Bioponics in Tomato Cultivation Toward Sustainable Farming: Evaluation of a Circular Tri-Trophic System Incorporating Aquaponics and Insects
Abstract
1. Introduction
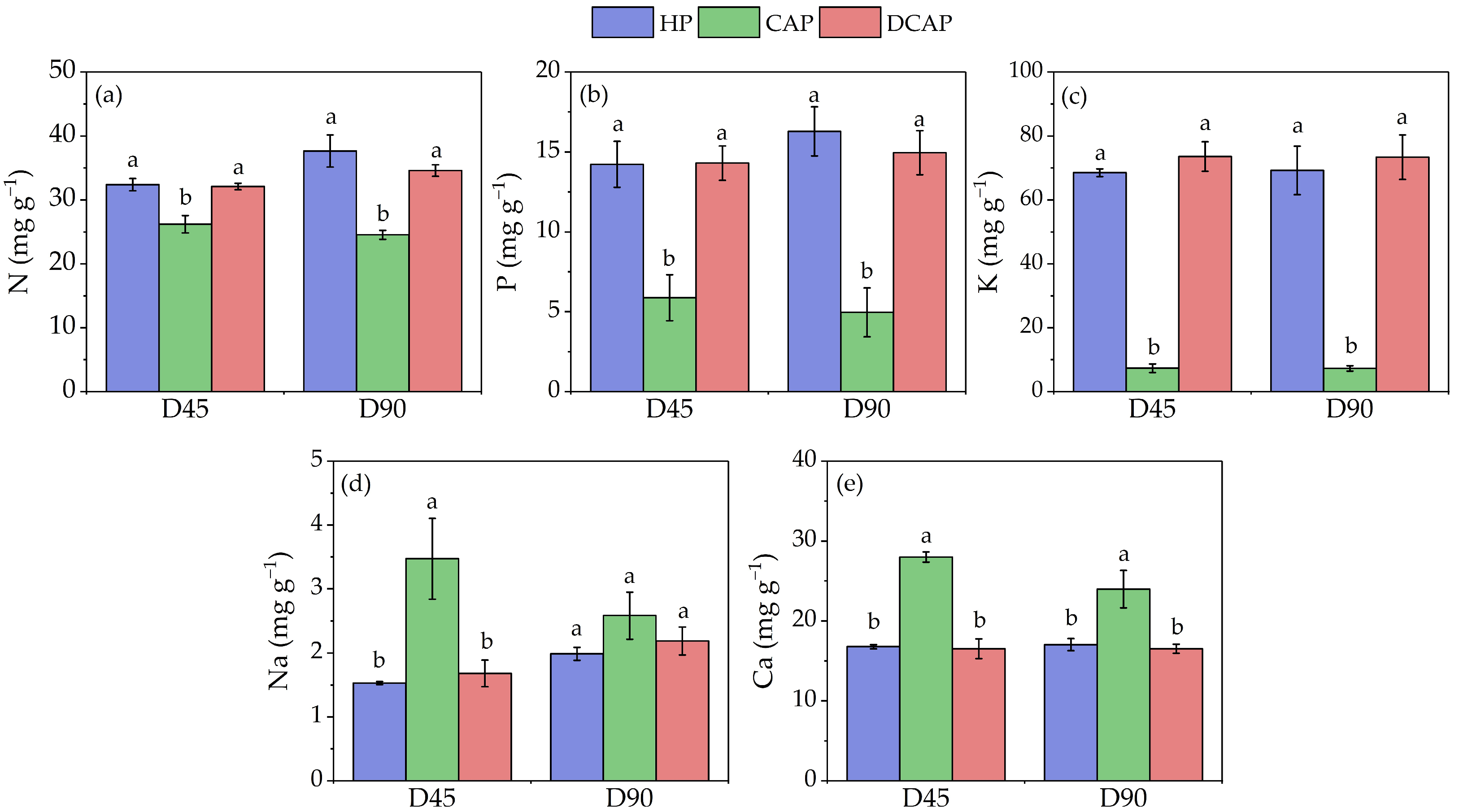
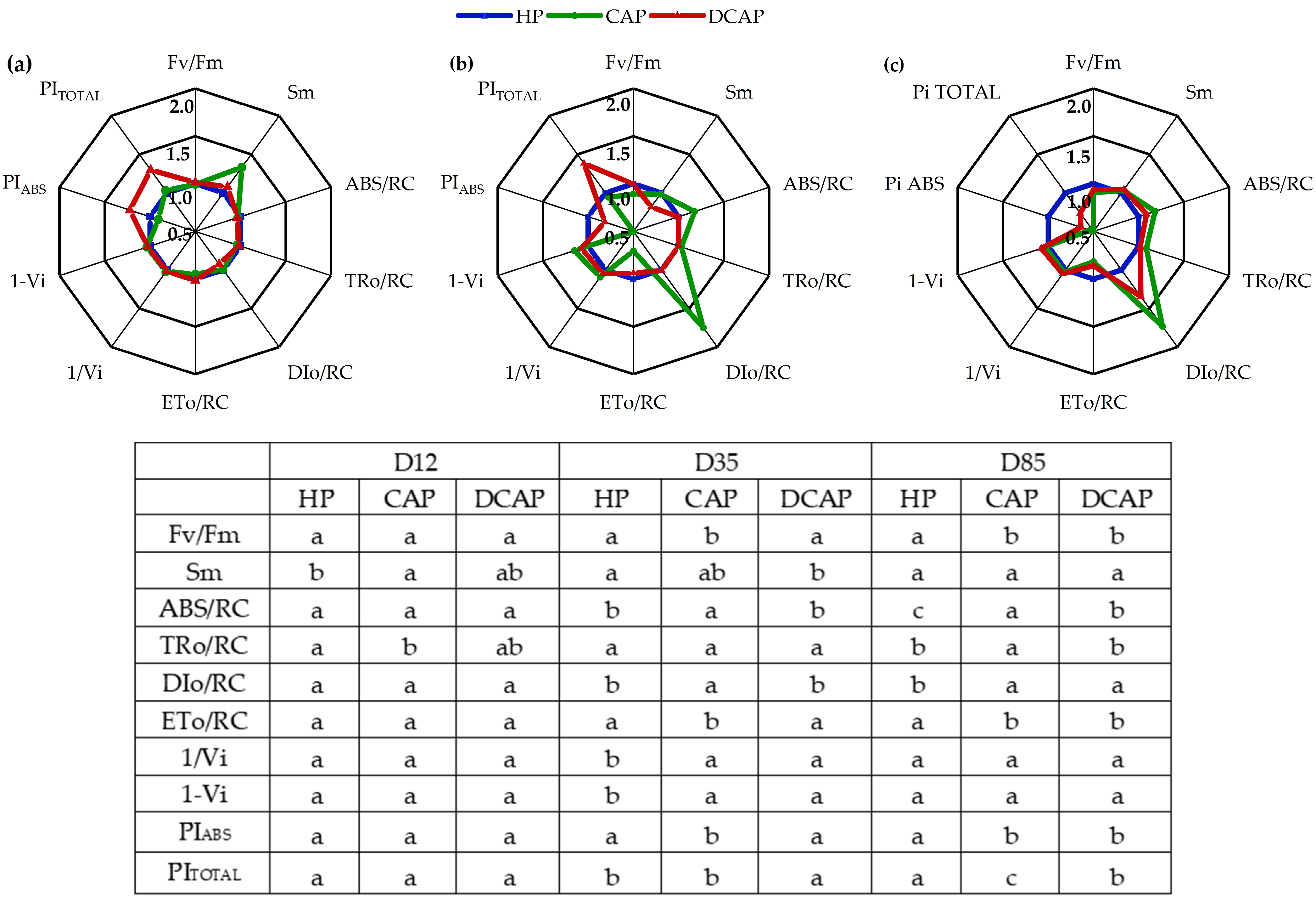
2. Results
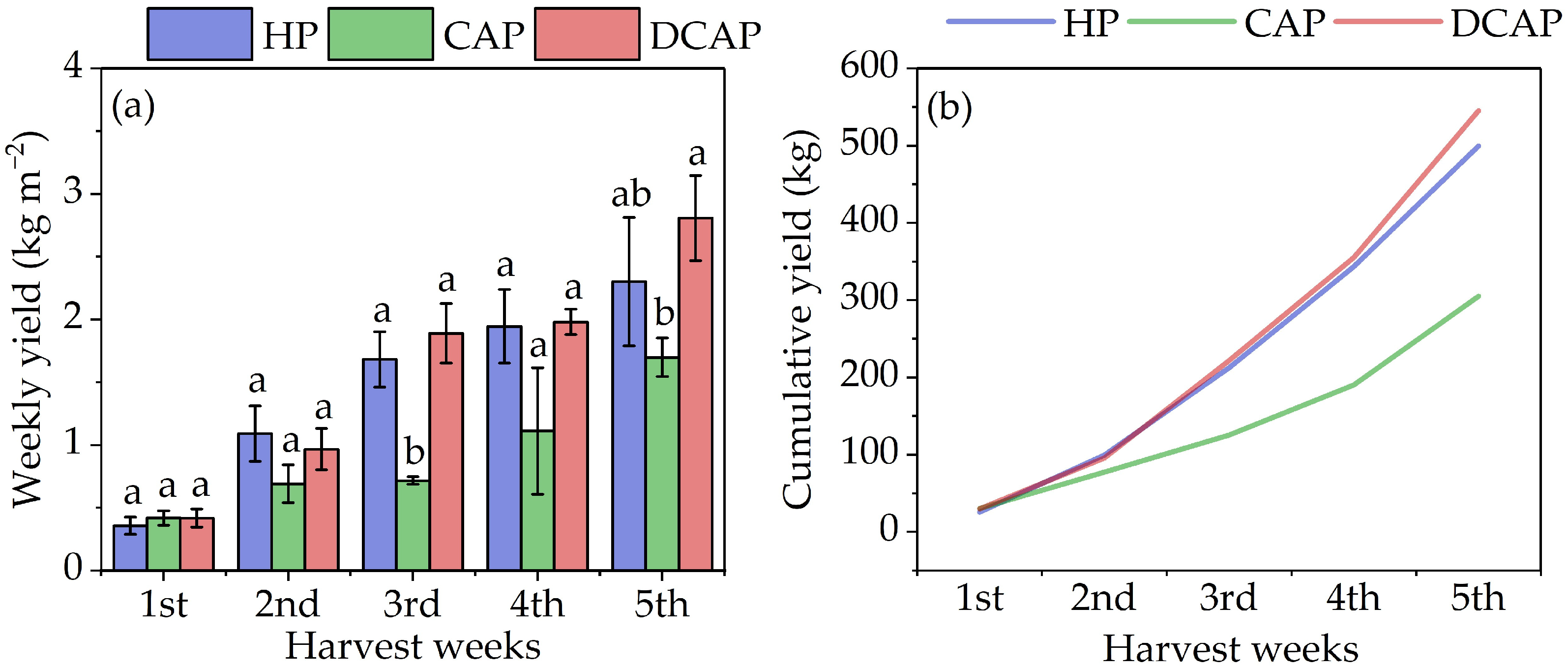
3. Discussion
4. Materials and Methods
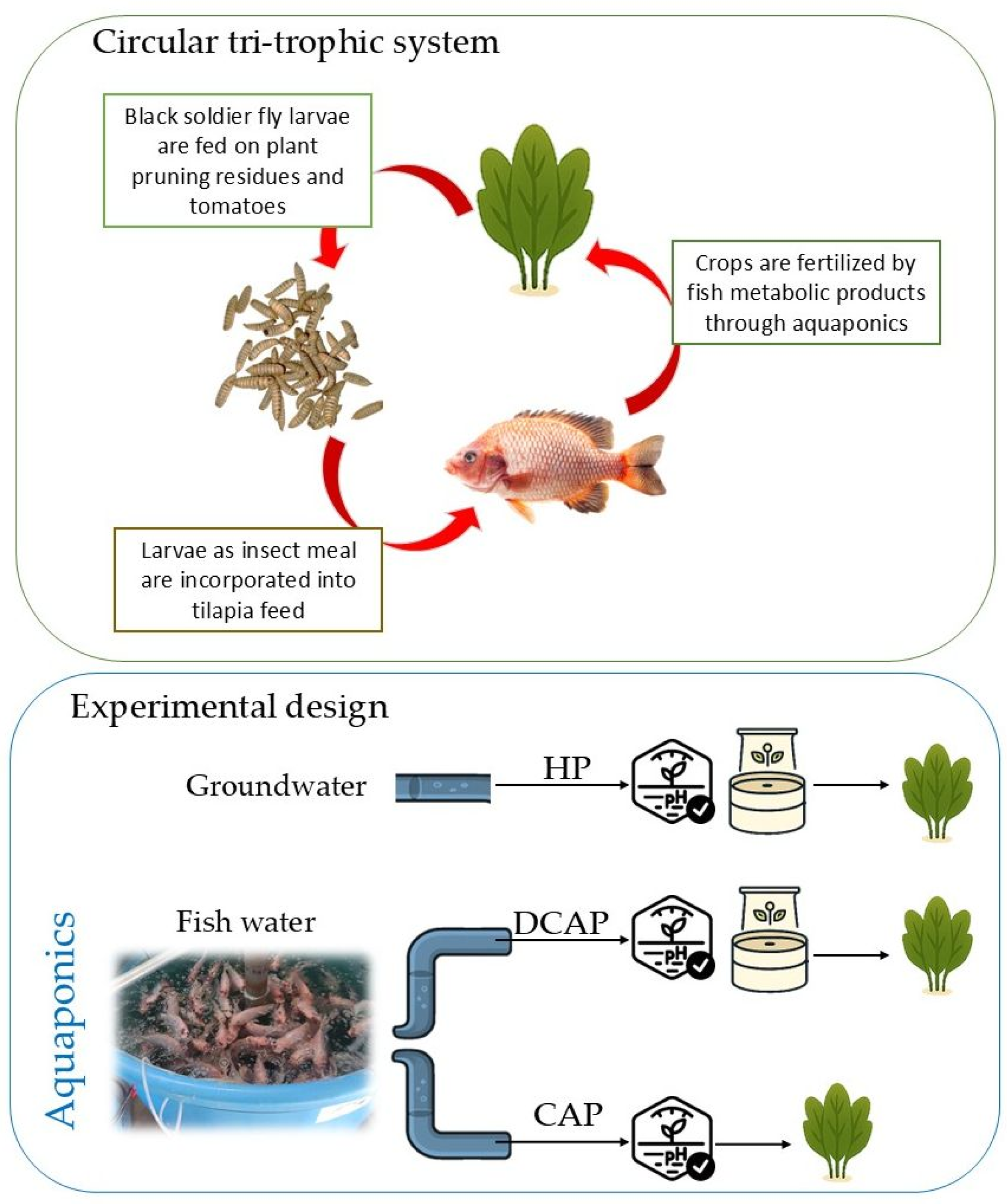
4.1. Experimental Setup
4.1.1. Recirculating Aquaculture System (RAS)
4.1.2. Insect Rearing
4.1.3. Crop Cultivation and Experimental Design
4.2. Physicochemical Parameters of the Irrigation Solutions
4.3. Crop Measurements
4.3.1. Plant Growth and Yield
4.3.2. Leaf Nutrient Concentration
4.3.3. Total Chlorophyll Content (SPAD Index)
4.3.4. Chlorophyll a Fluorescence In Vivo
4.3.5. Gas Exchange
4.4. Resource Use Efficiency Metrics
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marques, C.; Güneş, S.; Vilela, A.; Gomes, R. Life-Cycle Assessment in Agri-Food Systems and the Wine Industry—A Circular Economy Perspective. Foods 2025, 14, 1553. [Google Scholar] [CrossRef]
- Capaldi, G.; Binello, A.; Aimone, C.; Mantegna, S.; Grillo, G.; Cravotto, G. New Trends in Extraction-Process Intensification: Hybrid and Sequential Green Technologies. Ind. Crops Prod. 2024, 209, 117906. [Google Scholar] [CrossRef]
- Karachaliou, Z.; Naounoulis, I.; Katsoulas, N.; Levizou, E. Cascade Hydroponics as a Means to Increase the Sustainability of Cropping Systems: Evaluation of Functional, Growth, and Fruit Quality Traits of Melons. Sustainability 2025, 17, 4527. [Google Scholar] [CrossRef]
- Gruda, N.S.; Machado, R.M.A.; Van Os, E.A. Is Soilless Culture a Sustainable Form of Agriculture? Horticulturae 2023, 9, 1190. [Google Scholar] [CrossRef]
- Krastanova, M.; Sirakov, I.; Ivanova-Kirilova, S.; Yarkov, D.; Orozova, P. Aquaponic Systems: Biological and Technological Parameters. Biotechnol. Biotechnol. Equip. 2022, 36, 305–316. [Google Scholar] [CrossRef]
- Joyce, A.; Goddek, S.; Kotzen, B.; Wuertz, S. Aquaponics: Closing the Cycle on Limited Water, Land and Nutrient Resources. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 19–34. ISBN 978-3-030-15943-6. [Google Scholar]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. (Eds.) Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-15942-9. [Google Scholar]
- Tsoumalakou, E.; Mente, E.; Kormas, K.A.; Katsoulas, N.; Vlahos, N.; Kapsis, P.; Levizou, E. Precise Monitoring of Lettuce Functional Responses to Minimal Nutrient Supplementation Identifies Aquaponic System’s Nutrient Limitations and Their Time-Course. Agriculture 2022, 12, 1278. [Google Scholar] [CrossRef]
- Mourantian, A.; Aslanidou, M.; Mente, E.; Katsoulas, N.; Levizou, E. Basil Functional and Growth Responses When Cultivated via Different Aquaponic and Hydroponics Systems. PeerJ 2023, 11, e15664. [Google Scholar] [CrossRef] [PubMed]
- Monsees, H.; Suhl, J.; Paul, M.; Kloas, W.; Dannehl, D.; Würtz, S. Lettuce (Lactuca Sativa, Variety Salanova) Production in Decoupled Aquaponic Systems: Same Yield and Similar Quality as in Conventional Hydroponic Systems but Drastically Reduced Greenhouse Gas Emissions by Saving Inorganic Fertilizer. PLoS ONE 2019, 14, e0218368. [Google Scholar] [CrossRef]
- Aslanidou, M.; Elvanidi, A.; Mourantian, A.; Levizou, E.; Mente, E.; Katsoulas, N. Evaluation of Productivity and Efficiency of a Large-Scale Coupled or Decoupled Aquaponic System. Sci. Hortic. 2024, 337, 113552. [Google Scholar] [CrossRef]
- Rodgers, D.; Won, E.; Timmons, M.B.; Mattson, N. Complementary Nutrients in Decoupled Aquaponics Enhance Basil Performance. Horticulturae 2022, 8, 111. [Google Scholar] [CrossRef]
- Suhl, J.; Dannehl, D.; Kloas, W.; Baganz, D.; Jobs, S.; Scheibe, G.; Schmidt, U. Advanced Aquaponics: Evaluation of Intensive Tomato Production in Aquaponics vs. Conventional Hydroponics. Agric. Water Manag. 2016, 178, 335–344. [Google Scholar] [CrossRef]
- Kaushik, S.J.; Seiliez, I. Protein and Amino Acid Nutrition and Metabolism in Fish: Current Knowledge and Future Needs. Aquac. Res. 2010, 41, 322–332. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Neofytou, M.C.; Asimaki, A.; Daskalopoulou, E.; Psofakis, P.; Mente, E.; Rumbos, C.I.; Athanassiou, C.G. Fishmeal Replacement by Full-Fat and Defatted Hermetia illucens Prepupae Meal in the Diet of Gilthead Seabream (Sparus aurata). Sustainability 2023, 15, 786. [Google Scholar] [CrossRef]
- Henry, M.A.; Golomazou, E.; Asimaki, A.; Psofakis, P.; Fountoulaki, E.; Mente, E.; Rumbos, C.I.; Athanassiou, C.G.; Karapanagiotidis, I.T. Partial Dietary Fishmeal Replacement with Full-Fat or Defatted Superworm (Zophobas morio) Larvae Meals Modulates the Innate Immune System of Gilthead Seabream, Sparus Aurata. Aquac. Rep. 2022, 27, 101347. [Google Scholar] [CrossRef]
- Bordignon, F.; Trocino, A.; Gasco, L.; Oddon, S.B.; Xiccato, G.; Berton, M. Towards Circularity in Aquaculture Systems: Environmental Impact of Hermetia illucens Meal Inclusion in Diets for Rainbow Trout Reared in Aquaponics. J. Clean. Prod. 2024, 466, 142901. [Google Scholar] [CrossRef]
- Stathopoulou, P.; Asimaki, A.; Berillis, P.; Vlahos, N.; Levizou, E.; Katsoulas, N.; Karapanagiotidis, I.T.; Rumbos, C.I.; Athanassiou, C.G.; Mente, E. Aqua-Ento-Ponics: Effect of Insect Meal on the Development of Sea Bass, Dicentrarchus Labrax, in Co-Culture with Lettuce. Fishes 2022, 7, 397. [Google Scholar] [CrossRef]
- Quang Tran, H.; Van Doan, H.; Stejskal, V. Environmental Consequences of Using Insect Meal as an Ingredient in Aquafeeds: A Systematic View. Rev. Aquac. 2022, 14, 237–251. [Google Scholar] [CrossRef]
- Matsakidou, A.; Sarivasiliou, S.-I.; Pissia, M.-A.; Rumbos, C.I.; Athanassiou, C.G.; Paraskevopoulou, A. Compositional, Volatile, and Structural Features of Hermetia illucens (Black Soldier Fly) Flours: The Effect of Population and Life Stages. Future Foods 2024, 9, 100320. [Google Scholar] [CrossRef]
- Kuan, Z.-J.; Chan, B.K.-N.; Gan, S.K.-E. Worming the Circular Economy for Biowaste and Plastics: Hermetia illucens, Tenebrio Molitor, and Zophobas Morio. Sustainability 2022, 14, 1594. [Google Scholar] [CrossRef]
- Mielcarek, A.; Kłobukowska, K.; Rodziewicz, J.; Janczukowicz, W.; Bryszewski, K.Ł. Water Nutrient Management in Soilless Plant Cultivation Versus Sustainability. Sustainability 2023, 16, 152. [Google Scholar] [CrossRef]
- Zhu, Z.; Yogev, U.; Keesman, K.J.; Gross, A. Promoting Circular Economy: Comparison of Novel Coupled Aquaponics with Anaerobic Digestion and Conventional Aquaponic Systems on Nutrient Dynamics and Sustainability. Resour. Conserv. Recycl. 2024, 208, 107716. [Google Scholar] [CrossRef]
- Tsoumalakou, E.; Mente, E.; Vlahos, N.; Levizou, E. Spinach Responds to Minimal Nutrient Supplementation in Aquaponics by Up-Regulating Light Use Efficiency, Photochemistry, and Carboxylation. Horticulturae 2023, 9, 291. [Google Scholar] [CrossRef]
- Bittsánszky, A.; Uzinger, N.; Gyulai, G.; Mathis, A.; Junge, R.; Villarroel, M.; Kotzen, B.; Kőmíves, T. Nutrient Supply of Plants in Aquaponic Systems. Ecocycles 2016, 2, 17–20. [Google Scholar] [CrossRef]
- Khater, E.-S.; Bahnasawy, A.; Mosa, H.; Abbas, W.; Morsy, O. Nutrient Supply Systems and Their Effect on the Performance of the Nile Tilapia (Oreochromis niloticus) and Lettuce (Lactuca sativa) Plant Integration System. Sci. Rep. 2024, 14, 4229. [Google Scholar] [CrossRef] [PubMed]
- Tellbüscher, A.A.; Gebauer, R.; Mráz, J. Nutrients Revisited: Review and Meta-Data Analysis of Nutrient Inputs into Freshwater Aquaculture Systems. Aquaculture 2025, 595, 741633. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of Nutrient Deficiency in Maize and Tomato Plants by In Vivo Chlorophyll a Fluorescence Measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Leal, M.M.; Shaw, C.; Baganz, D.; Baganz, G.; Staaks, G.; Kloas, W.; Körner, O.; Monsees, H. Insect-Based Fish Feed in Decoupled Aquaponic Systems: Effect on Lettuce Production and Resource Use. PLoS ONE 2024, 19, e0295811. [Google Scholar] [CrossRef]
- Zeng, Q.; Ao, J.; Ling, Q.; Huang, Y.; Li, Q. Effects of K-Deficiency Stress on the Root Morphology and Nutrient Efficiency of Sugarcane. J. Plant Nutr. 2018, 41, 1425–1435. [Google Scholar] [CrossRef]
- Patel, M.; Fatnani, D.; Parida, A.K. Potassium Deficiency Stress Tolerance in Peanut (Arachis hypogaea) through Ion Homeostasis, Activation of Antioxidant Defense, and Metabolic Dynamics: Alleviatory Role of Silicon Supplementation. Plant Physiol. Biochem. 2022, 182, 55–75. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Nahar, K.; Hossain, M.d.; Mahmud, J.; Hossen, M.d.; Masud, A.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Waqas, M.; Yaning, C.; Iqbal, H.; Shareef, M.; Rehman, H.U.; Bilal, H.M. Synergistic Consequences of Salinity and Potassium Deficiency in Quinoa: Linking with Stomatal Patterning, Ionic Relations and Oxidative Metabolism. Plant Physiol. Biochem. 2021, 159, 17–27. [Google Scholar] [CrossRef]
- Chatzinikolaou, M.; Mourantian, A.; Feka, M.; Levizou, E. Lettuce Performance in a Tri-Trophic System Incorporating Crops, Fish and Insects Confirms the Feasibility of Circularity in Agricultural Production. Agronomy 2025, 15, 1782. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium Influences on Yield and Quality Production for Maize, Wheat, Soybean and Cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Bouain, N.; Krouk, G.; Lacombe, B.; Rouached, H. Getting to the Root of Plant Mineral Nutrition: Combinatorial Nutrient Stresses Reveal Emergent Properties. Trends Plant Sci. 2019, 24, 542–552. [Google Scholar] [CrossRef]
- Nozzi, V.; Graber, A.; Schmautz, Z.; Mathis, A.; Junge, R. Nutrient Management in Aquaponics: Comparison of Three Approaches for Cultivating Lettuce, Mint and Mushroom Herb. Agronomy 2018, 8, 27. [Google Scholar] [CrossRef]
- Roosta, H.R. Effects of Foliar Spray of K on Mint, Radish, Parsley and Coriander Plants in Aquaponic System. J. Plant Nutr. 2014, 37, 2236–2254. [Google Scholar] [CrossRef]
- Rurangwa, E.; Verdegem, M.C.J. Microorganisms in Recirculating Aquaculture Systems and Their Management. Rev. Aquac. 2015, 7, 117–130. [Google Scholar] [CrossRef]
- Schmautz, Z.; Graber, A.; Jaenicke, S.; Goesmann, A.; Junge, R.; Smits, T.H.M. Microbial Diversity in Different Compartments of an Aquaponics System. Arch. Microbiol. 2017, 199, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Grunert, O.; Hernandez-Sanabria, E.; Buysens, S.; De Neve, S.; Van Labeke, M.-C.; Reheul, D.; Boon, N. In-Depth Observation on the Microbial and Fungal Community Structure of Four Contrasting Tomato Cultivation Systems in Soil Based and Soilless Culture Systems. Front. Plant Sci. 2020, 11, 520834. [Google Scholar] [CrossRef]
- Chondrogiannis, C.; Grammatikopoulos, G. Photosynthesis in Developing Leaf of Juveniles and Adults of Three Mediterranean Species with Different Growth Forms. Photosynth. Res. 2016, 130, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Roosta, H.R.; Estaji, A.; Niknam, F. Effect of Iron, Zinc and Manganese Shortage-Induced Change on Photosynthetic Pigments, Some Osmoregulators and Chlorophyll Fluorescence Parameters in Lettuce. Photosynthetica 2018, 56, 606–615. [Google Scholar] [CrossRef]
- Kanai, S.; Moghaieb, R.E.; El-Shemy, H.A.; Panigrahi, R.; Mohapatra, P.K.; Ito, J.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Potassium Deficiency Affects Water Status and Photosynthetic Rate of the Vegetative Sink in Green House Tomato Prior to Its Effects on Source Activity. Plant Sci. 2011, 180, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Aslanidou, M.; Elvanidi, A.; Mourantian, A.; Levizou, E.; Mente, E.; Katsoulas, N. Nutrients Use Efficiency in Coupled and Decoupled Aquaponic Systems. Horticulturae 2023, 9, 1077. [Google Scholar] [CrossRef]
- Organisation des Nations Unies pour L’alimentation et L’agriculture; Société Internationale de la Science Horticole; Centre National pour la Recherche Agricole et la Vulgarisation (Eds.) Good Agricultural Pratices for Greenhouse Vegetable Crops: Principles for Mediterranean Climate Areas; FAO Plant Production and Protection Paper; FAO: Rome, Italy, 2013; ISBN 978-92-5-107649-1. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. ISBN 978-1-4020-3217-2. [Google Scholar]



| pH | EC | NO3− | PO43− | K+ | Ca2+ | Na+ | |
|---|---|---|---|---|---|---|---|
| HP | 5.70 ± 0.03 b | 2.54 ± 0.07 a | 12.99 ± 0.32 a | 1.07 ± 0.04 a | 6.09 ± 0.21 a | 4.04 ± 1.00 a | 1.61 ± 0.06 a |
| CAP | 6.17 ± 0.20 a | 1.15 ± 0.03 b | 6.22 ± 0.48 b | 0.64 ± 0.10 b | 0.05 ± 0.03 b | 0.22 ± 0.01 b | 0.13 ± 0.00 b |
| DCAP | 5.78 ± 0.06 ab | 2.41 ± 0.03 a | 12.54 ± 0.52 a | 1.12 ± 0.05 a | 6.67 ± 0.05 a | 4.07 ± 0.08 a | 1.70 ± 0.05 a |
| WUE (kg Tomato m−3 Water Used) | FUE (kg Tomato kg−1 Fertilizers Used) | |
|---|---|---|
| HP | 14.82 | 11.17 |
| CAP | 11.27 | |
| DCAP | 15.91 | 16.30 |
| Macronutrients | Concentration (mmol/L) | Micronutrients | Concentration (μmol/L) | ||
|---|---|---|---|---|---|
| Veg. Stage | Rep. Stage | Veg. Stage | Rep. Stage | ||
| NO3- | 14 | 14.5 | Fe | 15 | 20 |
| NH4+ | 0.7 | 1.3 | B | 35 | 40 |
| P | 1.2 | 1.4 | Cu | 0.8 | 0.8 |
| K | 7 | 6.8 | Mn | 5 | 6 |
| Ca | 5.1 | 5 | Zn | 10 | 12 |
| Mg | 2.4 | 3 | Mo | 0.5 | 0.5 |
| S | 3.6 | 4.5 | |||
| Fluorescence Parameters | |
|---|---|
| FM | Maximal fluorescence from a dark-adapted leaf |
| FV | Maximal variable fluorescence from a dark-adapted leaf. FV = FM − F0 |
| FV/FM | Maximum quantum efficiency of PSII photochemistry |
| Vi | Relative variable fluorescence at phase I of the fluorescence induction curve |
| 1-Vi | Measure of relative amplitude of the IP phase in OJIP transient, related to the size of the pools of final PSI electron acceptors |
| 1/Vi | Relative measure of the pool size of final electron acceptors of PSI |
| ABS/RC | Absorption flux (for PSII antenna chls) per reaction center (RC) |
| TR0/RC | Trapped energy flux per RC (at t = 0) |
| DI0/RC | Dissipated energy flux per RC (at t = 0) |
| PΙTOTAL | Performance index total for energy conservation from photons absorbed by PSII to the reduction of PSI end acceptors |
| PΙABS | Performance index for energy conservation from photons absorbed by PSII antenna |
| Sm | Normalized area above the OJIP curve |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourantian, A.; Chatzinikolaou, M.; Feka, M.; Levizou, E. Bioponics in Tomato Cultivation Toward Sustainable Farming: Evaluation of a Circular Tri-Trophic System Incorporating Aquaponics and Insects. Plants 2025, 14, 2882. https://doi.org/10.3390/plants14182882
Mourantian A, Chatzinikolaou M, Feka M, Levizou E. Bioponics in Tomato Cultivation Toward Sustainable Farming: Evaluation of a Circular Tri-Trophic System Incorporating Aquaponics and Insects. Plants. 2025; 14(18):2882. https://doi.org/10.3390/plants14182882
Chicago/Turabian StyleMourantian, Anastasia, Michalis Chatzinikolaou, Maria Feka, and Efi Levizou. 2025. "Bioponics in Tomato Cultivation Toward Sustainable Farming: Evaluation of a Circular Tri-Trophic System Incorporating Aquaponics and Insects" Plants 14, no. 18: 2882. https://doi.org/10.3390/plants14182882
APA StyleMourantian, A., Chatzinikolaou, M., Feka, M., & Levizou, E. (2025). Bioponics in Tomato Cultivation Toward Sustainable Farming: Evaluation of a Circular Tri-Trophic System Incorporating Aquaponics and Insects. Plants, 14(18), 2882. https://doi.org/10.3390/plants14182882







