Unveiling a Disease Complex Threatening Fig (Ficus carica L.) Cultivation in Southern Italy
Abstract
1. Introduction
2. Results
2.1. Field Survey, Fungal Isolation, and Morphological Characterization of the Isolates
2.2. Frequency and Relative Abundance of Isolated Morphological Groups
2.3. Phylogenetic Analyses and Species Identification
2.4. Pathogenicity Tests Results
2.5. Identification of Insects
3. Discussion
4. Materials and Methods
4.1. Field Sampling and Isolation
4.2. Morphological Characterization and Preliminary Identification of the Fungal Isolates
4.3. DNA Extraction and PCR Amplification and Sequencing
4.3.1. DNA Extraction
4.3.2. Amplification and Sequencing
4.4. Phylogenetic Analysis and Molecular Identification
4.5. Pathogenicity Tests
4.6. Identification of Scolytid Species
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kislev, M.E.; Hartmann, A.; Bar-Yosef, O. Early domesticated fig in the Jordan Valley. Science 2006, 312, 1372–1374. [Google Scholar] [CrossRef]
- Flaishman, M.; Rodov, V.; Stover, E. The fig: Botany, horticulture, and breeding. Hortic. Rev. 2008, 34, 113–197. [Google Scholar] [CrossRef]
- Condit, R. Research in large, long-term tropical forest plots. Trends Ecol. Evol. 1995, 10, 18–22. [Google Scholar] [CrossRef]
- Çaliskan, O.; Polat, A.A. Phytochemical and Antioxidant properties of selected fig (Ficus carica L.) accessions from the Eastern Mediterranean Region of Turkey. Sci. Hortic. 2011, 128, 473–478. [Google Scholar] [CrossRef]
- Mazzeo, A.; Magarelli, A.; Ferrara, G. The fig (Ficus carica L.): Varietal evolution from Asia to Puglia region, southeastern Italy. CABI Agric. Biosci. 2024, 5, 57. [Google Scholar]
- García, M.M.; Denno, B.D.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy, N.B. ScaleNet: A literature-based model of scale insect biology and systematics. Database 2016, 2016, bav118. [Google Scholar]
- Farina, P.; Mazza, G.; Benvenuti, C.; Cutino, I.; Giannotti, P.; Conti, B.; Bedini, S.; Gargani, E. Biological notes and distribution in Southern Europe of Aclees taiwanensis Kono, 1933 (Coleoptera: Curculionidae): A new pest of the fig tree. Insects 2021, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Faccoli, M.; Campo, G.; Perrotta, G.; Rassati, D. Two newly introduced tropical bark and ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) damaging figs (Ficus carica) in southern Italy. Zootaxa 2016, 4138, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- von Arx, J.A. Plant Pathogenic Fungi; J. Cramer: Berlin, Germany, 1987. [Google Scholar]
- Carlucci, A.; Raimondo, M.L.; Cibelli, F.; Phillips, J.L.; Lops, F. Pleurostomophora richardsiae, Neofusicoccum parvum and Phaeoacremonium aleophilum associated with a decline of olives in southern Italy. Phytopathol. Mediterr. 2013, 52, 517–527. [Google Scholar]
- Mondello, V.; Lo Piccolo, S.; Conigliaro, G.; Alfonzo, A.; Torta, L.; Burruano, S. First report of Neofusiccoccum vitifusiforme and presence of other Botryosphaeriaceae species associated with Botryosphaeria dieback of grapevine in Sicily (Italy). Phytopathol. Mediterr. 2013, 52, 388–396. [Google Scholar]
- Carlucci, A.; Cibelli, F.; Lops, F.; Raimondo, M.L. Characterization of Botryosphaeriaceae species as causal agents of trunk diseases on grapevines. Plant Dis. 2015, 99, 1678–1688. [Google Scholar] [CrossRef]
- Aiello, D.; Gusella, G.; Fiorenza, A.; Guarnaccia, V.; Polizzi, G. Identification of Neofusicoccum parvum causing canker and twig blight on Ficus carica in Italy. Phytopathol. Mediterr. 2020, 59, 213–218. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Li, M.; Ji, X.; Feng, C.; Wang, F. First Report of Ficus carica bot rot caused by Botryosphaeria dothidea in China. Plant Dis. 2020, 104, 1869. [Google Scholar] [CrossRef]
- Çeliker, N.M.; Michailides, T.J. First report of Lasiodiplodia theobromae causing canker and shoot blight of fig in Turkey. New Dis. Rep. 2012, 25, 12. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, H.; Du, G.; Zhu, L.; Song, Q.; Hu, Y.; Wang, E.; Wang, M.; Fan, X. First Report of Lasiodiplodia theobromae causing stem canker on common fig (Ficus carica) in Zhejiang Province of China. Plant Dis. 2018, 102, 2656–2657. [Google Scholar] [CrossRef]
- Seo, Y.; Back, C.G.; Park, M.J.; Park, J.H. First Report of Lasiodiplodia theobromae causing canker and dieback of common fig in Korea. Plant Dis. 2019, 103, 1023. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Vitale, A.; Cirvilleri, G.; Aiello, D.; Susca, A.; Epifani, F.; Perrone, G.; Polizzi, G. Characterisation and pathogenicity of fungal species associated with branch cankers and stem-end rot of avocado in Italy. Eur. J. Plant Pathol. 2016, 146, 963–976. [Google Scholar] [CrossRef]
- Ismail, A.M.; Cirvilleri, G.; Lombard, L.; Crous, P.W.; Groenewald, J.Z.; Polizzi, G. Characterisation of Neofusicoccum species causing mango dieback in Italy. Plant Pathol. J. 2013, 95, 549–557. [Google Scholar]
- Elshafie, E.A.; Ba-Omar, T. First report of Albizia lebbeck dieback caused by Scytalidium dimidiatum in Oman. Mycopathologia 2001, 154, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.D.; Burgess, T.; Lanoiselet, V.M. First record of Neoscytalidium dimidiatum and N. novaehollandiae on Mangifera indica and N. dimidiatum on Ficus carica in Australia. Australas. Plant Dis. Notes 2010, 5, 48–50. [Google Scholar] [CrossRef]
- Gusella, G.; Fiore, G.; Vitale, A.; Felts, D.G.; Michailides, T.J. New findings on the effects of different factors involved in fig limb dieback caused by Neoscytalidium dimidiatum in California. Eur. J. Plant Pathol. 2023, 167, 89–97. [Google Scholar] [CrossRef]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Fothergill, A.; McCarthy, D.; Rinaldi, M.G.; Brandt, M.E.; Zhang, N.; Geiser, D.M. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. Clin. Microbiol. J. 2008, 46, 2477–2490. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Guarnaccia, V.; Polizzi, G.; Crous, P.W. Symptomatic Citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia-Mol. Phylogeny Evol. Fungi 2018, 40, 1–25. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Lombard, L.; Crous, P.W. Back to the roots: A reappraisal of Neocosmospora. Persoonia-Mol. Phylogeny Evol. Fungi 2019, 43, 90–185. [Google Scholar] [CrossRef]
- Freeman, S.; Sharon, M.; Maymon, M.; Mendel, Z.; Protasov, A.; Aoki, T.; Eskalen, A.; O’Donnell, K. Fusarium euwallaceae sp. nov.—A symbiotic fungus of Euwallacea sp., an invasive ambrosia beetle in Israel and California. Mycologia 2013, 105, 1595–1606. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Sandoval-Denis, M.; Aiello, D.; Polizzi, G.; Crous, P.W. Neocosmospora perseae sp. nov., causing trunk cankers on avocado in Italy. Fungal Syst. Evol. 2018, 1, 131–140. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Aiello, D.; Polizzi, G.; Crous, P.W.; Sandoval-Denis, M. Soilborne diseases caused by Fusarium and Neocosmospora spp. on ornamental plants in Italy. Phytopathol. Mediterr. 2019, 58, 127–138. [Google Scholar]
- Guarnaccia, V.; Martino, A.; Brondino, L.; Gullino, M.L. Paraconiothyrium fuckelii, Diaporthe eres and Neocosmospora parceramosa causing cane blight of red raspberry in Northern Italy. Plant Pathol. 2022, 104, 683–698. [Google Scholar] [CrossRef]
- Bolboli, Z.; Mostowfizadeh-Ghalamfarsa, R.; Sandoval-Denis, M.; Jafari, M.; Crous, P.W. Neocosmospora caricae sp. nov. and N. metavorans, two new stem and trunk canker pathogens on Ficus carica in Iran. Mycol. Prog. 2022, 21, 89. [Google Scholar] [CrossRef]
- De Beer, Z.W.; Wingfield, M.J. Emerging lineages in the Ophiostomatales. In The Ophiostomatoid Fungi: Expanding Frontiers; Seifert, K.A., De Beer, Z.W., Wingfield, M.J., Eds.; CBA Biodiversity Series; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2013; pp. 21–46. [Google Scholar]
- Kajii, C.; Morita, T.; Jikumaru, S.; Kajimura, H.; Yamaoka, Y.; Kuroda, K. Xylem dysfunction in Ficus carica infected with wilt fungus Ceratocystis ficicola and the role of the vector beetle Euwallacea interjectus. IAWA J. 2013, 34, 301–312. [Google Scholar] [CrossRef]
- Moller, W.J.; Devay, J.E. Carrot as a species-selective isolation medium for Ceratocystis fimbriata. Phytopathology 1968, 58, 123–124. [Google Scholar]
- Favaro, A.; Battisti, A. Observation on elm bark beetle, Scolytus pymaeus (Fabricius) (Coleoptera, Scolytidae) as possible vectors of the fungus Ophiostoma ulmi (Schwarz) Nannfeldt. Redia 1993, 76, 459–466. [Google Scholar]
- Battisti, A.; Favaro, A.; Faccoli, M.; Masuitti, L. Suscettibilità di ceppi di Ulmus spp. all’attacco primario di Scolytus sp. Pl. (Coleoptera, Scolytidae). In Innovazioni e Prospettive Nella Difesa Fitosanitaria; Ist Sper. PAT. VEG: Roma, Italy, 1994; pp. 351–354. [Google Scholar]
- Faccoli, M.; Battisti, A. Observation on the transmission of Ophiostoma ulmi by the smaller Elm Bark Beetles (Scolytus spp.). In Integrating Cultural Tactics into the Management of Bark Beetles and Reforestation Pest; Geogoire, J.C., Liebhold, A.M., Stephen, F.M., Day, K.R., Salom, S.M., Eds.; Technical Report NE236; USDA Forest Service: Washington, DC, USA, 1997; pp. 172–176. [Google Scholar]
- Masood, A.; Saeed, S. Bark beetle, Hypocryphalus mangiferae stebbing (Coleoptera: Curculionidae: Scolytinae) is a vector of mango sudden death disease in Pakistan. Pak. J. Bot 2012, 44, 813–820. [Google Scholar]
- Johnson, A.J.; Li, Y.; Mandelshtam, M.Y.; Park, S.; Lin, C.S.; Gao, L.; Hulcr, J. East Asian Cryphalus Erichson (Curculionidae, Scolytinae): New species, new synonymy and redescriptions of species. ZooKeys 2020, 995, 15. [Google Scholar] [CrossRef] [PubMed]
- Habib, W.; Cavalieri, V.; Carlucci, M.; Dongiovanni, C.; Nigro, F. A new disease complex threatening fig (Ficus carica L.) in Southern Italy. 16th Congress of the Mediterranean Phytopathological Union, 4–8 April 2022, Limassol, Cyprus. Phytopathol. Mediterr. 2022, 61, 195. [Google Scholar]
- Gugliuzzo, A.; Gusella, G.; Leonardi, G.R.; Costanzo, M.B.; Ricupero, M.; Rassati, D.; Biondi, A.; Polizzi, G. From a cause of rapid fig tree dieback to a new threat to mango production: The invasive bark beetle Cryphalus dilutus Eichhoff (Coleoptera: Curculionidae, Scolytinae) and its associated fungi found on mango trees in Europe. EPPO Bull. 2023, 53, 663–670. [Google Scholar] [CrossRef]
- Kajitani, Y.; Masuya, H. Ceratocystis ficicola sp. nov., a causal fungus of fig canker in Japan. Mycoscience 2011, 52, 349–353. [Google Scholar] [CrossRef]
- Tsopelas, P.; Soulioti, N.; Wingfield, M.J.; Barnes, I.; Marincowitz, S.; Tjamos, E.C.; Paplomatas, E.J. Ceratocystis ficicola causing a serious disease of Ficus carica in Greece. Phytopathol. Mediterr. 2021, 60, 337–349. [Google Scholar] [CrossRef]
- Habib, W.; Carlucci, M.; Manco, L.; Altamura, G.; Delle Donne, A.G.; Nigro, F. First report of Ceratocystis ficicola causing canker and wilt disease on common fig (Ficus carica) in Italy. Plant Dis. 2023, 107, 3287. [Google Scholar] [CrossRef]
- Crous, P.W.; Akulov, A.; Balashov, S.; Boers, J.; Braun, U.; Castillo, J.; Delgado, M.A.; Denman, S.; Erhard, A.; Gusella, G.; et al. New and interesting Fungi. 6. Fungal Syst. Evol. 2023, 11, 109–156. [Google Scholar] [CrossRef]
- Hattori, Y.; Ando, Y.; Nakashima, C. Taxonomical re-examination of the genus Neofusicoccum in Japan. Mycoscience 2021, 62, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.; Groenewald, J.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef] [PubMed]
- De Beer, Z.W.; Duong, T.A.; Barnes, I.; Wingfield, B.D.; Wingfield, M.J. Redefining Ceratocystis and allied genera. Stud. Mycol. 2014, 79, 187–219. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Knížek, M.; Atkinson, T.H.; Jordal, B.H.; Ploetz, R.C.; Hulcr, J. Resolution of a global mango and fig pest identity crisis. Insect Syst. Divers. 2017, 1, ixx010. [Google Scholar] [CrossRef]
- Lombard, L.; Van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef]
- Coleman, J.J. The Fusarium solani species complex: Ubiquitous pathogens of agricultural importance. Mol. Plant. Pathol. 2016, 17, 146–158. [Google Scholar] [CrossRef]
- Polizzi, G.; Vitale, A. First report of Fusarium blight on majesty palm caused by Fusarium proliferatum in Italy. Plant Dis. 2003, 87, 1149. [Google Scholar] [CrossRef]
- Polizzi, G.; Aiello, D.; Guarnaccia, V.; Vitale, A.; Perrone, G.; Stea, G. First report of Fusarium wilt of paper flower (Bougainvillea glabra) caused by Fusarium oxysporum in Italy. Plant Dis. 2010, 94, 483. [Google Scholar] [CrossRef]
- Polizzi, G.; Aiello, D.; Guarnaccia, V.; Vitale, A.; Perrone, G.; Epifani, F. First report of Fusarium wilt on Eremophila spp. caused by Fusarium oxysporum in Italy. Plant Dis. 2010, 94, 1509. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, G.; Aiello, D.; Guarnaccia, V.; Vitale, A.; Perrone, G.; Stea, G. First report of Fusarium wilt on Philotheca myoporoides caused by Fusarium oxysporum in Italy. Plant Dis. 2011, 95, 877. [Google Scholar] [CrossRef]
- Bertoldo, C.; Gilardi, G.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Genetic diversity and virulence of Italian strains of Fusarium oxysporum isolated from Eustoma grandiflorum. Eur. J. Plant Pathol. 2015, 141, 83–97. [Google Scholar] [CrossRef]
- Jiang, Z.R.; Masuya, H.; Kajimura, H. Novel symbiotic association between Euwallacea ambrosia beetle and Fusarium fungus on fig trees in Japan. Front. Microbiol. 2021, 12, 725210. [Google Scholar] [CrossRef]
- Turco, E.; Marianelli, L.; Vizzuso, C.; Ragazzi, A.; Gini, R.; Selleri, B.; Tucci, R. First report of Botryosphaeria dothidea on sycamore, red oak, and english oak in North western Italy. Plant Dis. 2006, 90, 1106. [Google Scholar] [CrossRef]
- Deidda, A.; Buffa, F.; Linaldeddu, B.T.; Pinna, C.; Scanu, B.; Deiana, V.; Satta, A.; Franceschini, A.; Floris, I. Emerging pests and diseases threaten Eucalyptus camaldulensis plantations in Sardinia, Italy. Forest 2016, 9, 883–891. [Google Scholar] [CrossRef]
- Gusella, G.; Lawrence, D.P.; Aiello, D.; Luo, Y.; Polizzi, G.; Michailides, T. Etiology of Botryosphaeria panicle and shoot blight of pistachio (Pistacia vera) caused by Botryosphaeriaceae in Italy. Plant Dis. 2021, 106, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, V.; Martino, I.; Tabone, G.; Brondino, L.; Gullino, M.L. Fungal pathogens associated with stem blight and dieback of blueberry in northern Italy. Phytopathol. Mediterr. 2020, 59, 229–245. [Google Scholar] [CrossRef]
- Burruano, S.; Mondello, V.; Conigliaro, G.; Alfonzo, A.; Spagnolo, A.; Mugnai, L. Grapevine decline in Italy caused by Lasiodiplodia theobromae. Phytopathol. Mediterr. 2008, 47, 132–136. [Google Scholar]
- Garibaldi, A.; Bertetti, D.; Amatulli, M.T.; Cardinale, J.; Gullino, M.L. First report of postharvest fruit rot in avocado (Persea americana) caused by Lasiodiplodia theobromae in Italy. Plant Dis. 2012, 96, 460. [Google Scholar] [CrossRef]
- Fiorenza, A.; Aiello, D.; Costanzo, M.B.; Gusella, G.; Polizzi, G. A New Disease for Europe of Ficus microcarpa Caused by Botryosphaeriaceae Species. Plants 2022, 11, 727. [Google Scholar] [CrossRef]
- Berraf-Tebbal, A.; Guereiro, M.A.; PHilliPS, A.J.; Von Arx, J.A. Phylogeny of Neofusicoccum species associated with grapevine trunk diseases in Algeria, with description of Neofusicoccum algeriense sp. nov. Phytopath. Mediterr. 2014, 53, 416–427. [Google Scholar] [CrossRef]
- Barradas, C.; Phillips, A.J.; Correia, A.; Diogo, E.; Bragança, H.; Alves, A. Diversity and potential impact of Botryosphaeriaceae species associated with Eucalyptus globulus plantations in Portugal. Eur. J. Plant Pathol. 2016, 146, 245–257. [Google Scholar] [CrossRef]
- Serret-López, R.E.; Tlapal-Bolaños, B.; Leyva-Mir, S.G.; Correia, K.C.; Camacho-Tapia, M.; Méndez-Jaimes, F.; Tovar-Pedraza, J.M. First report of Neofusicoccum algeriense causing dieback of red raspberry in Mexico. Plant Dis. 2017, 101, 1673. [Google Scholar] [CrossRef]
- Masuya, H.; Yamaoka, Y.; Kaneko, S.; Yamaura, Y. Ophiostomatoid fungi isolated from Japanese red pine and their relationships with bark beetles. Mycoscience 2009, 50, 212–223. [Google Scholar] [CrossRef]
- Kato, K.; Hirota, K.; Miyagawa, T. A new disease, Ceratocystis canker of fig caused by Ceratocystis fimbriata Ellis et Halsted. Plant Prot. 1982, 36, 55–59. (In Japanese) [Google Scholar] [CrossRef]
- EPPO Alert List—Ceratocystis ficicola. 2022. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/alert_list_fungi/ceratocystis_ficicola (accessed on 20 August 2025).
- Mifsud, D.; Knizek, M. The Bark Beetles (Coleoptera: Scolytidae) of the Maltese Islands (Central Mediterranean). Bull. Ent. Soc. Malta 2009, 2, 25–52. [Google Scholar]
- Mifsud, D.; Annushka Falzon, A.; Malumphy, C.; de Lillo, E.; Vovlas, N.; Porcelli, F. On some arthropods associated with Ficus species (Moraceae) in the Maltese Islands. Bull. Ent. Soc. Malta 2012, 5, 5–34. [Google Scholar]
- Bonanno, G. Alien species: To remove or not to remove? That is the question. Environ. Sci. Policy 2016, 59, 67–73. [Google Scholar] [CrossRef]
- Barnouin, T.; Soldati, F.; Roques, A.; Faccoli, M.; Kirkendall, L.R.; Mouttet, R.; Daubree, J.B.; Noblecourt, T. Bark beetles and pinhole borers recently or newly introduced to France (Coleoptera: Curculionidae, Scolytinae and Platypodinae). Zootaxa 2020, 4877, 51–74. [Google Scholar] [CrossRef]
- Gaaliche, B.; Ben Abdelaali, N.; Mouttet, R.; Ben Halima-Kamel, M.; Hajlaoui, M.R. First report of Hypocryphalus scabricollis (Eichhoff, 1878), in Tunisia, an emerging pest on fig trees (Ficus carica L.). Bull. OEPP/EPPO Bull. 2018, 48, 164–166. [Google Scholar] [CrossRef]
- Leach, J.G. Insect Transmission of Plant Diseases; McGraw-Hill Book Company, Inc.: New York, NY, USA; London, UK, 1940. [Google Scholar]
- Whetzel, H.H. An Outline of the History of Phytopathology; W.B. Saunders: Philadelphia, PA, USA; London, UK, 1918. [Google Scholar] [CrossRef][Green Version]
- Slippers, B.; Crous, P.W.; Jami, F.; Groenewald, J.Z.; Wingfield, M.J. Diversity in the Botryosphaeriales: Looking back, looking forward. Fungal Biol. 2017, 121, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.S.J. Fungal Planet description sheets: 400–468. Persoonia 2016, 36, 316–458. [Google Scholar] [CrossRef]
- Habib, W.; Masiello, M.; El Ghorayeb, R.; Gerges, E.; Susca, A.; Meca, G.; Quiles, M.J.; Logrieco, F.A.; Moretti, A. Mycotoxin profile and phylogeny of pathogenic Alternaria species isolated from symptomatic tomato plants in Lebanon. Toxins 2021, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M.A. Method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- EPPO. PM 7/24 (4) Xylella fastidiosa. EPPO Bull. 2019, 49, 175–227. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [CrossRef] [PubMed]
- EPPO. PM 7/129 (2) DNA barcoding as an identification tool for a number of regulated pests. EPPO Bull. 2021, 51, 100–143. [Google Scholar] [CrossRef]



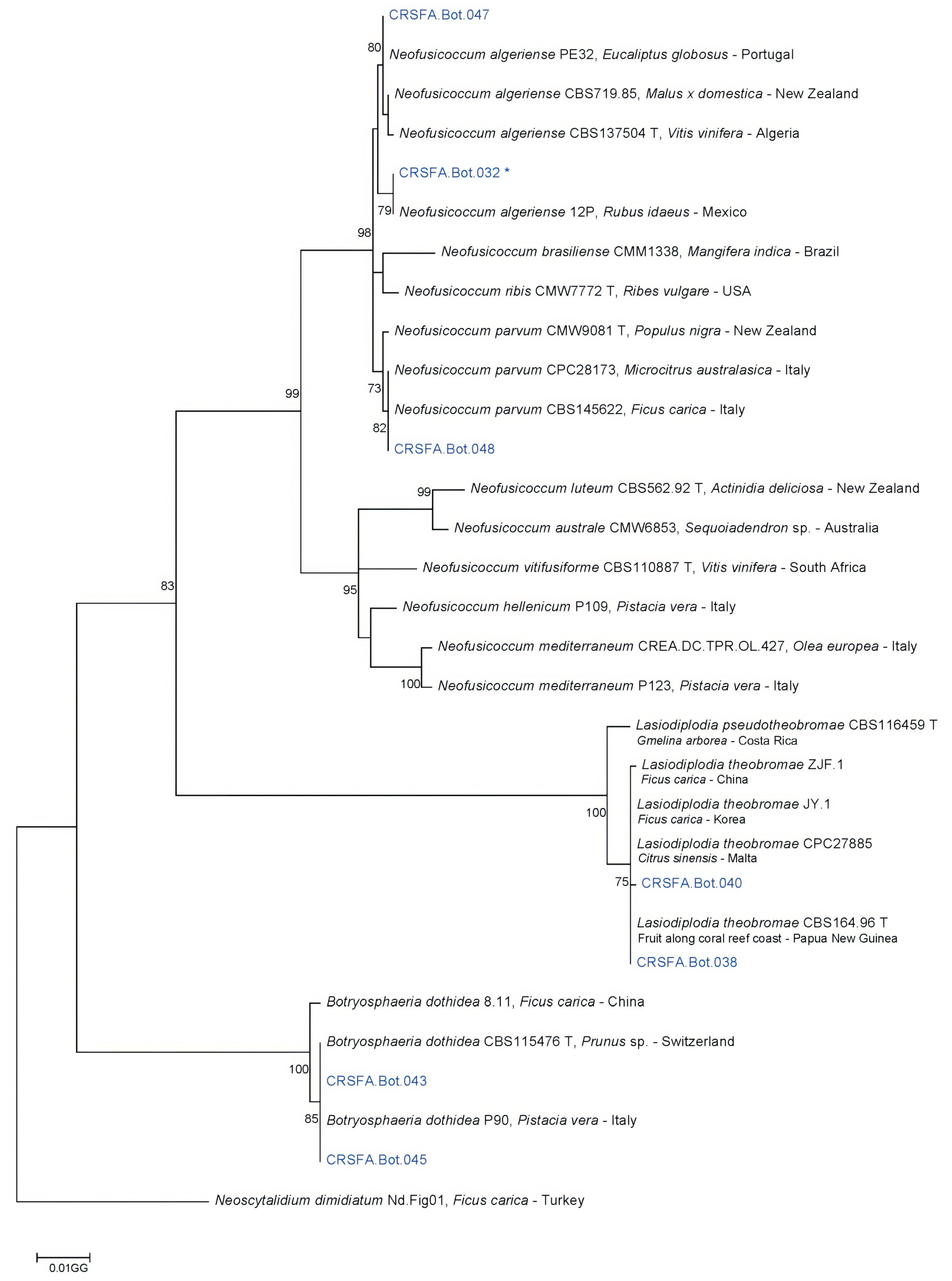
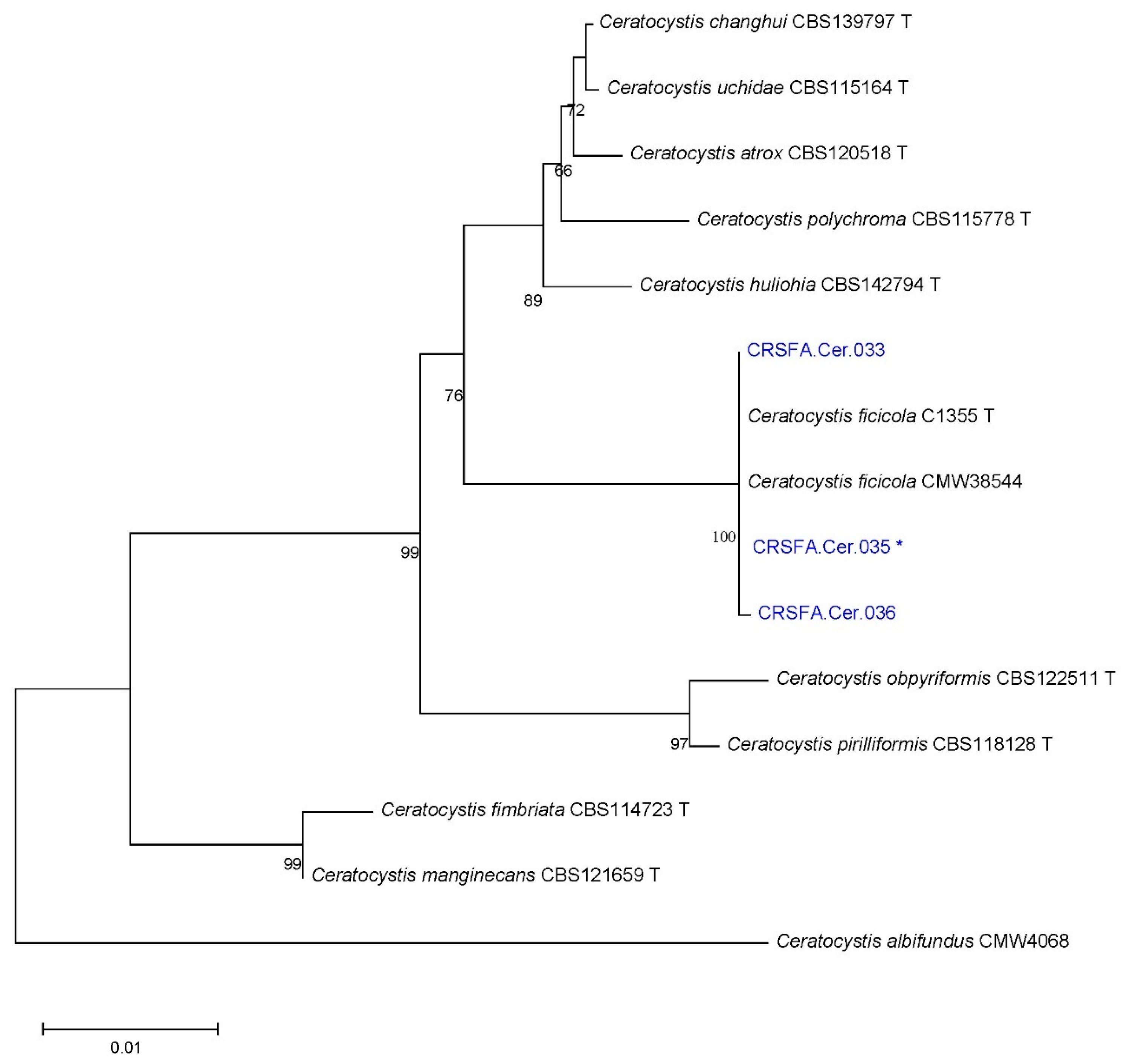

| Site | Locality | Province | Sample | Wood Fragments (#) | Adult Beetles (#) | Percentage of Fungal Colonies Recovered | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fusarium spp. | Ceratocystis spp. | Botryosphaeriaceae | Others | ||||||||||
| Wood | Beetles | Wood | Beetles | Wood | Beetles | Wood | Beetles | ||||||
| A | Salice Salentino | Lecce | A-1 | 30 | 9 | 46.67 | 66.67 | 6.00 | 0.00 | 3.33 | 0.00 | 13.33 | 0.00 |
| A-2 | 10 | 3 | 30.00 | 33.33 | 2.00 | 0.00 | 0.00 | 0.00 | 10.00 | 0.00 | |||
| B | Copertino | Lecce | B-1 | 40 | - | 12.50 | - | 0.00 | - | 77.50 | - | 0.00 | - |
| C | Guagnano | Lecce | C-1 | 30 | 30 | 0.00 | 0.00 | 0.00 | 0.00 | 93.33 | 12.00 | 0.00 | 0.00 |
| D | Ostuni | Brindisi | D-1 | 25 | - | 0.00 | - | 0.00 | - | 92.00 | - | 0.00 | - |
| E | Squinzano | Lecce | E-1 | 30 | 0 | 90.00 | 0.00 | 0.00 | 0.00 | 3.33 | 0.00 | 0.00 | 0.00 |
| E-2 | 10 | 5 | 90.00 | 30.00 | 2.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| E-3 | 5 | 5 | 0.00 | 20.00 | 5.00 | 20.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| E-4 | 20 | 0 | 55.00 | 0.00 | 1.00 | 0.00 | 20.00 | 0.00 | 15.00 | 0.00 | |||
| F | Ceglie Messapica | Brindisi | F-1 | 40 | 0 | 17.50 | 0.00 | 7.50 | 0.00 | 32.50 | 0.00 | 0.00 | 0.00 |
| F-2 | 30 | 2 | 36.67 | 50.00 | 6.67 | 50.00 | 36.67 | 0.00 | 0.00 | 0.00 | |||
| F-3 | 40 | 0 | 25.00 | 0.00 | 5.00 | 0.00 | 22.50 | 0.00 | 0.00 | 0.00 | |||
| Fungal Species (Isolate Code) | Colonized Twig Surface (%) ± SE | Notes/Figure Reference |
|---|---|---|
| Neocosmospora perseae (CRSFA.Fus.010) | 74.2 ± 3.2 (range: 65.5–81.5) | Symptoms in all replicates; see Figure 6C |
| Neocosmospora perseae (CRSFA.Fus.030) | 69.2 ± 3.0 (range 60.9–77.2) | Symptoms in all replicates; see Figure 6C |
| Neofusicoccum algeriense (CRSFA.Bot.032) | 61.5 ± 2.1 (range 54.0–68.0) | Confirmed pathogenicity; see Figure 6D |
| Ceratocystis ficicola (CRSFA.Cer.035) | 64.0 ± 1.8% (range 58–70%) | Symptoms in all replicates; see Figure 6A,B |
| Other fusarioid strains (various) | Non-pathogenic | No symptoms observed |
| Species | Culture Code | Host | Locality | Province | Site | Sample | GenBank Number | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ITS | TEF | RPB2 | TUB | LSU | |||||||
| Botryosphaeria dothidea | CRSFA.Bot.043 | F. carica | Copertino | Lecce | B | B-1 | OQ644314 | OQ716469 | - | OQ716476 | - |
| CRSFA.Bot.045 | F. carica | Ostuni | Brindisi | D | D-1 | OQ646770 | OQ716470 | - | OQ716477 | - | |
| Ceratocystis ficicola | CRSFA.Cer.033 | F. carica | Salice Salentino | Lecce | A | A-1 | OQ329983 | OQ352265 | OQ352268 | - | - |
| CRSFA.Cer.035 | F. carica | Squinzano | Lecce | E | E-4 | OQ335969 | OQ352266 | OQ352267 | - | - | |
| CRSFA.Cer.036 | Cryphalus dilutus | Squinzano | Lecce | E | E-3 | OQ939559 | OQ944935 | OQ944934 | - | - | |
| Lasiodiplodia theobromae | CRSFA.Bot.040 | C. dilutus | Guagnano | Lecce | C | C-1 | OQ644250 | OQ716472 | - | OQ716474 | - |
| CRSFA.Bot.038 | F. carica | Guagnano | Lecce | C | C-1 | OQ646769 | OQ657193 | - | OQ716475 | - | |
| Neofusicoccum algeriense | CRSFA.Bot.047 | F. carica | Squinzano | Lecce | E | E-1 | OQ642317 | OQ657192 | - | OQ657191 | - |
| CRSFA.Bot.032 | F. carica | Salice Salentino | Lecce | A | A-1 | OQ652029 | OQ716471 | - | OQ716478 | - | |
| N. macrospora | CRSFA.Fus.09 | F. carica | Salice Salentino | Lecce | A | A-1 | OQ780916 | OQ934042 | OQ944931 | OQ818831 | |
| N. parvum | CRSFA.Bot.048 | F. carica | Squinzano | Lecce | E | E-4 | OQ642323 | OQ674076 | - | OQ716473 | - |
| N. perseae | CRSFA.Fus.03 | F. carica | Copertino | Lecce | B | B-1 | OQ708344 | OR354697 | OQ984265 | - | OQ818623 |
| CRSFA.Fus.010 | F. carica | Salice Salentino | Lecce | A | A-1 | OQ785882 | OR294029 | OR003942 | - | OQ845689 | |
| CRSFA.Fus.014 | F. carica | Squinzano | Lecce | E | E-2 | OQ785883 | OR344502 | OR159909 | - | OQ842738 | |
| CRSFA.Fus.017 | C. dilutus | Squinzano | Lecce | E | E-2 | OQ818688 | OR344503 | OR168709 | - | OQ845690 | |
| CRSFA.Fus.018 | C. dilutus | Squinzano | Lecce | E | E-3 | OQ818687 | OR354698 | OR188777 | - | OQ845693 | |
| CRSFA.Fus.021 | F. carica | Squinzano | Lecce | E | E-4 | OQ818685 | OR344504 | OR188778 | - | OQ845692 | |
| CRSFA.Fus.025 | F. carica | Squinzano | Lecce | E | E-1 | OQ818690 | OR365292 | OR188779 | - | OQ845687 | |
| CRSFA.Fus.030 | F. carica | Squinzano | Lecce | E | E-1 | OQ818689 | OR365293 | OR188780 | - | OQ845691 | |
| Neocosmospora sp. (FSSC 24) | CRSFA.Fus.01 | F. carica | Copertino | Lecce | B | B-1 | OQ708490 | OQ885478 | OQ944929 | - | OQ818180 |
| Neocosmospora sp. (FSSC 25) | CRSFA.Fus.04 | C. dilutus | Salice Salentino | Lecce | A | A-1 | OQ708492 | OQ934040 | OQ984264 | - | OQ818664 |
| CRSFA.Fus.05 | C. dilutus | Salice Salentino | Lecce | A | A-1 | OQ750696 | OQ934041 | OQ944930 | - | OQ818686 | |
| Neocosmospora sp. (FSSC 14) | CRSFA.Fus.015 | F. carica | Squinzano | Lecce | E | E-2 | OQ790083 | OQ934043 | OQ944932 | - | OQ842766 |
| Neocosmospora sp. (FSSC 18) | CRSFA.Fus.024 | F. carica | Squinzano | Lecce | E | E-3 | OQ816805 | OQ934044 | OQ944933 | - | OQ845688 |
| Amplified Gene | Primers Pairs | Reference | Optimized PCR Protocols | Fungal Pathogens |
|---|---|---|---|---|
| ITS | ITS4 ITS5 | [82] | 95°: 5 min, (95° C: 1 min, 58° C: 1 min, 72° C: 1 min) × 25 cycles, 72 °C: 7 min | Fusarium spp. Botryosphaeriaceae Ceratocystis spp. |
| LSU | LR0R LR5 | [83] | 95 °C: 5 min, (95 °C: 1 min, 55 °C: 1 min, 72 °C: 1 min) × 25 cycles, 72 °C: 7 min | Fusarium spp. |
| TEF-1α | Tef1 Tef2 | [84] | 95 °C: 5 min, (95 °C: 1 min, 57 °C: 75 s, 72 °C: 1 min) × 30 cycles, 72 °C: 10 min | Fusarium spp. Ceratocystis spp. |
| ef1-986R ef1-728F | [85] | 95 °C: 5 min, (95 °C: 1 min, 57 °C: 75 s, 72 °C: 1 min) × 30 cycles, 72 °C: 10 min | Botryosphaeriaceae | |
| rpb2 | fRPB2-5F fRPB2-7cR | [86] | 95 °C: 5 min, (95 °C: 1 min, 55 °C: 1 min, 72 °C: 1 min) × 25 cycles, 72 °C: 7 min | Fusarium spp. Ceratocystis spp. |
| β-tubulin | Bt2a Bt2b | [87] | 94 °C: 3 min, (94 °C: 40 s, 58 °C: 1 min, 72 °C: 2 min) × 35 cycles, 72 °C: 10 min | Botryosphaeriaceae |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, W.; Carlucci, M.; Cavalieri, V.; Carbotti, C.; Nigro, F. Unveiling a Disease Complex Threatening Fig (Ficus carica L.) Cultivation in Southern Italy. Plants 2025, 14, 2865. https://doi.org/10.3390/plants14182865
Habib W, Carlucci M, Cavalieri V, Carbotti C, Nigro F. Unveiling a Disease Complex Threatening Fig (Ficus carica L.) Cultivation in Southern Italy. Plants. 2025; 14(18):2865. https://doi.org/10.3390/plants14182865
Chicago/Turabian StyleHabib, Wassim, Mariangela Carlucci, Vincenzo Cavalieri, Cecilia Carbotti, and Franco Nigro. 2025. "Unveiling a Disease Complex Threatening Fig (Ficus carica L.) Cultivation in Southern Italy" Plants 14, no. 18: 2865. https://doi.org/10.3390/plants14182865
APA StyleHabib, W., Carlucci, M., Cavalieri, V., Carbotti, C., & Nigro, F. (2025). Unveiling a Disease Complex Threatening Fig (Ficus carica L.) Cultivation in Southern Italy. Plants, 14(18), 2865. https://doi.org/10.3390/plants14182865








