Chemotyping of Koelreuteria paniculata Seed Cake with Bioactive and Feed Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. Basic Composition
2.2. Amino Acid Content
2.3. Fatty Acid Content
2.4. Composition of Individual Polyphenols
2.5. Antioxidative Potential
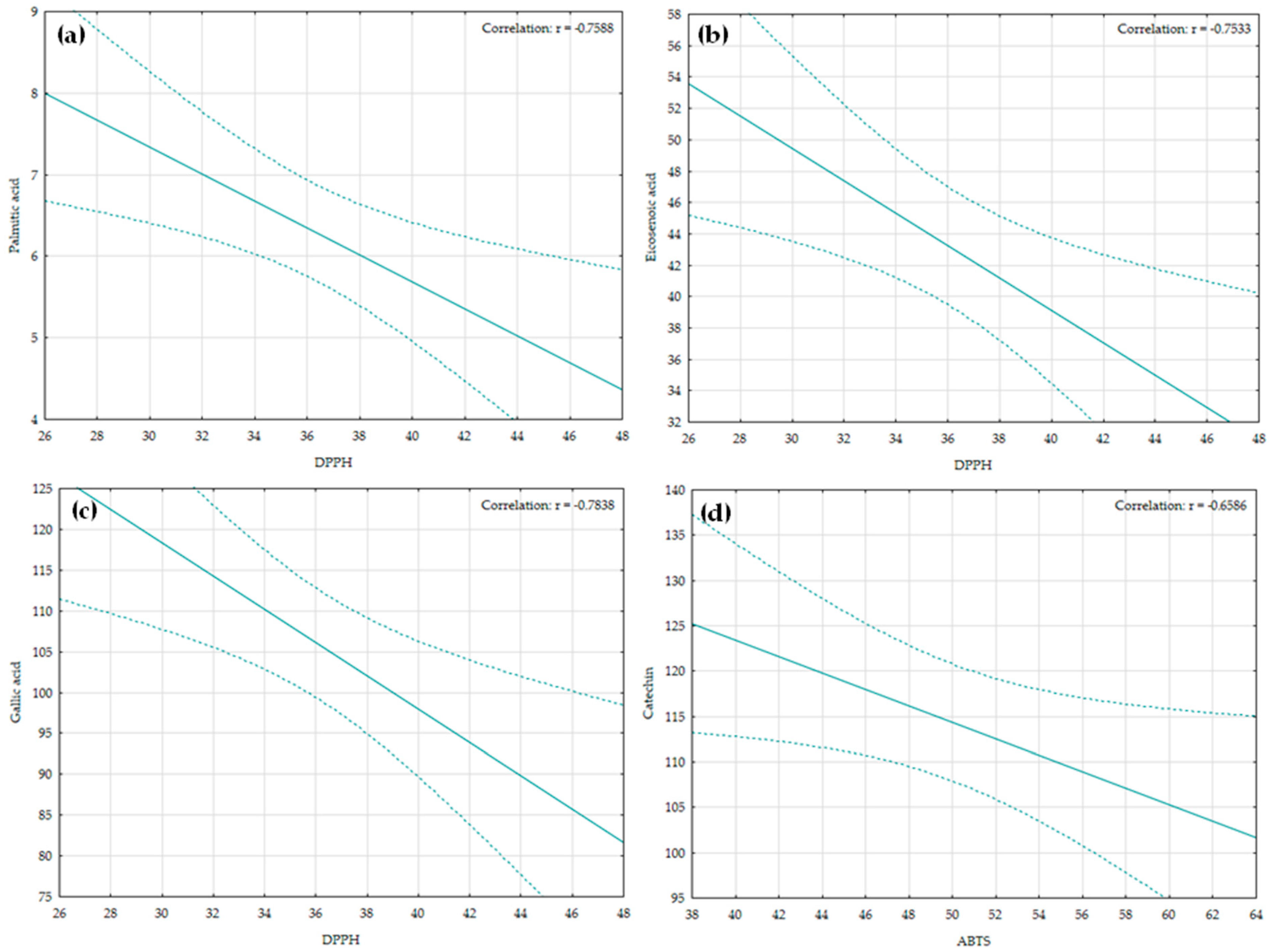
2.6. Correlation Analyses Within and Between Chemical and Phytochemical Parameters and Antioxydant Activity
3. Materials and Methods
3.1. Seed Cake Harnessing
3.2. Basic Chemical Composition and Amino Acid Content
3.3. Fatty Acid Content Analysis
3.4. HPLC Analysis
3.5. Antioxidative Activity
3.5.1. DPPH Radical Scavenging Assay
3.5.2. ABTS Radical Scavenging Activity Assay
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mostafa, A.E.; El-Hela, A.A.; Mohammad, A.-E.I.; Cutler, S.J.; Ross, S.A. New Triterpenoidal Saponins from Koelreuteria paniculata. Phytochem. Lett. 2016, 17, 213–218. [Google Scholar] [CrossRef]
- Shin, H.; Kwon, H.Y.; Choi, Y.I. Morphological characterization of inflorescence and trunk in golden-rain trees (Koelreuteria paniculata Laxm.). Acta Hortic. 2023, 1383, 345–348. [Google Scholar] [CrossRef]
- Ljubojević, M.; Tomić, M.; Simikić, M.; Savin, L.; Narandžić, T.; Pušić, M.; Grubač, M.; Vejnović, S.; Marinković, M. Koelreuteria paniculata invasiveness, yielding capacity and harvest date influence on biodiesel feedstock properties. J. Environ. Manag. 2021, 295, 113102. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y.; Zhang, Z.F.; Kou, Y.R. Nutritional characteristics of crude fat, crude protein and crude fiber in the fruits of Koelreuteria paniculata Laxm. Nonwood For. Res. 2009, 3, 61–65. [Google Scholar]
- Andonova, T.; Dimitrova-Dyulgerova, I.; Slavov, I.; Muhovski, Y.; Stoyanova, A. A comparative study of Koelreuteria paniculata Laxm. aerial parts essential oil composition. J. Essent. Oil Bear. Plants 2020, 23, 1363–1370. [Google Scholar] [CrossRef]
- Andonova, T.; Muhovski, Y.; Fidan, H.; Slavov, I.; Stoyanova, A.; Dimitrova-Dyulgerova, I. Chemical Compounds, Antitumor and Antimicrobial Activities of Dry Ethanol Extracts from Koelreuteria paniculata Laxm. Plants 2021, 10, 2715. [Google Scholar] [CrossRef]
- Andonova, T.; Muhovski, Y.; Vrancheva, R.; Slavov, I.; Apostolova, E.; Naimov, S.; Pavlov, A.; Dimitrova-Dyulgerova, I. Antioxidant and DNA-Protective Potentials, Main Phenolic Compounds, and Microscopic Features of Koelreuteria paniculata Aerial Parts. Antioxidants 2022, 11, 1154. [Google Scholar] [CrossRef]
- Andonova, T.; Muhovski, Y.; Apostolova, E.; Naimov, S.; Petkova, Z.; Teneva, O.; Antova, G.; Slavov, I.; Dimitrova-Dyulgerova, I. Koelreuteria paniculata Seed Oil—A Rich Natural Source of Unsaturated Fatty Acids and Phytocompounds with DNA Protective Potential. Foods 2023, 12, 2230. [Google Scholar] [CrossRef]
- Khan, I.U.; Yan, Z.; Chen, J. Production and Characterization of Biodiesel Derived from a Novel Source Koelreuteria paniculata Seed Oil. Energies 2020, 13, 791. [Google Scholar] [CrossRef]
- Tomić, M.; Ljubojević, M.; Mićić, R.; Simikić, M.; Dulić, J.; Narandžić, T.; Čukanović, J.; Sentić, I.; Dedović, N. Oil from Koelreuteria paniculata Laxm. 1772 as Possible Feedstock for Biodiesel Production. Fuel 2020, 277, 118162. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Wang, Y.; Liu, T.; Li, Z.; Jiang, L. Development, characterization and application of chitosan/locust bean gum based multifunctional green food packaging containing Koelreuteria paniculata Laxm. bracts extract and Ti-carbon dots. Int. J. Biol. Macromol. 2024, 278, 134610. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, L.; Chen, C.H.; Zhang, Y.Y.; Yang, Y.; Zhang, P.; Bao, G.H. Chemical Composition and Antibacterial Activity of 12 Medicinal Plant Ethyl Acetate Extracts Using LC–MS Feature-Based Molecular Networking. Phytochem. Anal. 2022, 33, 473–489. [Google Scholar] [CrossRef]
- Šarac, V.; Narandžić, T.; Rodić, V.; Popović, B.M.; Uka, D.; Tomaš Simin, M.; Ljubojević, M. Harnessing Koelreuteria paniculata Seed Extracts and Oil for Sustainable Woolly Apple Aphid Control. Horticulturae 2024, 10, 826. [Google Scholar] [CrossRef]
- Mahmoud, I.; Moharram, F.A.; Marzouk, M.S.; Soliman, H.S.; El-Dib, R.A. Two New Flavonol Glycosides from Leaves of Koelreuteria paniculata. Die Pharm. 2001, 56, 580–582. [Google Scholar]
- Seigler, D.S.; Butterfield, C.S. The Origin of Cyanolipids in Koelreuteria paniculata. Phytochemistry 1976, 15, 842–844. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Feed Demand Landscape and Implications of Food-Not Feed Strategy for Food Security and Climate Change. Animal 2018, 12, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Nehra, S.; Deen, M.K. Screening of Chinese Rain Tree (Koelreuteria elegans) Leaves, Bark and Defatted Seed Cake for Study of Their Total Phenolic Content, Flavonoids and Antioxidant Properties. J. Pharm. Phytopharmacol. 2019, 8, 1724–1728. [Google Scholar]
- Vichare, S.A.; Morya, S. Exploring waste utilization potential: Nutritional, functional and medicinal properties of oilseed cakes. Front. Food Sci. Technol. 2024, 4, 1441029. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.D. Dietary Fiber Utilization and Its Effects on Physiological Functions and Gut Health of Swine. Animal 2015, 9, 1441–1452. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.-Y.; Lin, L.-J.; Shih, H.-D.; Shy, Y.-M.; Chang, S.-C.; Lee, T.-T. The Potential Utilization of High-Fiber Agricultural By-Products as Monogastric Animal Feed and Feed Additives: A Review. Animals 2021, 11, 2098. [Google Scholar] [CrossRef]
- Aruwayo, A.; Maigandi, S.A. Neem (Azadirachta indica) Seed Cake/Kernel as Protein Source in Ruminants Feed. Am. J. Exp. Agric. 2013, 3, 482–494. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, V.; Thakur, A. An overview of anti-nutritional factors in food. Int. J. Chem. Stud. 2019, 7, 2472–2479. [Google Scholar]
- Kholif, A.E. A Review of Effect of Saponins on Ruminal Fermentation, Health and Performance of Ruminants. Vet. Sci. 2023, 10, 450. [Google Scholar] [CrossRef]
- Matthäus, B.; Zubr, J. Variability of Specific Components in Camelina sativa Oilseed Cakes. Ind. Crops Prod. 2000, 12, 9–18. [Google Scholar] [CrossRef]
- Ilić, P.N.; Rakita, S.M.; Spasevski, N.J.; Đuragić, O.M.; Marjanović Jeromela, A.M.; Cvejić, S.; Zanetti, F. Nutritivna Vrednost Srpskih Genotipova Lanika kao Alternativnih Sastojaka Hrane za Životinje. Food Feed Res. 2022, 49, 209–221. [Google Scholar] [CrossRef]
- Cardoso-Gutierrez, E.; Aranda-Aguirre, E.; Robles-Jimenez, L.E.; Castelán-Ortega, O.A.; Chay-Canul, A.J.; Foggi, G.; Angeles-Hernandez, J.C.; Vargas-Bello-Pérez, E.; González-Ronquillo, M. Effect of tannins from tropical plants on methane production from ruminants: A systematic review. Vet. Anim. Sci. 2021, 14, 100214. [Google Scholar] [CrossRef] [PubMed]
- Vujetić, J.C.; Spasevski, N.J.; Dragojlović, D.M. Tehnike Obrade Uklanjanja Antinutrijenata iz Uljanih Pogača kao Nusproizvoda koji se Koriste u Ishrani Životinja. Food Feed Res. 2025, 52, 37–51. [Google Scholar] [CrossRef]
- Maphosa, Y.; Jideani, V.A. Chapter 6: The Role of Legumes in Human Nutrition. In Functional Food—Improve Health Through Adequate Food; Hueda, M.C., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Manzoor, M.; Singh, D.; Aseri, G.K.; Sohal, J.S.; Vij, S.; Sharma, D. Role of Lacto-Fermentations in Reduction of Antinutrients in Plant-Based Foods. J. Appl. Biol. Biotechnol. 2021, 9, 7–16. [Google Scholar]
- Ancuța, P.; Sonia, A. Oil Press-Cakes and Meals Valorization through Circular Economy Approaches: A Review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Rakita, S.; Kokić, B.; Manoni, M.; Mazzoleni, S.; Lin, P.; Luciano, A.; Ottoboni, M.; Cheli, F.; Pinotti, L. Cold-Pressed Oilseed Cakes as Alternative and Sustainable Feed Ingredients: A Review. Foods 2023, 12, 432. [Google Scholar] [CrossRef]
- Kokić, B.; Rakita, S.; Vujetić, J. Impact of Using Oilseed Industry Byproducts Rich in Linoleic and Alpha-Linolenic Acid in Ruminant Nutrition on Milk Production and Milk Fatty Acid Profile. Animals 2024, 14, 539. [Google Scholar] [CrossRef]
- Maciorowski, K.G.; Herrera, P.; Jones, F.T.; Pillai, S.D.; Ricke, S.C. Effects on Poultry and Livestock of Feed Contamination with Bacteria and Fungi. Anim. Feed Sci. Technol. 2007, 133, 109–136. [Google Scholar] [CrossRef]
- Mahala, D.M.; Sharma, S.K.; Singh, U.B.; Sahu, P.K.; Singh, H.V.; Sharma, P.K. Microbial Transformation of Nutrients in Soil: An Overview. In Rhizosphere Microbes. Microorganisms for Sustainability; Sharma, S.K., Singh, U.B., Sahu, P.K., Singh, H.V., Sharma, P.K., Eds.; Springer: Singapore, 2020; Volume 23. [Google Scholar]
- Weimer, P.J. Degradation of Cellulose and Hemicellulose by Ruminal Microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Senarathna, S.C.; Malalgoda, M. Impact of Defatting Method on Oat Protein Isolate Structure–Function Characteristics. J. Cereal Sci. 2024, 117, 103876. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.; Xie, D.; Zheng, L.; Liu, X.; Wang, Z. The Underlying Reasons for the Efficient Extraction of Peanut Oil by Aqueous Ethanol Combined with Roasting Conditioning Pretreatment. Food Chem. 2024, 447, 138934. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, Z.; Spasevski, N.; Popović, S.; Banjac, V.; Đuragić, O.; Tomičić, R. By-Products of the Oil Industry as Sources of Amino Acids in Feed. Food Feed Res. 2020, 47, 131–137. [Google Scholar] [CrossRef]
- Lin, X.; Ruan, D.; Lin, Z.; Xiong, T.; Zhang, S.; Fan, Q.; Dong, X.; Deng, Y.; Jiang, Z.; Jiang, S. Effects of L-Methionine and DL-Methionine on Growth Performance, Methionine-Metabolizing Enzyme Activities, Feather Traits, and Intestinal Morphology of Medium-Growing, Yellow-Feathered Chickens between 1 and 30 Days of Age. Animals 2024, 14, 2135. [Google Scholar] [CrossRef]
- Yang, B.; Shen, Y.; Monroig, Ó.; Zhao, W.; Bao, Y.; Tao, S.; Jiao, L.; Zhou, Q.; Jin, M. The Ameliorative Role of Methionine in Hepatic Steatosis and Stress Response in Juvenile Black Seabream (Acanthopagrus schlegelii) Fed with a High-Fat Diet. Aquaculture 2024, 580, 740306. [Google Scholar] [CrossRef]
- Wan, M.; Yin, Y.; Duan, Y.; Chen, J. Methionine Metabolism, Functions, and Application in Swine. Anim. Nutr. 2025, in press. [Google Scholar] [CrossRef]
- Matthews, D.E. Review of lysine metabolism with a focus on humans. J. Nutr. 2020, 150, 2548S–2555S. [Google Scholar] [CrossRef]
- Monteiro, R.; Sousa, A.M.; Pereira, M.O. Aspartic Acid Unveils as Antibiofilm Agent and Tobramycin Adjuvant against Mucoid and Small Colony Variants of Pseudomonas aeruginosa Isolates In Vitro within Cystic Fibrosis Airway Mucus. Biofilm 2025, 9, 100252. [Google Scholar] [CrossRef]
- Lin, C.; Qin, H.; Liao, Y.; Chen, J.; Gao, B. Chemical Synthesis and Insecticidal Activity Research Based on α-Conotoxins. Molecules 2024, 29, 2846. [Google Scholar] [CrossRef]
- Abd El-Ghaffar, M.A.; Hashem, M.S.; Youssef, E.A.M.; Abdel-Aziz, M.S. Chitosan, Glutamic Acid/Monocarboxylic Cobalt-Phthalocyanine, and Carboxymethyl Cellulose as Innovative Antimicrobial Amide Biocomposites. J. Inorg. Organomet. Polym. 2024, 34, 5915–5924. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Z.; Yin, Q.; Shangguan, W.; Cao, C.; Huang, Q.; Cao, L. An Organic Solvent-Free Self-Assembly Strategy for Scalable Preparation of Nanobiopesticides with Enhanced Insecticidal Activity against Houseflies. Nanoscale 2025, 17, 9363–9373. [Google Scholar] [CrossRef]
- Aonishi, K.; Miyao, S.; Yokoi, L.; Kitaoka, N.; Koyama, K.; Matsuura, H.; Koseki, S. Isolation and identification of the antibacterial compounds produced by Maillard reaction of xylose with phenylalanine or proline. J. Agric. Food Chem. 2024, 72, 16010–16017. [Google Scholar] [CrossRef]
- Pawar, T.J.; Bravo-Espinoza, I.; Delgado-Alvarado, E.; Ramos-Morales, F.R.; Aguirre-Vidal, Y.; Olivares-Romero, J.L. Synthesis, insecticidal activities, toxicity assessment, and environmental implications of (R)- and (S)-proline-derived chiral neonicotinoid derivatives. ACS Agric. Sci. Technol. 2024, 4, 929–937. [Google Scholar] [CrossRef]
- Giordano, C.; Barnini, S. Glycine restores the sensitivity to antibiotics in multidrug-resistant bacteria. Microbiol. Spectr. 2024, 12, e00164-24. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, C.; Zhang, Y.; Liu, Y.; Pan, S.; Sun, W.; Zhang, X.; Zhong, Z.; Cao, C.; Chen, C.; Hassan, M.; et al. Aphicidal activity and biosafety assessment of small peptides in soybean plants. ACS Agric. Sci. Technol. 2025, 5, 1105–1115. [Google Scholar] [CrossRef]
- Khafoor, A.A.; Karim, A.S.; Sajadi, S.M. The effect of alanine and morine functional agents on antimicrobial potential of green synthesized CuO@Fe3O4@xanthan NCs using Pterocephalus nestorianus extract. Results Chem. 2024, 9, 101625. [Google Scholar] [CrossRef]
- Du, J.; Wu, Z.; Zhu, C.; Yang, H.; Zhao, F.; Fang, B. Exogenous cystine increases susceptibility of drug-resistant Salmonella to gentamicin by promoting oxidation of glutathione metabolism and imbalance of intracellular redox levels. Front. Microbiol. 2025, 16, 1527480. [Google Scholar] [CrossRef]
- Deegala, S.; Rathnapala, H.C.; Rajendran, S.; Hettiarachchi, C. Transgenic innovation: Harnessing cyclotides as next generation pesticides. ACS Omega 2025, 10, 6323–6336. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, Y.; Zhai, S.; Xiong, H.; Ming, Y.; Ma, Y. Preparation of valine–curcumin conjugate and its in vitro antibacterial and antitumor activity and in vivo biological effects on American eels (Anguilla rostrata). Fish Shellfish Immunol. 2024, 149, 109615. [Google Scholar] [CrossRef]
- Mori, G.; Rahimian, S.; Ozawa, R.; Murata, K.; Hachisu, M.; Arimura, G.-I. Development of menthyl esters of valine for pest control in tomato and lettuce crops. Plants 2024, 13, 1015. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, Y.; Xie, P.; Zhu, X.; Yang, C.; Wang, L.; Zhang, S. Antifungal Activities of L-Methionine and L-Arginine Treatment In Vitro and In Vivo against Botrytis cinerea. Microorganisms 2024, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Morimura, H.; Ishigami, K.; Kanie, S.; Sato, Y.; Kikuchi, Y. Antioxidant cysteine and methionine derivatives show trachea disruption in insects. PLoS ONE 2024, 19, e0310919. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Qin, Z.; Zhang, C.; Liu, H.; Zhou, T.; Wang, L.; Luo, Y.; Zeng, Z. Synthesis and antifungal activity of arecoline derivatives containing amino acid fragments. Mol. Divers. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.W.; Xu, L.; Zheng, M.X.; Shi, Y.; Zhu, Y.J. Engineering of Bacillus thuringiensis Cry2Ab toxin for improved insecticidal activity. AMB Express 2024, 14, 15. [Google Scholar] [CrossRef]
- Uçak, M.; Fırıncı, R.; Fırıncı, E.; Koca, S.; Yılmaz, A. Synthesis, characterization, antimicrobial and antioxidant evaluation of copper(II) complexes with a leucine-derived ligand. Monatsh. Chem. 2025, 156, 857–865. [Google Scholar] [CrossRef]
- Fatima, A.; Aslam, S.; Janiad, S.; Faisal, S.; Irfan, A.; Iqbal, J.; Shazly, G.A.; Zafar, A.M.; Shaheen, A.; Noreen, S.; et al. Synthesis and biological evaluation of rationally designed pyrazoles as insecticidal agents. Mol. Divers. 2025, in press. [Google Scholar] [CrossRef]
- Zhang, T.-C.; Fang, H.-B.; Gong, Y.-F.; Zhang, W.-F.; Liu, J.-C.; Chang, J.; Chen, Z.-F.; Zhao, L.-F.; Gu, Y.-C.; Hua, X. Evaluation of natural l-phenylalanine-derived amidohydrazide derivatives in ensuring agricultural production against phytopathogenic fungi. Pest Manag. Sci. 2025, 81, 5030–5042. [Google Scholar] [CrossRef]
- Kumar, V.; Nadarajan, S.; Boddupally, D.; Wang, R.; Bar, E.; Davidovich-Rikanati, R.; Doron-Faigenboim, A.; Alkan, N.; Lewinsohn, E.; Elad, Y.; et al. Phenylalanine treatment induces tomato resistance to Tuta absoluta via increased accumulation of benzenoid/phenylpropanoid volatiles serving as defense signals. Plant J. 2024, 119, 84–99. [Google Scholar] [CrossRef]
- Zhao, Z.; Wen, S.A.; Song, N.; Wang, L.; Zhou, Y.; Deng, X.; Li, H.; Chen, J.; Zhong, L.L. Arginine-enhanced antimicrobial activity of nanozymes against Gram-negative bacteria. Adv. Healthc. Mater. 2024, 13, 2301332. [Google Scholar] [CrossRef]
- Yousaf, I.; Tabassum, B.; Jabbar, B.; Amjad, M.A.; Qaisar, U.; Khan, A.; Khalid, R.; Adeyinka, O.S.; Nasir, I.A. Efficacy of arginine kinase as a promising RNAi target in Aphis gossypii genome as revealed through aphid bioassay on field-grown transgenic cotton plants. J. Plant Prot. Res. 2024, 64, 242–252. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Cao, S.; Liu, Z.; Tian, L.; Gao, Z.; Sun, M.; Zong, H.; Wang, D.; El-Sheikh, M.; et al. Integrated Physiological and Transcriptome Analyses of the Effects of Water-Soluble Amino Acid Fertilizer on Plant Growth. J. King Saud Univ. Sci. 2024, 36, 103504. [Google Scholar] [CrossRef]
- Gil, F.; Taf, R.; Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Moustakas, K.; Chojnacka, K. Advancing Sustainable Agriculture: Converting Dairy Wastes into Amino Acid Fertilizers. Sustain. Chem. Pharm. 2024, 42, 101782. [Google Scholar] [CrossRef]
- Aioub, A.A.; Ghosh, S.; Al-Farga, A.; Khan, A.N.; Bibi, R.; Elwakeel, A.M.; Ammar, E.E. Back to the Origins: Biopesticides as Promising Alternatives to Conventional Agrochemicals. Eur. J. Plant Pathol. 2024, 169, 697–713. [Google Scholar] [CrossRef]
- Lamberth, C. Naturally Occurring Amino Acid Derivatives with Herbicidal, Fungicidal or Insecticidal Activity. Amino Acids 2016, 48, 929–940. [Google Scholar] [CrossRef]
- Ansari, N.H.; Shahid, S.; Khan, M.S.; Rizvi, N.Z.; Shakeel Iqubal, S.M.; Bahafi, A. Amino Acid-Based Biosurfactants: Promising and Ecofriendly Biomolecules for Attaining Sustainable Agriculture and Environmental Safety. Colloid J. 2025, 87, 78–100. [Google Scholar] [CrossRef]
- Papazlatani, C.; Wagner, A.; Chen, Z.; Zweers, H.; de Boer, W.; Garbeva, P. Enhancement of Production of Pathogen-Suppressing Volatiles Using Amino Acids. Curr. Res. Microb. Sci. 2025, 8, 100385. [Google Scholar] [CrossRef] [PubMed]
- Irwansyah, I.; Li, Y.Q.; Shi, W.; Qi, D.; Leow, W.R.; Tang, M.B.; Li, S.; Chen, X. Gram-Positive Antimicrobial Activity of Amino Acid-Based Hydrogels. Adv. Mater. 2015, 27, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Maulina, D.; Amin, M.; Lestari, S.R.; Aziz, M. Alanine as Natural Biopesticide from Mirabilis jalapa and Its Interaction with Glutamate as an Inhibitor in Insects Immune System. Berk. Penelit. Hayati 2018, 23, 77–83. [Google Scholar] [CrossRef]
- Auttajinda, N.; Raviyan, P.; Ueno, W.K.; Jirarattanarangsri, W.; Osiriphun, S. Antibacterial activities of palmitic and lauric acids from palm kernel oil for the development of food-grade disinfectants. J. Rajamangala Univ. Technol. Sci. Math. Technol. 2023, 2, 1–15. [Google Scholar]
- Valdez-Ramírez, A.; Flores-Macías, A.; Ramos-López, M.Á.; Castañeda-Espinoza, J.D.; Rodríguez-González, F.; Herrera-Figueroa, L.E.; Figueroa-Brito, R. Effect of extracts and compounds of Jatropha curcas L. seeds against the fall armyworm Spodoptera frugiperda. Southwest. Entomol. 2024, 49, 120–132. [Google Scholar]
- Tahlan, S.; Kumar, P.; Narasimhan, B. Synthesis, antimicrobial evaluation and QSAR studies of stearic acid derivatives. Drug Res. 2014, 64, 98–103. [Google Scholar] [CrossRef]
- Bentrad, N.; Gaceb-Terrak, R.; Benmalek, Y.; Rahmania, F. Studies on chemical composition and antimicrobial activities of bioactive molecules from date palm (Phoenix dactylifera L.) pollens and seeds. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 242–256. [Google Scholar] [CrossRef]
- Ghavam, M.; Afzali, A.; Manca, M.L. Chemotype of damask rose with oleic acid (9-octadecenoic acid) and its antimicrobial effectiveness. Sci. Rep. 2021, 11, 8027. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Los, R.; Głowniak, K.; Malm, A. Antimicrobial activity of fatty acids from fruits of Peucedanum cervaria and P. alsaticum. Chem. Biodivers. 2010, 7, 2748–2754. [Google Scholar] [CrossRef]
- Mounisha, K.; Edward, Y.J.T.; Kannan, M.; Vellaikumar, S.; Uma, D.; Indiragandhi, P. Giant milkweed: A comprehensive review of chemical constituents and their insecticidal properties. Phytochem. Rev. 2025, 24, 1923–1940. [Google Scholar] [CrossRef]
- Perumalsamy, H.; Jang, M.J.; Kim, J.R.; Kadarkarai, M.; Ahn, Y.J. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasites Vectors 2015, 8, 237. [Google Scholar] [CrossRef]
- Kofi, A.; Stephen, G.; Francis, A. Antibacterial and radical scavenging activity of fatty acids from Paullinia pinnata L. Pharmacogn. Mag. 2009, 5, 119–123. [Google Scholar]
- Trigui, M.; Gasmi, L.; Zouari, I.; Tounsi, S. Seasonal variation in phenolic composition, antibacterial and antioxidant activities of Ulva rigida (Chlorophyta) and assessment of antiacetylcholinesterase potential. J. Appl. Phycol. 2013, 25, 319–328. [Google Scholar] [CrossRef]
- Neggaz, S.; Chenni, M.; Zitouni-Haouar, F.E.H.; Fernandez, X. Mycochemical composition and insecticidal bioactivity of Algerian desert truffles extract against two stored-product insects: Sitophilus oryzae (L.) (Coleoptera: Curculionidae) and Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae). 3 Biotech 2020, 10, 481. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Roszkowski, P.; Bielenica, A.; Olejarz, W.; Stępień, K.; Struga, M. Anticancer and antimicrobial effects of novel ciprofloxacin fatty acids conjugates. Eur. J. Med. Chem. 2020, 185, 111810. [Google Scholar] [CrossRef]
- Abdel-Aty, A.S. Insecticidal and phytocidal effects of Simmondsia chinensis constituents. J. Anim. Plant Sci. 2018, 28, 1746–1754. [Google Scholar]
- Jayaprakas, C.A.; Ratheesh, S.; Rajeswari, L.S. Biopesticidal Activity of Cassava (Manihot esculenta Crantz) Seed Oil Against Bihar Hairy Caterpillar (Spilarctia obliqua) and Cowpea Aphid (Aphis craccivora). J. Root Crops 2014, 39, 73–77. [Google Scholar]
- Farag, A.A.; Abd El-Rahman, H.A. Impact of some plant oils and hexaflumuron against Phenacoccus solenopsis (Hemiptera: Pseudococcidae) and Tetranychus urticae (Acari: Tetranychidae) on cotton plants. Egypt J. Plant Prot. Res. Inst. 2021, 4, 612–622. [Google Scholar]
- Altinoz, M.A.; Serdar, M.A.; Altinoz, S.M.; Eroglu, M.; Muhcu, M.; Kumru, P.; Ozpinar, A. ω9 Monounsaturated and Saturated Colostrum Fatty Acids May Benefit Newborns in General and Subtle Hypothyroid Stages. Nutrients 2025, 17, 2017. [Google Scholar] [CrossRef]
- Fan, G.; Li, Y.; Liu, Y.; Suo, X.; Jia, Y.; Yang, X. Gondoic acid alleviates LPS-induced Kupffer cells inflammation by inhibiting ROS production and PKCθ/ERK/STAT3 signaling pathway. Int. Immunopharmacol. 2022, 111, 109171. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.; Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. EFSA Panel on Contaminants in the Food Chain (CONTAM). Erucic acid in feed and food. EFSA J. 2016, 14, e04593. [Google Scholar]
- Commission Regulation (EU), No.1870/2019 of 7 November 2019 amending and correcting Regulation (EC) No 1881/2006 as regards maximum levels of erucic acid and hydrocyanic acid in certain foodstuffs. Off. J. Eur. Union 2019, L289/37, 37–40. Available online: https://eur-lex.europa.eu/eli/reg/2019/1870/oj (accessed on 20 August 2025).
- Galanty, A.; Grudzińska, M.; Paździora, W.; Paśko, P. Erucic Acid—Both Sides of the Story: A Concise Review on Its Beneficial and Toxic Properties. Molecules 2023, 28, 1924. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Sasaki, G.Y.; Wei, P.; Li, J.; Wang, L.; Zhu, J.; McTigue, D.; Yu, Z.; Bruno, R.S. Green tea extract prevents obesity in male mice by alleviating gut dysbiosis in association with improved intestinal barrier function that limits endotoxin translocation and adipose inflammation. J. Nutr. Biochem. 2019, 67, 78–89. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Li, Z.J.; Guo, X.; Dawuti, G.; Aibai, S. Antifungal activity of ellagic acid in vitro and in vivo. Phytother. Res. 2015, 29, 1019–1025. [Google Scholar] [CrossRef]
- De Filippis, A.; D’Amelia, V.; Folliero, V.; Zannella, C.; Franci, G.; Galdiero, M.; Di Donato, A.; Cafaro, V.; Rigano, M.M. Cistus incanus: A natural source of antimicrobial metabolites. Nat. Prod. Res. 2025, 39, 3396–3409. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.G.G.; Campos, C.D.L.; Pereira-Filho, J.L.; Pereira, A.P.A.; Reis, G.S.A.; Araújo, Á.W.d.M.S.; Monteiro, P.d.M.M.; Vidal, F.C.B.; Monteiro, S.G.; da Silva Figueiredo, I.F.; et al. Ellagic Acid Potentiates the Inhibitory Effects of Fluconazole Against Candida albicans. Antibiotics 2024, 13, 1174. [Google Scholar] [CrossRef]
- Khamis, W.M.; Behiry, S.I.; Marey, S.A.; Al-Askar, A.A.; Amer, G.; Heflish, A.A.; Elshamy, A.I.; Gaber, M.K. Phytochemical analysis and insight into insecticidal and antifungal activities of Indian hawthorn leaf extract. Sci. Rep. 2023, 13, 17194. [Google Scholar] [CrossRef]
- Punia, A.; Chauhan, N.S.; Kaur, S.; Sohal, S.K. Effect of ellagic acid on the larvae of Spodoptera litura (Lepidoptera: Noctuidae) and its parasitoid Bracon hebetor (Hymenoptera: Braconidae). J. Asia-Pac. Entomol. 2020, 23, 660–665. [Google Scholar] [CrossRef]
- Ajaegbu, E.E.; Danga, S.P.Y.; Chijoke, I.U.; Okoye, F.B.C. Mosquito adulticidal activity of the leaf extracts of Spondias mombin L. against Aedes aegypti L. and isolation of active principles. J. Vector Borne Dis. 2016, 53, 17–22. [Google Scholar] [CrossRef]
- Albrahim, J.S.; El-Fakharany, E.M.; El-Gendi, H.; Saleh, A.K.; El-Maradny, Y.A. Therapeutic perspectives of Mangifera indica L. peel extract: Phytochemical profile, antimicrobial, anticancer, and antiviral efficacy. Biomass Convers. Biorefin. 2025, 15, 11371–11394. [Google Scholar] [CrossRef]
- Punia, A.; Chauhan, N.S.; Singh, D.; Kesavan, A.K.; Kaur, S.; Sohal, S.K. Effect of gallic acid on the larvae of Spodoptera litura and its parasitoid Bracon hebetor. Sci. Rep. 2021, 11, 531. [Google Scholar] [CrossRef]
- Eldesouky, S.E.; Tawfeek, M.E.; Salem, M.Z. The toxicity, repellent, and biochemical effects of four wild plant extracts against Aphis gossypii Glover and Phenacoccus solenopsis Tinsley: HPLC analysis of phenolic compounds. Phytoparasitica 2024, 52, 98. [Google Scholar] [CrossRef]
- Naga, K.C.; Subramanian, S.; Sharma, R.K. Relative toxicity of phenolics against apple woolly aphid Eriosoma lanigerum. Indian J. Entomol. 2018, 80, 693–697. [Google Scholar] [CrossRef]
- Stec, K.; Kordan, B.; Gabryś, B. Quercetin and rutin as modifiers of aphid probing behavior. Molecules 2021, 26, 3622. [Google Scholar] [CrossRef] [PubMed]
- Riddick, E.W. Evaluating the Effects of Flavonoids on Insects: Implications for Managing Pests Without Harming Beneficials. Insects 2024, 15, 956. [Google Scholar] [CrossRef]
- Al-Majmaie, S.; Nahar, L.; Sharples, G.P.; Wadi, K.; Sarker, S.D. Isolation and antimicrobial activity of rutin and its derivatives from Ruta chalepensis (Rutaceae) growing in Iraq. Rec. Nat. Prod. 2019, 13, 64–70. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Mane, M.D.; Patole, N.S.; Kodalkar, V.N.; Metkari, S.A. An overview of antimicrobial properties of rutin. Int. J. Res. Pharm. Allied Sci. 2024, 3, 17–23. [Google Scholar]
- Goda, A.A.; Ayad, E.G.; Youssef, M.; Shi, J.; Xu, J.; Liu, X.; Zhou, Y.; Ramzy, S. Antimicrobial activity of licorice (Glycyrrhiza glabra) extract against some pathogenic microorganisms as a preservative agent in mango nectar as an alternative to sodium benzoate. J. Microbiol. Biotechnol. Food Sci. 2025, 14, e12298. [Google Scholar]
- Abdel-Baset, S.H.; Eltamany, E.E.; Elhady, S.S.; Abdelrazik, E. Nematicidal Activity of White Mustard Seeds as Promising Biofumigants for Control Root-Knot Nematodes, Meloidogyne spp., Infecting Solanum lycopersicum L. under Field Conditions. ACS Agric. Sci. Technol. 2025, 5, 113–124. [Google Scholar] [CrossRef]
- Contreras-Angulo, L.A.; Laaroussi, H.; Ousaaid, D.; Bakour, M.; Lyoussi, B.; Ferreira-Santos, P. Sustainable valorization of olive oil by-products: Green extraction of phytochemicals, encapsulation strategies, and food applications. J. Food Sci. 2025, 90, e70412. [Google Scholar] [CrossRef]
- Benvenuti, S.; Pellati, F.; Melegari, M.A.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, FCT164–FCT169. [Google Scholar] [CrossRef]
- Mariod, A.A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009, 116, 306–312. [Google Scholar] [CrossRef]
- Coelho, N.; Pereira, A.S.; Tavares, P. Moringa oleifera Seed Cake: A Review on the Current Status of Green Nanoparticle Synthesis. Appl. Biosci. 2024, 3, 197–212. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential distribution of amino acids in plants. Amino Acids 2017, 49, 821–869. [Google Scholar] [CrossRef]
- Andersen, P.C.; Gorbet, D.W. Influence of year and planting date on fatty acid chemistry of high oleic acid and normal peanut genotypes. J. Agric. Food Chem. 2002, 50, 1298–1305. [Google Scholar] [CrossRef]
- Malinda, K.; Sutanto, H.; Darmawan, A. Characterization and antioxidant activity of gallic acid derivative. AIP Conf. Proc. 2017, 1904, 020030. [Google Scholar] [CrossRef]
- Corradi, I.; De Souza, E.; Sande, D.; Takahashi, J.A. Correlation Between Phenolic Compounds Contents, Anti-tyrosinase and Antioxidant Activities of Plant Extracts. Chem. Eng. Trans. 2018, 64, 109–114. [Google Scholar]
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and Caffeine Content of Green Tea Dietary Supplements and Correlation with Antioxidant Capacity. J. Agric. Food Chem. 2006, 54, 1599–1603. [Google Scholar] [CrossRef]
- Vujasinovic, V.; Djilas, S.; Dimic, E.; Romanic, R.; Takaci, A. Shelf life of cold-pressed pumpkin (Cucurbita pepo L.) seed oil obtained with a screw press. J. Am. Oil Chem. Soc. 2010, 87, 1497–1505. [Google Scholar] [CrossRef]
- Akubude, V.C.; Maduako, J.N.; Egwuonwu, C.C.; Olaniyan, A.M.; Ozumba, I.C.; Nwosu, C.; Ajala, O.E. Effect of process parameters on oil yield mechanically expressed from almond seed (using response surface methodology). Am. J. Food Sci. Nutr. Res. 2017, 4, 1–8. [Google Scholar]
- ISO 6496:1999; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 5983-1:2005; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 1: Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 5984:2022; Animal Feeding Stuffs—Determination of Crude Ash. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 6492:1999; Animal Feeding Stuffs—Determination of Fat Content. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 6865:2000; Animal Feeding Stuffs—Determination of Crude Fiber Content. International Organization for Standardization: Geneva, Switzerland, 2000.
- SRPS EN ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. Institute for Standardization of Serbia: Belgrade, Serbia, 2017.
- Morales, F.J.; Jimenéz-Pérez, S. Free radical scavenging capacity of Maillard reaction products as related to color and fluorescence. Food Chem. 2001, 72, 119–125. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]



| Amino Acid | g/100 g of Protein | Antimicrobial Effect | Insecticidal Effect |
|---|---|---|---|
| Aspartic acid | 10.9 ± 0.16 | ✓ [44] | ✓ [45] |
| Threonine | 3.94 ± 0.16 | / | / |
| Serine | 5.06 ± 0.07 | / | / |
| Glutamic acid | 18.2 ± 0.65 | ✓ [46] | ✓ [47] |
| Proline | 2.17 ± 0.40 | ✓ [48] | ✓ [49] |
| Glycine | 5.92 ± 0.20 | ✓ [50] | ✓ [51] |
| Alanine | 4.08 ± 0.28 | ✓ [52] | / |
| Cystine | 0.68 ± 0.08 | ✓ [53] | ✓ [54] |
| Valine | 4.91 ± 0.65 | ✓ [55] | ✓ [56] |
| Methionine | 0.43 ± 0.22 | ✓ [57] | ✓ [58] |
| Isoleucine | 2.86 ± 0.42 | ✓ [59] | ✓ [60] |
| Leucine | 5.01 ± 0.54 | ✓ [61] | ✓ [62] |
| Tyrosine | 2.16 ± 0.20 | / | / |
| Phenylalanine | 4.15 ± 0.40 | ✓ [63] | ✓ [64] |
| Histidine | 2.04 ± 0.09 | / | / |
| Lysine | 5.52 ± 0.33 | / | / |
| Arginine | 12.1 ± 1.11 | ✓ [65] | ✓ [66] |
| Total amino acids (TAA) | 90.3 ± 5.63 |
| Fatty Acid | Content g/100 g Total FA | Antimicrobial Effect | Insecticidal Effect |
|---|---|---|---|
| Palmitic C16:0 | 6.30 ± 0.04 | ✓ [75] | ✓ [76] |
| Stearic C18:0 | 1.20 ± 0.06 | ✓ [77] | / |
| Oleic C18:1n9c | 31.1 ± 0.57 | ✓ [78,79] | ✓ [76] |
| Linoleic C18:2n6c | 12.8 ± 0.42 | ✓ [80] | ✓ [81] |
| Arachidic C20:0 | 2.60 ± 0.14 | / | ✓ [82] |
| Eicosenoic C20:1n9 | 43.0 ± 0.85 | ✓ [83,84] | ✓ [13] |
| Eicosadienoic C20:2n6 | 0.90 ± 0.00 | / | ✓ [85] |
| Erucic C22:1n9 | 1.70 ± 0.07 | ✓ [86] | ✓ [87] |
| Lignocerinic C24:0 | 0.40 ± 0.00 | / | / |
| Saturated fatty acids | 10.1 ± 0.28 | ||
| Monounsaturated fatty acids | 75.8 ± 1.06 | ||
| Polyunsaturated fatty acids | 13.7 ± 0.35 |
| Compound | Content (μg/g) |
|---|---|
| Quercetin | 5.11 ± 0.11 |
| Quercitrin | 14.28 ± 0.33 |
| Gallic acid | 749.32 ± 12.22 |
| Ellagic acid | 945.27 ± 16.98 |
| Rutin | 903.68 ± 19.21 |
| Catechin | 791.44 ± 13.24 |
| Compound | LOD (μg/mL) | LOQ (μg/mL) | Linear Range (μg/mL) | R2 | Equation of the Calibration Curve |
|---|---|---|---|---|---|
| Gallic acid | 6.25 | 18.50 | 15–800 | 0.9995 | y = 7222.3x − 2.2 |
| Ellagic acid | 12.50 | 30.20 | 15–560 | 0.9998 | y = 17369.0x + 81.4 |
| Rutin | 3.15 | 8.40 | 5–450 | 0.9995 | y = 15485.0x − 159.5 |
| Catechin | 5.25 | 10.10 | 10–800 | 0.9999 | y = 8655.4x − 15.5 |
| Quercetin | 3.45 | 5.65 | 5–450 | 0.9997 | y = 26656.5x + 50.6 |
| Quercitrin | 5.25 | 15.10 | 5–450 | 0.9998 | y = 17984.0x + 126.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šarac, V.; Šunjka, D.; Pušić Devai, M.; Sedlar, T.; Spasevski, N.; Rakita, S.; Dragojlović, D.; Tomičić, Z.; Šavikin, K.; Živković, J.; et al. Chemotyping of Koelreuteria paniculata Seed Cake with Bioactive and Feed Potential. Plants 2025, 14, 2873. https://doi.org/10.3390/plants14182873
Šarac V, Šunjka D, Pušić Devai M, Sedlar T, Spasevski N, Rakita S, Dragojlović D, Tomičić Z, Šavikin K, Živković J, et al. Chemotyping of Koelreuteria paniculata Seed Cake with Bioactive and Feed Potential. Plants. 2025; 14(18):2873. https://doi.org/10.3390/plants14182873
Chicago/Turabian StyleŠarac, Veljko, Dragana Šunjka, Magdalena Pušić Devai, Tea Sedlar, Nedeljka Spasevski, Slađana Rakita, Danka Dragojlović, Zorica Tomičić, Katarina Šavikin, Jelena Živković, and et al. 2025. "Chemotyping of Koelreuteria paniculata Seed Cake with Bioactive and Feed Potential" Plants 14, no. 18: 2873. https://doi.org/10.3390/plants14182873
APA StyleŠarac, V., Šunjka, D., Pušić Devai, M., Sedlar, T., Spasevski, N., Rakita, S., Dragojlović, D., Tomičić, Z., Šavikin, K., Živković, J., Čabarkapa, I., & Ljubojević, M. (2025). Chemotyping of Koelreuteria paniculata Seed Cake with Bioactive and Feed Potential. Plants, 14(18), 2873. https://doi.org/10.3390/plants14182873













