Comparative Physiological Responses of Lemna aequinoctialis and Spirodela polyrhiza to Mercury Stress: Implications for Biomonitoring and Phytoremediation
Abstract
1. Introduction
2. Results
2.1. Growth Parameters
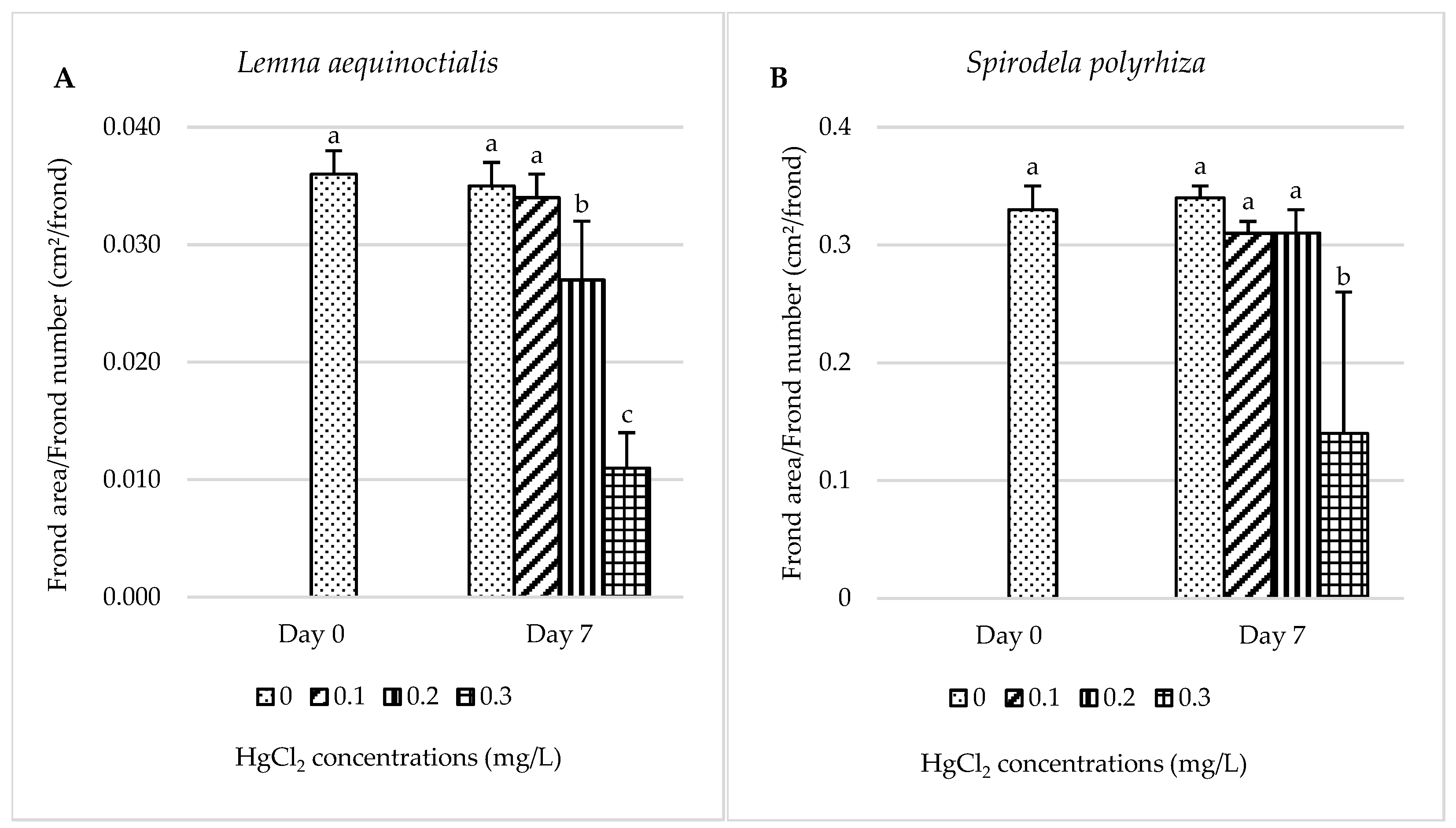
2.1.1. Frond Area
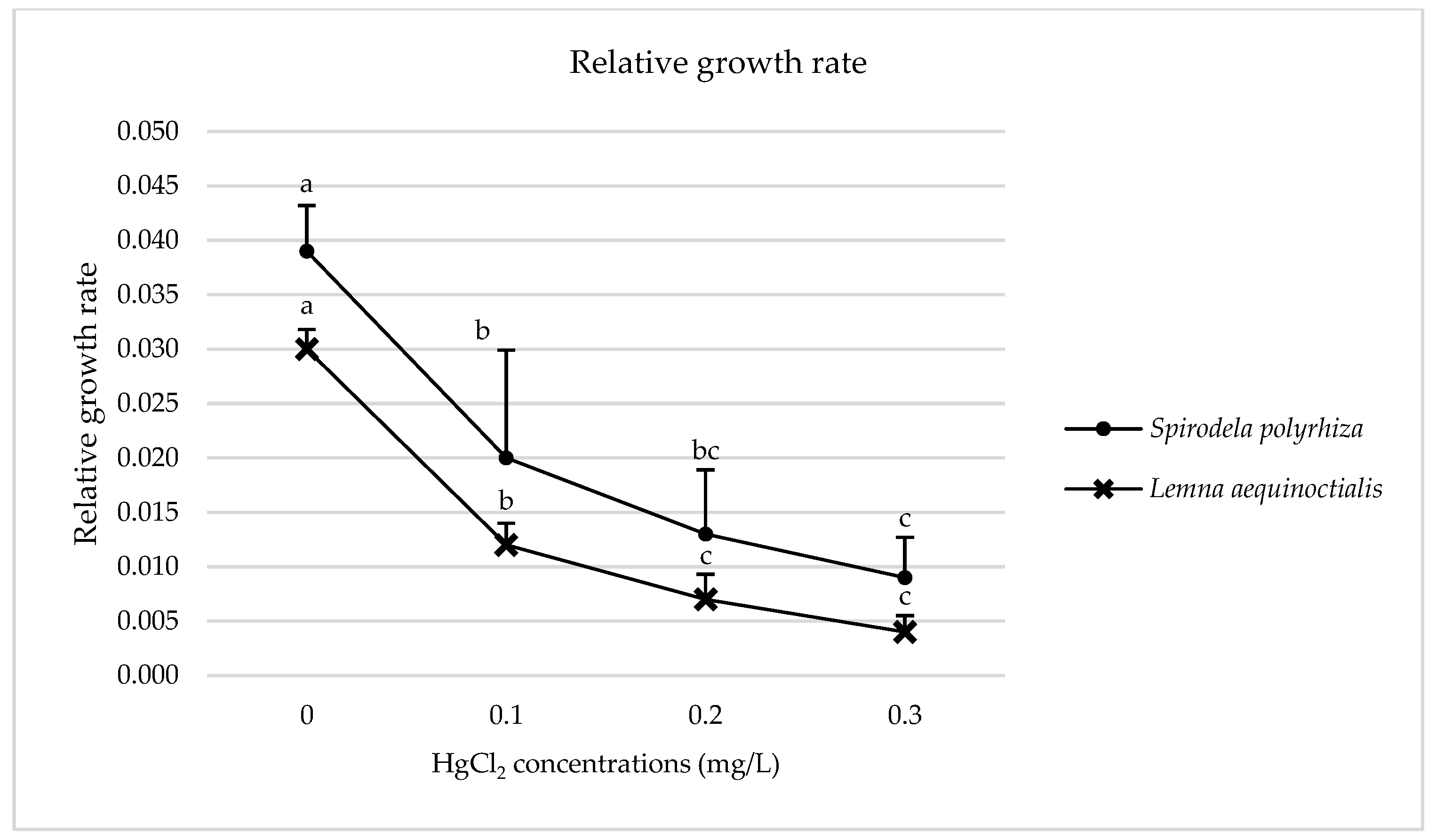
2.1.2. Relative Growth Rate
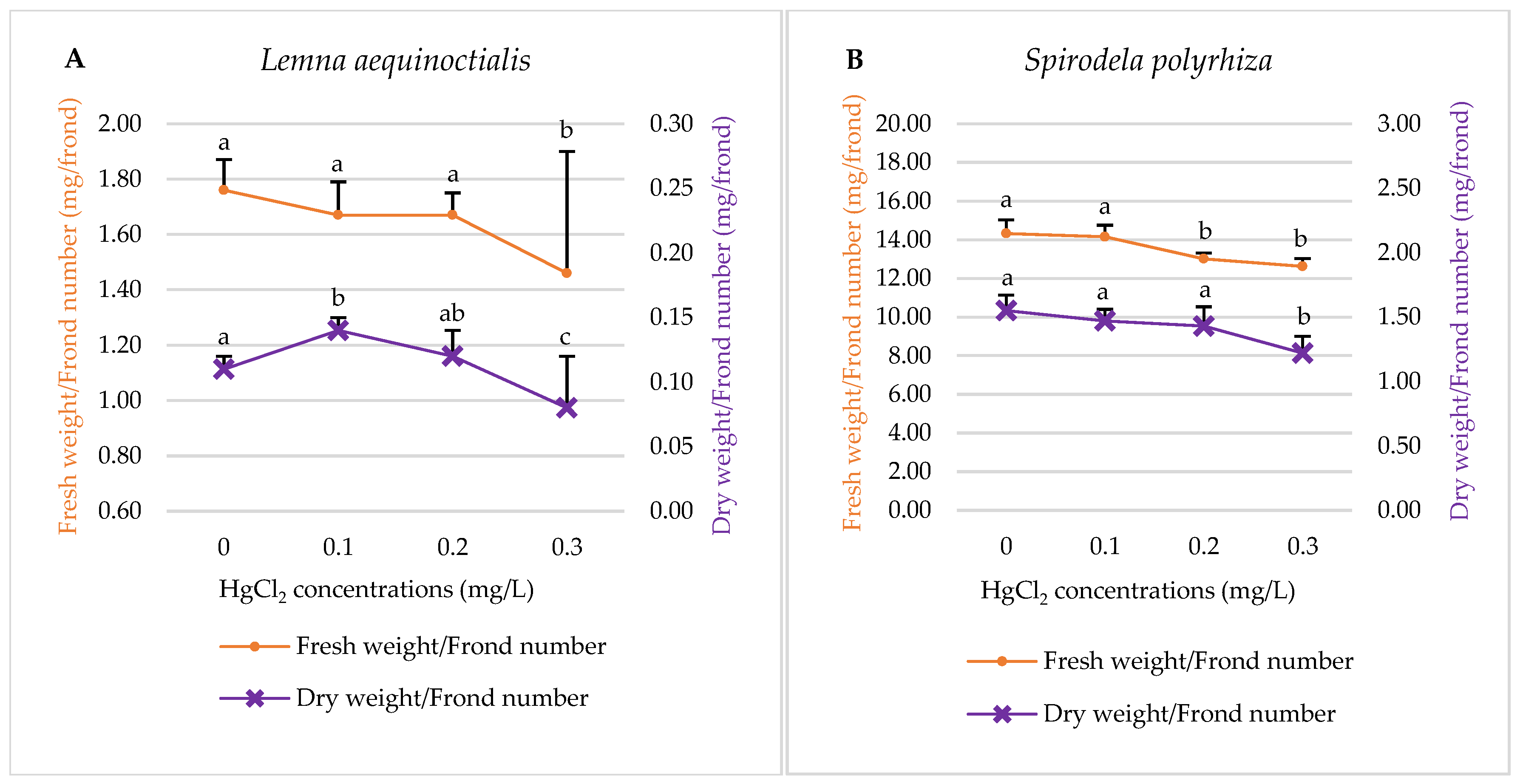
2.1.3. Fresh Weight and Dry Weight
2.1.4. Cumulative Colony Breakup
2.2. Pigments and Photosynthetic Parameters
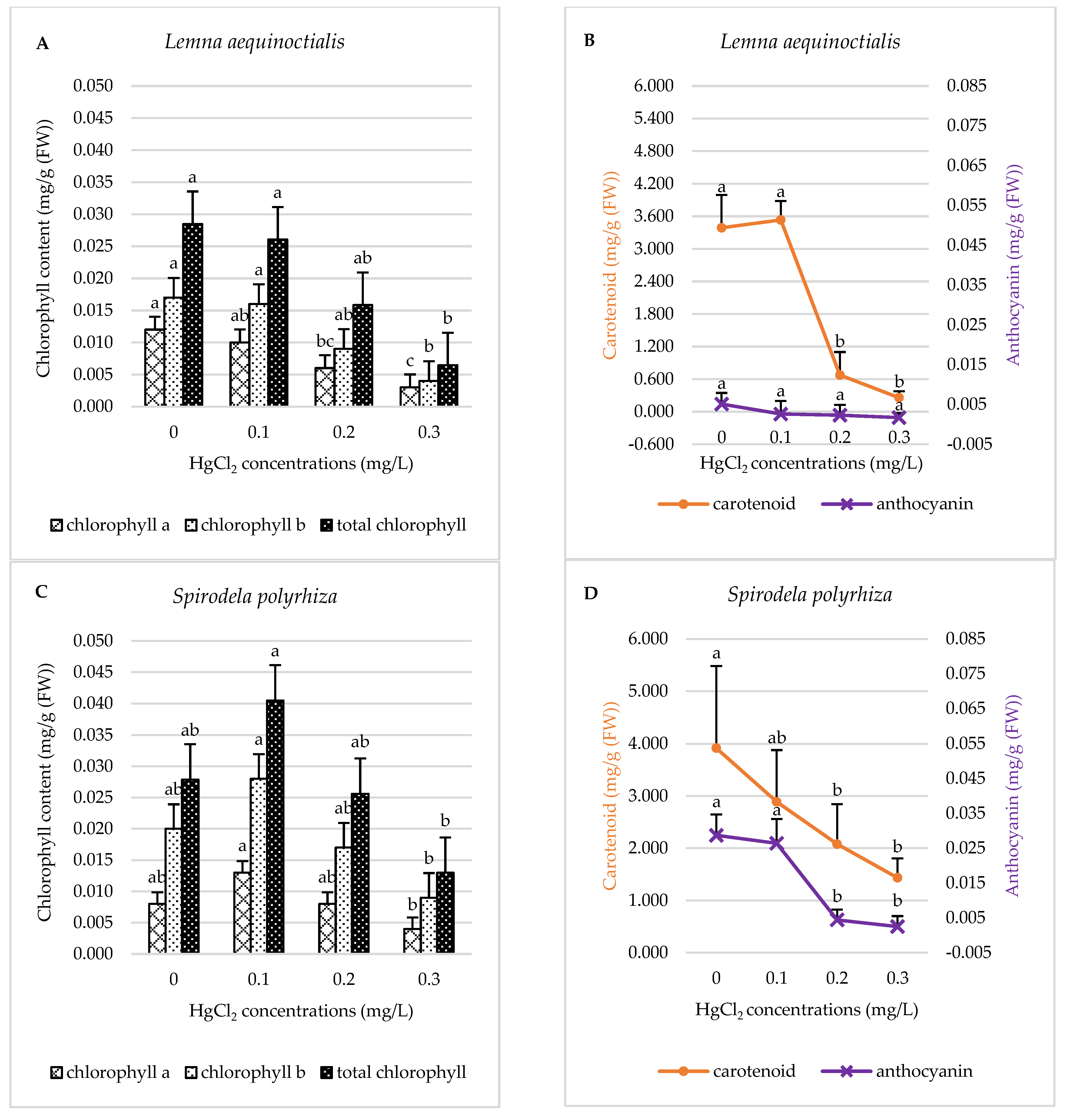
2.2.1. Pigment Content
2.2.2. Chlorophyll Fluorescence
3. Discussion
4. Materials and Methods
4.1. Growth Parameters
4.2. Pigment Content
4.3. Chlorophyll Fluorescence
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Yang, J.; Li, G.; Bishopp, A.; Heenatigala, P.; Hu, S.; Chen, Y.; Wu, Z.; Kumar, S.; Duan, P.; Yao, L. A comparison of growth on mercuric chloride for three Lemnaceae species reveals differences in growth dynamics that effect their suitability for use in either monitoring or remediating ecosystems contaminated with mercury. Front. Chem. 2018, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.M.; Walker Jr, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Mercury; U.S. Department of Health and Human Services: Washington, DC, USA, 2024.
- Oliveira, C.S.; Nogara, P.A.; Ardisson-Araújo, D.M.; Aschner, M.; Rocha, J.B.; Dórea, J.G. Neurodevelopmental effects of mercury. In Advances in Neurotoxicology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 27–86. [Google Scholar] [CrossRef]
- Business & Human Rights Resource Center. Gold Mines in Myanmar Caused Cross-Border Pollution, Discharging Poisonous Contaminants in Rivers in Thailand; Key Miners Reportedly Chinese. Available online: https://www.business-humanrights.org/en/latest-news/gold-mines-in-myanmar-caused-cross-border-pollution-discharging-poisonous-contaminants-in-rivers-in-thailand-key-miners-reportedly-chinese/ (accessed on 19 May 2025).
- Soe, P.S.; Kyaw, W.T.; Arizono, K.; Ishibashi, Y.; Agusa, T. Mercury pollution from artisanal and small-scale gold mining in Myanmar and other southeast asian countries. Int. J. Environ. Res. Public Health 2022, 19, 6290. [Google Scholar] [CrossRef]
- Qu, R.; Han, G.; Liu, M.; Li, X. The mercury behavior and contamination in soil profiles in mun river basin, Northeast Thailand. Int. J. Environ. Res. Public Health 2019, 16, 4131. [Google Scholar] [CrossRef]
- Pataranawat, P.; Parkpian, P.; Polprasert, C.; Delaune, R.D.; Jugsujinda, A. Mercury emission and distribution: Potential environmental risks at a small-scale gold mining operation, Phichit Province, Thailand. J. Environ. Sci. Health A 2007, 42, 1081–1093. [Google Scholar] [CrossRef]
- Zarcinas, B.A.; Pongsakul, P.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in southeast Asia 2. Thailand. Environ. Geochem. Health 2004, 26, 359–371. [Google Scholar] [CrossRef]
- Programme, U.N.E. Regional Study on Mercury Waste Management in the ASEAN Countries; United Nations Environment Programme: Nairobi, Kenya, 2017; Available online: https://wedocs.unep.org/20.500.11822/21135 (accessed on 6 May 2025).
- Kumar, M.; Seth, A.; Singh, A.K.; Rajput, M.S.; Sikandar, M. Remediation strategies for heavy metals contaminated ecosystem: A review. Environ. Sustain. Indic. 2021, 12, 100155. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Chokshi, K.; Kavanagh, K.; Khan, I.; Slaveykova, V.I.; Sieber, S. Surface displayed MerR increases mercury accumulation by green microalga Chlamydomonas reinhardtii. Environ. Int. 2024, 189, 108813. [Google Scholar] [CrossRef]
- Abdul Aziz, N.I.H.; Mohd Hanafiah, M.; Halim, N.H.; Fidri, P.A.S. Phytoremediation of TSS, NH3-N and COD from sewage wastewater by Lemna minor L., Salvinia minima, Ipomea aquatica and Centella asiatica. Appl. Sci. 2020, 10, 5397. [Google Scholar] [CrossRef]
- Cakaj, A.; Drzewiecka, K.; Hanć, A.; Lisiak-Zielińska, M.; Ciszewska, L.; Drapikowska, M. Plants as effective bioindicators for heavy metal pollution monitoring. Environ. Res. 2024, 256, 119222. [Google Scholar] [CrossRef]
- Tiwari, S.; Agrawal, S.B. New Paradigms in Environmental Biomonitoring Using Plants; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Senayai, A.; Harnvanichvech, Y.; Vajrodaya, S.; Oyama, T.; Kraichak, E. Genetic and morphological variation among populations of duckweed species in Thailand. Plants 2025, 14, 2030. [Google Scholar] [CrossRef]
- Ito, Y.; Barfod, A.S. An updated checklist of aquatic plants of Myanmar and Thailand. Biodivers. Data J. 2014, 2, e1019. [Google Scholar] [CrossRef]
- Khellaf, N.; Zerdaoui, M.; Faure, O.; Leclerc, J.C. Tolerance to heavy metals in the duckweed, Lemna minor. Environ. Int. 2008, 34, 1022–1026. [Google Scholar]
- Aslanzadeh, M.; Saboora, A.; Moradlou, O. Phytoremediation potential of duckweed (Lemna minor L.) for hexavalent chromium removal in synthetic wastewater: Unveiling physiological response and defense mechanisms against excessive heavy metal uptake. Int. J. Environ. Sci. Technol. 2024, 21, 10155–10174. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, D.; Soni, V. Copper and mercury induced oxidative stresses and antioxidant responses of Spirodela polyrhiza (L.) Schleid. Biochem. Biophys. Rep. 2020, 23, 100781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, Q.; Su, C.; Yang, Y.; Hu, D.; Xu, Q. Mercury induced oxidative stress, DNA damage, and activation of antioxidative system and Hsp70 induction in duckweed (Lemna minor). Ecotoxicol. Environ. Saf. 2017, 143, 46–56. [Google Scholar] [CrossRef]
- de Oliveira, E.A.; Borella, D.R.; Lopes, V.J.S.; Battirola, L.D.; Andrade, R.L.T.D.; Silva, A.C.D. Physiological effects of mercury on Handroanthus impetiginosus (Ipê Roxo) plants. Agronomy 2025, 15, 736. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, D.; Soni, V. Impact of mercury on photosynthetic performance of Lemna minor: A chlorophyll fluorescence analysis. Sci. Rep. 2023, 13, 12181. [Google Scholar] [CrossRef] [PubMed]
- Varga, M.; Horvatić, J.; Čelić, A. Short term exposure of Lemna minor and Lemna gibba to mercury, cadmium and chromium. Cent. Eur. J. Biol. 2013, 8, 1083–1093. [Google Scholar] [CrossRef]
- Lee, H.; De Saeger, J.; Bae, S.; Kim, M.; Depuydt, S.; Heynderickx, P.M.; Wu, D.; Han, T.; Park, J. Giant duckweed (Spirodela polyrhiza) root growth as a simple and sensitive indicator of copper and chromium contamination. Toxics 2023, 11, 788. [Google Scholar] [CrossRef]
- Khellaf, N.; Zerdaoui, M. Growth response of the duckweed Lemna minor to heavy metal pollution. J. Environ. Health Sci. Eng. 2009, 6, 161–166. Available online: https://ijehse.tums.ac.ir/index.php/jehse/article/view/207 (accessed on 19 May 2025).
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- de Barros, G.L.; Silva, F.; Teixeira, R.; Wagner, J.; Rombaldi, C.; Vizzotto, M.; Ubeyitogullari, A.; Nora, L. Anthocyanin extraction methods: Synthesis of morpho-anatomical knowledge for decision-making based on decision-tree. Int. J. Food Prop. 2024, 27, 1315–1346. [Google Scholar] [CrossRef]
- Dirilgen, N. Mercury and lead: Assessing the toxic effects on growth and metal accumulation by Lemna minor. Ecotoxicol. Environ. Saf. 2011, 74, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Jäger, T.; Würtenberger, S.; Baumgartner, S. Effects of homeopathic preparations of mercurius corrosivus on the growth rate of severely mercury-stressed duckweed Lemna gibba L. Homeopathy 2019, 108, 128–138. [Google Scholar] [CrossRef]
- Nasircilar, A.G.; Ulukapi, K.; Topcuoglu, B.; Kurubas, S.; Erkan, M. Salt and heavy metal stress responses and metal uptake potentials of some leafy vegetables. Agrosyst. Geosci. Environ. 2024, 7, e20487. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef]
- Zhu, T.; Li, L.; Duan, Q.; Liu, X.; Chen, M. Progress in our understanding of plant responses to the stress of heavy metal cadmium. Plant Signal. Behav. 2021, 16, 1836884. [Google Scholar] [CrossRef]
- Nishizono, H.; Ichikawa, H.; Suziki, S.; Suzuki, S.; Ishii, F. The role of the root cell wall in the heavy metal tolerance of Athyrium yokoscense. Plant Soil 1987, 101, 15–20. [Google Scholar] [CrossRef]
- Wang, C.; Wu, B.; Jiang, K.; Zhou, J. Effects of different types of heavy metal pollution on functional traits of invasive redroot pigweed and native red amaranth. Int. J. Environ. Res. 2018, 12, 419–427. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, H.; Kang, X.; Liu, C.; Chen, L.; Liang, X.; Jin, L. Inter-and intra-specific competition of duckweed under multiple heavy metal contaminated water. Aquat. Toxicol. 2017, 192, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-X.; Feng-Ying, Z.; Yang, H.; Jian-Cong, N. Thorough removal of inorganic and organic mercury from aqueous solutions by adsorption on Lemna minor powder. J. Hazard. Mater. 2011, 186, 423–429. [Google Scholar] [CrossRef]
- Li, T.; Xiong, Z. A novel response of wild-type duckweed (Lemna paucicostata Hegelm.) to heavy metals. Environ. Toxicol. 2004, 19, 95–102. [Google Scholar] [CrossRef]
- Topp, C.; Henke, R.; Keresztes, Á.; Fischer, W.; Eberius, M.; Appenroth, K.J. A novel mechanism of abscission in fronds of Lemna minor L. and the effect of silver ions. Plant Biol. 2011, 13, 517–523. [Google Scholar] [CrossRef]
- Li, T.; Xiong, Z. Cadmium-induced colony disintegration of duckweed (Lemna paucicostata Hegelm.) and as biomarker of phytotoxicity. Ecotoxicol. Environ. Saf. 2004, 59, 174–179. [Google Scholar] [CrossRef]
- Wang, X.; Wen, H.; Suprun, A.; Zhu, H. Ethylene signaling in regulating plant growth, development, and stress responses. Plants 2025, 14, 309. [Google Scholar] [CrossRef]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene signaling under stressful environments: Analyzing collaborative knowledge. Plants. 2022, 11, 2211. [Google Scholar] [CrossRef]
- Khan, S.; Alvi, A.F.; Khan, N.A. Role of ethylene in the regulation of plant developmental processes. Stresses 2024, 4, 28–53. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Roongsattham, P.; Morcillo, F.; Fooyontphanich, K.; Jantasuriyarat, C.; Tragoonrung, S.; Amblard, P.; Collin, M.; Mouille, G.; Verdeil, J.-L.; Tranbarger, T.J. Cellular and pectin dynamics during abscission zone development and ripe fruit abscission of the monocot oil palm. Front. Plant Sci. 2016, 7, 540. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Sharma, A.; Prasad, S.M.; Singh, V.P. A brief appraisal of ethylene signaling under abiotic stress in plants. Plant Signal. Behav. 2020, 15, 1782051. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Ethylene and Abiotic Stress Tolerance in Plants; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Druege, U. Ethylene and plant responses to abiotic stress. In Ethylene Action in Plants; Springer: New York, NY, USA, 2006; pp. 81–118. [Google Scholar] [CrossRef]
- Severi, A. Toxicity of selenium to Lemna minor in relation to sulfate concentration. Physiol. Plant. 2001, 113, 523–532. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef]
- He, C.; Zhao, Y.; Zhang, H.; Wang, Z.; Lü, J.; Ge, L.; Zhao, X.; Xu, C. Detoxification effect of selenium: Alleviating the inhibition of cadmium stress on the growth of rape (Brassica napus L.) by regulating photosynthetic pigments, metal complexation reaction, and antioxidant system. Agronomy 2025, 15, 541. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, H.; Liu, L.; Ruan, J.; Liu, M.; Zhang, Q. The physiological and flavonoid metabolism of the tea plant in response to nitrogen nutrition is regulated by the ultraviolet-B radiation environment. J. Plant Growth Regul. 2025, 44, 3884–3899. [Google Scholar] [CrossRef]
- Hou, W.; Chen, X.; Song, G.; Wang, Q.; Chang, C.C. Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol. Biochem. 2007, 45, 62–69. [Google Scholar] [CrossRef]
- Souri, Z.; Cardoso, A.A.; da-Silva, C.J.; de Oliveira, L.M.; Dari, B.; Sihi, D.; Karimi, N. Heavy Metals and Photosynthesis: Recent Developments; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Leblebici, Z.; Aksoy, A. Growth and lead accumulation capacity of Lemna minor and Spirodela polyrhiza (Lemnaceae): Interactions with nutrient enrichment. Water Air Soil Pollut. 2011, 214, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Pulatovna, V.N. Carotenoids, their sources and synthesis. Innov. Conf. 2025, 33–37. Available online: https://innovateconferences.org/index.php/ic/article/view/9 (accessed on 20 May 2025).
- Maslova, T.; Markovskaya, E.; Slemnev, N. Functions of carotenoids in leaves of higher plants. Biol. Bull. Rev. 2021, 11, 476–487. [Google Scholar] [CrossRef]
- Wang, S.; Duo, J.; Wufuer, R.; Li, W.; Pan, X. The binding ability of mercury (Hg) to photosystem I and II explained the difference in its toxicity on the two photosystems of Chlorella pyrenoidosa. Toxics 2022, 10, 455. [Google Scholar] [CrossRef]
- Sherin, G.; Aswathi, K.R.; Puthur, J.T. Photosynthetic functions in plants subjected to stresses are positively influenced by priming. Plant Stress 2022, 4, 100079. [Google Scholar] [CrossRef]
- Jat, M.; Ray, M.; Ahmad, M.A.; Prakash, P. Unravelling the photosynthetic dynamics and fluorescence parameters under ameliorative effects of 24-epibrassinolide in wheat (Triticum aestivum L.) grown under heat stress regime. Sci. Rep. 2024, 14, 30745. [Google Scholar] [CrossRef]
- Levin, G.; Yasmin, M.; Liran, O.; Hanna, R.; Kleifeld, O.; Horev, G.; Wollman, F.-A.; Schuster, G.; Nawrocki, W.J. Processes independent of nonphotochemical quenching protect a high-light-tolerant desert alga from oxidative stress. Plant Physiol. 2025, 197, kiae608. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A.; Nicola, S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol. Biochem. 2016, 106, 141–148. [Google Scholar] [CrossRef]
- Oláh, V.; Kosztankó, K.; Irfan, M.; Szabó, Z.B.; Jansen, M.A.; Szabó, S.; Mészáros, I. Frond-level analyses reveal functional heterogeneity within heavy metal-treated duckweed colonies. Plant Stress 2024, 11, 100405. [Google Scholar] [CrossRef]
- Rezania, S.; Taib, S.M.; Din, M.F.M.; Dahalan, F.A.; Kamyab, H. Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef]
- Zhou, Y.; Stepanenko, A.; Kishchenko, O.; Xu, J.; Borisjuk, N. Duckweeds for phytoremediation of polluted Water. Plants 2023, 12, 589. [Google Scholar] [CrossRef]
- Li, Z.; Xie, W.; Tian, Y.; Shen, J.; Su, X.; Yang, J.; Liang, M.; Qiao, X. Selenium-induced avoidance mechanism, ionic interactions, and antioxidant system to mitigate manganese toxicity in apple rootstock ‘Qingzhen 1’. Plant Stress 2025, 15, 100797. [Google Scholar] [CrossRef]
- Shivappa, S.; Amritha, K.; Nayak, S.; Chandrashekar, H.K.; Thorat, S.A.; Kaniyassery, A.; Govender, N.; Thiruvengadam, M.; Muthusamy, A. Integration of physio-biochemical, biological and molecular approaches to improve heavy metal tolerance in plants. 3 Biotech 2025, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 221: Lemna sp. Growth Inhibition Test; OECD: Paris, France, 2006. [Google Scholar]
- Lee, J.D.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Roháček, K. Chlorophyll fluorescence Parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Kingdom, S. Multiple Linear Regression Calculator. 2017. Available online: https://www.statskingdom.com/410multi_linear_regression.html (accessed on 29 April 2025).
- Ma, Y.; Wang, G.; Wang, Y.; Dai, W.; Luan, Y. Mercury uptake and transport by plants in aquatic environments: A meta-analysis. Appl. Sci. 2021, 11, 8829. [Google Scholar] [CrossRef]
| Time (h) | HgCl2 (mg/L) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 1.00 ± 0.00 | 0.67 ± 1.20 | 1.00 ± 1.00 | 3.67 ± 1.50 | 5.00 ± 2.00 * |
| 2 | 0 | 1.00 ± 0.00 | 1.00 ± 1.00 | 1.00 ± 0.00 | 5.67 ± 1.50 * | 8.67 ± 2.10 * |
| 3 | 0 | 1.00 ± 0.00 | 1.00 ± 1.00 | 2.00 ± 1.00 | 7.00 ± 1.00 * | 9.33 ± 2.30 * |
| 4 | 0 | 1.00 ± 0.00 | 1.00 ± 1.00 | 5.33 ± 0.60 * | 7.67 ± 0.60 * | 9.67 ± 2.10 * |
| 5 | 0 | 1.00 ± 0.00 | 1.00 ± 1.00 | 7.00 ± 0.00 * | 9.67 ± 3.10 * | 10.67 ± 1.50 * |
| 6 | 0 | 1.00 ± 0.00 | 1.00 ± 1.00 | 7.67 ± 1.20 * | 10.00 ± 2.60 * | 10.67 ± 1.50 * |
| 7 | 0 | 1.00 ± 0.00 | 2.67 ± 1.50 | 8.67 ± 0.60 * | 10.33 ± 2.50 * | 11.00 ± 1.70* |
| 8 | 0 | 1.00 ± 0.00 | 2.67 ± 1.50 | 10.00 ± 0.00 * | 10.67 ± 2.10 * | 11.00 ± 1.70 * |
| 24 | 0 | 2.00 ± 0.00 | 2.67 ± 1.50 | 10.00 ± 0.00 * | 11.00 ± 2.00 * | 11.33 ± 1.20 * |
| 48 | 0 | 2.00 ± 0.00 | 2.67 ± 1.50 | 10.00 ± 0.00 * | 11.00 ± 2.00 * | 11.33 ± 1.20 * |
| Time (h) | HgCl2 (mg/L) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 1.00 ± 1.00 |
| 8 | 0 | 0 | 0 | 0 | 0.33 ± 0.60 | 1.33 ± 1.50 |
| 24 | 0 | 0.33 ± 0.60 | 0.67 ± 1.20 | 1.00 ± 1.00 | 3.00 ± 1.00 | 4.00 ± 1.00 * |
| 48 | 0 | 0.67 ± 0.60 | 1.33 ± 1.50 | 2.00 ± 1.00 | 3.33 ± 1.50 | 4.00 ± 1.00 * |
| Fv/Fm | ||||
|---|---|---|---|---|
| HgCl2 (mg/L) | Day 0 | Day 3 | Day 5 | Day 7 |
| 0 | 0.773 ± 0.018 aA | 0.760 ± 0.025 aA | 0.770 ± 0.019 aA | 0.779 ± 0.035 aA |
| 0.1 | 0.780 ± 0.015 aA | 0.772 ± 0.033 aA | 0.726 ± 0.203 abA | 0.745 ± 0.060 abA |
| 0.2 | 0.779 ± 0.023 aA | 0.743 ± 0.085 aA | 0.737 ± 0.044 abA | 0.733 ± 0.046 bA |
| 0.3 | 0.773 ± 0.011 aA | 0.640 ± 0.102 bB | nd | nd |
| NPQ | ||||
| HgCl2 (mg/L) | Day 0 | Day 3 | Day 5 | Day 7 |
| 0 | 0.836 ± 0.633 aA | 0.858 ± 0.386 aA | 0.717 ± 0.540 aA | 0.827 ± 0.229 aA |
| 0.1 | 0.756 ± 0.404 aA | 1.457 ± 0.458 bB | 1.761 ± 0.421 bBC | 2.030 ± 0.290 bC |
| 0.2 | 0.770 ± 0.306 aA | 1.686 ± 0.545 bB | 2.019 ± 0.448 bBC | 2.241 ± 0.503 bC |
| 0.3 | 0.883 ± 0.262 aA | 3.712 ± 0.976 cB | 3.776 ± 0.839 cB | 3.692 ± 0.671 cB |
| Y (II) | ||||
| HgCl2 (mg/L) | Day 0 | Day 3 | Day 5 | Day 7 |
| 0 | 0.191 ± 0.043 aA | 0.172 ± 0.028 aA | 0.220 ± 0.054 aA | 0.177 ± 0.044 aA |
| 0.1 | 0.191 ± 0.035 aA | 0.150 ± 0.019 bB | 0.204 ± 0.072 aA | 0.169 ± 0.017 abA |
| 0.2 | 0.184 ± 0.060 aA | 0.138 ± 0.033 bB | 0.140 ± 0.010 bB | 0.146 ± 0.025 bB |
| 0.3 | 0.210 ± 0.030 aA | nd | nd | nd |
| Fv/Fm | ||||
|---|---|---|---|---|
| HgCl2 (mg/L) | Day 0 | Day 3 | Day 5 | Day 7 |
| 0 | 0.779 ± 0.021 aA | 0.786 ± 0.025 aAB | 0.802 ± 0.019 aB | 0.803 ± 0.017 aB |
| 0.1 | 0.785 ± 0.022 aA | 0.780 ± 0.046 aA | 0.773 ± 0.041 aA | 0.772 ± 0.037 aA |
| 0.2 | 0.787 ± 0.013 aA | 0.712 ± 0.035 bAB | 0.601 ± 0.079 bBC | 0.500 ± 0.302 bC |
| 0.3 | 0.795 ± 0.020 aA | 0.694 ± 0.020 bA | 0.383 ± 0.288 cB | 0.336 ± 0.287 bB |
| NPQ | ||||
| HgCl2 (mg/L) | Day 0 | Day 3 | Day 5 | Day 7 |
| 0 | 0.318 ± 0.165 aA | 0.326 ± 0.182 aA | 0.448 ± 0.163 aA | 0.442 ± 0.098 aA |
| 0.1 | 0.323 ± 0.204 aA | 0.364 ± 0.178 aA | 0.516 ± 0.223 aAB | 0.688 ± 0.295 bB |
| 0.2 | 0.319 ± 0.140 aA | 0.424 ± 0.343 aA | 0.985 ± 0.408 bB | 1.060 ± 0.285 cB |
| 0.3 | 0.322 ± 0.068 aA | 0.432 ± 0.098 aA | 1.230 ± 0.544 bB | 1.359 ± 0.375 dB |
| Y (II) | ||||
| HgCl2 (mg/L) | Day 0 | Day 3 | Day 5 | Day 7 |
| 0 | 0.336 ± 0.066 aA | 0.344 ± 0.032 aA | 0.353 ± 0.033 aA | 0.351 ± 0.045 aA |
| 0.1 | 0.345 ± 0.050 aA | 0.337 ± 0.062 abA | 0.282 ± 0.030 bB | 0.274 ± 0.027 bB |
| 0.2 | 0.329 ± 0.058 aA | 0.272 ± 0.089 bcA | 0.176 ± 0.118 cB | 0.086 ± 0.039 cC |
| 0.3 | 0.332 ± 0.044 aA | 0.243 ± 0.108 cB | 0.098 ± 0.106 dC | 0.043 ± 0.027 dC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruamsin, C.; Sonjaroon, W.; Khumwan, S.; Thamchaipenet, A.; Roongsattham, P. Comparative Physiological Responses of Lemna aequinoctialis and Spirodela polyrhiza to Mercury Stress: Implications for Biomonitoring and Phytoremediation. Plants 2025, 14, 2859. https://doi.org/10.3390/plants14182859
Ruamsin C, Sonjaroon W, Khumwan S, Thamchaipenet A, Roongsattham P. Comparative Physiological Responses of Lemna aequinoctialis and Spirodela polyrhiza to Mercury Stress: Implications for Biomonitoring and Phytoremediation. Plants. 2025; 14(18):2859. https://doi.org/10.3390/plants14182859
Chicago/Turabian StyleRuamsin, Chomphoonut, Weerasin Sonjaroon, Sirikorn Khumwan, Arinthip Thamchaipenet, and Peerapat Roongsattham. 2025. "Comparative Physiological Responses of Lemna aequinoctialis and Spirodela polyrhiza to Mercury Stress: Implications for Biomonitoring and Phytoremediation" Plants 14, no. 18: 2859. https://doi.org/10.3390/plants14182859
APA StyleRuamsin, C., Sonjaroon, W., Khumwan, S., Thamchaipenet, A., & Roongsattham, P. (2025). Comparative Physiological Responses of Lemna aequinoctialis and Spirodela polyrhiza to Mercury Stress: Implications for Biomonitoring and Phytoremediation. Plants, 14(18), 2859. https://doi.org/10.3390/plants14182859










