Overexpression of the Wild Soybean Expansin Gene GsEXPB1 Enhances Salt Stress Tolerance in Transgenic Soybeans
Abstract
1. Introduction
2. Results
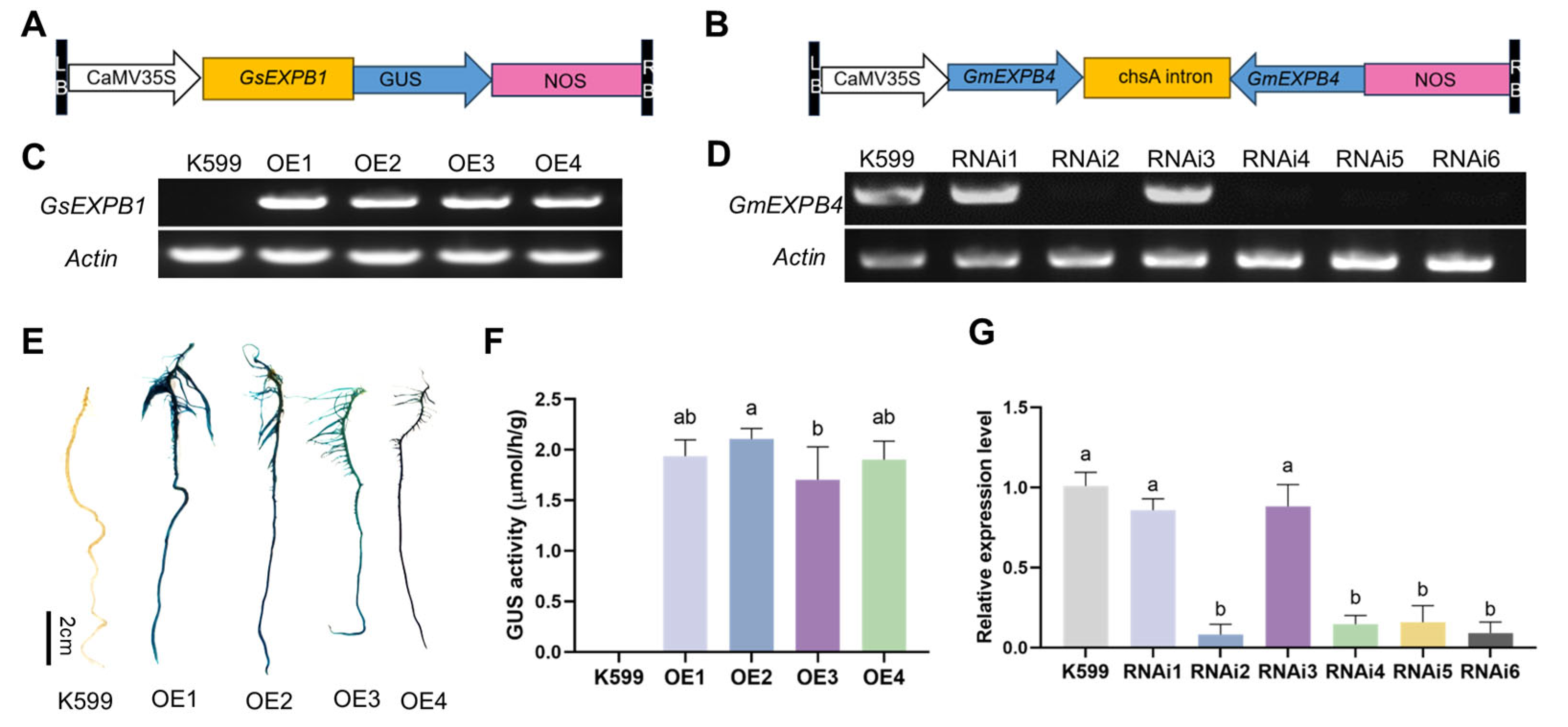
2.1. Phenotype Observation of Soybean Hairy Roots Overexpressing the GsEXPB1 Gene and Silenced by RNAi for the GmEXPB4 Gene
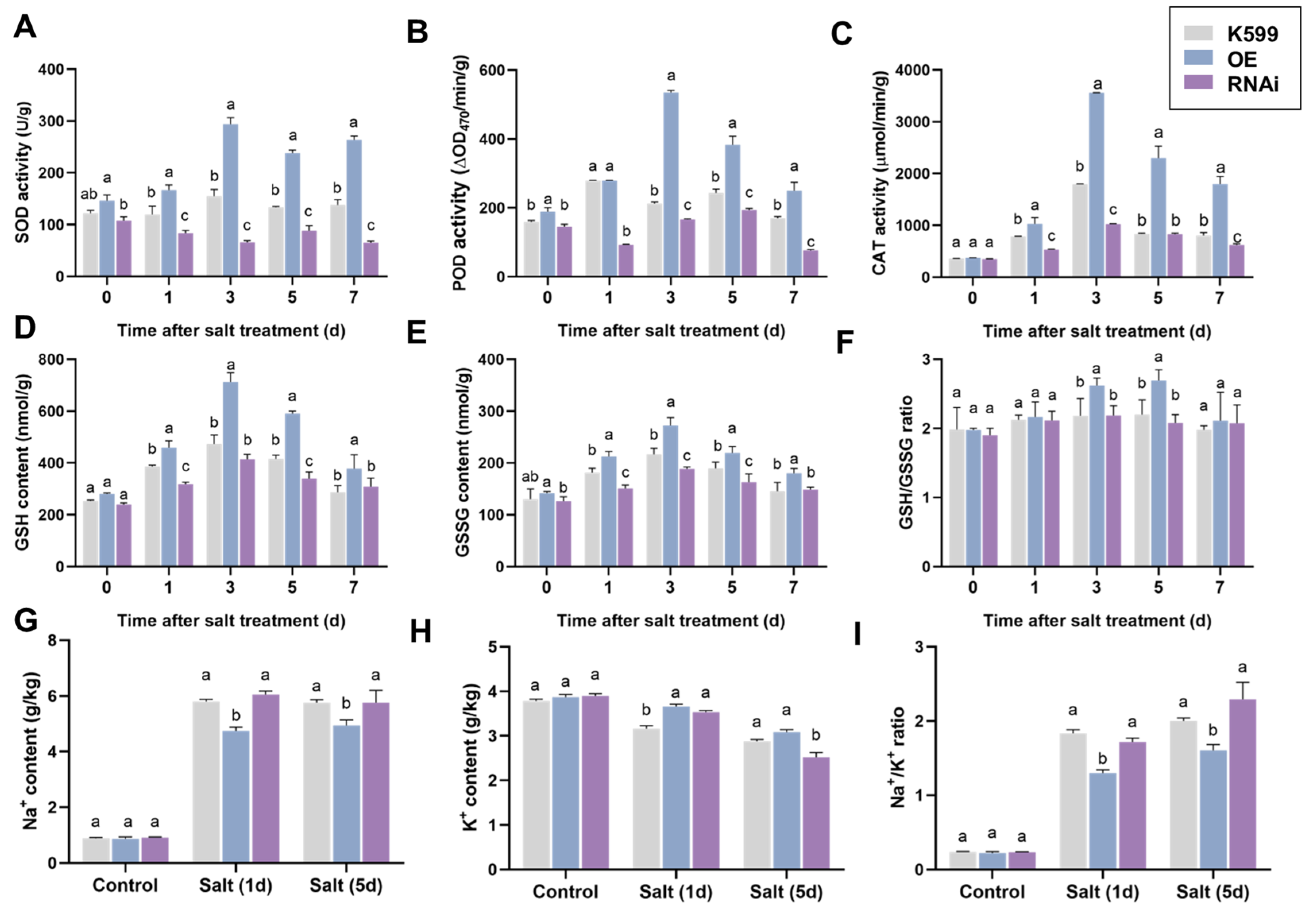
2.2. Detection of Physiological Indexes of Transgenic Hairy Roots Under Salt Stress
2.3. Construction of a Soybean Strain Overexpressing the GsEXPB1 Gene
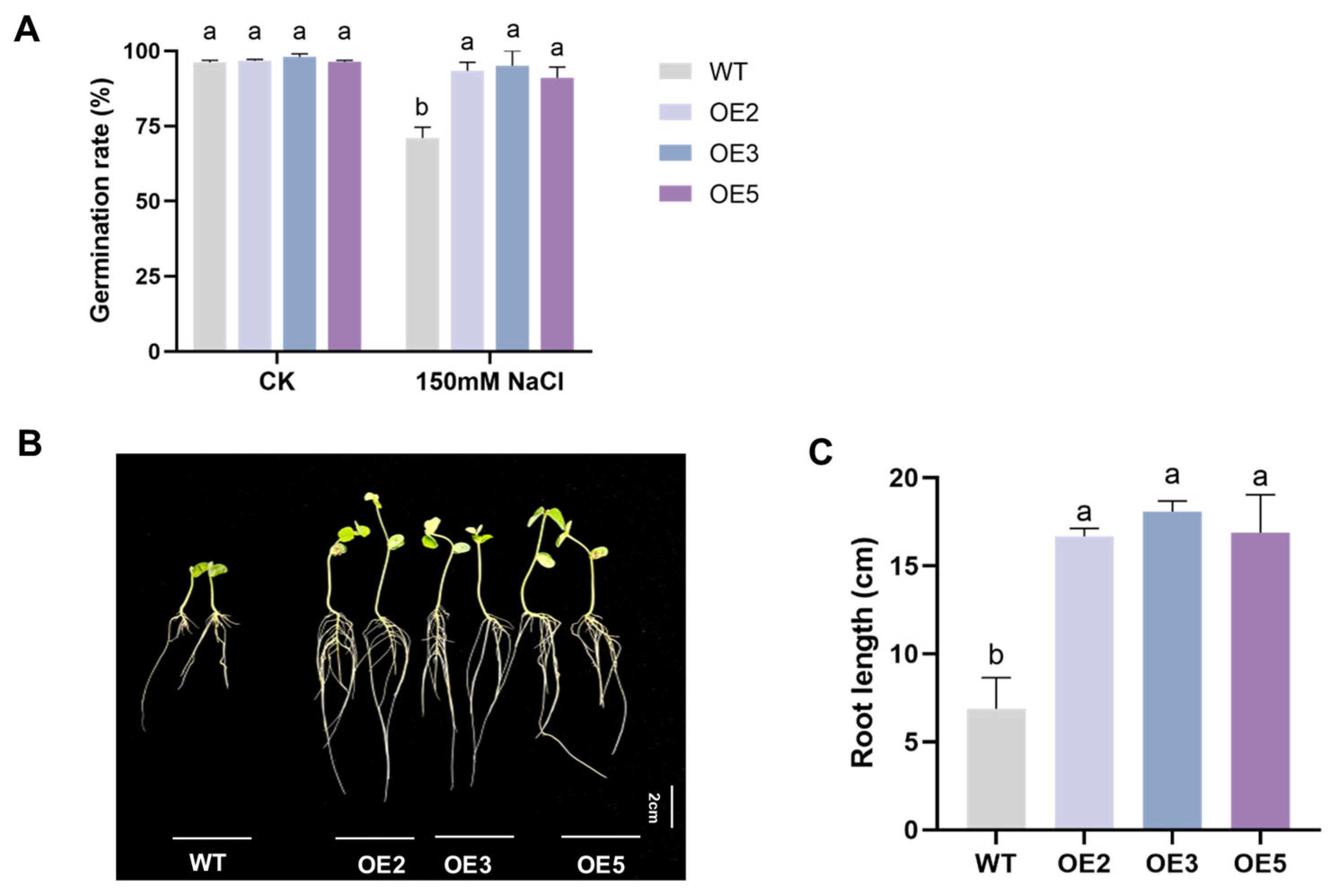
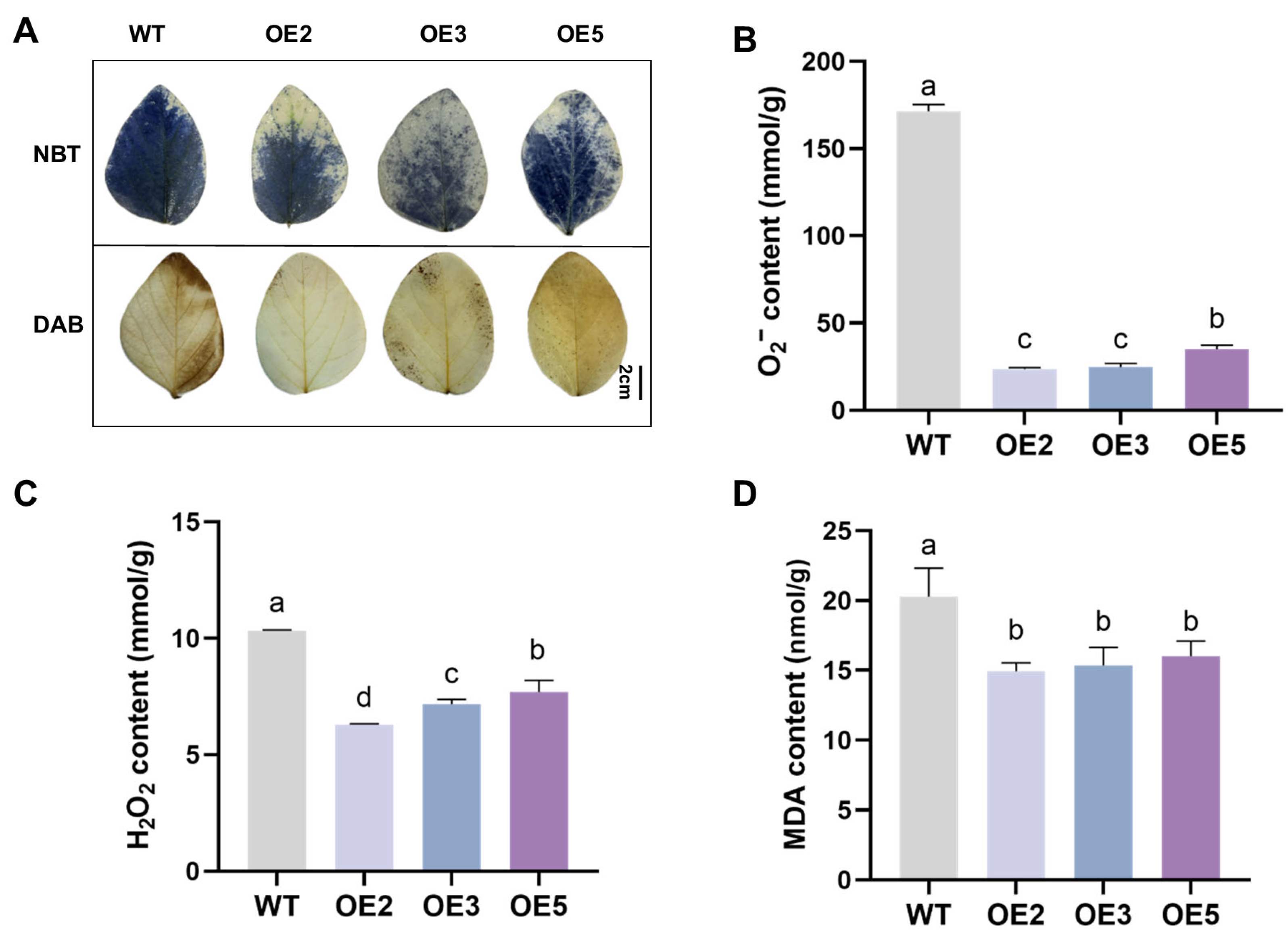
2.4. Analysis Results of Salt Tolerance of Transgenic Soybean Seeds
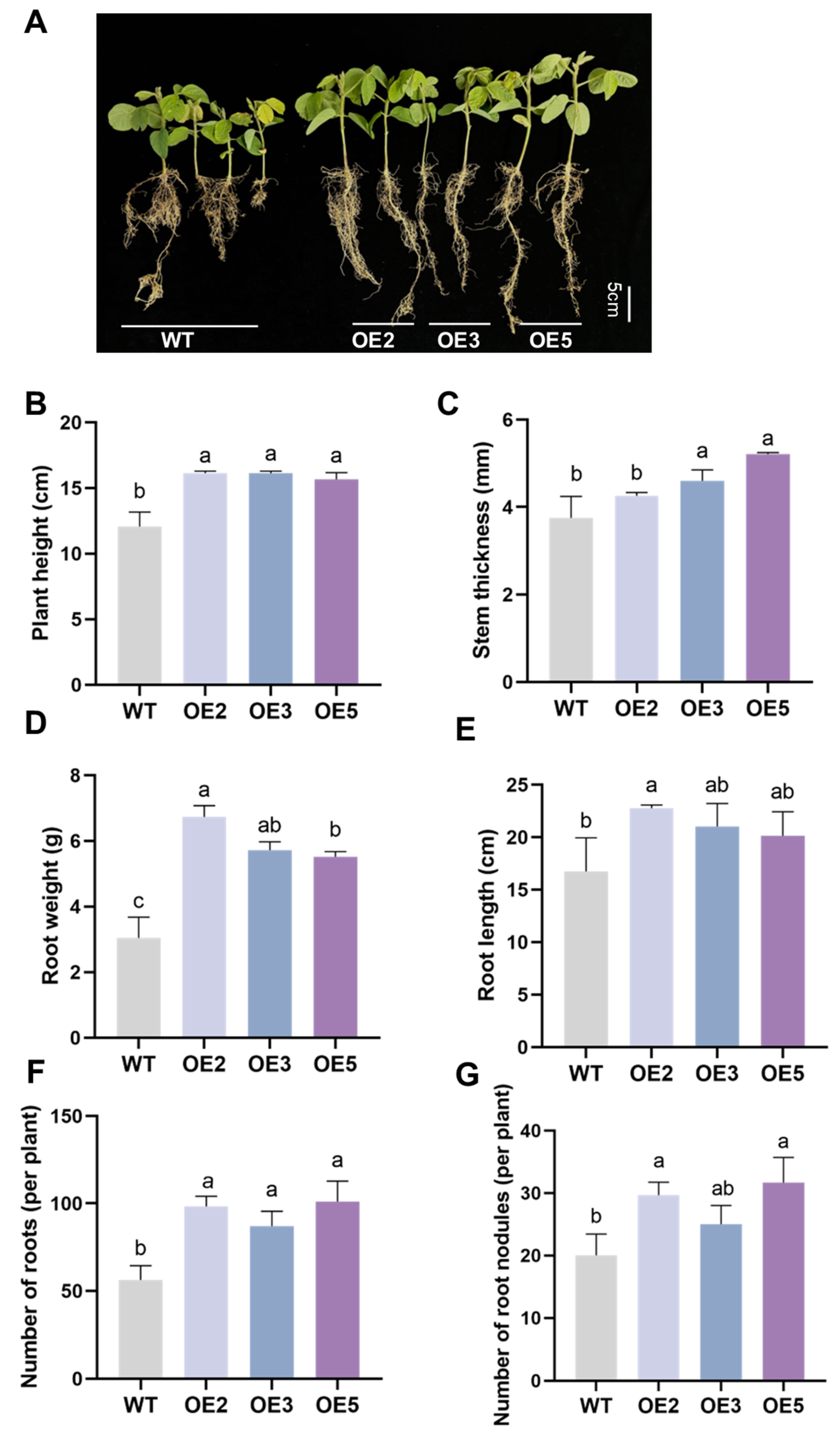
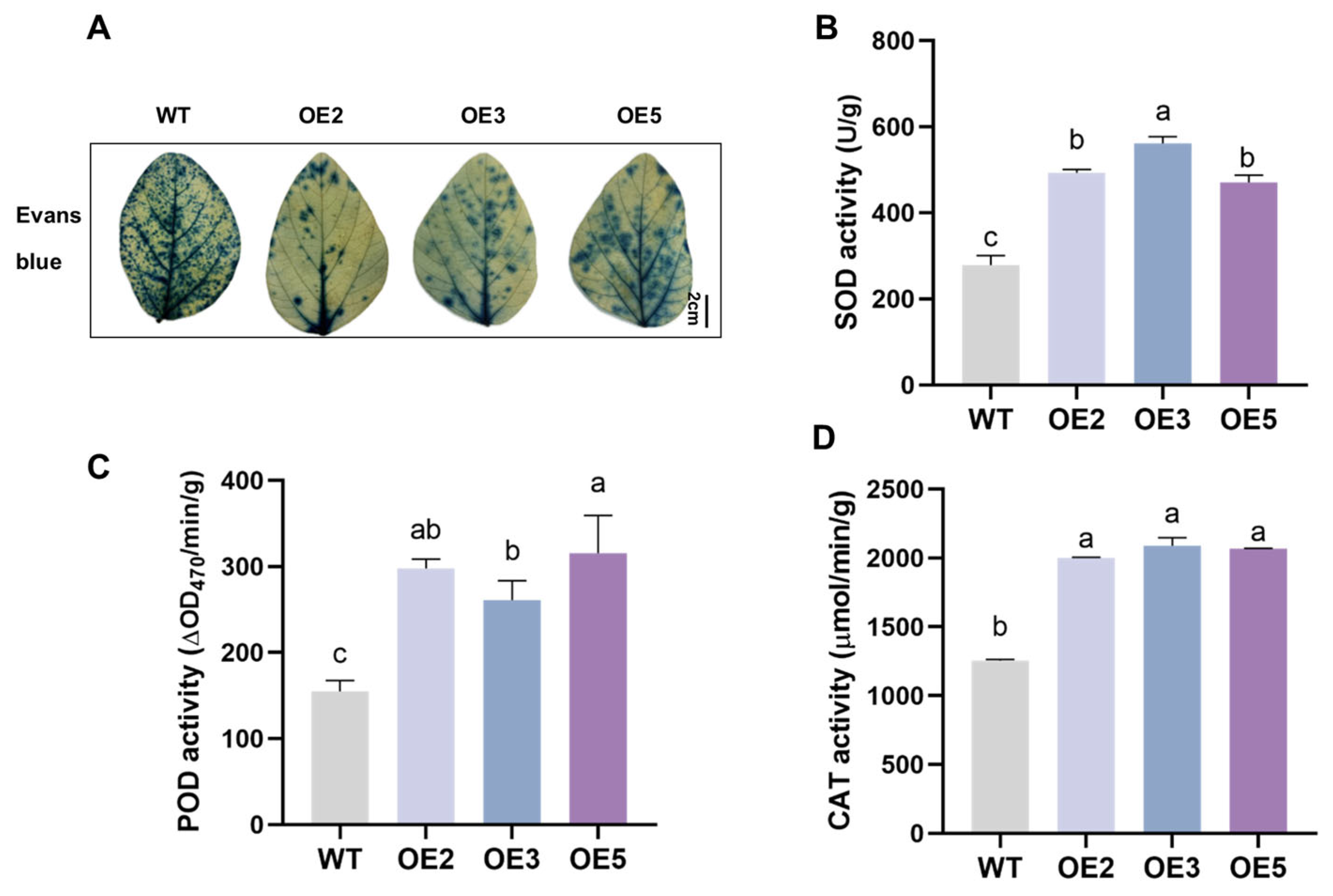
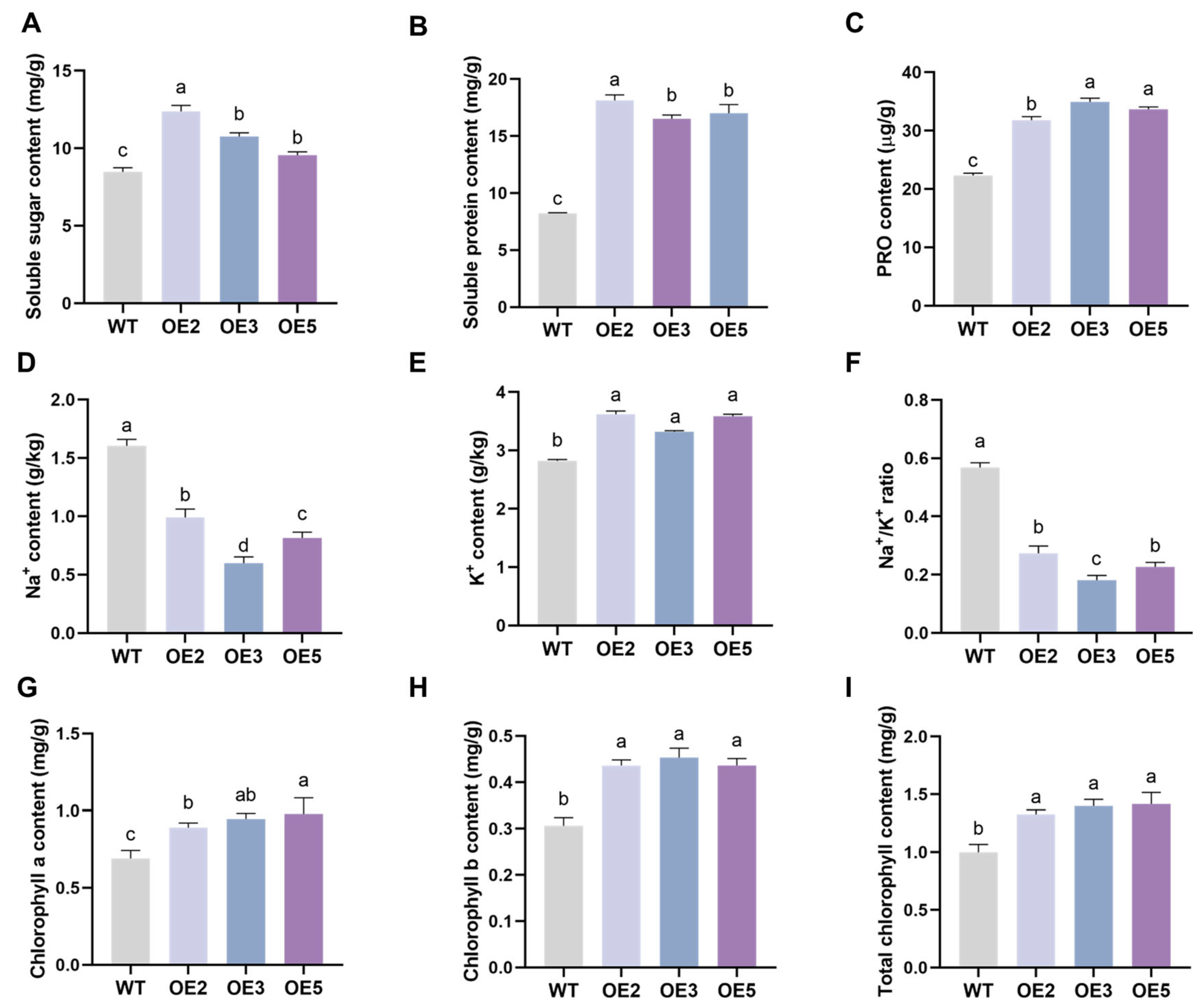
2.5. Analysis of Salt Tolerance of Transgenic Soybean Plants in the Seedling Stage
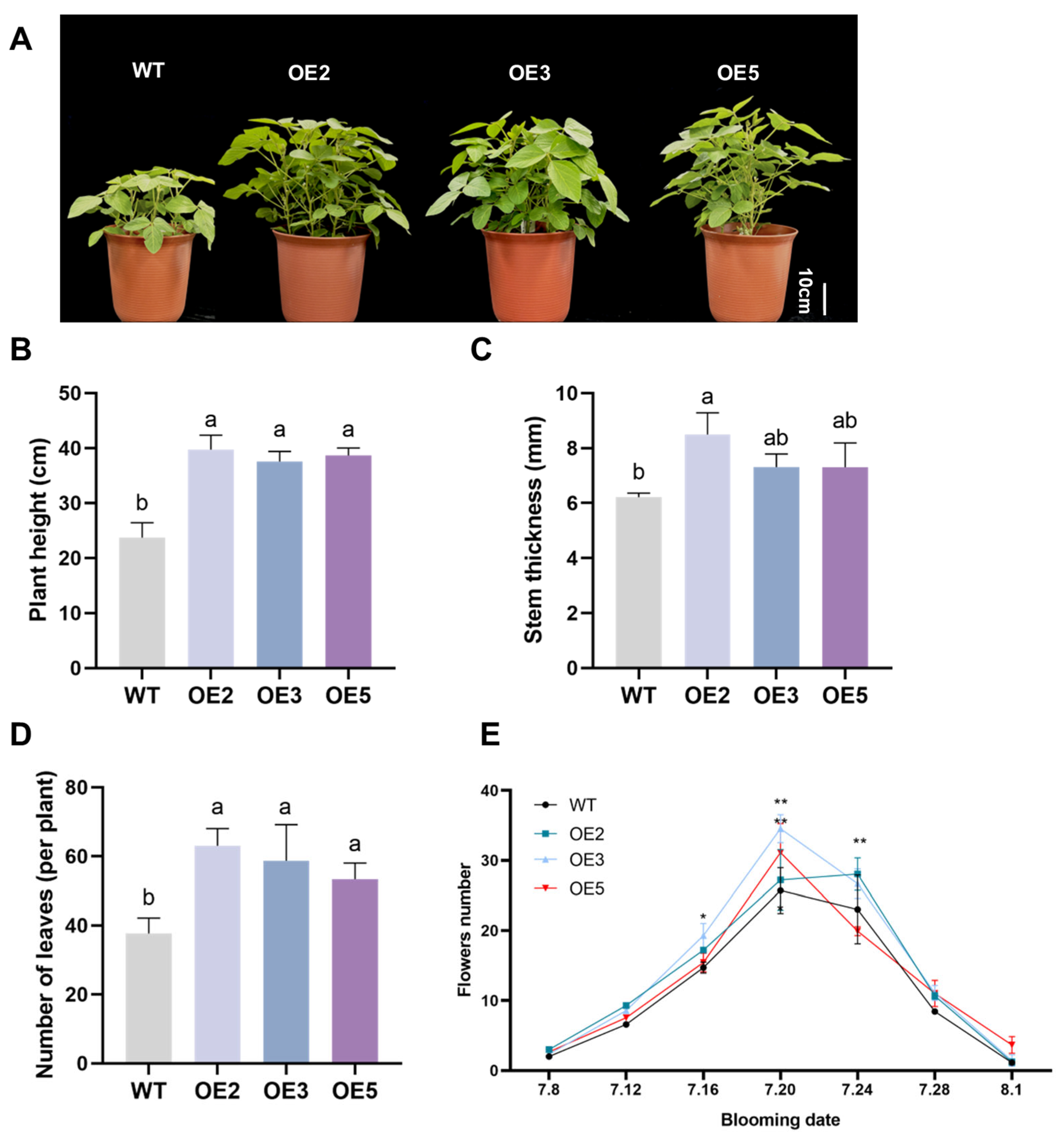
2.6. Phenotype Observation of Transgenic Soybeans During Flowering Period
2.7. Observation of Mature Transgenic Soybean Phenotype
3. Discussion
3.1. Function and Mechanism of the GsEXPB1 Gene in Regulating Soybean Root Growth
3.2. Function and Main Pathways of GsEXPB1 Gene Regulating Soybean Salt Stress Tolerance
3.3. The Application Value of the GsEXPB1 Gene in Soybean Breeding for Salt Stress Tolerance
4. Materials and Methods
4.1. Acquisition of Soybean Hairy Roots Through Overexpression of GsEXPB1 Gene and RNAi Silencing of GmEXPB4 Gene
4.2. Phenotype Observation of Hairy Roots
4.3. Measurement of Physiological Indicators of Hairy Roots
4.4. Acquisition of GsEXPB1-Overexpressing Cultivated Soybean Lines
4.5. Analysis of Salt Tolerance of Transgenic Soybean Seeds
4.6. Analysis of Salt Tolerance in Transgenic Soybean Plants
4.7. Data Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Zhang, M.; Feng, F.; Tian, Z. Toward a “Green Revolution” for Soybean. Mol. Plant 2020, 13, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Kofsky, J.; Zhang, H.; Song, B.H. The Untapped Genetic Reservoir: The Past, Current, and Future Applications of the Wild Soybean (Glycine soja). Front. Plant Sci. 2018, 9, 949. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Sedivy, E.J.; Wu, F.; Hanzawa, Y. Soybean domestication: The origin, genetic architecture and molecular bases. New Phytol. 2017, 214, 539–553. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, G.; Zhao, M.; Wang, M.; Jiang, M. Direct and indirect effects of soil salinization on soil seed banks in salinizing wetlands in the Songnen Plain, China. Sci. Total Environ. 2022, 819, 152035. [Google Scholar] [CrossRef]
- Meng, F.; Feng, N.; Zheng, D.; Liu, M.; Zhou, H.; Zhang, R.; Huang, X.; Huang, A. Exogenous Hemin enhances the antioxidant defense system of rice by regulating the AsA-GSH cycle under NaCl stress. PeerJ 2024, 12, e17219. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B. Protection of Halophytes and Their Uses for Cultivation of Saline-Alkali Soil in China. Biology 2021, 10, 353. [Google Scholar] [CrossRef]
- You, H.; Liu, Y.; Minh, T.N.; Lu, H.; Zhang, P.; Li, W.; Xiao, J.; Ding, X.; Li, Q. Genome-wide identification and expression analyses of nitrate transporter family genes in wild soybean (Glycine soja). J. Appl. Genet. 2020, 61, 489–501. [Google Scholar] [CrossRef]
- Hyten, D.L.; Song, Q.; Zhu, Y.; Choi, I.Y.; Nelson, R.L.; Costa, J.M.; Specht, J.E.; Shoemaker, R.C.; Cregan, P.B. Impacts of genetic bottlenecks on soybean genome diversity. Proc. Natl. Acad. Sci. USA 2006, 103, 16666–16671. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Bai, Z.; Xu, J.; Zhao, M.; Khan, Y.; Hu, Y.; Shi, L. Metabolomics and its physiological regulation process reveal the salt-tolerant mechanism in Glycine soja seedling roots. Plant Physiol. Biochem. 2018, 126, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Kumar, M.; Xu, L.; Wan, Q.; Huang, Y.H.; Xu, Z.L.; He, X.L.; Ma, J.B.; Pandey, G.K.; Shao, H.B. Genome-wide identification of Major Intrinsic Proteins in Glycine soja and characterization of GmTIP2;1 function under salt and water stress. Sci. Rep. 2017, 7, 4106. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Feng, P.; Yu, H.; Yu, X.; Sun, Q.; Liu, S.; Minh, T.N.; Chen, J.; Wang, D.; Zhang, Q.; et al. GsSnRK1 interplays with transcription factor GsERF7 from wild soybean to regulate soybean stress resistance. Plant Cell Environ. 2020, 43, 1192–1211. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Li, P.; Shen, Y.; Peng, H.; Liu, S.; Zhou, G.A.; Zhang, H.; Liu, Z.; Shi, M.; et al. Pan-Genome of Wild and Cultivated Soybeans. Cell 2020, 182, 162–176.e13. [Google Scholar] [CrossRef]
- Zheng, H.; Hou, L.; Xie, J.; Cao, F.; Wei, R.; Yang, M.; Qi, Z.; Zhu, R.; Zhang, Z.; Xin, D.; et al. Construction of Chromosome Segment Substitution Lines and Inheritance of Seed-Pod Characteristics in Wild Soybean. Front. Plant Sci. 2022, 13, 869455. [Google Scholar] [CrossRef] [PubMed]
- McQueen-Mason, S.; Durachko, D.M.; Cosgrove, D.J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell. 1992, 4, 1425–1433. [Google Scholar] [CrossRef]
- Li, M.; Liu, T.; Cao, R.; Cao, Q.; Tong, W.; Song, W. Evolution and Expression of the Expansin Genes in Emmer Wheat. Int. J. Mol. Sci. 2023, 24, 14120. [Google Scholar] [CrossRef]
- Sampedro, J.; Cosgrove, D.J. The expansin superfamily. Genome Biol. 2005, 6, 242. [Google Scholar] [CrossRef][Green Version]
- Guimaraes, L.A.; Mota, A.P.Z.; Araujo, A.C.G.; de Alencar Figueiredo, L.F.; Pereira, B.M.; de Passos Saraiva, M.A.; Silva, R.B.; Danchin, E.G.J.; Guimaraes, P.M.; Brasileiro, A.C.M. Genome-wide analysis of expansin superfamily in wild Arachis discloses a stress-responsive expansin-like B gene. Plant Mol. Biol. 2017, 94, 79–96. [Google Scholar] [CrossRef]
- Chen, F.; Bradford, K.J. Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol. 2000, 124, 1265–1274. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Lin, H.; Cao, J. Two Expansin Genes, AtEXPA4 and AtEXPB5, Are Redundantly Required for Pollen Tube Growth and AtEXPA4 Is Involved in Primary Root Elongation in Arabidopsis thaliana. Genes 2021, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Xu, Q.; Yang, Z.; Zhuang, L.; Yu, J.; Huang, B. Identification of expansin genes as promoting or repressing factors for leaf elongation in tall fescue. Physiol. Plant. 2023, 175, e13861. [Google Scholar] [CrossRef]
- Feng, X.; Xu, Y.; Peng, L.; Yu, X.; Zhao, Q.; Feng, S.; Zhao, Z.; Li, F.; Hu, B. TaEXPB7-B, a β-expansin gene involved in low-temperature stress and abscisic acid responses, promotes growth and cold resistance in Arabidopsis thaliana. J. Plant Physiol. 2019, 240, 153004. [Google Scholar] [CrossRef]
- Han, Z.; Liu, Y.; Deng, X.; Liu, D.; Liu, Y.; Hu, Y.; Yan, Y. Genome-wide identification and expression analysis of expansin gene family in common wheat (Triticum aestivum L.). BMC Genom. 2019, 20, 101. [Google Scholar] [CrossRef]
- Bernal-Gallardo, J.J.; González-Aguilera, K.L.; de Folter, S. EXPANSIN15 is involved in flower and fruit development in Arabidopsis. Plant Reprod. 2024, 37, 259–270. [Google Scholar] [CrossRef]
- Dong, C.; Zou, X.; Gao, Q.H. Genome-wide identification of expansin in Fragaria vesca and expression profiling analysis of the FvEXPs in different fruit development. Gene 2022, 814, 146162. [Google Scholar] [CrossRef]
- Narváez-Barragán, D.A.; Tovar-Herrera, O.E.; Segovia, L.; Serrano, M.; Martinez-Anaya, C. Expansin-related proteins: Biology, microbe-plant interactions and associated plant-defense responses. Microbiology 2020, 166, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Mühling, K.H.; Kutschera, U.; Geilfus, C.M. Salinity stiffens the epidermal cell walls of salt-stressed maize leaves: Is the epidermis growth-restricting? PLoS ONE 2015, 10, e0118406. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Wu, D.; Zhao, H.; Gong, L.; Xu, J. Regulation of SmEXPA13 expression by SmMYB1R1-L enhances salt tolerance in Salix matsudana Koidz. Int. J. Biol. Macromol. 2024, 270, 132292. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Y.; Kong, X.; Kang, H.; Ren, Y.; Wang, W. Ectopic expression of wheat expansin gene TaEXPA2 improved the salt tolerance of transgenic tobacco by regulating Na+/K+ and antioxidant competence. Physiol. Plant. 2017, 159, 161–177. [Google Scholar] [CrossRef]
- Kuluev, B.; Avalbaev, A.; Mikhaylova, E.; Nikonorov, Y.; Berezhneva, Z.; Chemeris, A. Expression profiles and hormonal regulation of tobacco expansin genes and their involvement in abiotic stress response. J. Plant Physiol. 2016, 206, 1–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, N.; Song, W.; Yin, G.; Qin, Y.; Yan, Y.; Hu, Y. Soybean (Glycine max) expansin gene superfamily origins: Segmental and tandem duplication events followed by divergent selection among subfamilies. BMC Plant Biol. 2014, 14, 93. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Li, C.; Zhou, C.; Liu, B.; Wu, Y.; He, F.; Xu, Y.; Li, F.; Feng, X. Overexpression of Wild Soybean Expansin Gene GsEXLB14 Enhanced the Tolerance of Transgenic Soybean Hairy Roots to Salt and Drought Stresses. Plants 2024, 13, 1656. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; He, F.; Xu, Y.; Li, L.; Wang, X.; Chen, Q.; Li, F. Genome-Wide Identification of Expansin Genes in Wild Soybean (Glycine soja) and Functional Characterization of Expansin B1 (GsEXPB1) in Soybean Hair Root. Int. J. Mol. Sci. 2022, 23, 5407. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Jiang, J.; Liu, Y.; Yu, L.; Chang, R.; Guan, R.; Qiu, L. Identification of a Novel Salt Tolerance-Related Locus in Wild Soybean (Glycine soja Sieb. & Zucc.). Front. Plant Sci. 2021, 12, 791175. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zeng, J.; Cao, F.; Ahmed, I.M.; Zhang, G.; Vincze, E.; Wu, F. HvEXPB7, a novel β-expansin gene revealed by the root hair transcriptome of Tibetan wild barley, improves root hair growth under drought stress. J. Exp. Bot. 2015, 66, 7405–7419. [Google Scholar] [CrossRef]
- ZhiMing, Y.; Bo, K.; XiaoWei, H.; ShaoLei, L.; YouHuang, B.; WoNa, D.; Ming, C.; Hyung-Taeg, C.; Ping, W. Root hair-specific expansins modulate root hair elongation in rice. Plant J. 2011, 66, 725–734. [Google Scholar] [CrossRef]
- Chen, S.; Luo, Y.; Wang, G.; Feng, C.; Li, H. Genome-wide identification of expansin genes in Brachypodium distachyon and functional characterization of BdEXPA27. Plant Sci. 2020, 296, 110490. [Google Scholar] [CrossRef]
- Won, S.K.; Choi, S.B.; Kumari, S.; Cho, M.; Lee, S.H.; Cho, H.T. Root hair-specific EXPANSIN B genes have been selected for Graminaceae root hairs. Mol. Cells 2010, 30, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kwasniewski, M.; Szarejko, I. Molecular cloning and characterization of beta-expansin gene related to root hair formation in barley. Plant Physiol. 2006, 141, 1149–1158. [Google Scholar] [CrossRef]
- Lee, D.K.; Ahn, J.H.; Song, S.K.; Choi, Y.D.; Lee, J.S. Expression of an expansin gene is correlated with root elongation in soybean. Plant Physiol. 2003, 131, 985–997. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Expanding wheat yields with expansin. New Phytol. 2021, 230, 403–405. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, H.; Chen, W.; Liu, J.; Jiang, C.; Jiang, H.; Zhu, S.; Cheng, B. Genome-wide identification and characterization of maize expansin genes expressed in endosperm. Mol. Genet. Genom. 2014, 289, 1061–1074. [Google Scholar] [CrossRef]
- Zou, X.; Liu, L.; Hu, Z.; Wang, X.; Zhu, Y.; Zhang, J.; Li, X.; Kang, Z.; Lin, Y.; Yin, C. Salt-induced inhibition of rice seminal root growth is mediated by ethylene-jasmonate interaction. J. Exp. Bot. 2021, 72, 5656–5672. [Google Scholar] [CrossRef]
- Jadamba, C.; Kang, K.; Paek, N.C.; Lee, S.I.; Yoo, S.C. Overexpression of Rice Expansin7 (Osexpa7) Confers Enhanced Tolerance to Salt Stress in Rice. Int. J. Mol. Sci. 2020, 21, 454. [Google Scholar] [CrossRef] [PubMed]
- Brasileiro, A.C.M.; Lacorte, C.; Pereira, B.M.; Oliveira, T.N.; Ferreira, D.S.; Mota, A.P.Z.; Saraiva, M.A.P.; Araujo, A.C.G.; Silva, L.P.; Guimaraes, P.M. Ectopic expression of an expansin-like B gene from wild Arachis enhances tolerance to both abiotic and biotic stresses. Plant J. 2021, 107, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wang, Q.; Zhou, D.; Wang, Y.; Miao, Y.; Zhong, S. Abiotic stress treatment reveals expansin like A gene OfEXLA1 improving salt and drought tolerance of Osmanthus fragrans by responding to abscisic acid. Hortic. Plant J. 2024, 10, 573–585. [Google Scholar] [CrossRef]
- Marowa, P.; Ding, A.; Xu, Z.; Kong, Y. Overexpression of NtEXPA11 modulates plant growth and development and enhances stress tolerance in tobacco. Plant Physiol. Biochem. 2020, 151, 477–485. [Google Scholar] [CrossRef]
- Liu, X.; Cai, Y.; Yao, W.; Chen, L.; Hou, W. The soybean NUCLEAR FACTOR-Y C4 and α-EXPANSIN 7 module influences phosphorus uptake by regulating root morphology. Plant Physiol. 2024, 197, kiae478. [Google Scholar] [CrossRef]
- Zhang, B.; Chang, L.; Sun, W.; Ullah, A.; Yang, X. Overexpression of an expansin-like gene, GhEXLB2 enhanced drought tolerance in cotton. Plant Physiol. Biochem. 2021, 162, 468–475. [Google Scholar] [CrossRef]
- Wei, P.C.; Zhang, X.Q.; Zhao, P.; Wang, X.C. Regulation of stomatal opening by the guard cell expansin AtEXPA1. Plant Signal Behav. 2011, 6, 740–742. [Google Scholar] [CrossRef]
- Montechiarini, N.H.; Delgado, L.; Morandi, E.N.; Néstor, J.C.; Gosparini, C.O. The expansin EXP1 gene in the elongation zone is induced during soybean embryonic axis germination and differentially expressed in response to ABA and PEG treatments. Seed Sci. Res. 2021, 31, 60–68. [Google Scholar] [CrossRef]
- Lizana, X.C.; Riegel, R.; Gomez, L.D.; Herrera, J.; Isla, A.; McQueen-Mason, S.J.; Calderini, D.F. Expansins expression is associated with grain size dynamics in wheat (Triticum aestivum L.). J. Exp. Bot. 2010, 61, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Arana, M.V.; de Miguel, L.C.; Sánchez, R.A. A phytochrome-dependent embryonic factor modulates gibberellin responses in the embryo and micropylar endosperm of Datura ferox seeds. Planta 2006, 223, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Mella, R.A.; Burgin, M.J.; Sánchez, R.A. Expansin gene expression in Datura ferox L. seeds is regulated by the low-fluence response, but not by the high-irradiance response of phytochromes. Seed Sci. Res. 2004, 14, 61–71. [Google Scholar] [CrossRef]
- Meir, S.; Hunter, D.A.; Chen, J.C.; Halaly, V.; Reid, M.S. Molecular changes occurring during acquisition of abscission competence following auxin depletion in Mirabilis jalapa. Plant Physiol. 2006, 141, 1604–1616. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Wang, X.; Liao, H. A comparison study of Agrobacterium-mediated transformation methods for root-specific promoter analysis in soybean. Plant Cell Rep. 2014, 33, 1921–1932. [Google Scholar] [CrossRef]
- Gao, L.; Ding, X.; Li, K.; Liao, W.; Zhong, Y.; Ren, R.; Liu, Z.; Adhimoolam, K.; Zhi, H. Characterization of Soybean mosaic virus resistance derived from inverted repeat-SMV-HC-Pro genes in multiple soybean cultivars. Theor. Appl. Genet. 2015, 128, 1489–1505. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Sun, Y.; Liu, W.; Shi, X.; Ma, J.; He, F.; Li, F.; Feng, X. Overexpression of the Wild Soybean Expansin Gene GsEXPB1 Enhances Salt Stress Tolerance in Transgenic Soybeans. Plants 2025, 14, 2851. https://doi.org/10.3390/plants14182851
Wang L, Sun Y, Liu W, Shi X, Ma J, He F, Li F, Feng X. Overexpression of the Wild Soybean Expansin Gene GsEXPB1 Enhances Salt Stress Tolerance in Transgenic Soybeans. Plants. 2025; 14(18):2851. https://doi.org/10.3390/plants14182851
Chicago/Turabian StyleWang, Linlin, Yanlin Sun, Wenxu Liu, Xiaolei Shi, Jing Ma, Fumeng He, Fenglan Li, and Xu Feng. 2025. "Overexpression of the Wild Soybean Expansin Gene GsEXPB1 Enhances Salt Stress Tolerance in Transgenic Soybeans" Plants 14, no. 18: 2851. https://doi.org/10.3390/plants14182851
APA StyleWang, L., Sun, Y., Liu, W., Shi, X., Ma, J., He, F., Li, F., & Feng, X. (2025). Overexpression of the Wild Soybean Expansin Gene GsEXPB1 Enhances Salt Stress Tolerance in Transgenic Soybeans. Plants, 14(18), 2851. https://doi.org/10.3390/plants14182851





