Analysis of Plant–Fungus Interactions in Calocybe gambosa Fairy Rings
Abstract
1. Introduction
2. Results
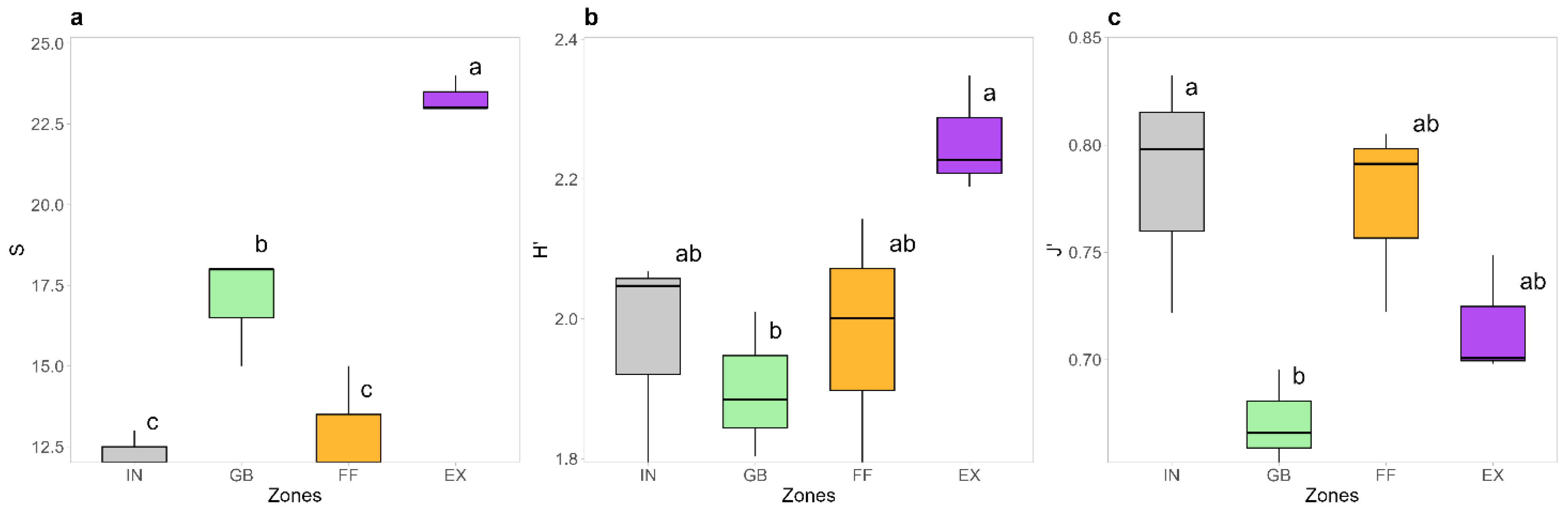
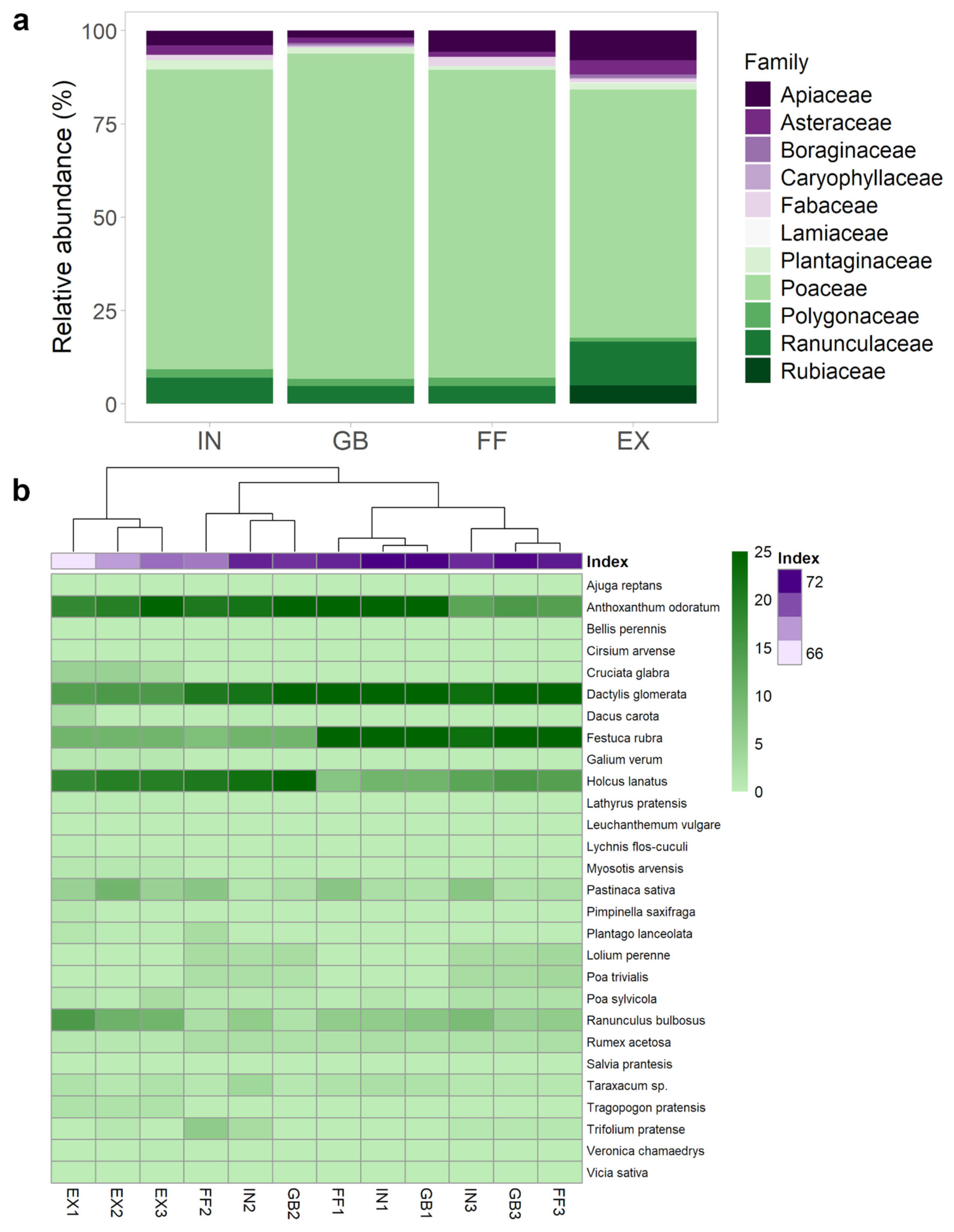
2.1. Plant Community Composition of Fairy Rings
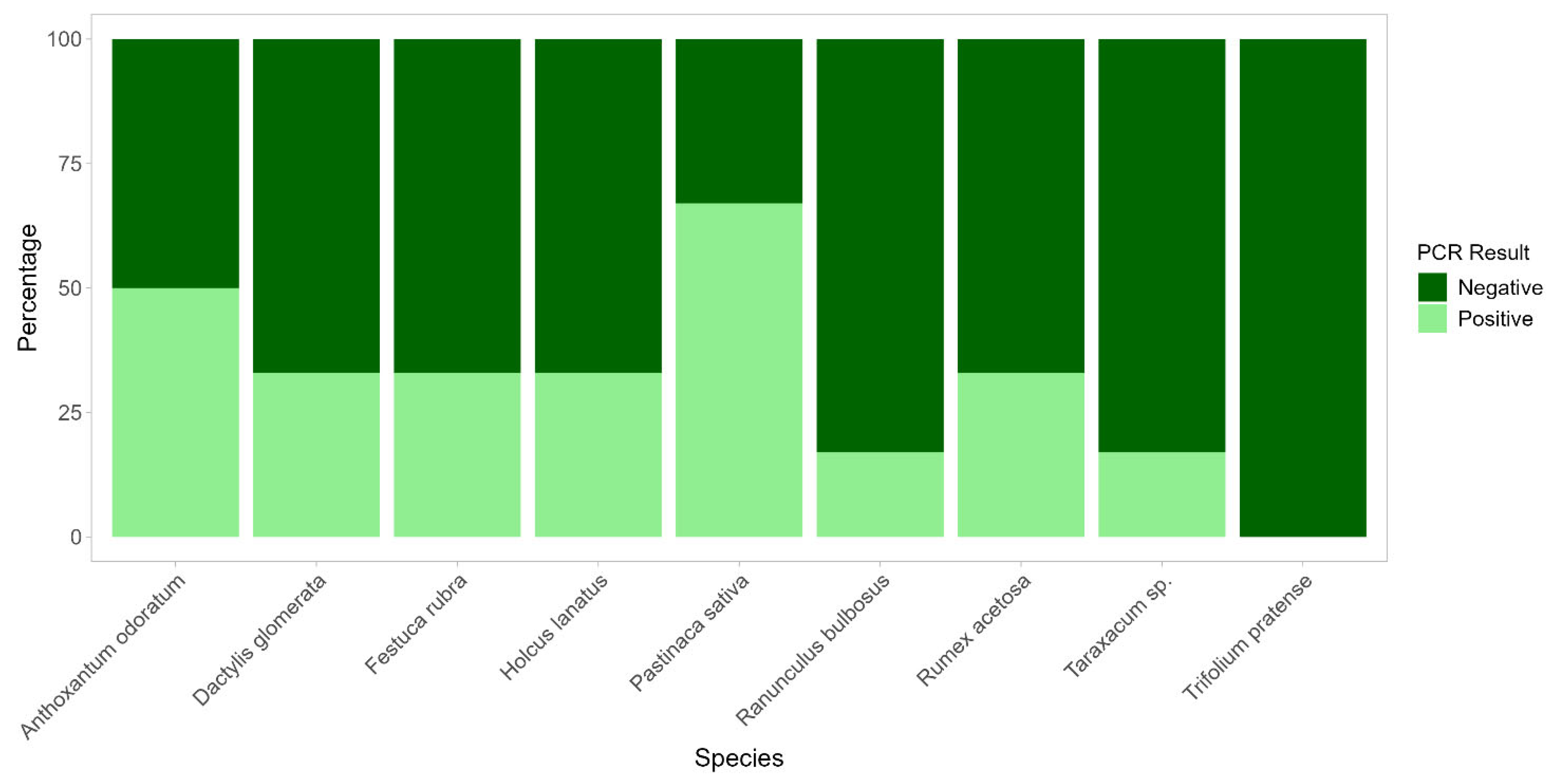
2.2. Verification of Radical Colonization by C. gambosa Mycelium Through PCR
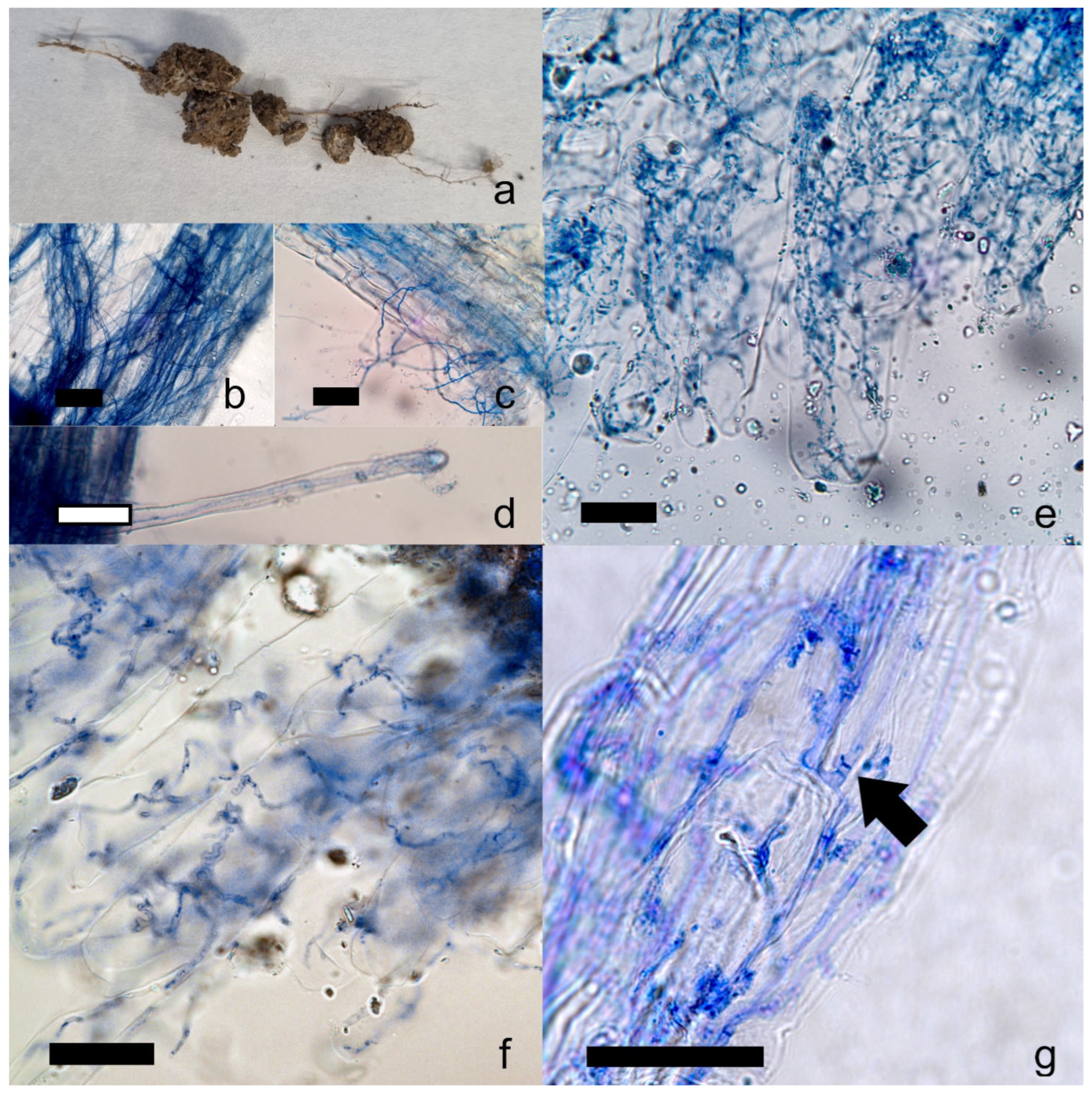
2.3. Microscopic Observations of In Situ and Ex Situ Plant Roots
2.4. Effects of Active Mycelium on Plant Growth
2.5. Effects of Soluble Substances and VOCs Produced by C. gambosa Mycelium on Plant Growth
3. Discussion
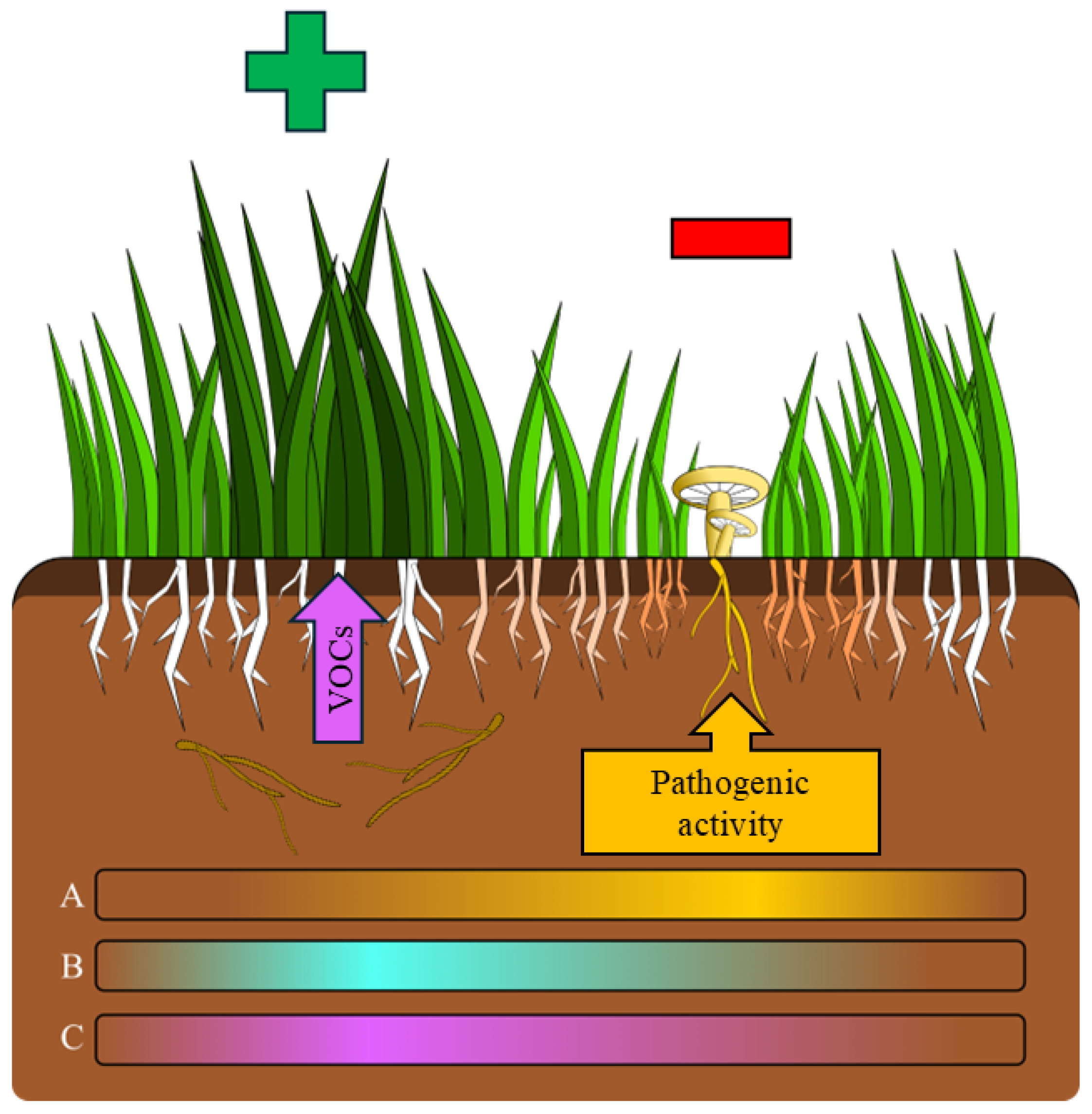
3.1. C. gambosa Influences Plant Community Composition Within FRs
3.2. Direct Pathogenicity of C. gambosa Mycelium on Plants in Fungal Front
3.3. Indirect Effects of C. gambosa Mycelium on Plants Mediated by VOCs in the Greener Belt
4. Materials and Methods
4.1. Description of Study Area
4.2. In Situ Characterization of Fairy Ring: Study Site and Vegetation Analysis
4.3. Mycelium Isolation
4.4. Selection of Species-Specific Primers
4.5. In Situ Detection of C. gambosa Radical Colonization: Plant Sampling and PCRs
4.6. Ex Situ Study of C. gambosa Mycelium Direct Effects on Plant Growth
4.7. Ex Situ Mycelial Enzyme Production
4.8. Microscope Observations of Ex Situ and In Situ C. gambosa Root Colonization
4.9. Ex Situ Influence of Soluble Substances Produced by the C. gambosa Mycelium on Plant Growth
4.10. Ex Situ Influence of VOCs Produced by the C. gambosa Mycelium on Plant Growth
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EX | External area |
| FF | Fungal front |
| FR | Fairy ring |
| GB | Greener belt |
| IN | Internal area |
| VOC | Volatile organic compound |
References
- Fidanza, M. Fairy ring 101. USGA Green Sect. Rec. 2009, 47, 8–10. [Google Scholar]
- Davidson, F.A.; Sleeman, B.D.; Rayner, A.D.M.; Crawford, J.W.; Ritz, K. Travelling waves and pattern formation in a model for fungal development. J. Math. Biol. 1997, 35, 589–608. [Google Scholar] [CrossRef]
- Karst, N.; Dralle, D.; Thompson, S. Spiral and Rotor Patterns Produced by Fairy Ring Fungi. PLoS ONE 2016, 11, e0149254. [Google Scholar] [CrossRef] [PubMed]
- Parker-Rhodes, A.F. Fairy ring kinetics. Trans. Br. Mycol. Soc. 1955, 38, 59–72. [Google Scholar] [CrossRef]
- Rayner, A.D. The challenge of the individualistic mycelium. Mycologia 1991, 83, 48–71. [Google Scholar] [CrossRef]
- Shantz, H.L.; Piemeisel, R.L. Fungus fairy rings in eastern Colorado and their effect on vegetation. J. Agr. Res. 1917, 11, 191–245. Available online: https://archivesspace.nal.usda.gov/repositories/4/archival_objects/21944 (accessed on 21 February 2025).
- Stevenson, D.R.; Thompson, C.J. Fairy ring kinetics. J. Theor. Biol. 1976, 58, 143–163. [Google Scholar] [CrossRef]
- Zotti, M.; De Filippis, F.; Cesarano, G.; Ercolini, D.; Tesei, G.; Allegrezza, M.; Giannino, F.; Mazzoleni, S.; Bonanomi, G. One ring to rule them all: An ecosystem engineer fungus fosters plant and microbial diversity in a Mediterranean grassland. New Phytol. 2020, 227, 884–898. [Google Scholar] [CrossRef]
- Duan, M.; Lu, J.; Yang, W.; Lu, M.; Wang, J.; Li, S.; Chen, Y.; Hu, L.; Wang, L. Metabarcoding and Metabolome Analyses Reveal Mechanisms of Leymus chinensis Growth Promotion by Fairy Ring of Leucocalocybe mongolica. J. Fungi 2022, 8, 944. [Google Scholar] [CrossRef]
- Duan, M.; Lu, M.; Lu, J.; Yang, W.; Li, B.; Ma, L.; Wang, L. Soil Chemical Properties, Metabolome, and Metabarcoding Give the New Insights into the Soil Transforming Process of Fairy Ring Fungi Leucocalocybe mongolica. J. Fungi 2022, 8, 680. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ibáñez, M.; Bol, R.; Brüggemann, N.; Lobo, A.; Jimenez, J.J.; Ruess, L.; Sebastià, M.T. Fairy ring-induced soil potassium depletion gradients reshape microbial community composition in a montane grassland. Eur. J. Soil Sci. 2022, 73, e13239. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Rong, Y.; Bei, Y.; Wei, Y.; Liu, N. Temporal variation of Q10 values in response to changes in soil physiochemical properties caused by fairy rings. Eur. J. Soil Biol. 2018, 86, 42–48. [Google Scholar] [CrossRef]
- Zotti, M.; Bonanomi, G.; Mancinelli, G.; Barquero, M.; De Filippis, F.; Giannino, F.; Mazzoleni, S.; González-Andrés, F. Riding the wave: Response of bacterial and fungal microbiota associated with the spread of the fairy ring fungus Calocybe gambosa. Appl. Soil Ecol. 2021, 163, 103963. [Google Scholar] [CrossRef]
- Kataoka, R.; Siddiqui, Z.A.; Kikuchi, J.; Ando, M.; Sriwati, R.; Nozaki, A.; Futai, K. Detecting nonculturable bacteria in the active mycorrhizal zone of the pine mushroom Tricholoma matsutake. J. Microbiol. 2012, 50, 199–206. [Google Scholar] [CrossRef]
- Kim, M.; Yoon, H.; You, Y.-H.; Kim, Y.-E.; Woo, J.-R.; Seo, Y.; Lee, G.-M.; Kong, W.-S.; Kim, J.-G. Metagenomic Analysis of Fungal Communities Inhabiting the Fairy Ring Zone of Tricholoma matsutake. J. Microbiol. Biotechnol. 2013, 23, 1347–1356. [Google Scholar] [CrossRef]
- Kim, M.; Yoon, H.; Kim, Y.E.; Kim, Y.J.; Kong, W.S.; Kim, J.G. Comparative analysis of bacterial diversity and communities inhabiting the fairy ring of Tricholoma matsutake by barcoded pyrosequencing. J. Appl. Microbiol. 2014, 117, 699–710. [Google Scholar] [CrossRef]
- Ohara, H.; Hamada, M. Disappearance of Bacteria from the Zone of Active Mycorrhizas in Tricholoma matsutake (S. Ito et Imai) Singer. Nature 1967, 213, 528–529. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Liu, N.; Zhang, Y. Effects of fairy ring fungi on plants and soil in the alpine and temperate grasslands of China. Plant Soil 2019, 441, 499–510. [Google Scholar] [CrossRef]
- Bonanomi, G.; Mingo, A.; Incerti, G.; Mazzoleni, S.; Allegrezza, M. Fairy rings caused by a killer fungus foster plant diversity in species-rich grassland. J. Veg. Sci. 2012, 23, 236–248. [Google Scholar] [CrossRef]
- Caesar-TonThat, T.C.; Espeland, E.; Caesar, A.J.; Sainju, U.M.; Lartey, R.T.; Gaskin, J.F. Effects of Agaricus lilaceps Fairy Rings on Soil Aggregation and Microbial Community Structure in Relation to Growth Stimulation of Western Wheatgrass (Pascopyrum smithii) in Eastern Montana Rangeland. Microb. Ecol. 2013, 66, 120–131. [Google Scholar] [CrossRef]
- Edwards, P.J. The Growth of Fairy Rings of Agaricus Arvensis and Their Effect Upon Grassland Vegetation and Soil. J. Ecol. 1984, 72, 505–513. [Google Scholar] [CrossRef]
- Fidanza, M. Characterization of soil properties associated with type-I fairy ring symptoms in turfgrass. Biologia 2007, 62, 533–536. [Google Scholar] [CrossRef]
- Fidanza, M.A.; Cisar, J.L.; Kostka, S.J.; Gregos, J.S.; Schlossberg, M.J.; Franklin, M. Preliminary investigation of soil chemical and physical properties associated with type-I fairy ring symptoms in turfgrass. Hydrol. Process. 2007, 21, 2285–2290. [Google Scholar] [CrossRef]
- Gramss, G.; Voigt, K.-D.; Bergmann, H. Factors influencing water solubility and plant availability of mineral compounds in the tripartite fairy rings of Marasmius oreades (Bolt.: Fr.) Fr. J. Basic Microbiol. 2005, 45, 41–54. [Google Scholar] [CrossRef]
- Salvatori, N.; Moreno, M.; Zotti, M.; Iuorio, A.; Cartenì, F.; Bonanomi, G.; Mazzoleni, S.; Giannino, F. Process based modelling of plants–fungus interactions explains fairy ring types and dynamics. Sci. Rep. 2023, 13, 19918. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.; Bonanomi, G.; Mazzoleni, S. Fungal fairy rings: History, ecology, dynamics and engineering functions. IMA fungus 2025, 16, e138320. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S. Fairy rings: Toadstools, stinkhorns, and puffballs. Plant Dis. 2008, 49, 1–9. Available online: http://www.ctahr.hawaii.edu/oc/freepubs/pdf/PD-49.pdf (accessed on 21 February 2025).
- Miller, G.L.; Grand, L.F.; Tredway, L.P. Identification and Distribution of Fungi Associated with Fairy Rings on Golf Putting Greens. Plant Dis. 2011, 95, 1131–1138. [Google Scholar] [CrossRef]
- Cao, M.; Liu, F.; Sun, L.; Wang, Y.; Wan, J.; Wang, R.; Zhou, H.; Wang, W.; Xu, J. Floccularia luteovirens modulates the growth of alpine meadow plants and affects soil metabolite accumulation on the Qinghai-Tibet Plateau. Plant Soil 2021, 459, 125–136. [Google Scholar] [CrossRef]
- Fisher, R.F. Nitrogen and phosphorus mobilization by the fairy ring fungus, Marasmius oreades (bolt.) fr. Soil Biol. Biochem. 1977, 9, 239–241. [Google Scholar] [CrossRef]
- Hardwick, N.V.; Heard, A.J. The Effect of Marasmius oreades in Pasture. Plant Pathol. 1978, 27, 53–57. [Google Scholar] [CrossRef]
- Narimatsu, M.; Koiwa, T.; Masaki, T.; Sakamoto, Y.; Ohmori, H.; Tawaraya, K. Relationship between climate, expansion rate, and fruiting in fairy rings (‘shiro’) of an ectomycorrhizal fungus Tricholoma matsutake in a Pinus densiflora forest. Fungal Ecol. 2015, 15, 18–28. [Google Scholar] [CrossRef]
- Mathur, S.P. Degradation of soil humus by the fairy ring mushroom. Plant Soil 1970, 33, 717–720. [Google Scholar] [CrossRef]
- Mitchinson, A. Fairy chemicals. Nature 2014, 505, 298. [Google Scholar] [CrossRef]
- Choi, J.-H.; Abe, N.; Tanaka, H.; Fushimi, K.; Nishina, Y.; Morita, A.; Kiriiwa, Y.; Motohashi, R.; Hashizume, D.; Koshino, H.; et al. Plant-Growth Regulator, Imidazole-4-Carboxamide, Produced by the Fairy Ring Forming Fungus Lepista sordida. J. Agric. Food Chem. 2010, 58, 9956–9959. [Google Scholar] [CrossRef]
- Kawagishi, H. Are fairy chemicals a new family of plant hormones? Proc. Jpn. Acad. Ser. B 2019, 95, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Choi, J.-H.; Takemura, H.; Kotajima, M.; Wu, J.; Tokuyama, S.; Hirai, H.; Asakawa, T.; Ouchi, H.; Inai, M.; et al. Biosynthesis of the Fairy Chemicals, 2-Azahypoxanthine and Imidazole-4-carboxamide, in the Fairy Ring-Forming Fungus Lepista sordida. J. Nat. Prod. 2020, 83, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Yamamoto, N.; Suzuki, T.; Dohra, H.; Choi, J.-H.; Terashima, Y.; Yokoyama, K.; Kawagishi, H.; Yano, K. Genome sequence analysis of the fairy ring-forming fungus Lepista sordida and gene candidates for interaction with plants. Sci. Rep. 2019, 9, 5888. [Google Scholar] [CrossRef]
- Elliott, J.S.B. Concerning Fairy Rings in Pastures. Ann. Appl. Biol. 1926, 13, 277–288. [Google Scholar] [CrossRef]
- Blenis, P.V.; Chow, P.S.; Duncan, I.; Knowles, N.R. Cyanide levels near fairy rings affect the growth of grasses and fungi. Can. J. Bot. 2004, 82, 1324–1329. [Google Scholar] [CrossRef]
- Edwards, P.J. Effects of the fairy ring fungus Agaricus arvensis on nutrient availability in grassland. New Phytol. 1988, 110, 377–381. [Google Scholar] [CrossRef]
- Smith, J.D.; Rupps, R. Antagonism in Marasmius oreades fairy rings. J. Sports Turf Res. Inst. 1978, 54, 97–105. [Google Scholar]
- Caspar, J.; Spiteller, P. A Free Cyanohydrin as Arms and Armour of Marasmius oreades. ChemBioChem 2015, 16, 570–573. [Google Scholar] [CrossRef]
- Lebeau, J.B.; Hawn, E.J. Fairy rings in Alberta. Can. Plant. Surv. 1961, 41, 317–320. [Google Scholar]
- Petrović, J.; Fernandes, Â.; Stojković, D.; Soković, M.; Barros, L.; Ferreira, I.; Shekhar, A.; Glamočlija, J. Step Forward Towards Exploring Nutritional and Biological Potential of Mushrooms: A Case Study of Calocybe gambosa (Fr.) Donk Wild Growing in Serbia. Pol. J. Food. Nutr. Sci. 2022, 72, 17–26. [Google Scholar] [CrossRef]
- Villares, A. Polysaccharides from the edible mushroom Calocybe gambosa: Structure and chain conformation of a (1→4),(1→6)-linked glucan. Carbohydr. Res. 2013, 375, 153–157. [Google Scholar] [CrossRef]
- Cairney, J.W.G. Translocation of solutes in ectomycorrhizal and saprotrophic rhizomorphs. Mycol. Res. 1992, 96, 135–141. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Druzhinina, I.S. Environmental and Microbial Relationships, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 287–300. [Google Scholar] [CrossRef]
- Cosby, H.E. Rings on The Range. Rangeland Ecol. Manag. 1960, 13, 283–288. [Google Scholar] [CrossRef]
- Fidanza, M.; Settle, D.; Wetzel, H. An Index for evaluating fairy ring symptoms in turfgrass. HortScience 2016, 51, 1194–1196. [Google Scholar] [CrossRef]
- Bonanomi, G.; Iacomino, G.; Di Costanzo, L.; Moreno, M.; Tesei, G.; Allegrezza, M.; Mazzoleni, S.; Idbella, M. Mechanisms and impacts of Agaricus urinascens fairy rings on plant diversity and microbial communities in a montane Mediterranean grassland. FEMS Microbiol. Ecol. 2025, 101, fiaf034. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Lian, L.; Zhang, J.; Liu, N.; Wilson, G.W.T.; Rillig, M.C.; Jia, S.; Yang, G.; Zhang, Y. Discovering the role of fairy ring fungi in accelerating nitrogen cycling to promote plant productivity in grasslands. Soil Biol. Biochem. 2024, 199, 109595. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulissen, D. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 1991, 18, 1–248. [Google Scholar]
- Ardelean, D.; Paltinean, M.; Petrescu, I.; Sarac, I.; Onișan, E.; Stroia, C. Enhancing sorrel growing rate: Insights from soil variability and adaptability study in Arad county. J. Hortic. For. Biotechnol. 2023, 27, 161–165. Available online: https://journal-hfb.usab-tm.ro (accessed on 21 February 2025).
- Korpelainen, H.; Pietiläinen, M. Sorrel (Rumex acetosa L.): Not Only a Weed but a Promising Vegetable and Medicinal Plant. Bot. Rev. 2020, 86, 234–246. [Google Scholar] [CrossRef]
- Weigel, R.; Werner, A.; Würzberg, L.; Zembold, K.; Angulo, K.; Bach, W.; Hémonnet-Dal, C.; Nähring, T.; Hiebenga, M.; Muffler, L. Root and shoot traits of two common herbs respond differently to drought and fertilization in a multifactorial global change experiment. Plant Soil 2025, 512, 1–16. [Google Scholar] [CrossRef]
- Lima, M.A.S.S.; Fontanetti, A.; Soares, M.Ã.; Gazaffi, R.; de Souza Gallo, A. Vegetative Development and Nutrient Absorption March of Sorrel (Rumex acetosa L.). J. Agric. Sci. 2019, 11, 262–270. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation classification by reference to strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies and Vegetation Processes; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Lawniczak, A.; Drapikowska, M.; Celka, Z.; Szkudlarz, P.; Jackowiak, B. Response of Anthoxanthum odoratum and A. aristatum to the different habitat types and nutrient concentration in soil. Fresen. Environ. Bull 2011, 20, 2465–2474. [Google Scholar]
- Qian, F.; Su, X.; Zhang, Y.; Bao, Y. Variance of soil bacterial community and metabolic profile in the rhizosphere vs. non-rhizosphere of native plant Rumex acetosa L. from a Sb/As co-contaminated area in China. J. Hazard. Mater. 2023, 456, 131681. [Google Scholar] [CrossRef]
- Filer, T.H. Parasitic Aspects of a Fairy Ring Fungus, Marasmius oreades. Phytopathology 1965, 55, 1132–1134. Available online: https://research.fs.usda.gov/treesearch/43154 (accessed on 21 February 2025).
- Terashima, Y.; Fukiharu, T.; Fujiie, A. Morphology and comparative ecology of the fairy ring fungi, Vascellum curtisii and Bovista dermoxantha, on turf of bentgrass, bluegrass, and Zoysiagrass. Mycoscience 2004, 45, 251–260. [Google Scholar] [CrossRef]
- Carroll, G. Fungal endophytes in stems and leaves: From latent pathogen to mutualistic symbiont. Ecology 1988, 69, 2–9. [Google Scholar] [CrossRef]
- Clay, K. Fungal endophytes of grasses: A defensive mutualism between plants and fungi. Ecology 1988, 69, 10–16. [Google Scholar] [CrossRef]
- Faeth, S.H. Are endophytic fungi defensive plant mutualists? Oikos 2002, 98, 25–36. [Google Scholar] [CrossRef]
- Ballesteros, G.I.; Acuña-Rodríguez, I.S.; Barrera, A.; Gundel, P.E.; Newsham, K.K.; Molina-Montenegro, M.A. Seed fungal endophytes promote the establishment of invasive Poa annua in maritime Antarctica. Plant. Ecol. Divers. 2022, 15, 199–212. [Google Scholar] [CrossRef]
- Gundel, P.E.; Sun, P.; Charlton, N.D.; Young, C.A.; Miller, T.E.X.; Rudgers, J.A. Simulated folivory increases vertical transmission of fungal endophytes that deter herbivores and alter tolerance to herbivory in Poa autumnalis. Ann. Bot. 2020, 125, 981–991. [Google Scholar] [CrossRef]
- Tello, S.A.; Silva-Flores, P.; Agerer, R.; Halbwachs, H.; Beck, A.; Peršoh, D. Hygrocybe virginea is a systemic endophyte of Plantago lanceolata. Mycol. Prog. 2014, 13, 471–475. [Google Scholar] [CrossRef]
- Douaiher, M.N.; Nowak, E.; Durand, R.; Halama, P.; Reignault, P. Correlative analysis of Mycosphaerella graminicola pathogenicity and cell wall-degrading enzymes produced in vitro: The importance of xylanase and polygalacturonase. Plant Pathol. 2007, 56, 79–86. [Google Scholar] [CrossRef]
- Jorge, I.; Navas-Cortés, J.A.; Jiménez-Díaz, R.M.; Tena, M. Cell wall degrading enzymes in fusarium wilt of chickpea: Correlation between pectinase and xylanase activities and disease development in plants infected with two pathogenic races of Fusarium oxysporum f. sp. ciceris. Can. J. Bot. 2006, 84, 1395–1404. [Google Scholar] [CrossRef]
- Roberti, R.; Osti, F.; Innocenti, G.; Rombolà, A.D.; Di Marco, S. An environmentally sustainable approach for the management of Phaeoacremonium minimum, the main agent of wood diseases in Actinidia deliciosa. Eur. J. Plant. Pathol. 2017, 148, 151–162. [Google Scholar] [CrossRef]
- Cheaib, A.; Killiny, N. Photosynthesis Responses to the Infection with Plant Pathogens. Mol. Plant-Microbe Interact. 2024, 38, 9–29. [Google Scholar] [CrossRef]
- Saleem, A.; El-Said, A.H.M.; Maghraby, T.A.; Hussein, M.A. Pathogenicity and Pectinase Activity of Some Facultative Mycoparasites Isolated from Vicia faba Diseased Leaves in Relation to Photosynthetic Pigments of Plant. J. Plant Pathol. Microbiol. 2012, 3, 141. [Google Scholar] [CrossRef]
- Boccardo, F.; Traverso, M.; Vizzini, A.; Zotti, M. Funghi d’Italia; Zanichelli: Bologna, Italy, 2008; pp. 184–185. [Google Scholar]
- Angelini, P.; Tirillini, B.; Venanzoni, R. In vitro antifungal activity of Calocybe gambosa extracts against yeasts and filamentous fungi. Afr. J. Microbiol. Res. 2012, 6, 1810–1814. [Google Scholar] [CrossRef]
- Schlunegger, U.P.; Kuchen, A.; Clemencon, H. Mycelium products in higher fungi. I. phenoxazine derivatives in Calocybe gambosa. Helv. Chim. Acta 1976, 59, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.; Lee, S.; Bennett, J.W. Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 2015, 99, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef]
- Naznin, H.A.; Kimura, M.; Miyazawa, M.; Hyakumachi, M. Analysis of Volatile Organic Compounds Emitted by Plant Growth-Promoting Fungus Phoma sp. GS8-3 for Growth Promotion Effects on Tobacco. Microbes Environ. 2013, 28, 42–49. [Google Scholar] [CrossRef]
- You, J.; Li, G.; Li, C.; Zhu, L.; Yang, H.; Song, R.; Gu, W. Biological Control and Plant Growth Promotion by Volatile Organic Compounds of Trichoderma koningiopsis T-51. J. Fungi 2022, 8, 131. [Google Scholar] [CrossRef]
- Jiang, L.; Lee, M.H.; Kim, C.Y.; Kim, S.W.; Kim, P.I.; Min, S.R.; Lee, J. Plant Growth Promotion by Two Volatile Organic Compounds Emitted from the Fungus Cladosporium halotolerans NGPF1. Front. Plant. Sci. 2021, 12, 794349. [Google Scholar] [CrossRef]
- Sun, L.; Cao, M.; Liu, F.; Wang, Y.; Wan, J.; Wang, R.; Zhou, H.; Wang, W.; Xu, J. The volatile organic compounds of Floccularia luteovirens modulate plant growth and metabolism in Arabidopsis thaliana. Plant Soil 2020, 456, 207–221. [Google Scholar] [CrossRef]
- Fratianni, S.; Acquaotta, F. The Climate of Italy. In Landscapes and Landforms of Italy; Soldati, M., Marchetti, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 29–38. [Google Scholar] [CrossRef]
- AMINT APS. Available online: https://www.funghiitaliani.it/ (accessed on 17 April 2024).
- ARPAE EMILIA-ROMAGNA. Available online: https://www.arpae.it/ (accessed on 21 February 2025).
- Google Earth. Available online: https://earth.google.com/web/ (accessed on 21 February 2025).
- ACTA PLANTARUM “IPFI: Index Plantarum”. Available online: https://www.actaplantarum.org/flora/flora.php (accessed on 17 April 2024).
- Braun-Blanquet, J. Plant Sociology; McGraw-Hill Book, Co.: New York, NY, USA, 1932. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie; Springer: Vienna, Austria, 1951. [Google Scholar] [CrossRef]
- Marx, D.H. The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 1969, 59, 153–163. [Google Scholar] [CrossRef]
- Wang, R.; Guerin-Laguette, A.; Huang, L.L.; Wang, X.H.; Butler, R.; Wang, Y.; Yu, F.Q. Mycorrhizal syntheses between Lactarius spp. section Deliciosi and Pinus spp. and the effects of grazing insects in Yunnan, China. Can. J. For. Res. 2019, 49, 616–627. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. Mol Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Iotti, M.; Zambonelli, A. A quick and precise technique for identifying ectomycorrhizas by PCR. Mycol. Res. 2006, 110, 60–65. [Google Scholar] [CrossRef]
- NCBI. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 14 January 2024).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Cao, L.; Qiu, Z.; You, J.; Tan, H.; Zhou, S. Isolation and characterization of endophytic Streptomyces strains from surface-sterilized tomato (Lycopersicon esculentum) roots. Lett. Appl. Microbiol. 2004, 39, 425–430. [Google Scholar] [CrossRef]
- Atak, A. Determination of Downy mildew and Powdery Mildew Resistance of Some Grape Cultivars and Genotypes. S. Afr. J. Enol. Vitic. 2017, 38, 11–17. [Google Scholar] [CrossRef][Green Version]
- Taksonyi, P.; Kocsis, L.; Mátyas, K.K.; Taller, J. The effect of quinone outside inhibitor fungicides on powdery mildew in a grape vineyard in Hungary. Sci. Hortic. 2013, 161, 233–238. [Google Scholar] [CrossRef]
- Townsend, G.R.; Heuberger, J.W. Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis. Rep. 1943, 27, 340–343. [Google Scholar]
- Righini, H.; Tedeschi, P.; Maietti, A.; Francioso, O.; Quintana, A.M.; Zuffi, V.; Ciurli, A.; Roberti, R. Enhanced Growth and Nutritional Properties of Radish Sprouts Using Extracts from Anabaena minutissima and Sargassum vulgare. ACS Agric. Sci. Technol. 2024, 4, 700–710. [Google Scholar] [CrossRef]
- Mugnai, L.; Surico, G. Produzione di enzimi esocellulari da parte di funghi del legno di viti colpite dal mal dell’esca. Micol. Ital. 1996, 26, 11–22. Available online: https://hdl.handle.net/2158/214097 (accessed on 21 February 2025).
- St Leger, R.J.; Joshi, L.; Roberts, D.W. Adaptation of proteases and carbohydrases of saprophytic, phytopathogenic and entomopathogenic fungi to the requirements of their ecological niches. Microbiology 1997, 143, 1983–1992. [Google Scholar] [CrossRef]
- Jiménez-Guerrero, I.; Pérez-Montaño, F.; Zdyb, A.; Beutler, M.; Werner, G.; Göttfert, M.; Ollero, F.J.; Vinardell, J.M.; López-Baena, F.J. GunA of Sinorhizobium (Ensifer) fredii HH103 is a T3SS-secreted cellulase that differentially affects symbiosis with cowpea and soybean. Plant Soil 2019, 435, 15–26. [Google Scholar] [CrossRef]
- Eriksson, K.; Pettersson, B. Extracellular Enzyme System Utilized by the Fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the Breakdown of Cellulose. Eur. J. Biochem. 1975, 51, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Ayers, W.; Papavizas, G.; Diem, A. Polygalacturonate trans-eliminase and polygalacruronase production by Rhizoctonia solani. Phytopathology 1966, 56, 1006–1011. [Google Scholar]
- Durrands, P.K.; Cooper, R.M. Selection and characterization of pectinase-deficient mutants of Verticillium albo-atrum. Physiol. Mol. Plant. Pathol. 1988, 32, 343–362. [Google Scholar] [CrossRef]
- Marchi, G.; Roberti, S.; D’Ovidio, R.; Surico, G. Pectic enzymes production by Phaeomoniella chlamydospora. Phytopathol. Mediterr. 2001, 40, S407–S416. Available online: https://digital.casalini.it/10.1400/14657 (accessed on 21 February 2025).
- Graziosi, S.; Puliga, F.; Iotti, M.; Amicucci, A.; Zambonelli, A. In vitro interactions between Bradyrhizobium spp. and Tuber magnatum mycelium. Environ. Microbiol. Rep. 2024, 16, e13271. [Google Scholar] [CrossRef]
- Tryfinopoulou, P.; Chourdaki, A.; Nychas, G.-J.E.; Panagou, E.Z. Competitive yeast action against Aspergillus carbonarius growth and ochratoxin A production. Int. J. Food Microbiol. 2020, 317, 108460. [Google Scholar] [CrossRef]
- Barrow, J.R.; Aaltonen, R. A staining method for systemic endophytic fungi in plants. Emerg. Concepts Plant Health Manag. Res. Signpost 2004, 61–67. [Google Scholar]

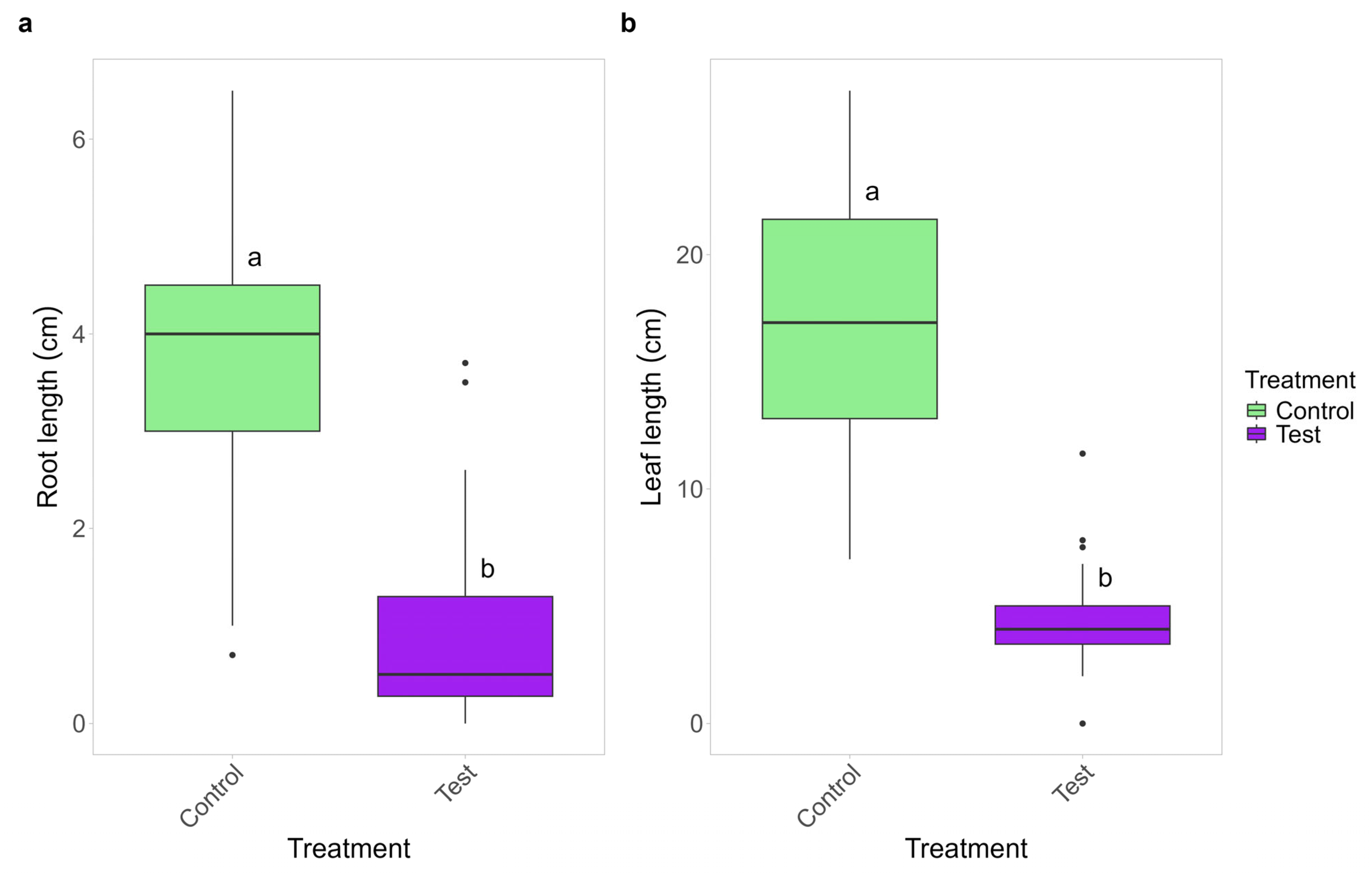


| Treatment | Average Weight of Dry Plant (g) | Chlorophyll-a (µg/mg) | Chlorophyll-b (µg/mg) | Carotenoid (µg/mg) |
|---|---|---|---|---|
| Control | 0.069 ± 0.0084 a | 1.17 ± 0.060 a | 0.57 ± 0.023 a | 0.18 ± 0.0079 a |
| Co-cultured | 0.024 ± 0.0012 b | 0.22 ± 0.017 b | 0.17 ± 0.017 b | 0.031 ± 0.0073 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graziosi, S.; Lombini, A.; Puliga, F.; Righini, H.; Dalla Pozza, L.; Zuffi, V.; Iotti, M.; Francioso, O.; Roberti, R.; Zambonelli, A. Analysis of Plant–Fungus Interactions in Calocybe gambosa Fairy Rings. Plants 2025, 14, 2884. https://doi.org/10.3390/plants14182884
Graziosi S, Lombini A, Puliga F, Righini H, Dalla Pozza L, Zuffi V, Iotti M, Francioso O, Roberti R, Zambonelli A. Analysis of Plant–Fungus Interactions in Calocybe gambosa Fairy Rings. Plants. 2025; 14(18):2884. https://doi.org/10.3390/plants14182884
Chicago/Turabian StyleGraziosi, Simone, Alessandra Lombini, Federico Puliga, Hillary Righini, Ludovico Dalla Pozza, Veronica Zuffi, Mirco Iotti, Ornella Francioso, Roberta Roberti, and Alessandra Zambonelli. 2025. "Analysis of Plant–Fungus Interactions in Calocybe gambosa Fairy Rings" Plants 14, no. 18: 2884. https://doi.org/10.3390/plants14182884
APA StyleGraziosi, S., Lombini, A., Puliga, F., Righini, H., Dalla Pozza, L., Zuffi, V., Iotti, M., Francioso, O., Roberti, R., & Zambonelli, A. (2025). Analysis of Plant–Fungus Interactions in Calocybe gambosa Fairy Rings. Plants, 14(18), 2884. https://doi.org/10.3390/plants14182884










