Long-Term Nitrogen Fertilization Increases Soil Organic Carbon and Wheat Yields on Purple Soil in China
Abstract
1. Introduction
2. Results
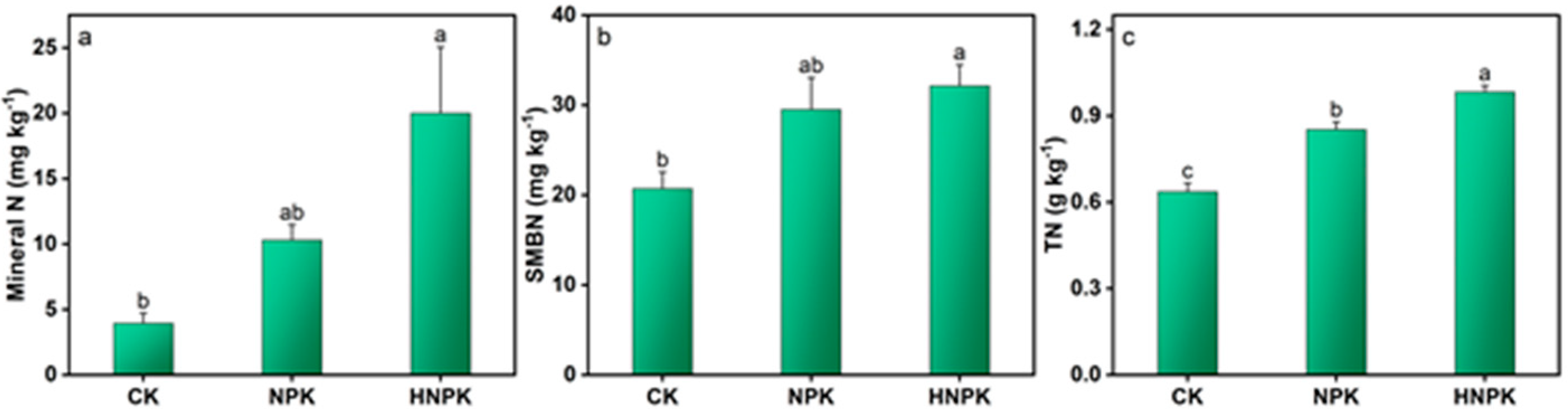
2.1. Effects of N Fertilization on Soil N and C Pools
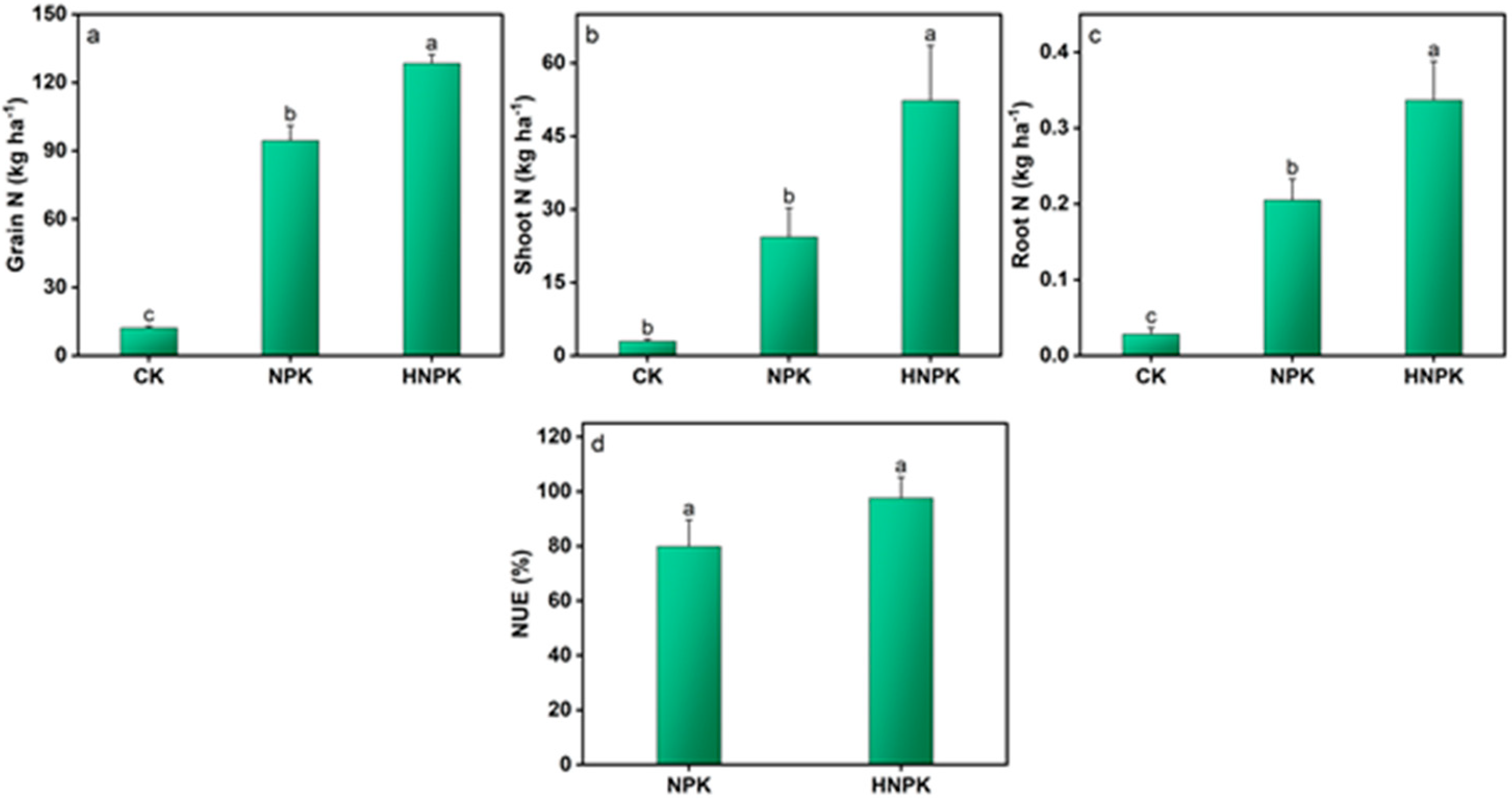
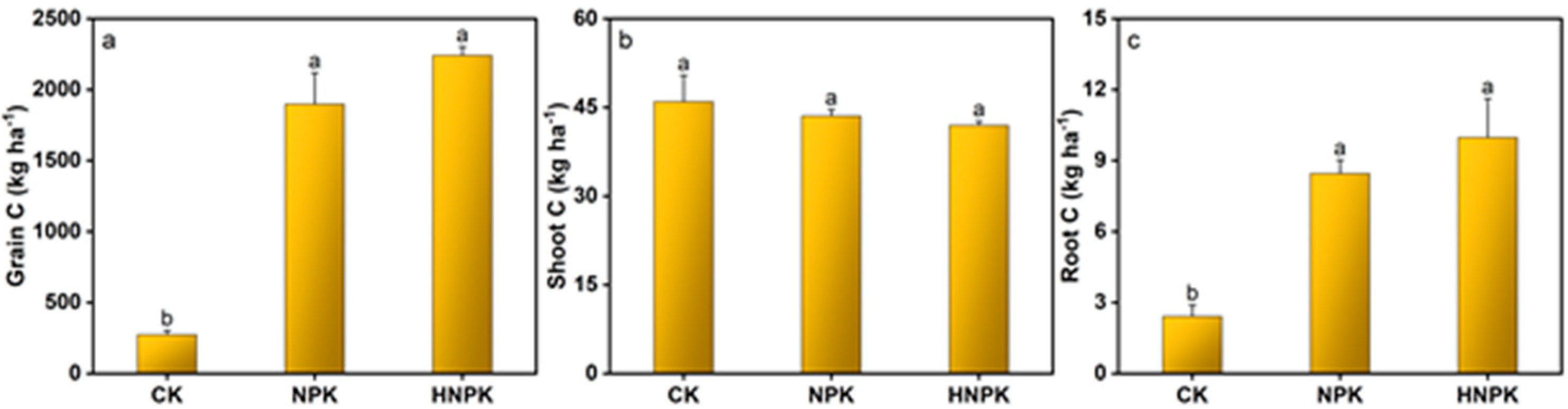
2.2. Effects of N Fertilization on Plant Nutrients and NUE
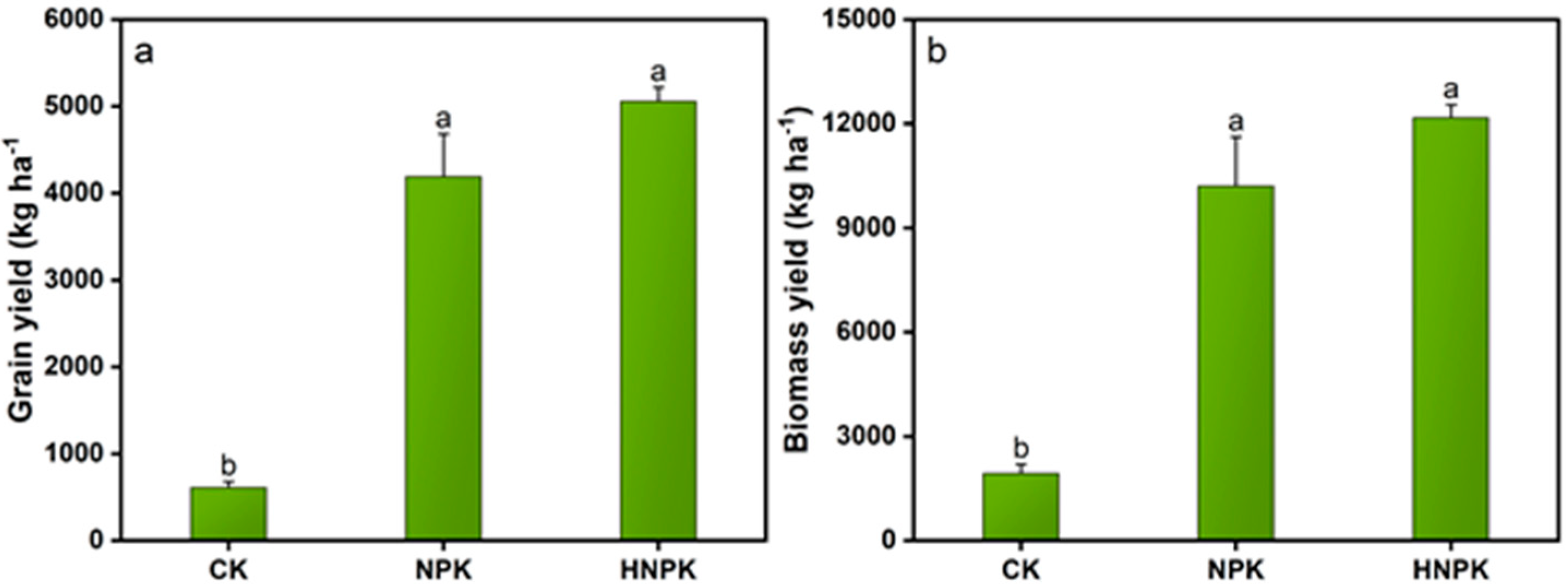
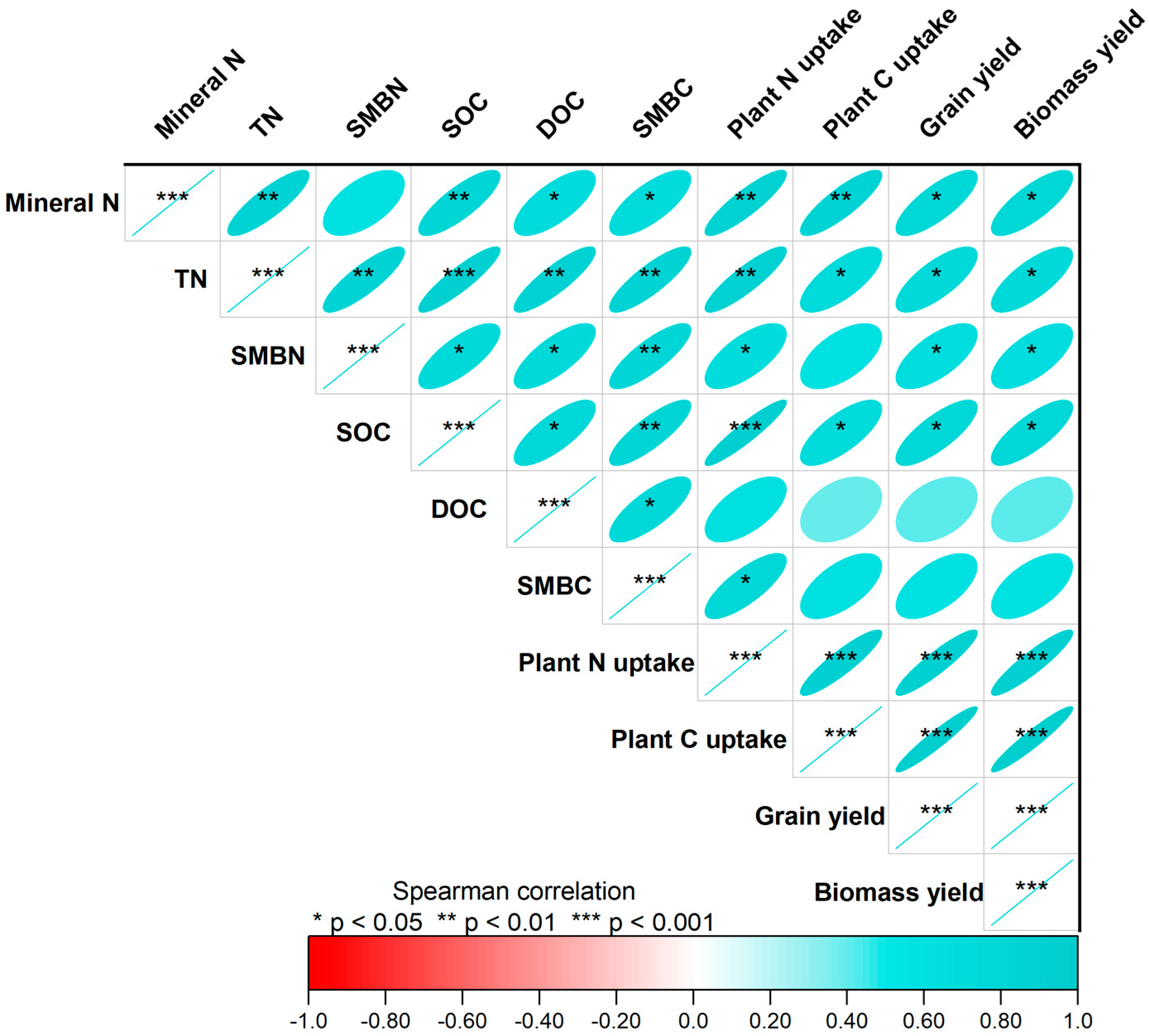
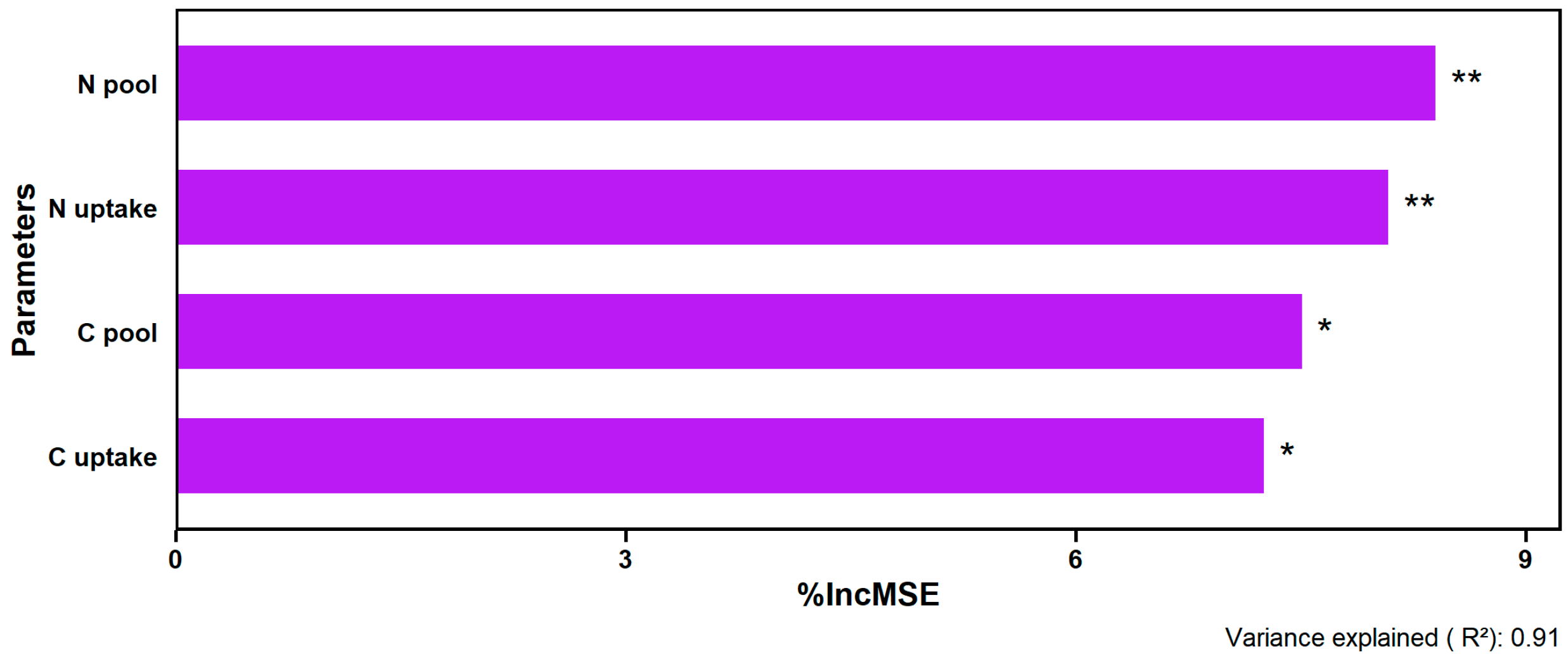
2.3. Effects of N Fertilization on Yields and Their Relationship with Soil and Plant Nutrients
3. Discussion
3.1. N Fertilization Effects on Soil N and C Pools
3.2. N Fertilization Effects on Plant Nutrient Uptake and NUE
3.3. N Fertilization Effects on Yields and Their Relationship with Plant and Soil Nutrients
4. Materials and Methods
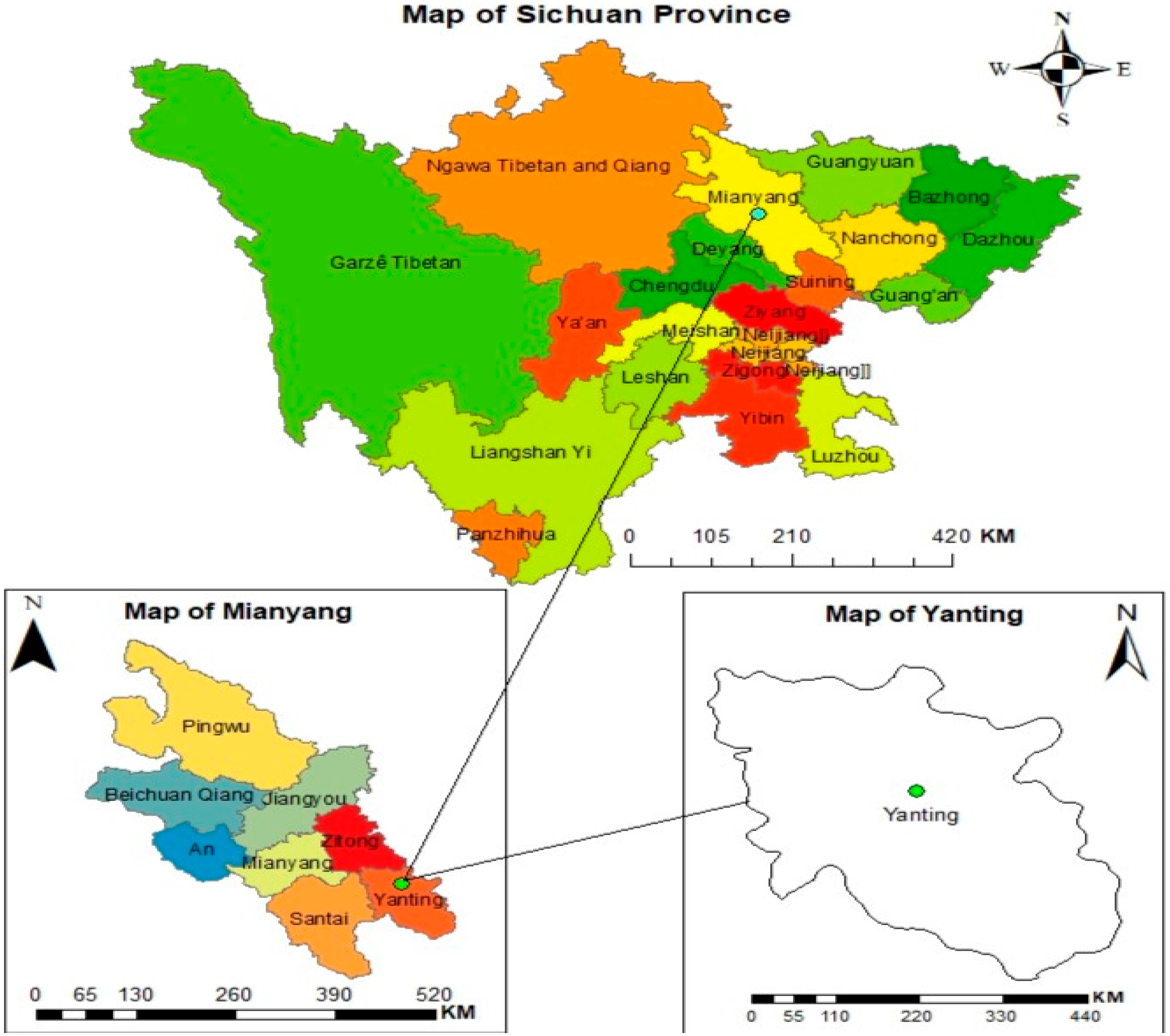
4.1. Experimental Site Characteristics
| Parameter | Value | Unit |
|---|---|---|
| pH | 8.30 | |
| Mineral N | 42.29 | mg kg−1 |
| Total N | 0.62 | g kg−1 |
| Unsaturated hydraulic conductivity | 16.80 | mm h−1 |
| Organic matter | 8.75 | g kg−1 |
4.2. Experimental Design
4.3. Soil Sampling and Analysis
4.4. Plant Nutrient Content and Yield Determination
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhtar, S.; Mekonnen, T.W.; Mashingaidze, K.; Osthoff, G.; Labuschagne, M. Heterosis and Combining Ability of Iron, Zinc and Their Bioavailability in Maize Inbred Lines under Low Nitrogen and Optimal Environments. Heliyon 2023, 9, e14177. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a Century of Ammonia Synthesis Changed the World. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Ghose, B. Food Security and Food Self-sufficiency in China: From Past to 2050. Food Energy Secur. 2014, 3, 86–95. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H. Global Nitrogen and Phosphorus Fertilizer Use for Agriculture Production in the Past Half Century: Shifted Hot Spots and Nutrient Imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef]
- Stewart, W.M.; Dibb, D.W.; Johnston, A.E.; Smyth, T.J. The Contribution of Commercial Fertilizer Nutrients to Food Production. Agron. J. 2005, 97, 1–6. [Google Scholar] [CrossRef]
- Du, C.; Liu, Y.; Guo, J.; Zhang, W.; Xu, R.; Zhou, B.; Xiao, X.; Zhang, Z.; Gao, Z.; Zhang, Y.; et al. Novel Annual Nitrogen Management Strategy Improves Crop Yield and Reduces Greenhouse Gas Emissions in Wheat-Maize Rotation Systems under Limited Irrigation. J. Environ. Manag. 2024, 353, 120236. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; van Grinsven, H.; Winiwarter, W. Too Much of a Good Thing. Nature 2011, 472, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, T.; Kuang, F.; Luo, Z.; Tang, J.; Xu, T. Measurements of Nitrate Leaching from a Hillslope Cropland in the Central Sichuan Basin, China. Soil Sci. Soc. Am. J. 2009, 73, 1419–1426. [Google Scholar] [CrossRef]
- Hina, N. A Comprehensive Meta-Analysis of Nitrate Leaching from Wheat, Rice, and Maize Cultivation on a Global Scale. J. Agric. Food Res. 2025, 19, 101722. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Díaz, R.J.; Levin, L.A.; Turner, R.E.; Gilbert, D.; Zhang, J. Dynamics and Distribution of Natural and Human-Caused Hypoxia. Biogeosciences 2010, 7, 585–619. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Passioura, J.B.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Nutrient Availability Limits Carbon Sequestration in Arable Soils. Soil Biol. Biochem. 2014, 68, 402–409. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Gebremikael, M.T.; Wu, H.; Cai, D.; Wang, B.; Li, B.; Zhang, J.; Li, Y.; Xi, J. Response of Soil Organic Carbon Fractions, Microbial Community Composition and Carbon Mineralization to High-Input Fertilizer Practices under an Intensive Agricultural System. PLoS ONE 2018, 13, e0195144. [Google Scholar] [CrossRef]
- Kamran, M.; Huang, L.; Nie, J.; Geng, M.; Lu, Y.; Liao, Y.; Zhou, F.; Xu, Y. Effect of Reduced Mineral Fertilization (NPK) Combined with Green Manure on Aggregate Stability and Soil Organic Carbon Fractions in a Fluvo-Aquic Paddy Soil. Soil Tillage Res. 2021, 211, 105005. [Google Scholar] [CrossRef]
- Powlson, D.S.; Whitmore, A.P.; Goulding, K.W.T. Soil Carbon Sequestration to Mitigate Climate Change: A Critical Re-examination to Identify the True and the False. Eur. J. Soil Sci. 2011, 62, 42–55. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, X.; Tang, W.; Li, C.; Wang, K.; Li, W.; Cheng, K.; Pan, X. Long-Term Effects of NPK Fertilizers and Organic Manures on Soil Organic Carbon and Carbon Management Index under a Double-Cropping Rice System in Southern China. Commun. Soil Sci. Plant Anal. 2018, 49, 1976–1989. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J.; Hu, T.; Gong, Y. Long-Term Manuring and Fertilization Effects on Soil Organic Carbon Pools under a Wheat–Maize Cropping System in North China Plain. Plant Soil 2009, 314, 67–76. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R.; Boast, C.W. The Myth of Nitrogen Fertilization for Soil Carbon Sequestration. J. Environ. Qual. 2007, 36, 1821–1832. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, S.; Lu, C.; Li, X.; Li, F.; Wang, T. Effects of Different Tillage and Fertilization Management Practices on Soil Organic Carbon and Aggregates under the Rice–Wheat Rotation System. Soil Tillage Res. 2021, 212, 105071. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-Term Effects of Mineral Fertilizers on Soil Microorganisms–A Review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Chen, L.; Li, K.K.; Shi, W.J.; Wang, X.L.; Wang, E.T.; Liu, J.F.; Sui, X.H.; Mi, G.H.; Tian, C.F.; Chen, W.X. Negative Impacts of Excessive Nitrogen Fertilization on the Abundance and Diversity of Diazotrophs in Black Soil under Maize Monocropping. Geoderma 2021, 393, 114999. [Google Scholar] [CrossRef]
- Huddell, A.; Ernfors, M.; Crews, T.; Vico, G.; Menge, D.N.L. Nitrate Leaching Losses and the Fate of 15N Fertilizer in Perennial Intermediate Wheatgrass and Annual Wheat—A Field Study. Sci. Total Environ. 2023, 857, 159255. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Xia, L.; Akiyama, H.; Chen, X.; Chen, J.; Fang, Y.; Vancov, T.; Li, Y.; Yao, Y.; et al. Accounting for Differences between Crops and Regions Reduces Estimates of Nitrate Leaching from Nitrogen-Fertilized Soils. Commun. Earth Environ. 2025, 6, 29. [Google Scholar] [CrossRef]
- Xia, L.; Lam, S.K.; Chen, D.; Wang, J.; Tang, Q.; Yan, X. Can Knowledge-Based N Management Produce More Staple Grain with Lower Greenhouse Gas Emission and Reactive Nitrogen Pollution? A Meta-Analysis. Glob. Change Biol. 2017, 23, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Bah, H.; Zhou, M.; Ren, X.; Hu, L.; Dong, Z.; Zhu, B. Effects of Organic Amendment Applications on Nitrogen and Phosphorus Losses from Sloping Cropland in the Upper Yangtze River. Agric. Ecosyst. Environ. 2020, 302, 107086. [Google Scholar] [CrossRef]
- Zhu, B.; Yao, Z.; Hu, D.; Bah, H. Effects of Substitution of Mineral Nitrogen with Organic Amendments on Nitrogen Loss from Sloping Cropland of Purple Soil. Front. Agric. Sci. Eng. 2022, 9, 396–406. [Google Scholar] [CrossRef]
- He, M.; Xin, X.; Meng, L.; Yan, X.; Zhao, C.; Cai, Z.; Zhu, A.; Zhang, J.; Müller, C. Long-Term Appropriate N Management Can Continuously Enhance Gross N Mineralization Rates and Crop Yields in a Maize-Wheat Rotation System. Biol. Fertil. Soils 2023, 59, 501–511. [Google Scholar] [CrossRef]
- Ros, G.H.; Hanegraaf, M.C.; Hoffland, E.; Van Riemsdijk, W.H. Predicting Soil N Mineralization: Relevance of Organic Matter Fractions and Soil Properties. Soil Biol. Biochem. 2011, 43, 1714–1722. [Google Scholar] [CrossRef]
- Dalias, P.; Christou, A. Secondary Immobilization as a Phase of N Mineralization Dynamics of Soil Organic Inputs. Nitrogen 2022, 3, 600–607. [Google Scholar] [CrossRef]
- Morais, F.A.; Gatiboni, L.C. Phosphorus Availability and Microbial Immobilization in a Nitisol with the Application of Mineral and Organo-Mineral Fertilizers. An. Acad. Bras. Ciênc. 2015, 87, 2289–2299. [Google Scholar] [CrossRef]
- Zheng, X.; Oba, B.T.; Wang, H.; Shen, C.; Zhao, R.; Zhao, D.; Ding, H. Organo-Mineral Complexes Alter Bacterial Composition and Induce Carbon and Nitrogen Cycling in the Rhizosphere. Sci. Total Environ. 2022, 836, 155671. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Fierer, N. Microbial Nitrogen Limitation Increases Decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of Microbial Nitrogen Use Efficiency to Carbon: Nitrogen Imbalances Regulates Soil Nitrogen Cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Xu, X. Competition between Roots and Microorganisms for Nitrogen: Mechanisms and Ecological Relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zeng, Z.; Song, Z.; Wang, F.; Tian, D.; Mi, W.; Huang, X.; Wang, J.; Song, L.; Yang, Z.; et al. Vital Roles of Soil Microbes in Driving Terrestrial Nitrogen Immobilization. Glob. Change Biol. 2021, 27, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P.; Bennett, J. Nitrogen Mineralization: Challenges of A Changing Paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Gupta, V.V.S.R.; Germida, J.J. Soil Aggregation: Influence on Microbial Biomass and Implications for Biological Processes. Soil Biol. Biochem. 2015, 80, A3–A9. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The Interplay between Microbial Communities and Soil Properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Wu, S.; Konhauser, K.O.; Chen, B.; Huang, L. “Reactive Mineral Sink” Drives Soil Organic Matter Dynamics and Stabilization. Npj Mater. Sustain. 2023, 1, 3. [Google Scholar] [CrossRef]
- Christopher, S.F.; Lal, R. Nitrogen Management Affects Carbon Sequestration in North American Cropland Soils. Crit. Rev. Plant Sci. 2007, 26, 45–64. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Xu, F.; Du, J.; Tian, X.; Shi, J.; Wei, G. Organic Amendments Affect Soil Organic Carbon Sequestration and Fractions in Fields with Long-Term Contrasting Nitrogen Applications. Agric. Ecosyst. Environ. 2021, 322, 107643. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, M.; Zeng, F.; Xu, P.; Ma, S.; Zhang, B.; Li, Z.; Wang, Y.; Zhu, B. How Do Soil Organic Carbon Pool, Stock and Their Stability Respond to Crop Residue Incorporation in Subtropical Calcareous Agricultural Soils? Agric. Ecosyst. Environ. 2022, 332, 107927. [Google Scholar] [CrossRef]
- Mo, F.; Yang, D.; Wang, X.; Crowther, T.W.; Vinay, N.; Luo, Z.; Yu, K.; Sun, S.; Zhang, F.; Xiong, Y.; et al. Nutrient Limitation of Soil Organic Carbon Stocks under Straw Return. Soil Biol. Biochem. 2024, 192, 109360. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) Framework Integrates Plant Litter Decomposition with Soil Organic Matter Stabilization: Do Labile Plant Inputs Form Stable Soil Organic Matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Ma, X.; Ma, W.; Wang, C.; Xu, Y. Nitrogen and Phosphorus Supply Controls Stability of Soil Organic Carbon in Alpine Meadow of the Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2025, 379, 109336. [Google Scholar] [CrossRef]
- Manzoni, S.; Cotrufo, M.F. Mechanisms of Soil Organic Carbon and Nitrogen Stabilization in Mineral-Associated Organic Matter–Insights from Modeling in Phase Space. Biogeosciences 2024, 21, 4077–4098. [Google Scholar] [CrossRef]
- Li, L.-J.; Zhu-Barker, X.; Ye, R.; Doane, T.A.; Horwath, W.R. Soil Microbial Biomass Size and Soil Carbon Influence the Priming Effect from Carbon Inputs Depending on Nitrogen Availability. Soil Biol. Biochem. 2018, 119, 41–49. [Google Scholar] [CrossRef]
- Wang, W.; Chen, G.-R.; Li, M.-Y.; Chen, Y.; Wang, Y.; Tao, H.-Y.; Hou, H.-Z.; Rehman, M.M.U.; Ashraf, M.; Song, Y.; et al. Long-Term Cereal-Legume Intercropping Accelerates Soil Organic Carbon Loss in Subsoil of Dryland. Resour. Conserv. Recycl. 2024, 211, 107898. [Google Scholar] [CrossRef]
- Liu, F.; Wang, D.; Zhang, B.; Huang, J. Concentration and Biodegradability of Dissolved Organic Carbon Derived from Soils: A Global Perspective. Sci. Total Environ. 2021, 754, 142378. [Google Scholar] [CrossRef] [PubMed]
- Yeerken, S.; Deng, M.; Li, L.; Thi Kinh, C.; Wang, Z.; Huang, Y.; Xiao, Y.; Song, K. Evaluating the Role of High N2O Affinity Complete Denitrifiers and Non-Denitrifying N2O Reducing Bacteria in Reducing N2O Emissions in River. J. Hazard. Mater. 2024, 479, 135602. [Google Scholar] [CrossRef]
- Gao, H.; Song, X.; Wu, X.; Zhang, N.; Liang, T.; Wang, Z.; Yu, X.; Duan, C.; Han, Z.; Li, S. Interactive Effects of Soil Erosion and Mechanical Compaction on Soil DOC Dynamics and CO2 Emissions in Sloping Arable Land. CATENA 2024, 238, 107906. [Google Scholar] [CrossRef]
- Ren, T.; Ukalska-Jaruga, A.; Smreczak, B.; Cai, A. Dissolved Organic Carbon in Cropland Soils: A Global Meta-Analysis of Management Effects. Agric. Ecosyst. Environ. 2024, 371, 109080. [Google Scholar] [CrossRef]
- Zhang, P.; Jiao, R.; Gong, C.; Wang, F.; Yan, W.; Li, Q.; Wang, D. Turnover of Dissolved Organic Carbon Fuels Nocturnal CO2 Emissions from a Headwater Catchment Reservoir, Southeastern China: Effects of Ecosystem Metabolism on Source Partitioning of CO2 Emissions. J. Environ. Sci. 2022, 121, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Z.; Zhang, X.; Xu, X.; Chen, H.; Xiong, Z. Net Global Warming Potential and Greenhouse Gas Intensity from the Double Rice System with Integrated Soil–Crop System Management: A Three-Year Field Study. Atmos. Environ. 2015, 116, 92–101. [Google Scholar] [CrossRef]
- Pei, Y.; Chen, X.; Niu, Z.; Su, X.; Wang, Y.; Wang, X. Effects of Nitrogen Fertilizer Substitution by Cow Manure on Yield, Net GHG Emissions, Carbon and Nitrogen Footprints in Sweet Maize Farmland in the Pearl River Delta in China. J. Clean. Prod. 2023, 399, 136676. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen Uptake, Assimilation and Remobilization in Plants: Challenges for Sustainable and Productive Agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Zhao, D.; Li, X.; Liu, Y.; Shi, S.; Wang, G. A Comparative Study on the Characteristics of Material Transport and Their Effects on Single Spike Yield in Different Winter Wheat Varieties under Drought Stress 2025. Adv. Resour. Res. 2025, 5, 1049–1064. [Google Scholar]
- Abid, M.; Tian, Z.; Ata-Ul-Karim, S.T.; Cui, Y.; Liu, Y.; Zahoor, R.; Jiang, D.; Dai, T. Nitrogen Nutrition Improves the Potential of Wheat (Triticum aestivum L.) to Alleviate the Effects of Drought Stress during Vegetative Growth Periods. Front. Plant Sci. 2016, 7, 981. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, X.-Y.; Ren, H.; Zhang, J.-W.; Zhao, B.; Ren, B.-Z.; Liu, P. Deep Nitrogen Fertilizer Placement Improves the Yield of Summer Maize (Zea mays L.) by Enhancing Its Photosynthetic Performance after Silking. BMC Plant Biol. 2025, 25, 172. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shi, Y.; Yu, Z.; Zhang, Y.; Zhang, Z. Optimizing Nitrogen Application Strategies Can Improve Grain Yield by Increasing Dry Matter Translocation, Promoting Grain Filling, and Improving Harvest Indices. Front. Plant Sci. 2025, 16, 1565446. [Google Scholar] [CrossRef]
- Posada, J.M.; Schuur, E.A.G. Relationships among Precipitation Regime, Nutrient Availability, and Carbon Turnover in Tropical Rain Forests. Oecologia 2011, 165, 783–795. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Zhang, L.; Chen, Y.; Baskin, C.C. Effects of Increased Precipitation on C, N and P Stoichiometry at Different Growth Stages of a Cold Desert Annual. Glob. Ecol. Conserv. 2022, 37, e02158. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, N.; Kumar, A. Enhancing Resource Use Efficiency in Crops Through Plant Functional Traits. In Plant Functional Traits for Improving Productivity; Springer: Singapore, 2024; pp. 97–117. [Google Scholar]
- Gonzalez-Dugo, V.; Durand, J.-L.; Gastal, F. Water Deficit and Nitrogen Nutrition of Crops. A Review. Agron. Sustain. Dev. 2010, 30, 529–544. [Google Scholar] [CrossRef]
- Lawlor, D.W. Carbon and Nitrogen Assimilation in Relation to Yield: Mechanisms Are the Key to Understanding Production Systems. J. Exp. Bot. 2002, 53, 773–787. [Google Scholar] [CrossRef]
- Liu, S.; An, X.; Xu, C.; He, D.; Li, X.; Chen, C.; Guo, B.; Xu, D.; Huang, J. Integrative Transcriptomic-Physiological Analysis Deciphers Nitrogen-Mediated Carbon Reallocation Balancing Growth and Flavonoid Metabolism in Epimedium Pubescens. Front. Plant Sci. 2025, 16, 1539445. [Google Scholar] [CrossRef]
- Makino, A. Photosynthesis, Grain Yield, and Nitrogen Utilization in Rice and Wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Li, L.; Xie, J.; Fudjoe, S.K.; Zhang, R.; Luo, Z.; Anwar, S. Nitrogen Supply Affects Grain Yield by Regulating Antioxidant Enzyme Activity and Photosynthetic Capacity of Maize Plant in the Loess Plateau. Agronomy 2021, 11, 1094. [Google Scholar] [CrossRef]
- Luo, W.; Li, M.; Sardans, J.; Lü, X.; Wang, C.; Peñuelas, J.; Wang, Z.; Han, X.; Jiang, Y. Carbon and Nitrogen Allocation Shifts in Plants and Soils along Aridity and Fertility Gradients in Grasslands of China. Ecol. Evol. 2017, 7, 6927–6934. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Shi, S.; Rong, Y.; Liu, J.; Zhang, L. Contrasting Allocation Patterns in Wheat and Weeds: Allometric Belowground and Reproductive Investment versus Optimal Partitioning Adaptations. Front. Plant Sci. 2025, 16, 1542205. [Google Scholar] [CrossRef]
- Chinthalapudi, D.P.M.; Kingery, W.; Ganapathi Shanmugam, S. A Review of Plant-Mediated and Fertilization-Induced Shifts in Ammonia Oxidizers: Implications for Nitrogen Cycling in Agroecosystems. Land 2025, 14, 1182. [Google Scholar] [CrossRef]
- Drinkwater, L.E.; Snapp, S.S. Nutrients in Agroecosystems: Rethinking the Management Paradigm. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2007; Volume 92, pp. 163–186. [Google Scholar]
- Nabi, F.; Yi, Z.; Kama, R.; Sajid, S.; Li, H. Optimizing Nitrogen Management to Enhance Growth and Minimize Pollution Risk in Pennisetum Hydridum Cultivation. Agronomy 2025, 15, 1452. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, Nitrogen-Use Efficiency, and Nitrogen Management. AMBIO J. Hum. Environ. 2002, 31, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.J. Genetic Variation in Traits for Nitrogen Use Efficiency in Wheat. J. Exp. Bot. 2017, 68, 2627–2632. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Brüggemann, N.; Dannenmann, M.; Wang, Y.; Butterbach-Bahl, K. Sustaining Crop Productivity While Reducing Environmental Nitrogen Losses in the Subtropical Wheat-Maize Cropping Systems: A Comprehensive Case Study of Nitrogen Cycling and Balance. Agric. Ecosyst. Environ. 2016, 231, 1–14. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Wang, X.; Wang, Y. Long-Term Field Measurements of Annual Methane and Nitrous Oxide Emissions from a Chinese Subtropical Wheat-Rice Rotation System. Soil Biol. Biochem. 2017, 115, 21–34. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, Y.; Zhu, T.; Zhao, F. Response Patterns of Soil Nitrogen Cycling to Crop Residue Addition: A Review. J. Soil Sci. Plant Nutr. 2024, 24, 1761–1774. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 88, pp. 97–185. [Google Scholar]
- Wang, J.; Sun, N.; Xu, M.; Wang, S.; Zhang, J.; Cai, Z.; Cheng, Y. The Influence of Long-Term Animal Manure and Crop Residue Application on Abiotic and Biotic N Immobilization in an Acidified Agricultural Soil. Geoderma 2019, 337, 710–717. [Google Scholar] [CrossRef]
- Hirsch, P.R.; Mauchline, T.H. The Importance of the Microbial N Cycle in Soil for Crop Plant Nutrition. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 93, pp. 45–71. [Google Scholar]
- Imran; Ortas, I. Mechanism in Soil Health Improvement with Soil Microbial Actions and Its Role in Potential Agriculture. Commun. Soil Sci. Plant Anal. 2025, 56, 2066–2087. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Z.; Li, J.; Li, J.; Xu, M. Fertilization Decreases Microbial CUE via Enhancing Soil Properties and Microbial Respiration in Coal Mine Reclamation Area. Appl. Soil Ecol. 2025, 211, 106145. [Google Scholar] [CrossRef]
- Zang, H.; Wang, J.; Kuzyakov, Y. N Fertilization Decreases Soil Organic Matter Decomposition in the Rhizosphere. Appl. Soil Ecol. 2016, 108, 47–53. [Google Scholar] [CrossRef]
- Jia, H.; Lei, A.; Lei, J.; Ye, M.; Zhao, J. Effects of Hydrological Processes on Nitrogen Loss in Purple Soil. Agric. Water Manag. 2007, 89, 89–97. [Google Scholar] [CrossRef]
- Yao, Z.; Hu, H.; Li, Y.; Sun, X.; Adl, S.; Wang, X.; Zhang, Y.; Zhu, B. Soil Micro-Food Webs at Aggregate Scale Are Associated with Soil Nitrogen Supply and Crop Yield. Geoderma 2024, 442, 116801. [Google Scholar] [CrossRef]
- Luo, X.; Shi, S.; Liu, Y.; Yang, H.; Li, N.; Dong, Z.; Zhu, B.; He, X. Arbuscular Mycorrhizal Fungal Communities of Topsoil and Subsoil of an Annual Maize-Wheat Rotation after 15-Years of Differential Mineral and Organic Fertilization. Agric. Ecosyst. Environ. 2021, 315, 107442. [Google Scholar] [CrossRef]
- McLauchlan, K.K.; Hobbie, S.E. Comparison of Labile Soil Organic Matter Fractionation Techniques. Soil Sci. Soc. Am. J. 2004, 68, 1616–1625. [Google Scholar] [CrossRef]
- Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Jones, D.L.; Willett, V.B. Experimental Evaluation of Methods to Quantify Dissolved Organic Nitrogen (DON) and Dissolved Organic Carbon (DOC) in Soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen—Inorganic Forms. In Methods of Soil Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1996; pp. 1123–1184. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform Fumigation and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of Soil Microbial Biomass C by Fumigation-Extraction—An Automated Procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2021. Available online: http://www.R-project.org/ (accessed on 22 June 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, J.; Yao, Z.; Raza, S.T.; Iqbal, H.; Zhu, B. Long-Term Nitrogen Fertilization Increases Soil Organic Carbon and Wheat Yields on Purple Soil in China. Plants 2025, 14, 2866. https://doi.org/10.3390/plants14182866
Iqbal J, Yao Z, Raza ST, Iqbal H, Zhu B. Long-Term Nitrogen Fertilization Increases Soil Organic Carbon and Wheat Yields on Purple Soil in China. Plants. 2025; 14(18):2866. https://doi.org/10.3390/plants14182866
Chicago/Turabian StyleIqbal, Jasim, Zhiyuan Yao, Syed Turab Raza, Hassan Iqbal, and Bo Zhu. 2025. "Long-Term Nitrogen Fertilization Increases Soil Organic Carbon and Wheat Yields on Purple Soil in China" Plants 14, no. 18: 2866. https://doi.org/10.3390/plants14182866
APA StyleIqbal, J., Yao, Z., Raza, S. T., Iqbal, H., & Zhu, B. (2025). Long-Term Nitrogen Fertilization Increases Soil Organic Carbon and Wheat Yields on Purple Soil in China. Plants, 14(18), 2866. https://doi.org/10.3390/plants14182866








