 1665 | 1665 | 0.84 | 1.26 | - | [71] |
| 30 | 32.903 | α-cadinol | 1672 | 1673 | 1.15 | 1.70 | - | [72] |
| 31 | 34.052 | Aromadendrene oxide-(2) | 1679 | 1678 | 0.59 | - | - | [73] |
| 32 | 34.076 | 6-epi-shyobunol | 1890 | 1881 | 0.54 | 1.89 | 0.42 | [74] |
| 33 | 48.170 | Phytol | 2112 | 2112 | 0.17 | 0.56 | - | [75] |
| Total Identified | 98.58 | 99.16 | 98.00 | |||||
| Hydrocarbon Monoterpenes | 1.34 | 0.86 | 5.55 | |||||
| Oxygenated Monoterpenes | 71.33 | 53.83 | 79.95 | |||||
| Hydrocarbon Sesquiterpenes | 18.04 | 34.34 | 9.97 | |||||
| Oxygenated Sesquiterpenes | 5.36 | 7.32 | 1.81 | |||||
| Oxygenated Diterpene | 0.17 | 0.56 | - | |||||
| Other Compounds | 2.34 | 2.25 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreiro, G.d.O.; Magalhães, H.M.; Pinc, M.M.; de Souza, S.G.H.; Gazim, Z.C.; Canonico Silva, G.C.; Gonçalves, J.E.; Alberton, O. Substrates in Organic Mint Cultivation: Growth, Phytochemistry and Biological Activities. Plants 2025, 14, 2886. https://doi.org/10.3390/plants14182886
Carreiro GdO, Magalhães HM, Pinc MM, de Souza SGH, Gazim ZC, Canonico Silva GC, Gonçalves JE, Alberton O. Substrates in Organic Mint Cultivation: Growth, Phytochemistry and Biological Activities. Plants. 2025; 14(18):2886. https://doi.org/10.3390/plants14182886
Chicago/Turabian StyleCarreiro, Gilcielen de Oliveira, Hélida Mara Magalhães, Mariana Moraes Pinc, Silvia Graciele Hulse de Souza, Zilda Cristiani Gazim, Gabriela Catuzo Canonico Silva, José Eduardo Gonçalves, and Odair Alberton. 2025. "Substrates in Organic Mint Cultivation: Growth, Phytochemistry and Biological Activities" Plants 14, no. 18: 2886. https://doi.org/10.3390/plants14182886
APA StyleCarreiro, G. d. O., Magalhães, H. M., Pinc, M. M., de Souza, S. G. H., Gazim, Z. C., Canonico Silva, G. C., Gonçalves, J. E., & Alberton, O. (2025). Substrates in Organic Mint Cultivation: Growth, Phytochemistry and Biological Activities. Plants, 14(18), 2886. https://doi.org/10.3390/plants14182886







