Geographical-Scale Evidence Reveals Plant Nutrient as an Effective Indicator for Coastal Carbon Emissions
Abstract
1. Introduction
2. Materials and Methods
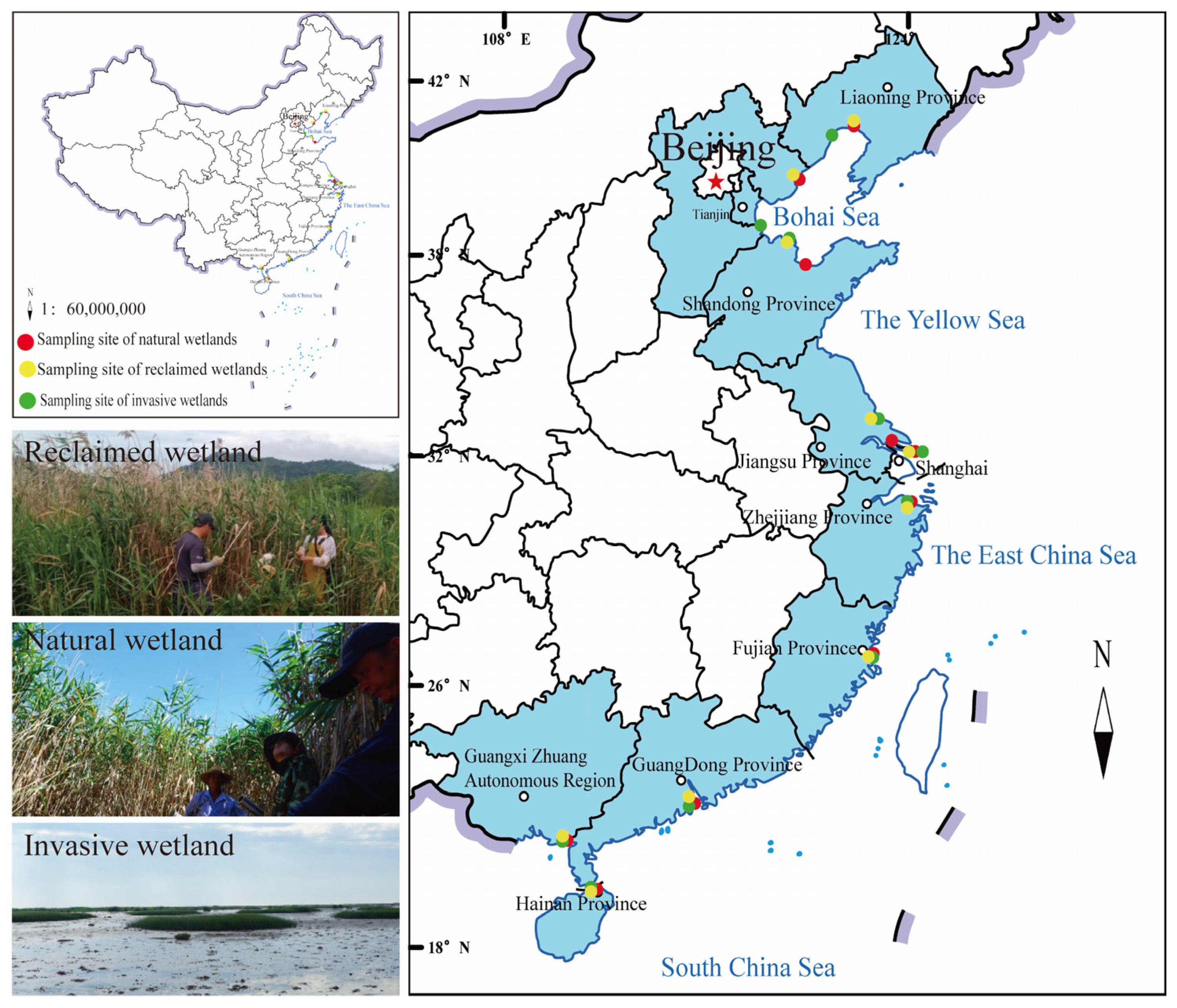
2.1. Site Description
2.2. Sampling and Measurement
2.3. Incubation Experiments
2.4. Statistical Analysis
3. Results
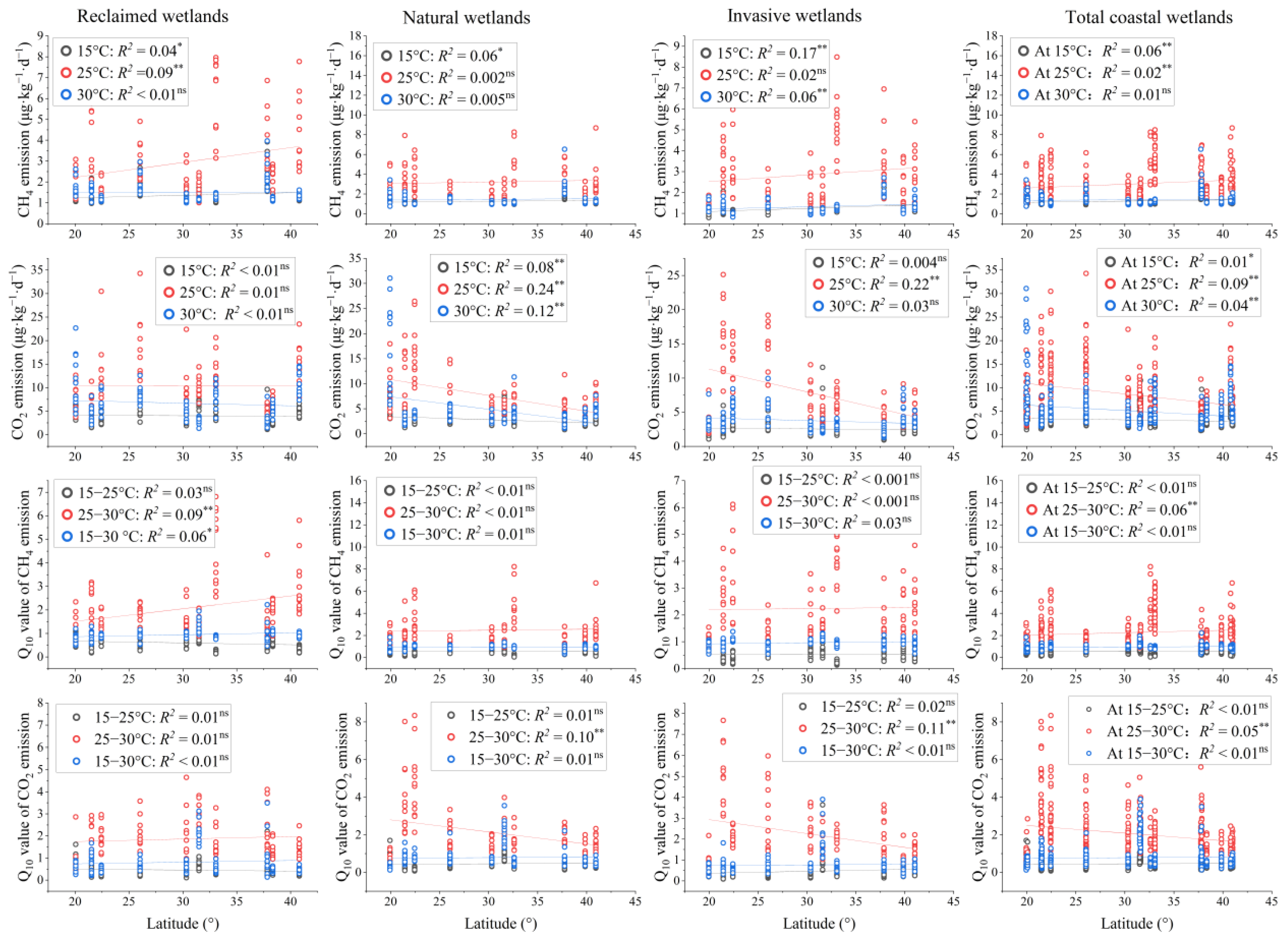
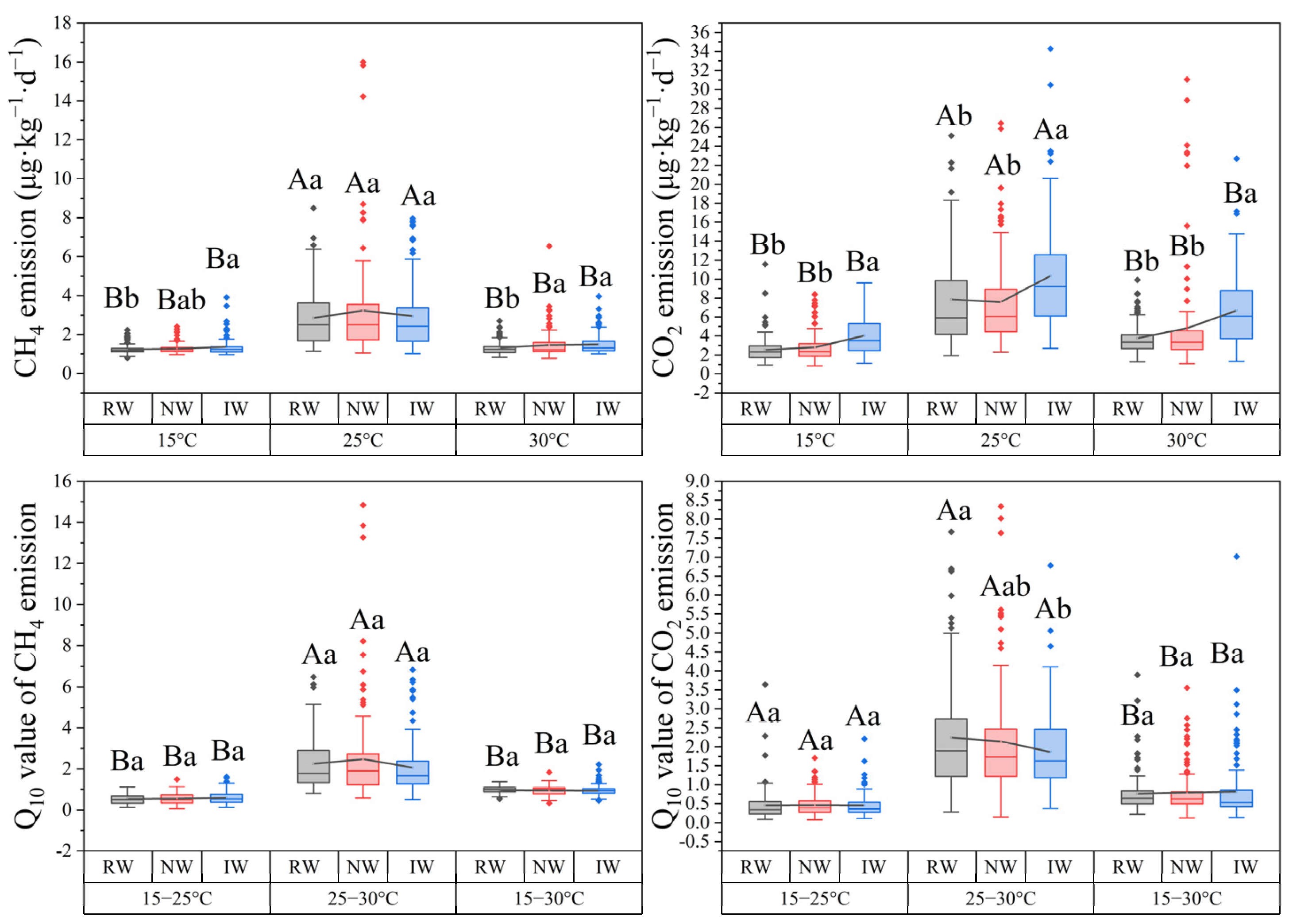
3.1. Latitudinal Patterns and Distributions of Carbon Emission
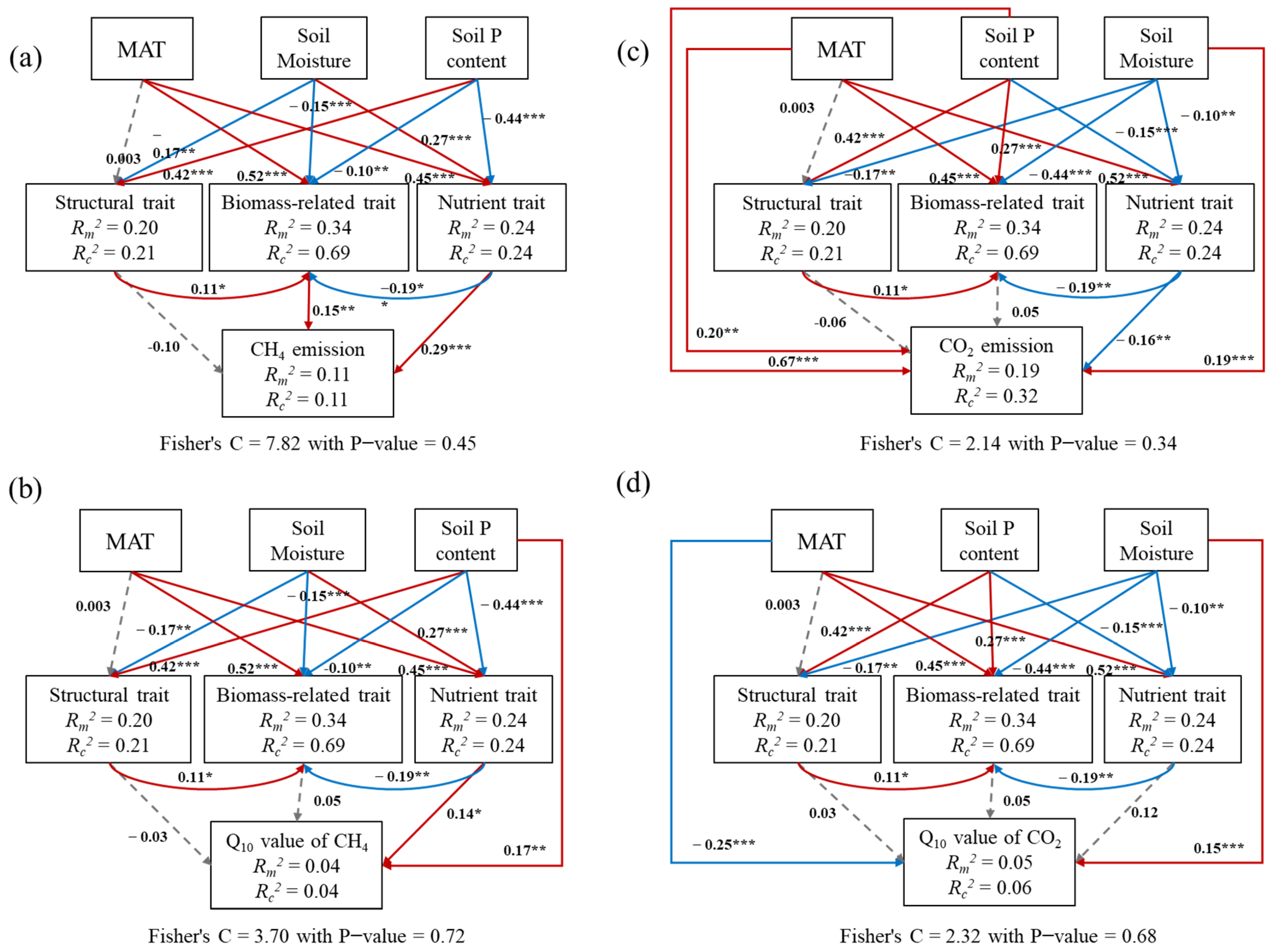
3.2. The Response–Effect Trait Framework to Carbon Emission
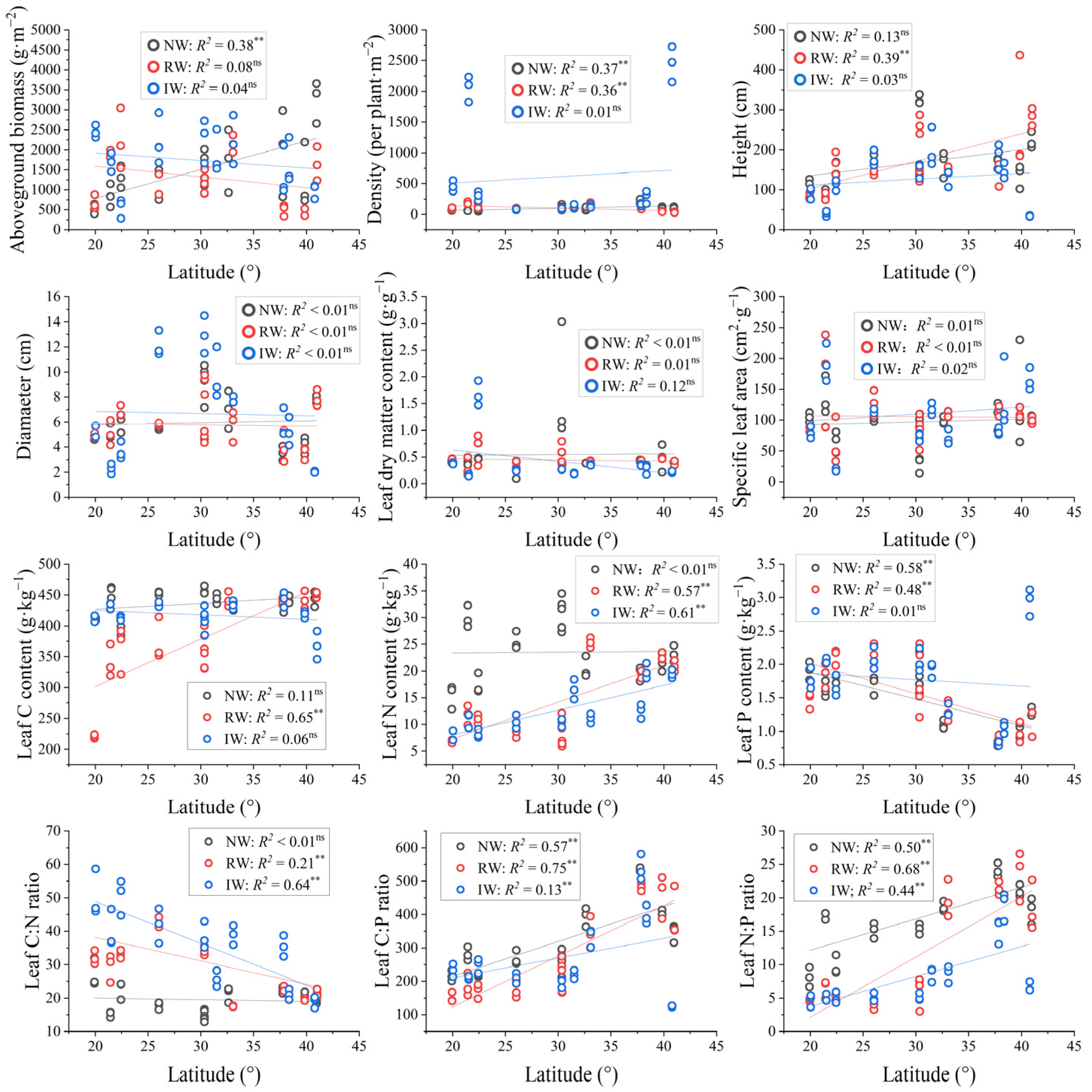
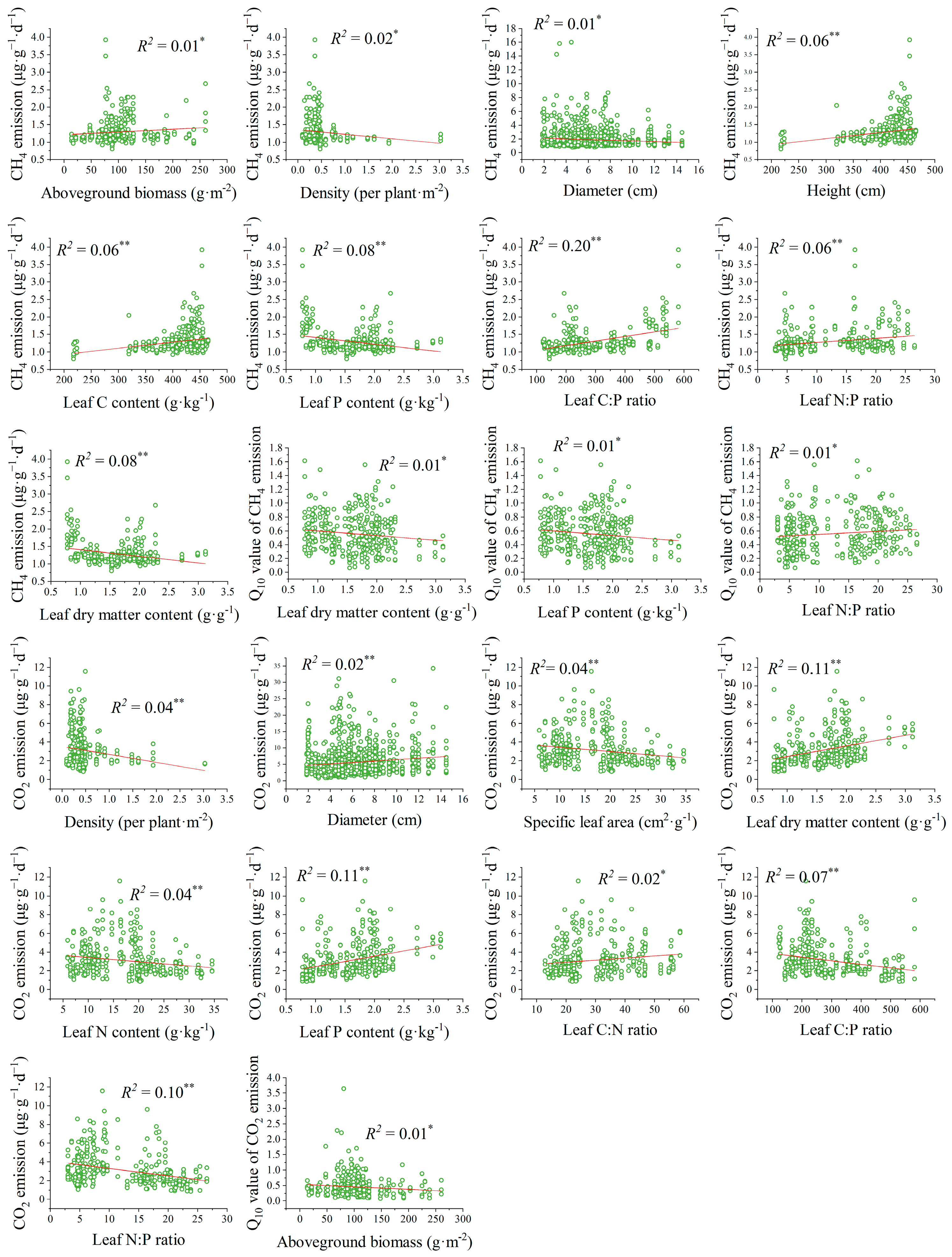
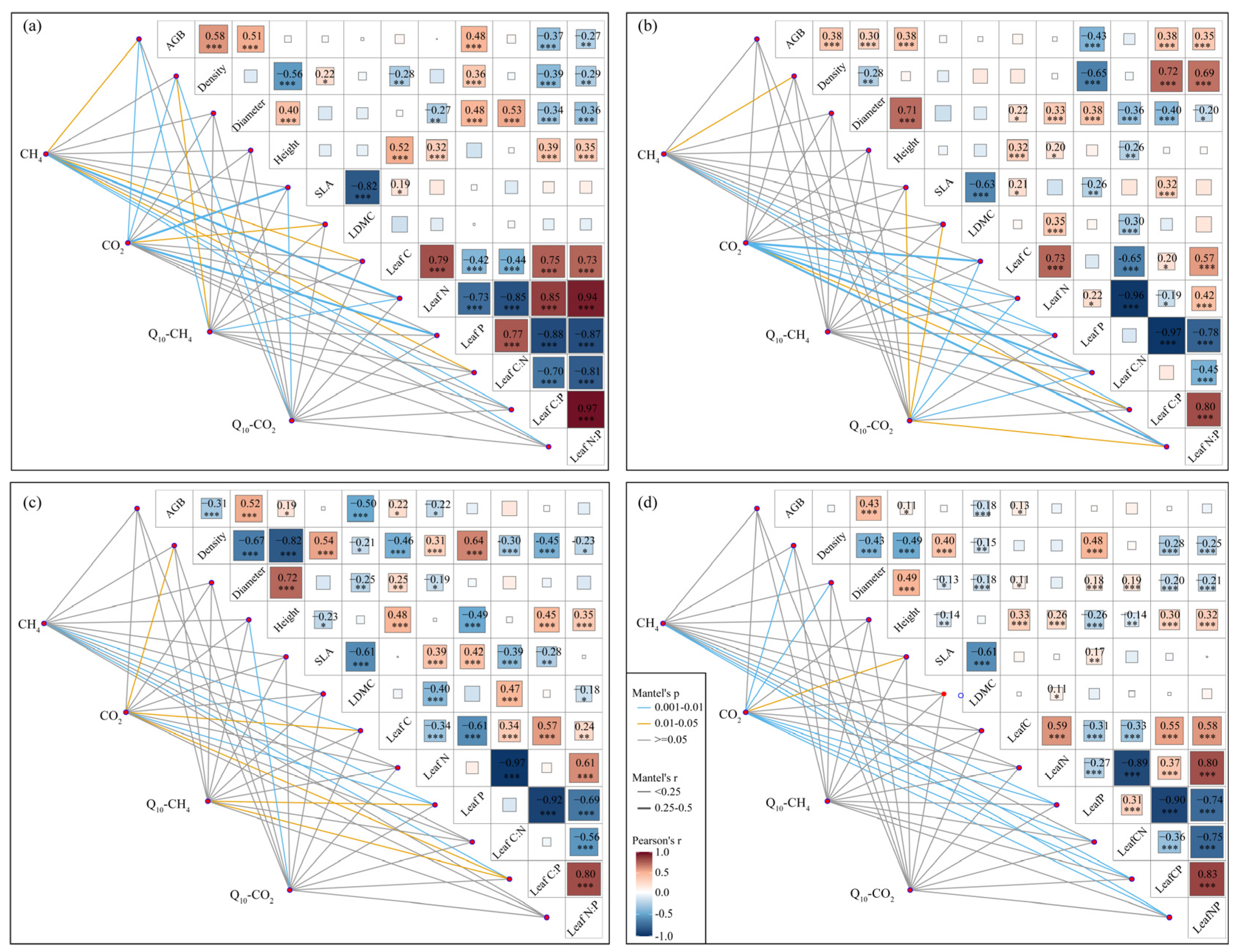
3.3. The Relationships Between Plant Traits and Carbon Emissions
4. Discussion
4.1. The Response–Effect Trait Framework to Carbon Emissions
4.2. Effects of Environmental Conditions
4.3. Effects of Land Uses on Carbon Emission
4.4. Limitations and Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, F.M.; Liu, J.H.; Qin, G.M.; Zhang, J.F.; Zhou, J.G.; Wu, J.T.; Zhang, L.L.; Thapa, P.; Sanders, C.J.; Santos, I.R.; et al. Coastal blue carbon in China as a nature-based solution towards carbon neutrality. Innovation 2023, 4, 100481. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Hansen, J.E.; Lacis, A.A. Sun and dust versus greenhouse gases: An assessment of their relative roles in global climate change. Nature 1990, 346, 713–719. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Montoya, J.M.; Woodward, G.; Jones, J.I.; Trimmer, M. Warming increases the proportion of primary production emitted as methane from freshwater mesocosms. Glob. Change Biol. 2011, 17, 1225–1234. [Google Scholar] [CrossRef]
- Yang, H.L.; Tang, J.W.; Zhang, C.S.; Dai, Y.H.; Zhou, C.; Xu, P.; Perry, D.C.; Chen, X.C. Enhanced carbon uptake and reduced methane emissions in a newly restored wetland. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005222. [Google Scholar] [CrossRef]
- IPCC. Climate change 2014: Synthesis report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- Inglett, K.S.; Inglett, P.W.; Reddy, K.R.; Osborne, T.Z. Temperature sensitivity of greenhouse gas production in wetland soils of different vegetation. Biogeochemistry 2012, 108, 77–90. [Google Scholar] [CrossRef]
- Chen, H.Y.; Xu, X.; Fang, C.M.; Li, B.; Nie, M. Differences in the temperature dependence of wetland CO2 and CH4 emissions vary with water table depth. Nat. Clim. Change 2021, 11, 766–771. [Google Scholar] [CrossRef]
- Nie, M. Hydrospheric methane emission and its microbiological mechanisms under climate warming. Acta Microbiol. Sin. 2020, 60, 1821–1833. [Google Scholar]
- Li, X.F.; Sardans, J.; Hou, L.J.; Liu, M.; Xu, C.B.; Peñuelas, J. Climatic temperature controls the geographical patterns of coastal marshes greenhouse gases emissions over China. J. Hydrol. 2020, 590, 125378. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zhu, T.; Li, B.; Fang, C.M.; Nie, M. The thermal response of soil microbial methanogenesis decreases in magnitude with changing temperature. Nat. Commun. 2020, 11, 5733. [Google Scholar] [CrossRef]
- Zhang, H.J.; Yao, X.D.; Zeng, W.J.; Fang, Y.; Wang, W. Depth dependence of temperature sensitivity of soil carbon dioxide, nitrous oxide and methane emissions. Soi. Biol. Biochem. 2020, 149, 107956. [Google Scholar] [CrossRef]
- Hu, M.J.; Sardans, J.; Yang, X.Y.; Peñuelas, J.; Tong, C. Patterns and environmental drivers of greenhouse gas fluxes in the coastal wetlands of China: A systematic review and synthesis. Environ. Res. 2020, 186, 109576. [Google Scholar] [CrossRef] [PubMed]
- Girkin, N.T.; Dhandapani, S.; Evers, S.; Ostle, N.; Turner, B.L.; Sjögerste, S. Interactions between labile carbon, temperature and land use regulate carbon dioxide and methane production in tropical peat. Biogeochemistry 2020, 147, 87–97. [Google Scholar] [CrossRef]
- Kooch, Y.; Ghorbanzadeh, N.; Francaviglia, R. Soil carbon stocks can be negatively affected by land use and climate change in natural ecosystems of semi-arid environment of Iran. Geoderma Reg. 2022, 31, e00591. [Google Scholar] [CrossRef]
- Davidson, N.C. How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshw. Res. 2014, 65, 934–941. [Google Scholar] [CrossRef]
- Ma, Z.J.; Melville, D.S.; Liu, J.G.; Chen, Y.; Yang, H.Y.; Ren, W.W.; Zhang, Z.W.; Piersma, T.; Li, B. Ecosystems management rethinking China’s new great wall. Science 2014, 346, 912–914. [Google Scholar] [CrossRef]
- Hu, Y.K.; Tian, B.; Yuan, L.; Li, X.Z.; Huang, Y.; Shi, R.H.; Jiang, X.Y.; Wang, L.H.; Sun, C. Mapping coastal salt marshes in China using time series of Sentinel-1 SAR. ISPRS J. Photogramm. Remote Sens. 2021, 173, 122–134. [Google Scholar] [CrossRef]
- Yuan, J.J.; Liu, D.Y.; Ji, Y.; Xiang, J.; Lin, Y.X.; Wu, M.; Ding, W.X. Spartina alterniflora invasion drastically increases methane production potential by shifting methanogenesis from hydrogenotrophic to methylotrophic pathway in a coastal marsh. J. Ecol. 2019, 107, 2436–2450. [Google Scholar] [CrossRef]
- Sutton-Grier, A.E.; Megonigal, J.P. Plant species traits regulate methane production in freshwater wetland soils. Soil Biol. Biochem. 2011, 43, 413–420. [Google Scholar] [CrossRef]
- Happonen, K.; Virkkala, A.M.; Kemppinen, J.; Niittynen, P.; Luoto, M. Relationships between above-ground plant traits and carbon cycling in tundra plant communities. J. Ecol. 2021, 110, 700–716. [Google Scholar] [CrossRef]
- Goud, E.M.; Moore, T.R.; Roulet, N.T. Predicting peatland carbon fluxes from non-destructive plant traits. Funct. Ecol. 2017, 3, 1824–1833. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.; Wright, I.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Writh, C.; Prentice, I.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive la difference: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Minden, V.; Kleyer, M. Ecosystem multifunctionality of coastal marshes is determined by key plant traits. J. Veg. Sci. 2015, 26, 651–662. [Google Scholar] [CrossRef]
- Zibel, C.R.; Bassett, T.; German, E.; Brudvig, L.A. Plant functional traits and environmental conditions shape community assembly and ecosystem functioning during restoration. J. Appl. Ecol. 2017, 54, 1070–1079. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Comelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.; Schweiger, A.K.; Carteron, A.; Kalacska, M.; Laliberté, E. Foliar spectra and traits of bog plants across nitrogen deposition gradients. Remote Sens. 2020, 12, 2448. [Google Scholar] [CrossRef]
- Xiong, J.; Shao, X.X.; Yuan, H.J.; Liu, E.J.; Xu, H.D.; Wu, M. Effect of human reclamation and Spartina alterniflora invasion on C-N-P stoichiometry in plant organs across coastal wetlands over China. Plant Soil. 2024, 494, 167–183. [Google Scholar] [CrossRef]
- Xiong, J.; Shao, X.X.; Li, N.; Yuan, H.J.; Liu, E.J.; Wu, M. Effects of land-use on soil C, N, and P stocks and stoichiometry in coastal wetlands dependent on soil depth and latitude. Catena 2024, 240, 107999. [Google Scholar] [CrossRef]
- Ren, G.Q.; Cui, M.M.; Yu, H.C.; Fan, X.; Zhu, Z.Q.; Zhang, H.Y.; Dai, Z.C.; Sun, J.F.; Yang, B.; Du, D.L. Global environmental change shifts ecological stoichiometry coupling between plant and soil in early-stage invasions. J. Soil. Sci. Plant Nutr. 2024, 24, 2402–2412. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice hall INC: New York, NY, USA, 1958. [Google Scholar]
- Wang, W.; Zeng, C.; Sardans, J.; Wang, C.; Tong, C.; Peñuelas, J. Soil methane production, anaerobic and aerobic oxidation in porewater of wetland soils of the Minjiang River Estuarine, China. Wetlands 2018, 38, 627–640. [Google Scholar] [CrossRef]
- Reinhardt, C.H.; Cole, C.A.; Stover, L.R. A method for coring inland, freshwater wetland soils. Wetlands 2000, 20, 422–426. [Google Scholar] [CrossRef]
- Xu, X.; Liu, H.; Liu, Y.Z.; Zhou, C.H.; Pan, L.H.; Fang, C.M.; Nie, M.; Li, B. Human eutrophication drives biogeographic saltmarsh productivity patterns in China. Ecol. Appl. 2020, 30, e02045. [Google Scholar] [CrossRef] [PubMed]
- Megonigal, J.P.; Hines, M.E.; Visscher, P.T. Anaerobic metabolism: Linkages to trace gases and aerobic processes. In Biogeochemistry; Schlesinger, W.H., Ed.; Elsevier-Pergamon: Oxford, UK, 2004; p. 317. [Google Scholar]
- Xiong, J.; Shao, X.; Yuan, H.; Liu, E.; Xu, H.; Wu, M. Carbon, nitrogen, and phosphorus stoichiometry and plant growth strategy as related to land-use in Hangzhou Bay coastal wetland, China. Front. Plant Sci. 2022, 13, 946949. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Wei, T.Y.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix, Version 0.92; R Development Core Team: Vienna, Austria, 2021.
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package, R package version 2.6-4; R Development Core Team: Vienna, Austria, 2022.
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research, (R Package Version 2.4.3); Northwestern University: Evanston, IL, USA, 2022.
- Goud, E.M.; Touchette, S.; Strachan, I.B.; Strack, M. Graminoids vary in functional traits, carbon dioxide and methane fluxes in a restored peatland: Implications for modelling carbon storage. J. Ecol. 2022, 110, 2105–2117. [Google Scholar] [CrossRef]
- Xu, Z.W.; Yu, G.R.; Wang, Q.F.; Zhang, X.Y.; Wang, R.L.; Zhao, N.; He, N.P.; Liu, Z.P. Plant functional traits determine latitudinal variations in soil microbial function: Evidence from forests in China. Biogeosciences 2019, 16, 3333–3349. [Google Scholar] [CrossRef]
- Lau, J.A. Aboveground–Belowground Linkages: Biotic Interactions, Ecosystem processes, and Global Change. Q. Rev. Biol. 2011, 86, 340. [Google Scholar]
- Reich, P.B.; Ellsworth, D.S.; Uhl, C. Leaf carbon and nutrient assimilation and conservation in species of differing successional status in an oligotrophic Amazonian forest. Funct. Ecol. 1995, 9, 65–76. [Google Scholar] [CrossRef]
- Li, X.Q.; Xia, J.B.; Zhao, X.M.; Chen, Y.P. Effects of planting Tamarix chinensis on shallow soil water and salt content under different groundwater depths in the Yellow River Delta. Geoderma 2019, 335, 104–111. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A. Pattern and variation of C: N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Zhu, D.; Wu, N.; Bhattarai, N.; Oil, K.P.; Chen, H.; Rawat, G.S.; Rashid, I.; Dhakal, M.; Joshi, S.; Tian, J.Q.; et al. Methane emissions respond to soil temperature in convergent patterns but divergent sensitivities across wetlands along altitude. Glob. Change Biol. 2021, 27, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.F.; Middelburg, J.J.; Röckmann, T.; Aerts, R.; Blauw, L.G.; Egger, M.; Jetten, M.S.M.; de Jong, A.E.E.; Msisel, O.H.; Rasigraf, O.; et al. Methane feedbacks to the global climate system in a warmer world. Rev. Geophys. 2018, 56, 207–250. [Google Scholar] [CrossRef]
- Mishra, S.R.; Pattnaik, P.; Sethunathan, N.; Adhya, T.K. Anion-mediated salinity affecting methane production in a flooded alluvial soil. Geomicrobiol. J. 2003, 20, 579–586. [Google Scholar] [CrossRef]
- Segarra, K.E.A.; Schubotz, F.; Samarkin, V.; Yoshinaga, M.Y.; Hinrichs, K.U.; Joye, S.B. High rates of anaerobic methane oxidation in freshwater wetlands reduce potential atmospheric methane emissions. Nat. Comm. 2015, 6, 7477. [Google Scholar] [CrossRef]
- Shen, L.D.; Hu, B.L.; Liu, S.; Chai, X.P.; He, Z.F.; Ren, H.X.; Liu, Y.; Geng, S.; Wang, W.; Tang, J.L.; et al. Anaerobic methane oxidation coupled to nitrite reduction can be a potential methane sink in coastal environments. Appl. Genet. Mol. Biotechnol. 2016, 100, 7171–7180. [Google Scholar] [CrossRef]
- Xiong, J.; Sheng, X.C.; Wang, M.; Wu, M.; Shao, X.X. Comparative study of methane emission in the reclamation-restored wetlands and natural marshes in the Hangzhou Bay coastal wetland. Ecol. Eng. 2022, 175, 106473. [Google Scholar] [CrossRef]
- Yan, Z.B. Effects of nitrogen and phosphorus addition on the transgenerational growth and stoichiometry of Arabidopsis thaliana. Ph.D. dissertation, Peking University, Beijing, China, 2017. [Google Scholar]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- van Dijk, G.; Lamers, L.P.M.; Loeb, R.; Westendorp, P.J.; Kuiperij, R.; van Kleef, H.H.; Klinge, M.; Smolders, A.J.P. Salinization lowers nutrient availability in formerly brackish freshwater wetlands; unexpected results from a long-term field experiment. Biogeochemistry 2019, 143, 67–83. [Google Scholar] [CrossRef]
- Dierberg, F.E.; DeBusk, T.A.; Larson, N.R.; Kharbanda, M.D.; Chan, N.; Gabriel, M.C. Effects of sulfate amendments on mineralization and phosphorus release from South Florida (USA) wetland soils under anaerobic conditions. Soil. Biol. Biochem. 2011, 43, 31–45. [Google Scholar] [CrossRef]
| Distribution | Unit | |
|---|---|---|
| (a) Plant traits | ||
| Specific leaf area (SLA) | Structural trait | cm2·g−1 |
| Leaf dry matter content (LDMC) | Structural trait | g·g−1 |
| Density | Biomass-related trait | per plant·m−2 |
| Diameter | Biomass-related trait | cm |
| Height | Biomass-related trait | cm |
| Aboveground biomass (AGB) | Biomass-related trait | g·m−2 |
| Leaf C content | Nutrient trait | g·kg−1 |
| Leaf N content | Nutrient trait | g·kg−1 |
| Leaf P content | Nutrient trait | g·kg−1 |
| Leaf C:N ratio | Nutrient trait | —— |
| Leaf C:P ratio | Nutrient trait | —— |
| Leaf N:P ratio | Nutrient trait | —— |
| (b) Environmental conditions | ||
| Mean annual temperature (MAT) | Climate | °C |
| Mean annual precipitation (MATP) | Climate | mm |
| Moisture | Soil properties | % |
| pH | Soil properties | —— |
| Salinity | Soil properties | g·kg−1 |
| Sulfate concentration | Soil properties | mg·g−1 |
| Dissolved carbon content (DOC) | Soil properties | mg·kg−1 |
| Soil N content | Soil properties | g·kg−1 |
| Soil P content | Soil properties | g·kg−1 |
| (c) Soil carbon emissions | ||
| CH4 emission | Carbon emission | μg·g−1·d−1 |
| CO2 emission | Carbon emission | μg·g−1·d−1 |
| Q10 value of CH4 emission (Q10-CH4) | Temperature sensitivity | —— |
| Q10 value of CO2 emission (Q10-CO2) | Temperature sensitivity | —— |
| CH4 | CO2 | Q10-CH4 | Q10-CO2 | CH4 | CO2 | Q10-CH4 | Q10-CO2 | ||
|---|---|---|---|---|---|---|---|---|---|
| Intercept (NW) | 2.076 | −0.358 | 1.391 | 0.571 | Intercept (NW) | 2.236 | 1.417 | 1.232 | 0.675 |
| RW | 0.183 | 1.585 | −0.067 | 0.104 | RW | −0.088 | 0.675 | −0.065 | 0.230 |
| IW | 0.489 | 7.122 | 0.307 | 0.572 | IW | 0.256 | 4.777 | 0.223 | 0.462 |
| MAT | −0.005 | 0.315 | −0.004 | 0.033 | MAP | <0.001 | 0.003 | <0.001 | <0.001 |
| RW × MAT | −0.022 | −0.110 | −0.001 | −0.004 | RW × MAP | <0.001 | −0.001 | <0.001 | <0.001 |
| IW × MAT | −0.032 | −0.300 | −0.026 | −0.038 | IW × MAP | <0.001 | −0.002 | <0.001 | <0.001 |
| R2adj-squared | 0.030 | 0.259 | 0.022 | 0.038 | R2adj-squared | 0.055 | 0.273 | 0.014 | 0.042 |
| F-statistic | 3.227 | 26.170 | 2.584 | 3.811 | F-statistic | 5.191 | 27.890 | 2.027 | 4.122 |
| p-value | 0.007 | <0.001 | 0.026 | 0.002 | p-value | 0.001 | <0.001 | 0.074 | 0.001 |
| Natural Wetlands | Reclaimed Wetlands | Invasive Wetlands | |
|---|---|---|---|
| Aboveground biomass (g·m−2) | 1518.95 ± 857.52 ab | 1318.38 ± 686.23 b | 1730.69 ± 702.62 a |
| Density (per plant·m−2) | 102.17 ± 43.40 b | 101.90 ± 49.50 b | 614.13 ± 841.12 a |
| Diameter (cm) | 5.95 ± 1.92 a | 5.77 ± 1.76 a | 6.68 ± 3.78 a |
| Height (cm) | 168.40 ± 66.15 a | 173.46 ± 80.84 a | 127.20 ± 58.21 b |
| Specific leaf area (cm2·g−1) | 97.14 ± 38.98 a | 105.68 ± 37.95 a | 110.14 ± 58.60 a |
| Leaf dry matter content (g·g−1) | 0.55 ± 0.51 a | 0.45 ± 0.15 a | 0.42 ± 0.43 a |
| Leaf C content (g·kg−1) | 436.82 ± 20.98 a | 379.66 ± 69.51 b | 417.72 ± 22.37 a |
| Leaf N content (g·kg−1) | 23.54 ± 5.66 a | 14.36 ± 6.73 b | 12.80 ± 4.23 b |
| Leaf P content (g·kg−1) | 1.48 ± 0.39 b | 1.55 ± 0.48 ab | 1.77 ± 0.59 a |
| Leaf C:N ratio | 19.51 ± 4.19 b | 30.97 ± 11.46 a | 36.15 ± 11.13 a |
| Leaf C:P ratio | 321.47 ± 101.59 a | 278.84 ± 128.30 a | 271.01 ± 115.87 a |
| Leaf N:P ratio | 16.94 ± 4.88 a | 11.38 ± 8.05 b | 8.32 ± 4.79 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, J.; Shao, X.; Xu, H.; Wu, M. Geographical-Scale Evidence Reveals Plant Nutrient as an Effective Indicator for Coastal Carbon Emissions. Plants 2025, 14, 2852. https://doi.org/10.3390/plants14182852
Xiong J, Shao X, Xu H, Wu M. Geographical-Scale Evidence Reveals Plant Nutrient as an Effective Indicator for Coastal Carbon Emissions. Plants. 2025; 14(18):2852. https://doi.org/10.3390/plants14182852
Chicago/Turabian StyleXiong, Jing, Xuexin Shao, Haidong Xu, and Ming Wu. 2025. "Geographical-Scale Evidence Reveals Plant Nutrient as an Effective Indicator for Coastal Carbon Emissions" Plants 14, no. 18: 2852. https://doi.org/10.3390/plants14182852
APA StyleXiong, J., Shao, X., Xu, H., & Wu, M. (2025). Geographical-Scale Evidence Reveals Plant Nutrient as an Effective Indicator for Coastal Carbon Emissions. Plants, 14(18), 2852. https://doi.org/10.3390/plants14182852






