5-AzaC Facilitates Somatic Embryogenesis and Germination Across Two Embryogenic Lines in Larix olgensis
Abstract
1. Introduction
2. Results
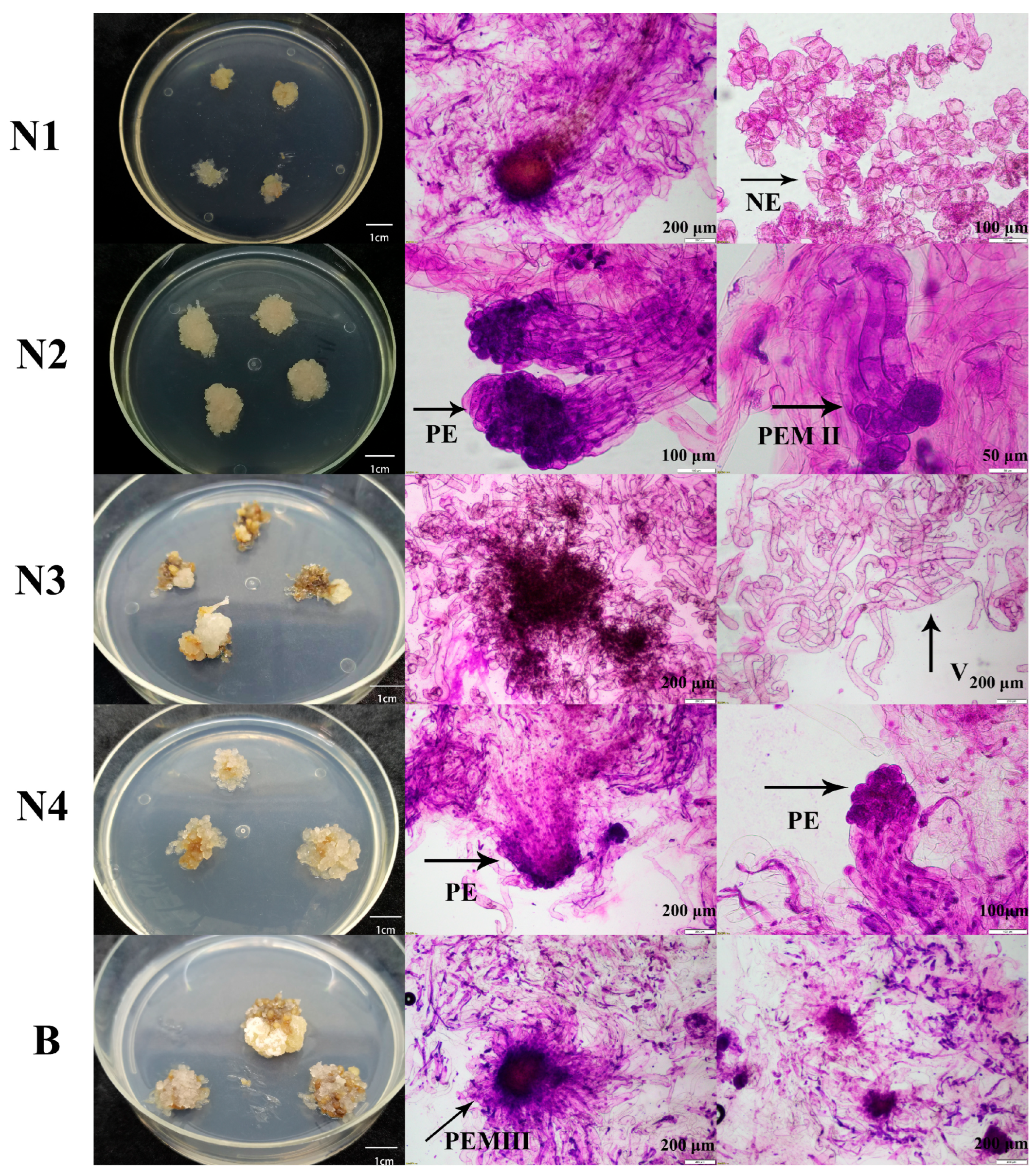
2.1. The Selection of the Materials
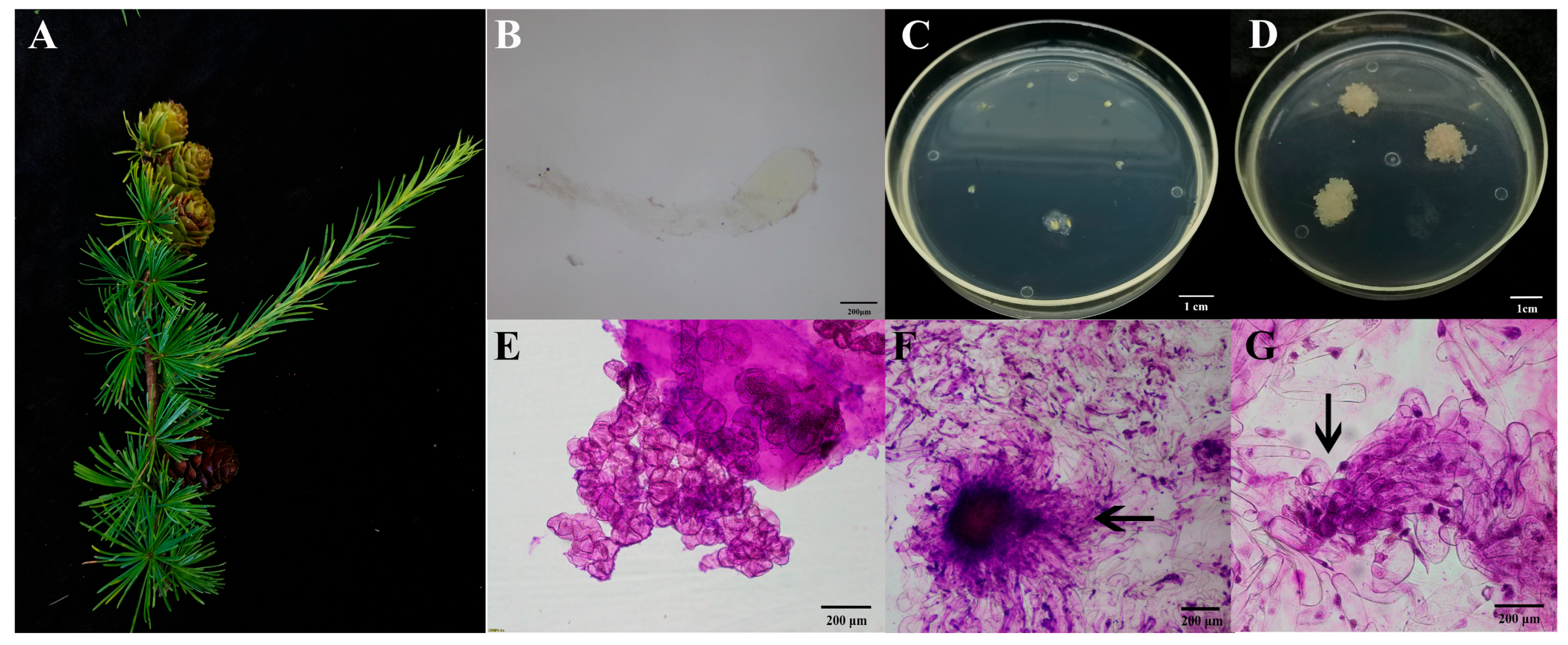
2.1.1. Microscopic Examination of Embryogenic Callus
2.1.2. Screening of the Embryogenic Lines
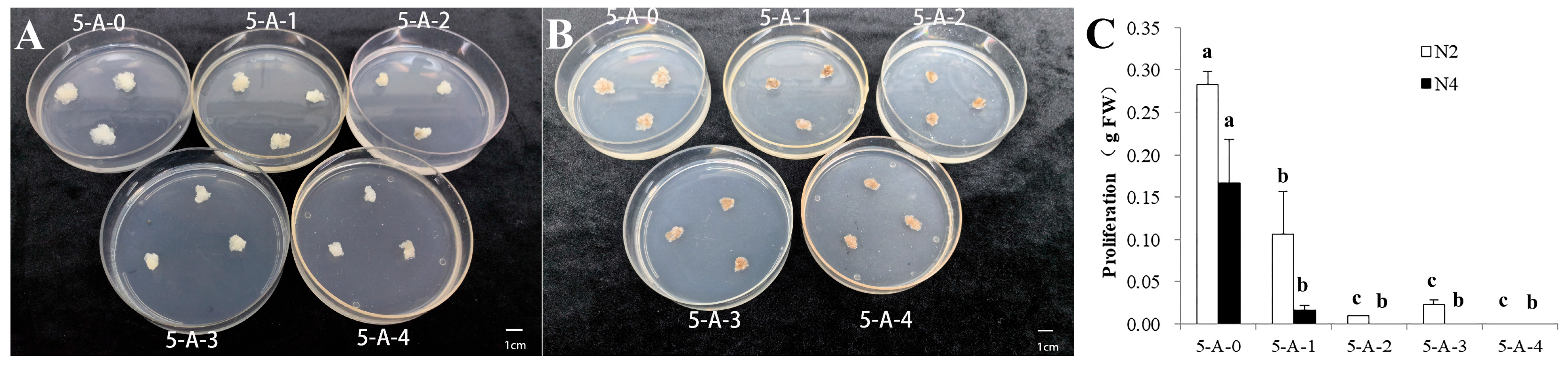
2.2. The Effects of 5-AzaC on Proliferation and Somatic Embryogenesis
2.2.1. The Effects of 5-AzaC on the Proliferation of Different Embryogenic Lines
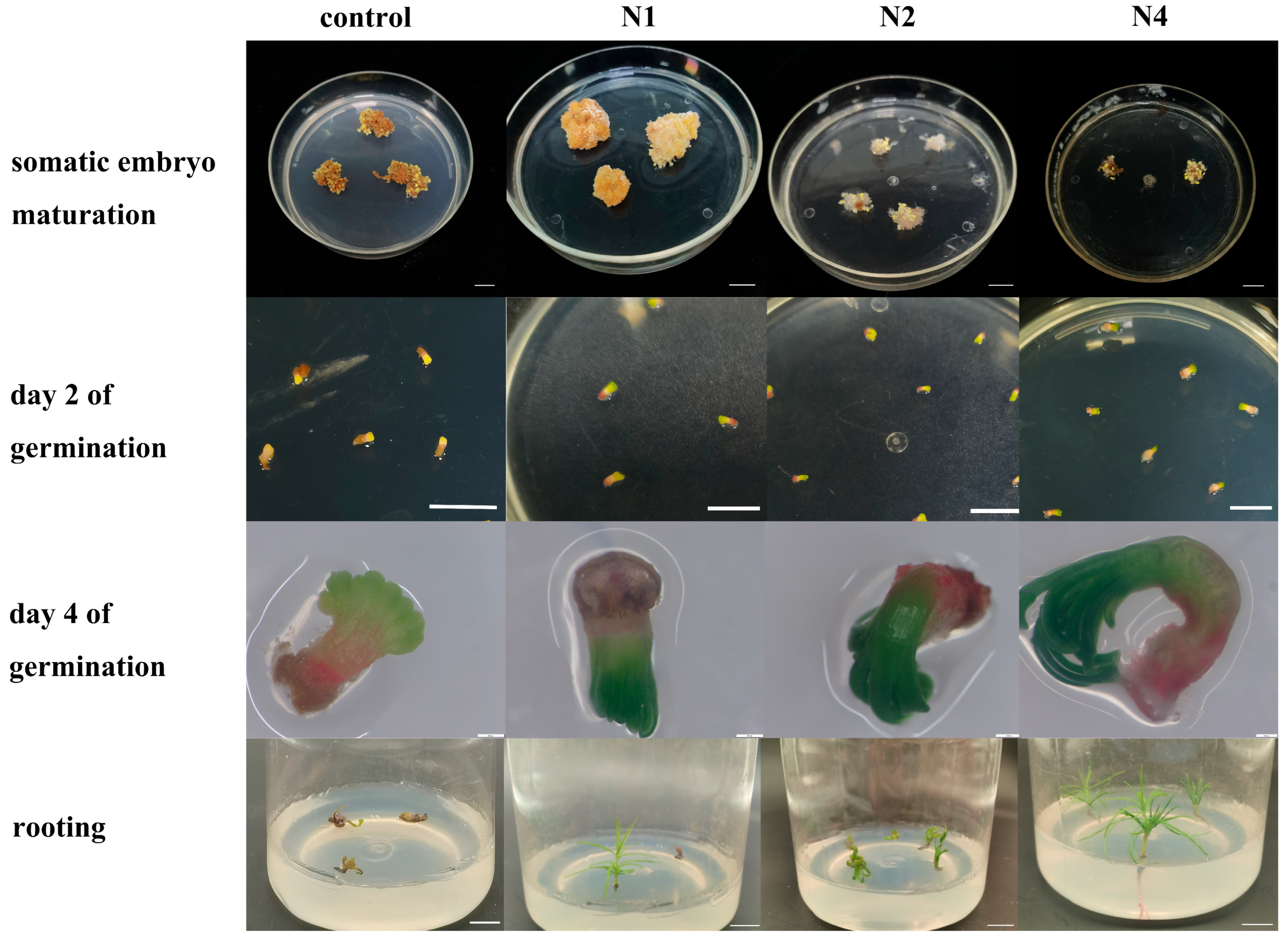
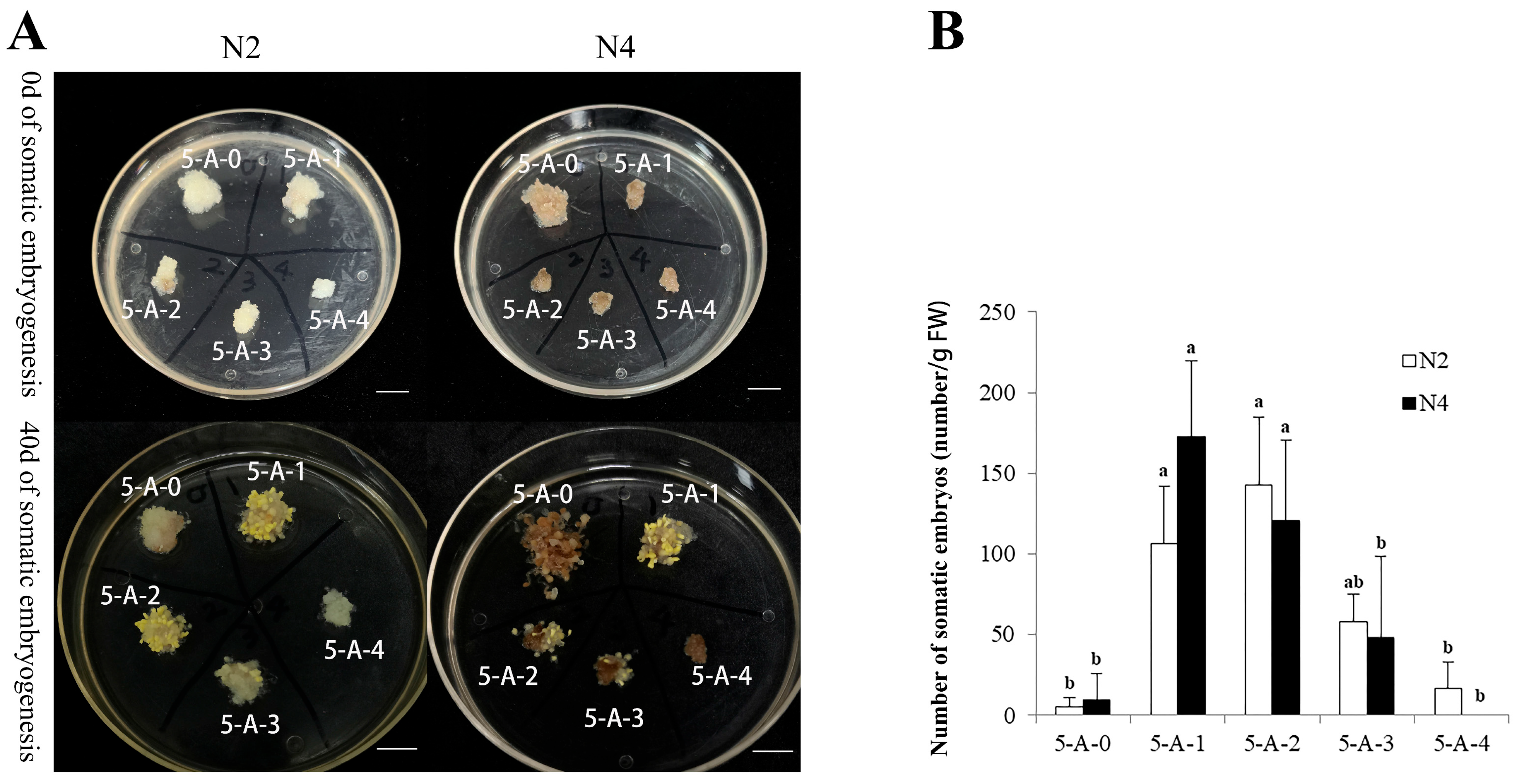
2.2.2. The Effects of 5-AzaC on Somatic Embryo Maturation
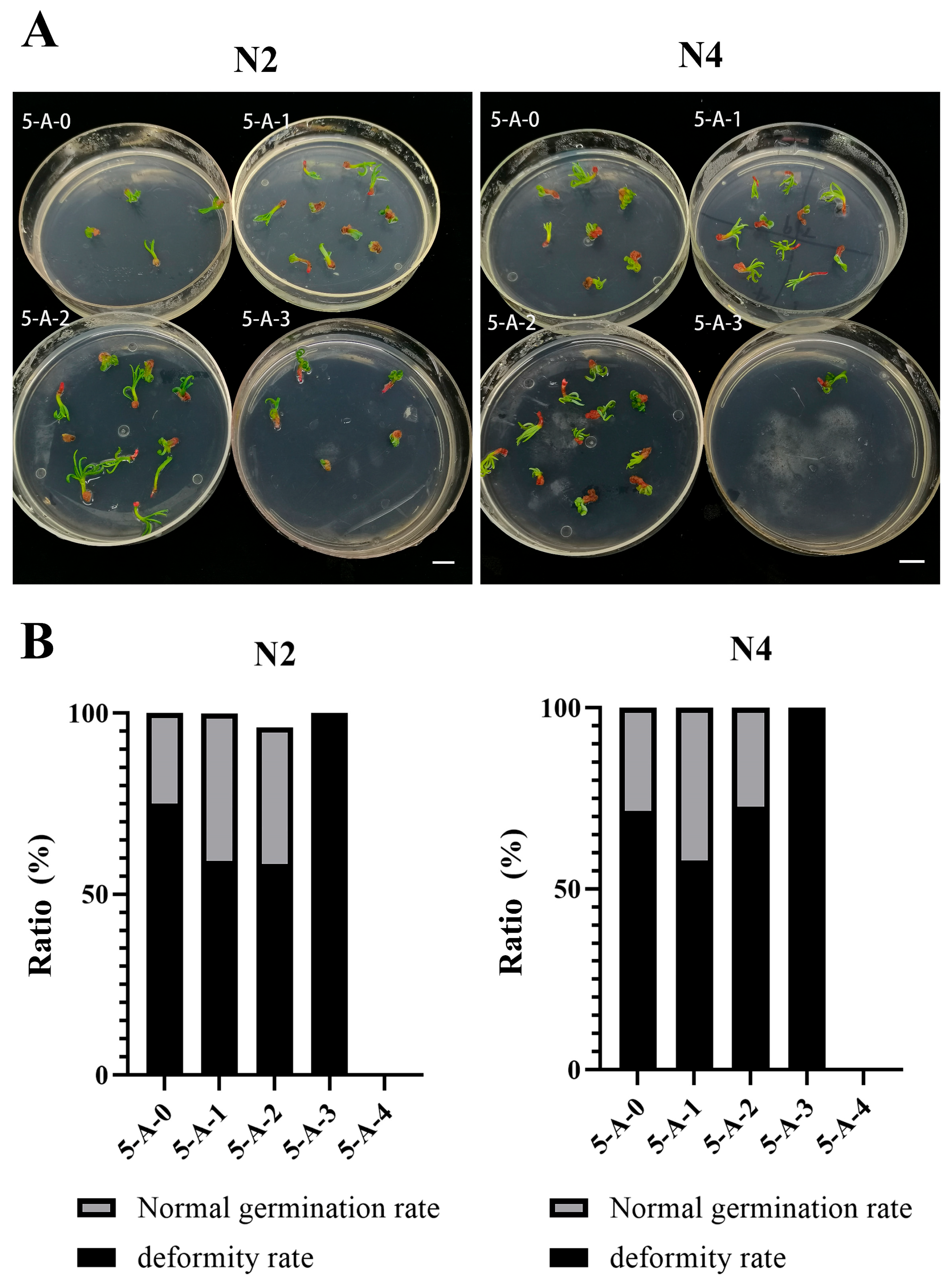
2.2.3. The Effect of 5-AzaC on Somatic Embryo Germination
3. Discussion
3.1. The Decline in the Somatic Embryogenesis Potential Following Prolonged Subculture
3.2. Restored Embryogenic Potential via 5-AzaC Treatment
4. Materials and Methods
4.1. The Experimental Materials
4.2. Experimental Methods
4.2.1. The Selection of the Experimental Materials
4.2.2. The Effect of 5-AzaC on Somatic Embryogenesis
4.3. The Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, A.; Xu, M.S.; Hou, D.; Zhao, Y.T.; Zhang, Q.Q.; Zhou, L.L.; Yang, X.D.; Yan, E.R. Allometric biomass equations for shrub and small tree species in subtropical China. Silva Fenn. 2015, 36, 434–443. [Google Scholar] [CrossRef]
- Wang, W.D.; Li, C.H.; Yang, J.L.; Zhang, H.G.; Zhang, S.L. Somatic Embryogenesis and Plantlet Regeneration from Immature Zygotic Embryos of Hybrid Larch. Sci. Silvae Sin. 2009, 45, 34–38. [Google Scholar]
- Song, Y.; Li, S.J.; Bai, X.M.; Zhang, H.G. Response Surface Optimization of Culture Conditions for the Formation of Early Embryos in Larix olgensis. Bull. Bot. Res. 2017, 37, 613–620. [Google Scholar]
- Välimäki, S.; Teyssier, C.; Tikkinen, M.; Delile, A.; Boizot, N.; Varis, S.; Lelu-Walter, M.-A.; Aronen, T. Norway spruce somatic embryogenesis benefits from proliferation of embryogenic tissues on filter discs and cold storage of cotyledonary embryos. Front Plant Sci. 2022, 13, 1031686. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhen, C.; Zhang, H.G.; Li, S.J. Embryogenic Callus Induction and Somatic Embryogenesis from Immature Zygotic Embryos of Larix olgensis. Sci. Silvae Sin. 2016, 52, 45–54. [Google Scholar]
- Fehér, A. Somatic embryogenesis-Stress-induced remodeling of plant cell fate. Bba Gene Regul. Mech. 2015, 1849, 385–402. [Google Scholar] [CrossRef]
- Breton, D.; Harvengt, L.; Trontin, J.F.; Bouvet, A.; Favre, J.M. Long-term subculture randomly affects morphology and subsequent maturation of early somatic embryos in maritime pine. Plant Cell Tiss. Organ. Cult. 2006, 87, 95–108. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Noceda, C.; Pelletier, G.; Label, P.; Rodriguez, R.; Lelu-Walter, M.A. Biological characterization of young and aged embryogenic cultures of Pinus pinaster (Ait.). In Vitro Cel. Dev. Biol.-Plant 2009, 45, 20–33. [Google Scholar] [CrossRef]
- Dos, S.E.L.; Filho, J.C.B.; Hansel, F.A.; Sousa, J.A.T.; Auer, C.G.; Steiner, N.; Degenhardt, J. 5-Azacytidine affects gene expression and metabolic profile of Pinus elliottii × Pinus caribaea var. hondurensis embryogenic cell lines. Plant Cell Tiss. Organ. Cult. 2023, 155, 637–651. [Google Scholar]
- Karim, R.; Tan, Y.S.; Singh, P.; Khalid, N.; Harikrishna, J.A. Expression and DNA methylation of SERK, BBM, LEC2 and WUS genes in in vitro cultures of Boesenbergia rotunda (L.) Mansf. Physiol. Mol. Biol. Plants 2018, 24, 741–751. [Google Scholar] [CrossRef]
- Hao, Y.J.; Deng, X.X. Stress Treatments and DNA Methylation Affected the Somatic Embryogenesis of Citrus Callus. Acta Bot. Sin. 2002, 44, 673. [Google Scholar]
- Tokuji, Y.; Takano, S.; Tonomura, M.; Tanaka, S.; Igari, K.; Watanabe, T. Influence of 5′-azacitidine on promoting recovery of cell competence for shoot organogenesis in Arabidopsis. Plant Cell Tiss. Organ. Cult. 2011, 106, 289–297. [Google Scholar] [CrossRef]
- Pila Quinga, L.A.; Pacheco de Freitas Fraga, H.; do Nascimento Vieira, L.; Pedro Guerra, M. Epigenetics of long-term somatic embryogenesis in Theobroma cacao L.: DNA methylation and recovery of embryogenic potential. Plant Cell Tiss. Organ. Cult. 2017, 131, 295–305. [Google Scholar] [CrossRef]
- Valledor, L.; Hasbún, R.; Meijón, M.; Rodríguez, J.L.; Santamaría, E.; Viejo, M.; Berdasco, M.; Feito, I.; Fraga, M.F.; Cañal, M.J.; et al. Involvement of DNA methylation in tree development and micropropagation. Plant Cell Tiss. Organ. Cult. 2007, 91, 75–86. [Google Scholar] [CrossRef]
- Nie, L.J.; Wang, Z.C. The molecular mechanism and application of DNA methylation inhibitor in the developmental biology of plants. Acta Agric. Nucl. Sin. 2007, 21, 362–365. [Google Scholar]
- Leljak-Levanić, D.; Bauer, N.; Mihaljević, S.; Jelaska, S. Changes in DNA methylation during somatic embryogenesis in Cucurbita pepo L. Plant Cell Rep. 2004, 23, 120–127. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kobayashi, H.; Togashi, T.; Mori, Y.; Kikuchi, K.; Kuriyama, K.; Tokuji, Y. Formation of embryogenic cell clumps from carrot epidermal cells is suppressed by 5-azacytidine, a DNA methylation inhibitor. J. Plant Physiol. 2005, 162, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Montalvo, P.; De-la-Peña, C.; Oropeza, C.; Nic-Can, G.; Córdova-Lara, I.; Castillo-Castro, E.; Sáenz-Carbonell, L. A peak in global DNA methylation is a key step to initiate the somatic embryogenesis of coconut palm (Cocos nucifera L.). Plant Cell Rep. 2020, 39, 1345–1357. [Google Scholar] [CrossRef]
- Chen, R.; Chen, X.; Huo, W.; Zheng, S.; Lin, Y.; Lai, Z. Transcriptome analysis of azacitidine (5-AzaC)-treatment affecting the development of early somatic embryogenesis in longan. J. Hortic. Sci. Biotech. 2020, 96, 311–323. [Google Scholar] [CrossRef]
- Leljak-Levanic, D.; Mihaljevic, S.; Jelaska, S. Variations in dna methylation in Picea omorika (Panč) purk. embryogenic tissue and the ability for embryo maturation. Propag. Ornam. Plants 1992, 70, 199–206. [Google Scholar]
- Teyssier, C.; Maury, S.; Beaufour, M.; Grondin, C.; Delaunay, A.; Metté, C.L.; Ader, K.; Cadene, M.; Label, P.; Lelu-Walter, M.A. In search of markers for somatic embryo maturation in hybrid larch (Larix × eurolepis): Global DNA methylation and proteomic analyses. Physio Plant. 2014, 150, 271–291. [Google Scholar] [CrossRef]
- Yuan, G.Y.; Wang, D.; Yu, C.G.; Hua, J.F.; Yin, Y.L.; Chen, T.T. 5-AzaCytidine Promotes Somatic Embryogenesis of Taxodium Hybrid ‘Zhongshanshan’ by Regulating Redox Homeostasis. Plants 2025, 14, 1354. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.R.; Qin, W.Y.; Li, Y.C. Effects of demethylating reagent 5-aza-2′-deoxycytidine on the growth and cephalotaxine production in Cephalotaxus mannii suspension cells. Plant Cell Tiss. Organ. Cult. 2019, 139, 359–368. [Google Scholar] [CrossRef]
- Qi, L.W. Studies on the Somatic Embryogenesis and Establishment of Experimental System in Larix principis-Rupprechtii. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2000. [Google Scholar]
- Jones, N.B.; Van Staden, J. Plantlet Production from Somatic Embryos of Pinus patula. J. Plant Physiol. 1995, 145, 519–525. [Google Scholar] [CrossRef]
- Montalbán, I.A.; De Diego, N.; Aguirre Igartua, E.; Setién, A.; Moncaleán, P. A combined pathway of somatic embryogenesis and organogenesis to regenerate radiata pine plants. Plant Biotechnol. Rep. 2011, 5, 177–186. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, X.; Xing, H.; Tretyakova, I.N.; Nosov, A.M.; Yang, L.; Shen, H. Interaction of Subculture Cycle, Hormone Ratio, and Carbon Source Regulates Embryonic Differentiation of Somatic Cells in Pinus koraiensis. Forests 2022, 13, 1557. [Google Scholar] [CrossRef]
- Mamedes-Rodrigues, T.C.; Batista, D.S.; Vieira, N.M.; Matos, E.M.; Fernandes, D.; Nunes-Nesi, A.; Cruz, C.D.; Viccini, L.F.; Nogueira, F.T.S.; Otoni, W.C. Regenerative potential, metabolic profile, and genetic stability of Brachypodium distachyon embryogenic calli as affected by successive subcultures. Protoplasma 2018, 255, 655–667. [Google Scholar] [CrossRef]
- Passamani, L.Z.; Reis, R.S.; Vale, E.M.; Sousa, K.R.; Aragao, V.P.M.; Santa-Catarina Silveira, V. Long-term culture with 2,4-dichlorophenoxyacetic acid affects embryogenic competence in sugarcane callus via changes in starch, polyamine and protein profiles. Plant Cell Tiss. Organ. Cult. 2020, 140, 415–429. [Google Scholar] [CrossRef]
- Ree, J.F.; Polesi, L.G.; Back, F.; Bertolazi, A.A.; Silveira, V.; Guerra, M.P. Aging peach palm (Bactris gasipaes Kunth) cultures lose embryogenic potential and metabolic cellular function due to continuous culture in hypoxic environments. Plant Cell Tiss. Organ. Cult. 2020, 140, 49–67. [Google Scholar] [CrossRef]
- Cordeiro, D.; Canhoto, J.; Correia, S. Analysing the loss of embryogenic competence in long-term cell lines of Solanum betaceum Cav.: Involvement of miR827, phosphate and sugar. BMC Plant Biol. 2025, 25, 851. [Google Scholar] [CrossRef]
- Pasternak, T.; Dudits, D. Epigenetic Clues to Better Understanding of the Asexual Embryogenesis in planta and in vitro Front. Plant Sci. 2019, 10, 778. [Google Scholar]
- Luan, A.; Chen, C.; Xie, T.; He, J.; He, Y. Methylation Analysis of CpG Islands in Pineapple SERK1 Promoter. Genes 2020, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Betekhtin, A.; Milewska-Hendel, A.; Chajec, L.; Rojek, M.; Nowak, K.; Kwasniewska, J.; Wolny, E.; Kurczynska, E.; Hasterok, R. 5-Azacitidine Induces Cell Death in a Tissue Culture of Brachypodium distachyon. Int. J. Mol. Sci. 2018, 19, 1806. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Liu, Y.; Wang, C.; Ning, Y.; Cui, C.; Zhang, H.; Li, M.; Li, S. 5-AzaC Facilitates Somatic Embryogenesis and Germination Across Two Embryogenic Lines in Larix olgensis. Plants 2025, 14, 2818. https://doi.org/10.3390/plants14182818
Zhao W, Liu Y, Wang C, Ning Y, Cui C, Zhang H, Li M, Li S. 5-AzaC Facilitates Somatic Embryogenesis and Germination Across Two Embryogenic Lines in Larix olgensis. Plants. 2025; 14(18):2818. https://doi.org/10.3390/plants14182818
Chicago/Turabian StyleZhao, Wenna, Yu Liu, Chen Wang, Yajing Ning, Chengpeng Cui, Hanguo Zhang, Meng Li, and Shujuan Li. 2025. "5-AzaC Facilitates Somatic Embryogenesis and Germination Across Two Embryogenic Lines in Larix olgensis" Plants 14, no. 18: 2818. https://doi.org/10.3390/plants14182818
APA StyleZhao, W., Liu, Y., Wang, C., Ning, Y., Cui, C., Zhang, H., Li, M., & Li, S. (2025). 5-AzaC Facilitates Somatic Embryogenesis and Germination Across Two Embryogenic Lines in Larix olgensis. Plants, 14(18), 2818. https://doi.org/10.3390/plants14182818





