Advances in Cell Wall Dynamics and Gene Expression in Postharvest Fruit Softening
Abstract
1. Introduction
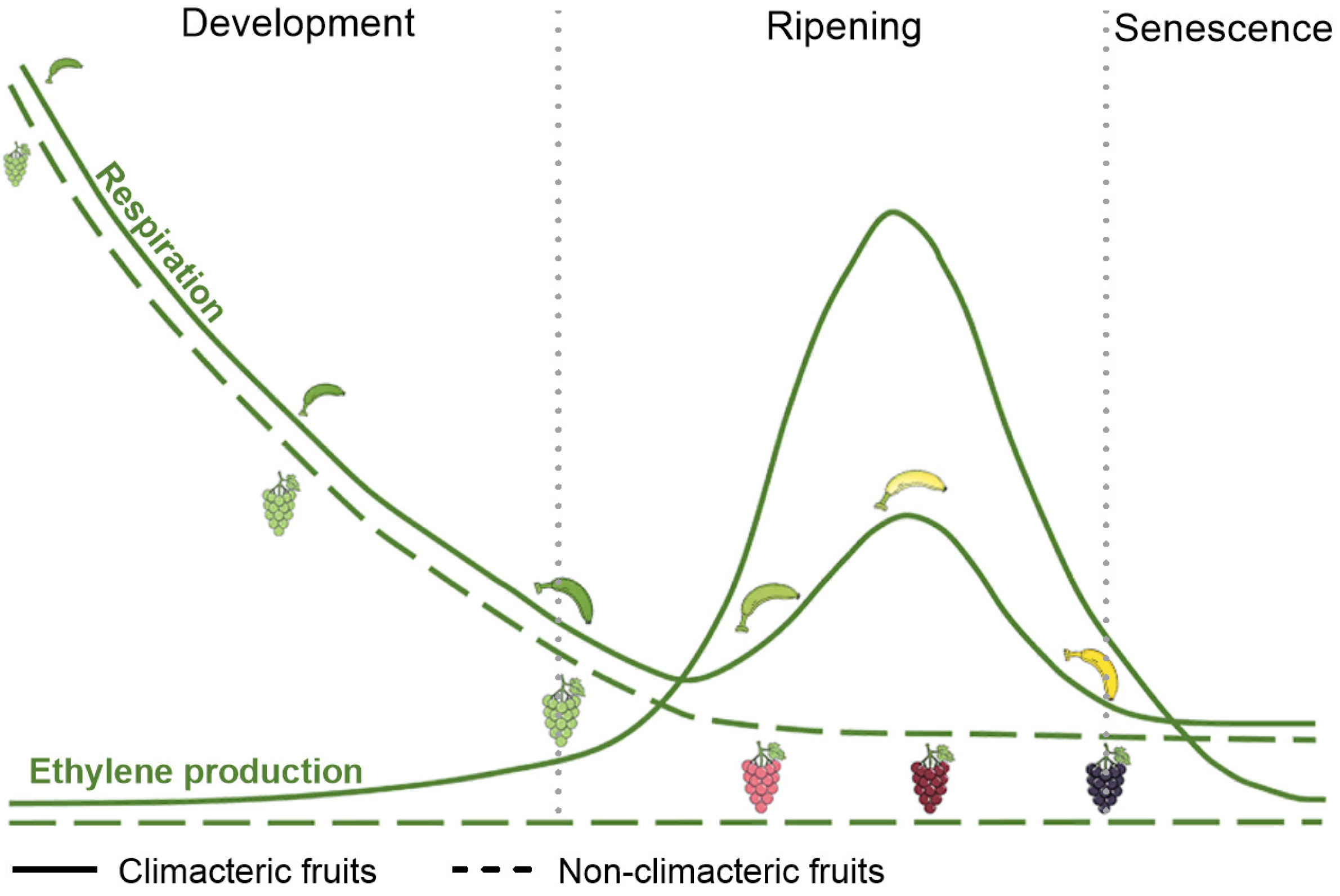
2. The Process of Fruit Softening
3. Cell Wall and Fruits Softening
3.1. Composition of the Cell Wall
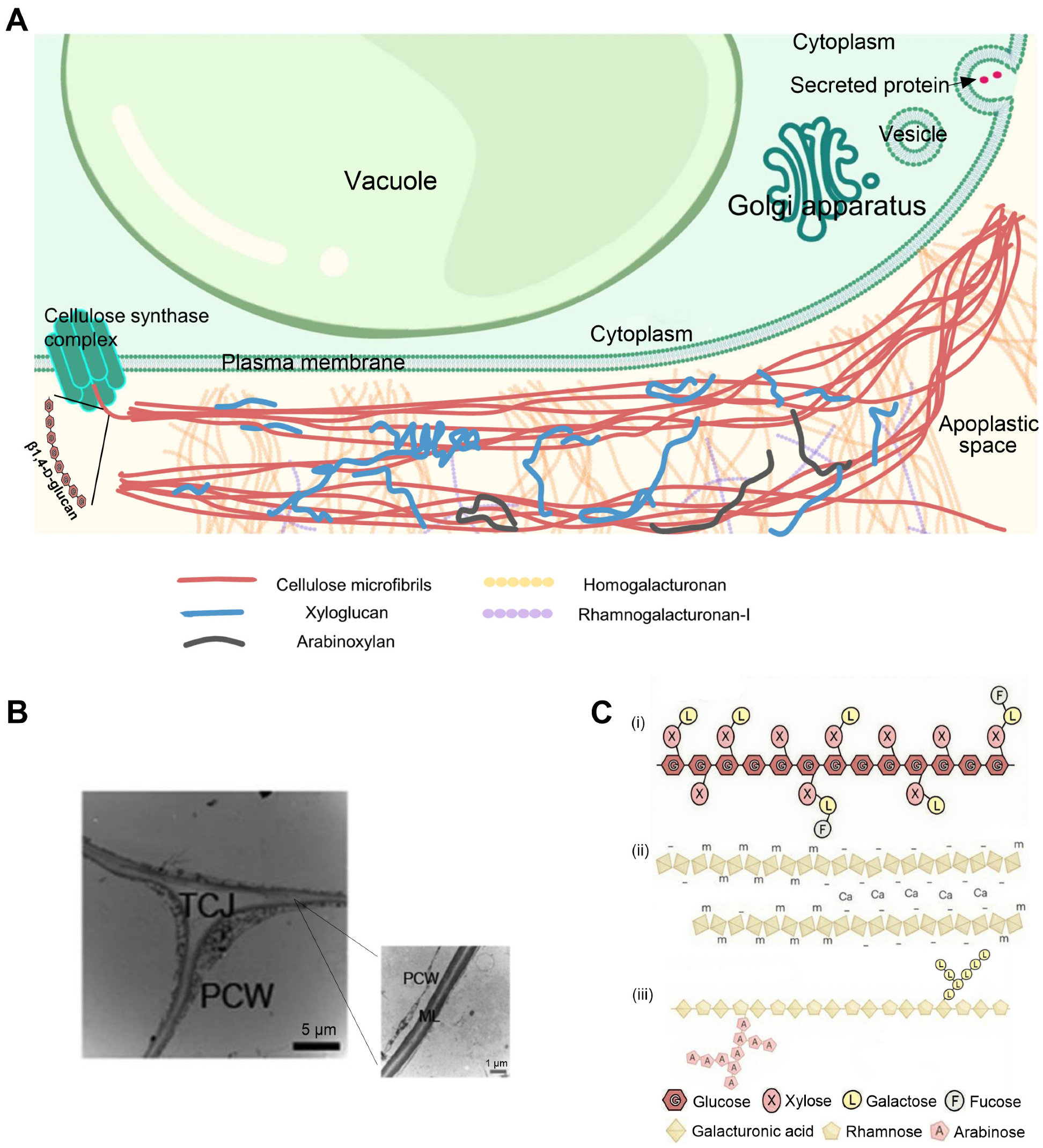
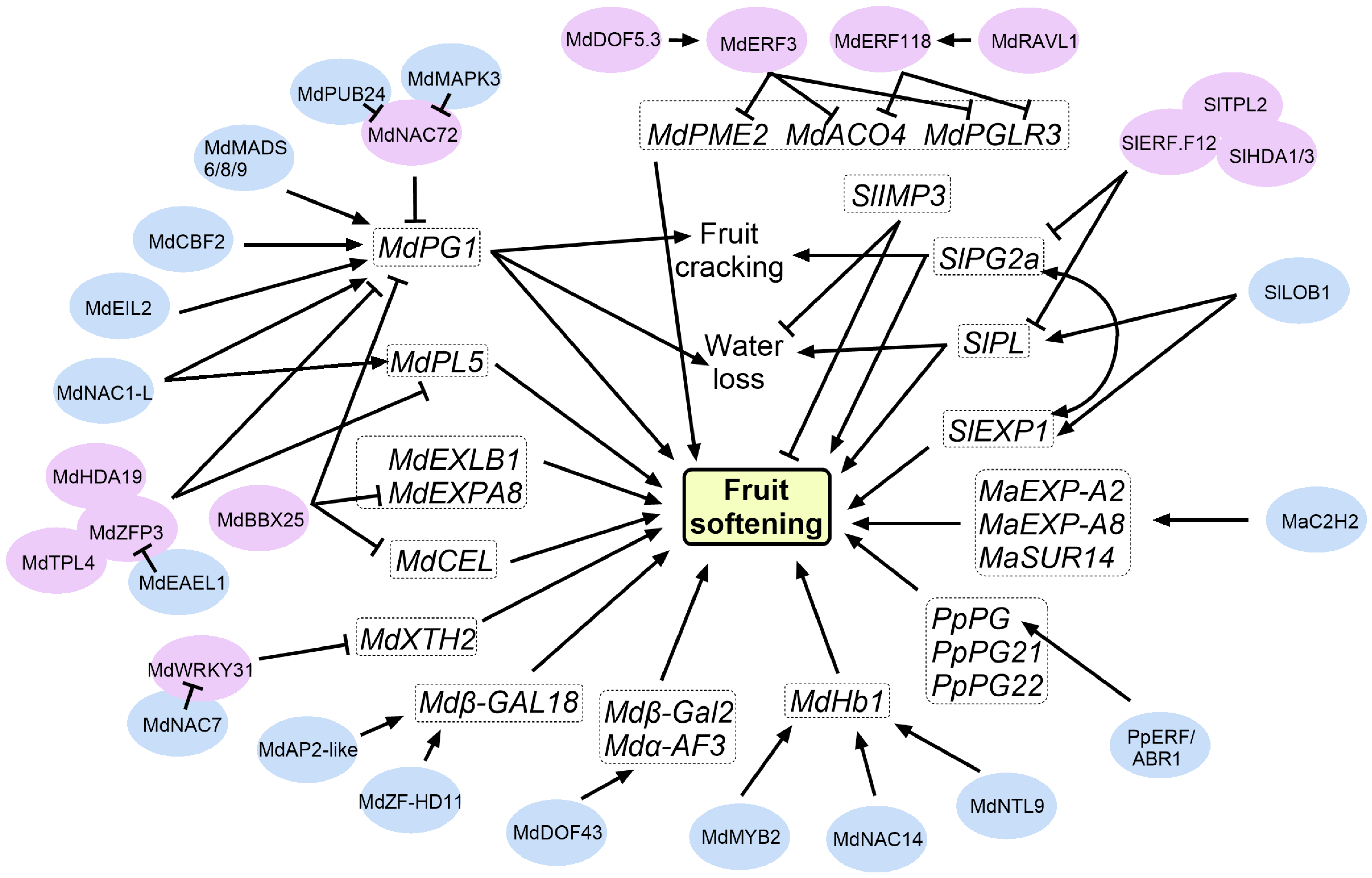
3.2. Cell Wall Remodeling Proteins
3.3. Modulators Involved in the CWRPs-Mediated Regulatory Network of Fruit Softening
4. Water Loss
5. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Migicovsky, Z.; Yeats, T.H.; Watts, S.; Song, J.; Forney, C.F.; Burgher-MacLellan, K.; Somers, D.J.; Gong, Y.; Zhang, Z.; Vrebalov, J. Apple ripening is controlled by a NAC transcription factor. Front. Genet. 2021, 12, 671300. [Google Scholar] [CrossRef]
- Brummell, D.A.; Bowen, J.K.; Gapper, N.E. Biotechnological approaches for controlling postharvest fruit softening. Curr. Opin. Biotechnol. 2022, 78, 102786. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, Z.; Lv, T.; Xu, Y.; Wei, Y.; Liu, W.; Liu, L.; Wang, A.; Li, T. Ethylene enhances MdMAPK3-mediated phosphorylation of MdNAC72 to promote apple fruit softening. Plant Cell 2023, 35, 2887–2909. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, L.; Yang, G.; Cai, Y.; Wang, Y.; Sun, B.; Sun, L.; Liu, W.; Wang, A. Ethylene-Activated E3 Ubiquitin Ligase MdEAEL1 Promotes Apple Fruit Softening by Facilitating the Dissociation of Transcriptional Repressor Complexes. Adv. Sci. 2025, 12, e2417393. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Kwon, S.-I.; Watkins, C.B.; Kang, I.-K. Characterization of fruit quality attributes and cell wall metabolism in 1-methylcyclopropene (1-MCP)-treated ‘Summer King’ and ‘Green Ball’ apples during cold storage. Front. Plant Sci. 2019, 10, 1513. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Zhan, W.; Wang, H.; Chen, M.; Li, T.; Bai, T.; Jiao, J.; Song, C.; Song, S. The apple transcription factor MdZF-HD11 regulates fruit softening by promoting Mdβ-GAL18 expression. J. Exp. Bot. 2024, 75, 819–836. [Google Scholar] [CrossRef]
- Johnston, J.W.; Hewett, E.W.; Hertog, M.L. Postharvest softening of apple (Malus domestica) fruit: A review. N. Z. J. Crop Hortic. Sci. 2002, 30, 145–160. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, M.; Wang, A. Recent advances in the regulation of climacteric fruit ripening: Hormone, transcription factor and epigenetic modifications. Front. Agric. Sci. Eng. 2021, 8, 314–334. [Google Scholar] [CrossRef]
- Kevany, B.M.; Tieman, D.M.; Taylor, M.G.; Cin, V.D.; Klee, H.J. Ethylene receptor degradation controls the timing of ripening in tomato fruit. Plant J. 2007, 51, 458–467. [Google Scholar] [CrossRef]
- Alexander, L.; Grierson, D. Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef]
- Zhu, P.; Xu, L.; Zhang, C.; Toyoda, H.; Gan, S.-S. Ethylene produced by Botrytis cinerea can affect early fungal development and can be used as a marker for infection during storage of grapes. Postharvest Biol. Technol. 2012, 66, 23–29. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Catalysts of plant cell wall loosening. F1000Research 2016, 5, 119. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef] [PubMed]
- Daher, F.B.; Braybrook, S.A. How to let go: Pectin and plant cell adhesion. Front. Plant Sci. 2015, 6, 523. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lin, Y.-C.J.; Chen, Y.-L.; Zhou, C.; Li, S.; De Ridder, N.; Oliveira, D.M.; Zhang, L.; Zhang, B.; Wang, J.P. Woody plant cell walls: Fundamentals and utilization. Mol. Plant 2024, 17, 112–140. [Google Scholar] [CrossRef]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, G.B.; Blaschek, L.; Frandsen, K.E.; Noack, L.C.; Persson, S. Cellulose synthesis in land plants. Mol. Plant 2023, 16, 206–231. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Temple, H.; Phyo, P.; Yang, W.; Lyczakowski, J.J.; Echevarría-Poza, A.; Yakunin, I.; Parra-Rojas, J.P.; Terrett, O.M.; Saez-Aguayo, S.; Dupree, R. Golgi-localized putative S-adenosyl methionine transporters required for plant cell wall polysaccharide methylation. Nat. Plants 2022, 8, 656–669. [Google Scholar] [CrossRef]
- Shi, D.; Ren, A.; Tang, X.; Qi, G.; Xu, Z.; Chai, G.; Hu, R.; Zhou, G.; Kong, Y. MYB52 negatively regulates pectin demethylesterification in seed coat mucilage. Plant Physiol. 2018, 176, 2737–2749. [Google Scholar] [CrossRef] [PubMed]
- Gunaseelan, K.; Schröder, R.; Rebstock, R.; Ninan, A.S.; Deng, C.; Khanal, B.P.; Favre, L.; Tomes, S.; Dragulescu, M.A.; O’Donoghue, E.M. Constitutive expression of apple endo-POLYGALACTURONASE1 in fruit induces early maturation, alters skin structure and accelerates softening. Plant J. 2024, 117, 1413–1431. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Ray, D.; Aswal, V.K.; Deshpande, A.P.; Varughese, S. Pectin self-assembly and its disruption by water: Insights into plant cell wall mechanics. Phys. Chem. Chem. Phys. 2022, 24, 22691–22698. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; Orfila, C.; Limberg, G.; Buchholt, H.C.; van Alebeek, G.-J.W.; Voragen, A.G.; Marcus, S.E.; Christensen, T.M.; Mikkelsen, J.D.; Murray, B.S. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls: Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J. Biol. Chem. 2001, 276, 19404–19413. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, L.; Black, I.; Glushka, J.; Urbanowicz, B.; Heiss, C.; Azadi, P. Most of the rhamnogalacturonan-I from cultured Arabidopsis cell walls is covalently linked to arabinogalactan-protein. Carbohydr. Polym. 2023, 301, 120340. [Google Scholar] [CrossRef]
- Saez-Aguayo, S.; Largo-Gosens, A. Rhamnogalacturonan-I forms mucilage: Behind its simplicity, a cutting-edge organization. J. Exp. Bot. 2022, 73, 3299–3303. [Google Scholar] [CrossRef]
- Park, Y.B.; Cosgrove, D.J. A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol. 2012, 158, 1933–1943. [Google Scholar] [CrossRef]
- Jarvis, M.C. Forces on and in the cell walls of living plants. Plant Physiol. 2024, 194, 8–14. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Building an extensible cell wall. Plant Physiol. 2022, 189, 1246–1277. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Wang, X.; Durachko, D.M.; Zhang, S.; Cosgrove, D.J. Molecular insights into the complex mechanics of plant epidermal cell walls. Science 2021, 372, 706–711. [Google Scholar] [CrossRef]
- San Clemente, H.; Kolkas, H.; Canut, H.; Jamet, E. Plant cell wall proteomes: The core of conserved protein families and the case of non-canonical proteins. Int. J. Mol. Sci. 2022, 23, 4273. [Google Scholar] [CrossRef]
- Su, G.; Lin, Y.; Wang, C.; Lu, J.; Liu, Z.; He, Z.; Shu, X.; Chen, W.; Wu, R.; Li, B.; et al. Expansin SlExp1 and endoglucanase SlCel2 synergistically promote fruit softening and cell wall disassembly in tomato. Plant Cell 2023, 36, 709–726. [Google Scholar] [CrossRef]
- Steinbrecher, T.; Leubner-Metzger, G. Tissue and cellular mechanics of seeds. Curr. Opin. Genet. Dev. 2018, 51, 1–10. [Google Scholar] [CrossRef]
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.-L. Microalgae disruption techniques for product recovery: Influence of cell wall composition. J. Appl. Phycol. 2019, 31, 61–88. [Google Scholar] [CrossRef]
- Burton, R.A.; Gidley, M.J.; Fincher, G.B. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 2010, 6, 724–732. [Google Scholar] [CrossRef]
- Sarath, G.; Akin, D.E.; Mitchell, R.B.; Vogel, K.P. Cell-wall composition and accessibility to hydrolytic enzymes is differentially altered in divergently bred switchgrass (Panicum virgatum L.) genotypes. Appl. Biochem. Biotechnol. 2008, 150, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ma, F.; Shi, S.; Qi, X.; Zhu, X.; Yuan, J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 2010, 56, 147–154. [Google Scholar] [CrossRef]
- Longhi, S.; Hamblin, M.T.; Trainotti, L.; Peace, C.P.; Velasco, R.; Costa, F. A candidate gene based approach validates Md-PG1 as the main responsible for a QTL impacting fruit texture in apple (Malus × domestica Borkh). BMC Plant Biol. 2013, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Goulao, L.F.; Santos, J.; de Sousa, I.; Oliveira, C.M. Patterns of enzymatic activity of cell wall-modifying enzymes during growth and ripening of apples. Postharvest Biol. Technol. 2007, 43, 307–318. [Google Scholar] [CrossRef]
- Ng, J.K.T.; Schröder, R.; Sutherland, P.W.; Hallett, I.C.; Hall, M.I.; Prakash, R.; Smith, B.G.; Melton, L.D.; Johnston, J.W. Cell wall structures leading to cultivar differences in softening rates develop early during apple (Malus × domestica) fruit growth. BMC Plant Biol. 2013, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Sitrit, Y.; Bennett, A.B. Regulation of tomato fruit polygalacturonase mRNA accumulation by ethylene: A re-examination. Plant Physiol. 1998, 116, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Tacken, E.; Ireland, H.; Gunaseelan, K.; Karunairetnam, S.; Wang, D.; Schultz, K.; Bowen, J.; Atkinson, R.G.; Johnston, J.W.; Putterill, J. The role of ethylene and cold temperature in the regulation of the apple POLYGALACTURONASE1 gene and fruit softening. Plant Physiol. 2010, 153, 294–305. [Google Scholar] [CrossRef]
- Wakasa, Y.; Kudo, H.; Ishikawa, R.; Akada, S.; Senda, M.; Niizeki, M.; Harada, T. Low expression of an endopolygalacturonase gene in apple fruit with long-term storage potential. Postharvest Biol. Technol. 2006, 39, 193–198. [Google Scholar] [CrossRef]
- Li, J.; Zhu, H.; Yuan, R. Profiling the expression of genes related to ethylene biosynthesis, ethylene perception, and cell wall degradation during fruit abscission and fruit ripening in apple. J. Am. Soc. Hortic. Sci. 2010, 135, 391–401. [Google Scholar] [CrossRef]
- Atkinson, R.G.; Sutherland, P.W.; Johnston, S.L.; Gunaseelan, K.; Hallett, I.C.; Mitra, D.; Brummell, D.A.; Schröder, R.; Johnston, J.W.; Schaffer, R.J. Down-regulation of POLYGALACTURONASE1 alters firmness, tensile strength and water loss in apple (Malus × domestica) fruit. BMC Plant Biol. 2012, 12, 129. [Google Scholar] [CrossRef]
- Ireland, H.S.; Yao, J.L.; Tomes, S.; Sutherland, P.W.; Nieuwenhuizen, N.; Gunaseelan, K.; Winz, R.A.; David, K.M.; Schaffer, R.J. Apple SEPALLATA1/2-like genes control fruit flesh development and ripening. Plant J. 2013, 73, 1044–1056. [Google Scholar] [CrossRef]
- Wu, B.; Shen, F.; Wang, X.; Zheng, W.Y.; Xiao, C.; Deng, Y.; Wang, T.; Yu Huang, Z.; Zhou, Q.; Wang, Y. Role of MdERF3 and MdERF118 natural variations in apple flesh firmness/crispness retainability and development of QTL-based genomics-assisted prediction. Plant Biotechnol. J. 2021, 19, 1022–1037. [Google Scholar] [CrossRef]
- Su, Q.; Yang, H.; Li, X.; Zhong, Y.; Feng, Y.; Li, H.; Tahir, M.M.; Zhao, Z. Upregulation of PECTATE LYASE5 by a NAC transcription factor promotes fruit softening in apple. Plant Physiol. 2024, 196, 1887–1907. [Google Scholar] [CrossRef]
- Brummell, D.A. Sensing when the wall comes tumbling down. J. Exp. Bot. 2020, 71, 6865–6868. [Google Scholar] [CrossRef]
- Paniagua, C.; Ric-Varas, P.; García-Gago, J.A.; López-Casado, G.; Blanco-Portales, R.; Muñoz-Blanco, J.; Schückel, J.; Knox, J.P.; Matas, A.J.; Quesada, M.A. Elucidating the role of polygalacturonase genes in strawberry fruit softening. J. Exp. Bot. 2020, 71, 7103–7117. [Google Scholar] [CrossRef]
- Quesada, M.A.; Blanco-Portales, R.; Posé, S.; García-Gago, J.A.; Jiménez-Bermudez, S.; Munoz-Serrano, A.; Caballero, J.L.; Pliego-Alfaro, F.; Mercado, J.A.; Munoz-Blanco, J. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol. 2009, 150, 1022–1032. [Google Scholar] [CrossRef]
- Jiménez-Bermudez, S.; Redondo-Nevado, J.; Munoz-Blanco, J.; Caballero, J.L.; López-Aranda, J.M.; Valpuesta, V.; Pliego-Alfaro, F.; Quesada, M.A.; Mercado, J.A. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol. 2002, 128, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, C.; Wei, H.; Qian, J.; Xie, J.; Li, Z.; Zheng, X.; Tan, B.; Li, J.; Cheng, J. VvPL11 is a key member of the pectin lyase gene family involved in grape softening. Horticulturae 2023, 9, 182. [Google Scholar] [CrossRef]
- Qian, M.; Xu, Z.; Zhang, Z.; Li, Q.; Yan, X.; Liu, H.; Han, M.; Li, F.; Zheng, J.; Zhang, D. The downregulation of PpPG21 and PpPG22 influences peach fruit texture and softening. Planta 2021, 254, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, W.; Xiong, F.; Xian, Z.; Su, D.; Ren, M.; Li, Z. Silencing of Sl PL, which encodes a pectate lyase in tomato, confers enhanced fruit firmness, prolonged shelf-life and reduced susceptibility to grey mould. Plant Biotechnol. J. 2017, 15, 1544–1555. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, J.; Wang, X.; Wang, Y.; Yuan, Y.; Yang, S. PpERF/ABR1 functions as an activator to regulate PpPG expression resulting in fruit softening during storage in peach (Prunus persica). Postharvest Biol. Technol. 2022, 189, 111919. [Google Scholar] [CrossRef]
- Deng, H.; Chen, Y.; Liu, Z.; Liu, Z.; Shu, P.; Wang, R.; Hao, Y.; Su, D.; Pirrello, J.; Liu, Y. SlERF. F12 modulates the transition to ripening in tomato fruit by recruiting the co-repressor TOPLESS and histone deacetylases to repress key ripening genes. Plant Cell 2022, 34, 1250–1272. [Google Scholar] [CrossRef]
- Wang, D.; Samsulrizal, N.H.; Yan, C.; Allcock, N.S.; Craigon, J.; Blanco-Ulate, B.; Ortega-Salazar, I.; Marcus, S.E.; Bagheri, H.M.; Perez Fons, L. Characterization of CRISPR mutants targeting genes modulating pectin degradation in ripening tomato. Plant Physiol. 2019, 179, 544–557. [Google Scholar] [CrossRef]
- Shi, Y.; Li, B.-J.; Grierson, D.; Chen, K.-S. Insights into cell wall changes during fruit softening from transgenic and naturally occurring mutants. Plant Physiol. 2023, 192, 1671–1683. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H.; Civello, P.M.; Palys, J.M.; Bennett, A.B.; Dunsmuir, P. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell 1999, 11, 2203–2216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Bin, X.; An, X.-H.; Zhao, D.-Y.; Cheng, C.-G.; Li, E.-M.; Zhou, J.-T.; Kang, G.-D.; Zhang, Y.-Z. Overexpression of the apple expansin-like gene MdEXLB1 accelerates the softening of fruit texture in tomato. J. Integr. Agric. 2022, 21, 3578–3588. [Google Scholar] [CrossRef]
- Costa, F.; Van de Weg, W.; Stella, S.; Dondini, L.; Pratesi, D.; Musacchi, S.; Sansavini, S. Map position and functional allelic diversity of Md-Exp7, a new putative expansin gene associated with fruit softening in apple (Malus × domestica Borkh.) and pear (Pyrus communis). Tree Genet. Genomes 2008, 4, 575–586. [Google Scholar] [CrossRef]
- Lai, X.; Zhu, X.; Chen, H.; Pang, X.; Chen, W.; Li, X.; Song, Z. The MaC2H2-like zinc finger protein is involved in ripening and ripening disorders caused by chilling stress via the regulation of softening-related genes in ‘Fenjiao’ banana. Postharvest Biol. Technol. 2022, 186, 111817. [Google Scholar] [CrossRef]
- Shi, Y.; Vrebalov, J.; Zheng, H.; Xu, Y.; Yin, X.; Liu, W.; Liu, Z.; Sorensen, I.; Su, G.; Ma, Q. A tomato LATERAL ORGAN BOUNDARIES transcription factor, SlLOB1, predominantly regulates cell wall and softening components of ripening. Proc. Natl. Acad. Sci. USA 2021, 118, e2102486118. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, D.; Wang, X.; Xu, X.; Yu, W.; Wang, C.; Yuan, Y.; Yang, S.; Cheng, C. Integrated analysis of postharvest storage characteristics of seven apple cultivars and transcriptome data identifies MdBBX25 as a negative regulator of fruit softening during storage in apples. Postharvest Biol. Technol. 2024, 207, 112646. [Google Scholar] [CrossRef]
- Percy, A.E.; O’Brien, I.E.; Jameson, P.E.; Melton, L.D.; MacRae, E.A.; Redgwell, R.J. Xyloglucan endotransglycosylase activity during fruit development and ripening of apple and kiwifruit. Physiol. Plant. 1996, 96, 43–50. [Google Scholar] [CrossRef]
- Han, Y.; Ban, Q.; Li, H.; Hou, Y.; Jin, M.; Han, S.; Rao, J. DkXTH8, a novel xyloglucan endotransglucosylase/hydrolase in persimmon, alters cell wall structure and promotes leaf senescence and fruit postharvest softening. Sci. Rep. 2016, 6, 39155. [Google Scholar] [CrossRef]
- Witasari, L.D.; Huang, F.C.; Hoffmann, T.; Rozhon, W.; Fry, S.C.; Schwab, W. Higher expression of the strawberry xyloglucan endotransglucosylase/hydrolase genes Fv XTH 9 and Fv XTH 6 accelerates fruit ripening. Plant J. 2019, 100, 1237–1253. [Google Scholar] [CrossRef]
- Wang, D.; Lu, Q.; Wang, X.; Ling, H.; Huang, N. Elucidating the role of SlXTH5 in tomato fruit softening. Hortic. Plant J. 2023, 9, 777–788. [Google Scholar] [CrossRef]
- Ma, M.; Yuan, Y.; Cheng, C.; Zhang, Y.; Yang, S. The MdXTHB gene is involved in fruit softening in ‘Golden Del. Reinders’ (Malus pumila). J. Sci. Food Agric. 2021, 101, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, N.; Jiang, S.; Xu, H.; Wang, Y.; Wang, C.; Li, M.; Liu, J.; Qu, C.; Liu, W. Analysis of the xyloglucan endotransglucosylase/hydrolase gene family during apple fruit ripening and softening. J. Agric. Food Chem. 2017, 65, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Sun, Q.; Ma, C.N.; Wei, M.M.; Wang, C.K.; Zhao, Y.W.; Wang, W.Y.; Hu, D.G. MdWRKY31-MdNAC7 regulatory network: Orchestrating fruit softening by modulating cell wall-modifying enzyme MdXTH2 in response to ethylene signalling. Plant Biotechnol. J. 2024, 22, 3244–3261. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cong, P.; He, J.; Bu, H.; Qin, S.; Lyu, D. Differential pulp cell wall structures lead to diverse fruit textures in apple (Malus domestica). Protoplasma 2022, 259, 1205–1217. [Google Scholar] [CrossRef]
- Liu, H.; Qian, M.; Song, C.; Li, J.; Zhao, C.; Li, G.; Wang, A.; Han, M. Down-regulation of PpBGAL10 and PpBGAL16 delays fruit softening in peach by reducing polygalacturonase and pectin methylesterase activity. Front. Plant Sci. 2018, 9, 1015. [Google Scholar] [CrossRef]
- Ban, Q.; Han, Y.; He, Y.; Jin, M.; Han, S.; Suo, J.; Rao, J. Functional characterization of persimmon β-galactosidase gene DkGAL1 in tomato reveals cell wall modification related to fruit ripening and radicle elongation. Plant Sci. 2018, 274, 109–120. [Google Scholar] [CrossRef]
- Smith, D.L.; Abbott, J.A.; Gross, K.C. Down-regulation of tomato β-galactosidase 4 results in decreased fruit softening. Plant Physiol. 2002, 129, 1755–1762. [Google Scholar] [CrossRef]
- Paniagua, C.; Blanco-Portales, R.; Barceló-Muñoz, M.; García-Gago, J.A.; Waldron, K.W.; Quesada, M.A.; Muñoz-Blanco, J.; Mercado, J.A. Antisense down-regulation of the strawberry β-galactosidase gene FaβGal4 increases cell wall galactose levels and reduces fruit softening. J. Exp. Bot. 2016, 67, 619–631. [Google Scholar] [CrossRef]
- Wang, M.; Zhan, W.; Chen, M.; Guo, Y.; Wang, H.; Wu, Y.; Bai, T.; Jiao, J.; Song, C.; Shi, J. MdAP2-like, a new regulator in apple, simultaneously modulates fruit softening and size. Postharvest Biol. Technol. 2024, 217, 113123. [Google Scholar] [CrossRef]
- Nobile, P.M.; Wattebled, F.; Quecini, V.; Girardi, C.L.; Lormeau, M.; Laurens, F. Identification of a novel α-L-arabinofuranosidase gene associated with mealiness in apple. J. Exp. Bot. 2011, 62, 4309–4321. [Google Scholar] [CrossRef]
- Yang, H.; Liu, X.; Hu, W.; Yang, Z.; Zhao, Z. Apple transcription factor MdDof43 promotes fruit softening by regulating cell wall-modifying genes Mdβ-Gal2 and Mdα-AF3. Hortic. Plant J. 2025; in press. [Google Scholar] [CrossRef]
- Wang, W.; Yu, J.; Du, M.; Wang, J.; Hu, D. Basic helix-loop-helix (bHLH) transcription factor MdbHLH3 negatively affects the storage performance of postharvest apple fruit. Hortic. Plant J. 2022, 8, 700–712. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, F.; Ji, S.; Dai, H.; Zhou, X.; Wei, B.; Cheng, S.; Wang, A. Abscisic acid accelerates postharvest blueberry fruit softening by promoting cell wall metabolism. Sci. Hortic. 2021, 288, 110325. [Google Scholar] [CrossRef]
- Wu, M.; Liu, K.; Li, H.; Li, Y.; Zhu, Y.; Su, D.; Zhang, Y.; Deng, H.; Wang, Y.; Liu, M. Gibberellins involved in fruit ripening and softening by mediating multiple hormonal signals in tomato. Hortic. Res. 2023, 11, uhad275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-W.; Zhao, T.-T.; Sun, Q.; Liu, X.-L.; Huang, X.-Y.; Li, L.-G.; Wang, H.-B.; Li, W.-K.; Wang, C.-K.; Wang, W.-Y. Enrichment of two important metabolites D-galacturonic acid and D-glucuronic acid inhibits MdHb1-mediated fruit softening in apple. Nat. Plants 2025, 11, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Feng, Y.; Yuan, S.; Zhao, X.; Wu, C.; Wang, C.; Xue, Z. Different regulatory mechanisms of plant hormones in the ripening of climacteric and non-climacteric fruits: A review. Plant Mol. Biol. 2021, 107, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Saladié, M.; Matas, A.J.; Isaacson, T.; Jenks, M.A.; Goodwin, S.M.; Niklas, K.J.; Xiaolin, R.; Labavitch, J.M.; Shackel, K.A.; Fernie, A.R. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol. 2007, 144, 1012–1028. [Google Scholar] [CrossRef]
- Veraverbeke, E.A.; Verboven, P.; Van Oostveldt, P.; Nicolaı, B.M. Prediction of moisture loss across the cuticle of apple (Malus sylvestris subsp. Mitis (Wallr.)) during storage: Part 1. Model development and determination of diffusion coefficients. Postharvest Biol. Technol. 2003, 30, 75–88. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; Hagenmaier, R.H. Alternatives to shellac coatings provide comparable gloss, internal gas modification, and quality for ‘Delicious’ apple fruit. HortScience 2002, 37, 559–563. [Google Scholar] [CrossRef]
- Shackel, K.A.; Greve, C.; Labavitch, J.M.; Ahmadi, H. Cell turgor changes associated with ripening in tomato pericarp tissue. Plant Physiol. 1991, 97, 814–816. [Google Scholar] [CrossRef]
- Fernández, V.; Khayet, M.; Montero-Prado, P.; Heredia-Guerrero, J.A.; Liakopoulos, G.; Karabourniotis, G.; Del Rio, V.; Domínguez, E.; Tacchini, I.; Nerín, C. New insights into the properties of pubescent surfaces: Peach fruit as a model. Plant Physiol. 2011, 156, 2098–2108. [Google Scholar] [CrossRef]
- Mukhtar, A.; Damerow, L.; Blanke, M. Non-invasive assessment of glossiness and polishing of the wax bloom of European plum. Postharvest Biol. Technol. 2014, 87, 144–151. [Google Scholar] [CrossRef]
- Wang, J.; Hao, H.; Liu, R.; Ma, Q.; Xu, J.; Chen, F.; Cheng, Y.; Deng, X. Comparative analysis of surface wax in mature fruits between Satsuma mandarin (Citrus unshiu) and ‘Newhall’ navel orange (Citrus sinensis) from the perspective of crystal morphology, chemical composition and key gene expression. Food Chem. 2014, 153, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, S.; Chen, H.; Chu, W.; Mu, H.; Ge, L. Epicuticular wax’s effect on fruit softening of blueberry. J. Chin. Inst. Food Sci. Technol. 2014, 14, 103–108. [Google Scholar]
- Özgen, M.; Palta, J.P.; Smith, J.D. Ripeness stage at harvest influences postharvest life of cranberry fruit: Physiological and anatomical explanations. Postharvest Biol. Technol. 2002, 24, 291–299. [Google Scholar] [CrossRef]
- Dong, X.; Rao, J.; Huber, D.J.; Chang, X.; Xin, F. Wax composition of ‘Red Fuji’ apple fruit during development and during storage after 1-methylcyclopropene treatment. Hortic. Environ. Biotechnol. 2012, 53, 288–297. [Google Scholar] [CrossRef]
- Dong, X.; Rao, J.; Zhu, S.; Yang, Q. Combination of modified atmosphere packaging and 1-methylcyclopropene treatment suppress decreasing of wax composition of apples during cold storage. Trans. Chin. Soc. Agric. Eng. 2013, 29, 269–277. [Google Scholar]
- Cajuste, J.F.; González-Candelas, L.; Veyrat, A.; García-Breijo, F.J.; Reig-Armiñana, J.; Lafuente, M.T. Epicuticular wax content and morphology as related to ethylene and storage performance of ‘Navelate’ orange fruit. Postharvest Biol. Technol. 2010, 55, 29–35. [Google Scholar] [CrossRef]
- Curry, E. Effects of 1-MCP applied postharvest on epicuticular wax of apples (Malus domestica Borkh.) during storage. J. Sci. Food Agric. 2008, 88, 996–1006. [Google Scholar] [CrossRef]
- Knoche, M.; Lang, A. Ongoing growth challenges fruit skin integrity. Crit. Rev. Plant Sci. 2017, 36, 190–215. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, M.; Ren, L.; Li, A.; Chen, G.; Hu, Z. The SlFSR gene controls fruit shelf-life in tomato. J. Exp. Bot. 2018, 69, 2897–2909. [Google Scholar] [CrossRef]
- Jiang, F.; Lopez, A.; Jeon, S.; de Freitas, S.T.; Yu, Q.; Wu, Z.; Labavitch, J.M.; Tian, S.; Powell, A.L.; Mitcham, E. Disassembly of the fruit cell wall by the ripening-associated polygalacturonase and expansin influences tomato cracking. Hortic. Res. 2019, 6, 17. [Google Scholar] [CrossRef]
- Moctezuma, E.; Smith, D.L.; Gross, K.C. Antisense suppression of a β-galactosidase gene (TB G6) in tomato increases fruit cracking. J. Exp. Bot. 2003, 54, 2025–2033. [Google Scholar] [CrossRef]
- Yu, J.; Wang, R.; Ma, W.; Lei, S.; Zhu, M.; Yang, G. Pectate lyase gene VvPL1 plays a role in fruit cracking of table grapes. J. Agric. Food Chem. 2023, 71, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Maguire, K.M.; Lang, A.; Banks, N.H.; Hall, A.; Hopcroft, D.; Bennett, R. Relationship between water vapour permeance of apples and micro-cracking of the cuticle. Postharvest Biol. Technol. 1999, 17, 89–96. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, Y.; Huang, B.; Hu, X.; Tang, Y.; Xu, X.; Wu, M.; Gong, Z.; Luo, Y.; Gong, M. Control of fruit softening and ascorbic acid accumulation by manipulation of SlIMP3 in tomato. Plant Biotechnol. J. 2022, 20, 1213–1225. [Google Scholar] [CrossRef]
- Catiempo, R.L.; Photchanachai, S.; Powell, A.F.; Strickler, S.R.; Wongs-Aree, C. Transcriptome analysis suggests the role of expansin genes in the improved germination of sunflower (Helianthus annuus L.) seeds after hydropriming. Crop Sci. 2024, 64, 1862–1873. [Google Scholar] [CrossRef]
- Cao, X.; Li, M.; Li, J.; Song, Y.; Zhang, X.; Yang, D.; Li, M.; Wei, J. Co-expression of hydrolase genes improves seed germination of Sinopodophyllum hexandrum. Ind. Crops Prod. 2021, 164, 113414. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, G.; Li, H.; Wang, Y.; Gao, H.; Jemrić, T.; Fu, D. Molecular and genetic events determining the softening of fleshy fruits: A comprehensive review. Int. J. Mol. Sci. 2022, 23, 12482. [Google Scholar] [CrossRef]
- Chen, D.-M.; Jia, L.-G.; Zhao, G.-D.; Zhang, C.-H.; Yang, F.-Q.; Zhang, X.-S.; Zhao, T.-S.; Li, C.-M.; Zhao, Y.-B. ‘Yuguan’, a Late-Ripening Apple Cultivar in China. HortScience 2022, 57, 1148–1149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, D.; Liu, T.; Yan, Z.; Ji, X.; Li, Y.; Wu, Y.; Cheng, H.; Wang, Y.; Cui, J.; et al. Advances in Cell Wall Dynamics and Gene Expression in Postharvest Fruit Softening. Plants 2025, 14, 2831. https://doi.org/10.3390/plants14182831
Wang X, Zhang D, Liu T, Yan Z, Ji X, Li Y, Wu Y, Cheng H, Wang Y, Cui J, et al. Advances in Cell Wall Dynamics and Gene Expression in Postharvest Fruit Softening. Plants. 2025; 14(18):2831. https://doi.org/10.3390/plants14182831
Chicago/Turabian StyleWang, Xumin, Da Zhang, Tiantian Liu, Zhuo Yan, Xinmei Ji, Yusheng Li, Yaqin Wu, Hehe Cheng, Yingjie Wang, Jianchao Cui, and et al. 2025. "Advances in Cell Wall Dynamics and Gene Expression in Postharvest Fruit Softening" Plants 14, no. 18: 2831. https://doi.org/10.3390/plants14182831
APA StyleWang, X., Zhang, D., Liu, T., Yan, Z., Ji, X., Li, Y., Wu, Y., Cheng, H., Wang, Y., Cui, J., Wu, Y., & Chen, L. (2025). Advances in Cell Wall Dynamics and Gene Expression in Postharvest Fruit Softening. Plants, 14(18), 2831. https://doi.org/10.3390/plants14182831







