Functional Traits of Native Plant Species That Inhibit the Seedling Growth of the Exotic Invader Solidago canadensis
Abstract
1. Introduction
2. Results
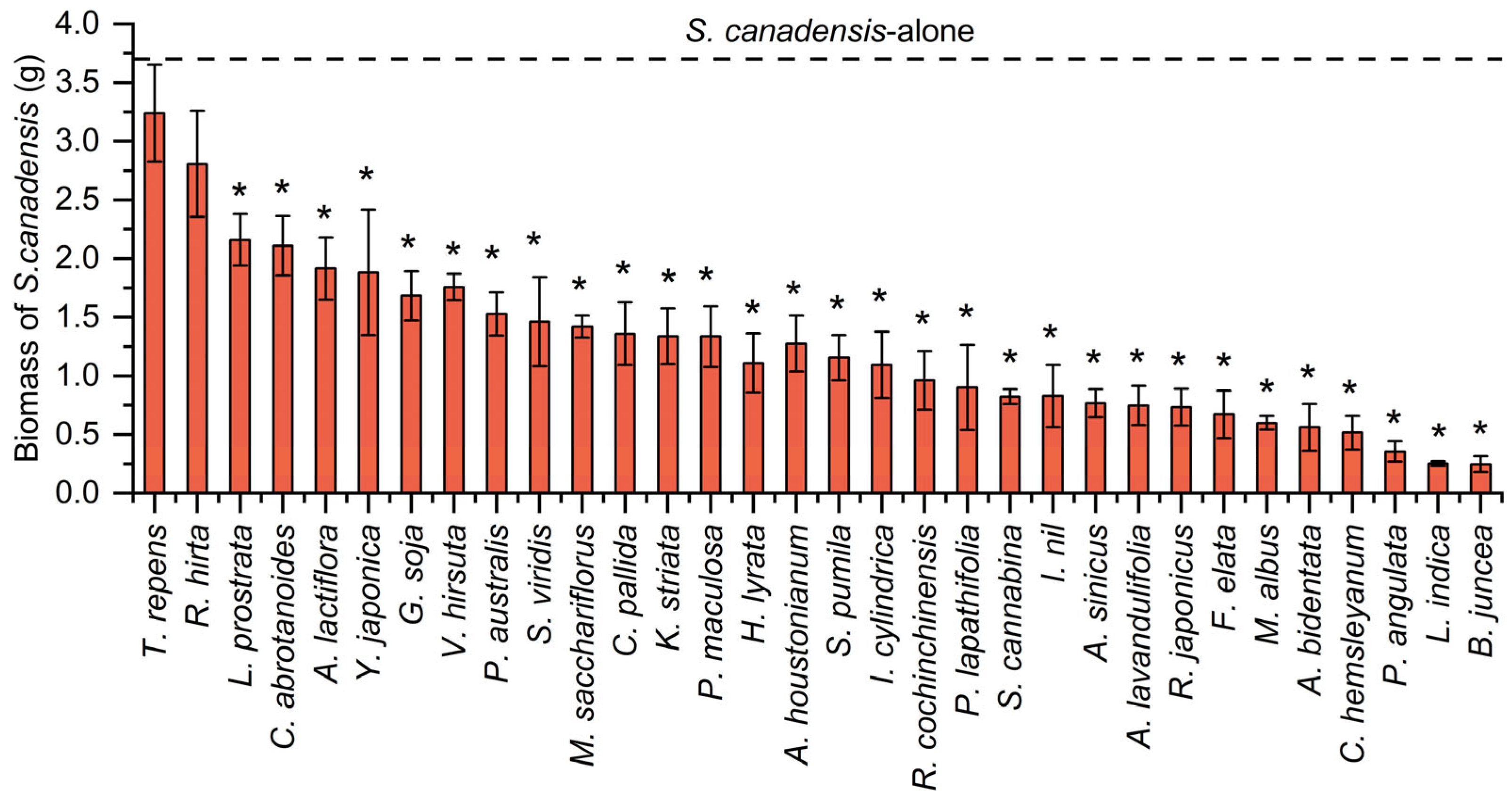
2.1. Changes in Biomass of Native Plants and S. canadensis Before and After Competition
2.2. Correlation Analysis of CRCI with Different Functional Traits in Native Plants and S. canadensis
2.3. Stepwise Regression Modeling of CRCI and Functional Traits in Native Plants and S. canadensis
3. Discussion
3.1. Variation of Competitive Effects Between S. canadensis and Native Plants
3.2. Differences in Functional Traits Associated with Competitive Effects of Native Plants on S. canadensis
3.3. Optimizing Invasion Control Through Native Plant Traits Suppressing S. canadensis Growth
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design
4.3. Measurements
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRCI | Corrected index of relative competitive intensity |
| RL | Root length |
| RT | Root tip |
| RD | Root diameter |
| RSA | Root surface area |
| RSR | Root–shoot ratio |
| SRL | Specific root length |
| LA | Leaf area |
| Mroot | Biomass of root |
| Mstem | Biomass of stem |
| Mleaf | Biomass of leaf |
| H10–70 | Plant height of 10–70 days |
| M | Total biomass |
References
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; van Kleunen, M.; Winter, M.; et al. Global rise in emerging alien species results from increased accessibility of new source pools. Proc. Natl. Acad. Sci. USA 2018, 115, E2264–E2273. [Google Scholar] [CrossRef]
- Culliney, T.W. benefits of classical biological control for managing invasive plants. Crit. Rev. Plant Sci. 2005, 24, 131–150. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Hulme, P.E.; Bacher, S.; Kenis, M.; Klotz, S.; Kühn, I.; Minchin, D.; Nentwig, W.; Olenin, S.; Panov, V.; Pergl, J.; et al. Grasping at the routes of biological invasions: A framework for integrating pathways into policy. J. Appl. Ecol. 2008, 45, 403–414. [Google Scholar] [CrossRef]
- Oduor, A.M.O. Native plant species show evolutionary responses to invasion by Parthenium hysterophorus in an African savanna. New Phytol. 2021, 233, 983–994. [Google Scholar] [CrossRef]
- Pearson, D.E.; Ortega, Y.K.; Eren, Ö.; Hierro, J.L. Community assembly theory as a framework for biological invasions. Trends Ecol. Evol. 2018, 33, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Duda, J.J.; Freeman, D.C.; Emlen, J.M.; Belnap, J.; Kitchen, S.G.; Zak, J.C.; Sobek, E.; Tracy, M.; Montante, J. Differences in native soil ecology associated with invasion of the exotic annual chenopod, Halogeton glomeratus. Biol. Fertil. Soils 2003, 38, 72–77. [Google Scholar] [CrossRef]
- Xu, S.; Li, K.; Li, G.; Hu, Z.; Zhang, J.; Iqbal, B.; Du, D. Canada goldenrod invasion regulates the effects of soil moisture on soil respiration. Int. J. Environ. Res. Public Health 2022, 19, 15446. [Google Scholar] [CrossRef]
- D’Antonio, C.; Meyerson, L.A. Exotic Plant Species as Problems and Solutions in Ecological Restoration: A Synthesis. Restor. Ecol. 2002, 10, 703–713. [Google Scholar] [CrossRef]
- Johnstone, I.M. Plant invasion windows: A time-based classification of invasion potential. Biol. Rev. 1986, 61, 369–394. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Du, Y.; Yang, B.; Wang, J.; Cui, M.; Dai, Z.; Adomako, M.O.; Rutherford, S.; Du, D. Influence of precipitation dynamics on plant invasions: Response of alligator weed (Alternanthera philoxeroides) and co-occurring native species to varying water availability across plant communities. Biol. Invasions 2022, 25, 519–532. [Google Scholar] [CrossRef]
- Wang, L.W.; Li, W.R.; Ding, J.Q.; Siemann, E. Dual drivers of plant invasions: Enemy release and enhanced mutualisms. J. Ecol. 2025, 113, 1997–2012. [Google Scholar] [CrossRef]
- Atkinson, M.S.; Savage, A.E. Invasive amphibians alter host-pathogen interactions with primarily negative outcomes for native species. Biol. Conserv. 2023, 286, 110310. [Google Scholar] [CrossRef]
- Green, J.L.; Waller, L.P.; Allen, W.J.; Orwin, K.H.; Pelser, P.B.; Smaill, S.; Dickie, I.A. Plant-soil feedback from non-native communities increases pine invasion and re-invasion potential. Plant Soil 2025. [Google Scholar] [CrossRef]
- Wang, Q.; He, J.Q.; Chen, Q.; Xiao, L.; Dong, Z.G. Biological characteristics and feeding habit of Argyrogramma albostriata feeding on Solidago canadensis. Chin. Agric. Sci. Bull. 2011, 27, 60–65. [Google Scholar]
- Schuster, M.J.; Wragg, P.D.; Reich, P.B.; Pauchard, A. Using revegetation to suppress invasive plants in grasslands and forests. J. Appl. Ecol. 2018, 55, 2362–2373. [Google Scholar] [CrossRef]
- Cutting, K.J.; Hough-Goldstein, J. Integration of biological control and native seeding to restore invaded plant communities. Restor. Ecol. 2013, 21, 648–655. [Google Scholar] [CrossRef]
- Price, J.N.; Pärtel, M. Can limiting similarity increase invasion resistance? A meta-analysis of experimental studies. Oikos 2012, 122, 649–656. [Google Scholar] [CrossRef]
- Austin, M.P.; Groves, R.H.; Fresco, L.M.F.; Kaye, P.E. Relative growth of six thistle species along a nutrient gradient with multispecies competition. J. Ecol. 1985, 73, 667–684. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Grace, J.; Tilman, D. Components of resource competition in plant communities. Perspect. Plant Compet. 1990, 27–49. [Google Scholar] [CrossRef]
- Goldberg, D.E. Competitive Ability: Definitions, Contingency and correlated traits. R. Soc. 1996, 351, 1377–1385. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Landa, K. Competitive effect and response: Hierarchies and correlated traits in the early stages of competition. J. Ecol. 1991, 79, 1013–1030. [Google Scholar] [CrossRef]
- Thomsen, M.A.; Corbin, J.D.; D’Antonio, C.M. The effect of soil nitrogen on competition between native and exotic perennial grasses from northern coastal California. J. Plant Ecol. 2006, 186, 23–35. [Google Scholar] [CrossRef]
- Wang, P.; Stieglitz, T.; Zhou, D.W.; Cahill Jr, J.F. Are competitive effect and response two sides of the same coin, or fundamentally different? Funct. Ecol. 2010, 24, 196–207. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef]
- Rolhauser, A.G.; Nordenstahl, M.; Aguiar, M.R.; Pucheta, E.; Schwinning, S. Community-level natural selection modes: A quadratic framework to link multiple functional traits with competitive ability. J. Ecol. 2018, 107, 1457–1468. [Google Scholar] [CrossRef]
- Mokany, K.; Raison, R.J.; Prokushkin, A.S. Critical analysis of root: shoot ratios in terrestrial biomes. Glob. Change Biol. 2005, 12, 84–96. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 595–607. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Keddy, P.; Nielsen, K.; Weiher, E.; Lawson, R. Relative competitive performance of 63 species of terrestrial herbaceous plants. J. Veg. Sci. 2002, 13, 5–16. [Google Scholar] [CrossRef]
- Kunstler, G.; Falster, D.; Coomes, D.A.; Hui, F.; Kooyman, R.M.; Laughlin, D.C.; Poorter, L.; Vanderwel, M.; Vieilledent, G.; Wright, S.J.; et al. Plant functional traits have globally consistent effects on competition. Nature 2015, 529, 204–207. [Google Scholar] [CrossRef]
- Nuñez, M.A.; Chiuffo, M.C.; Torres, A.; Paul, T.; Dimarco, R.D.; Raal, P.; Policelli, N.; Moyano, J.; García, R.A.; van Wilgen, B.W.; et al. Ecology and management of invasive Pinaceae around the world: Progress and challenges. Biol. Invasions 2017, 19, 3099–3120. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Grewell, B.J.; D’Antonio, C.M.; Funk, J.L.; James, J.J.; Molinari, N.; Parker, I.M.; Richards, C.L. A functional trait perspective on plant invasion. Ann. Bot. 2012, 110, 141–153. [Google Scholar] [CrossRef]
- van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef]
- Muth, N.Z.; Pigliucci, M. Traits of invasives reconsidered: Phenotypic comparisons of introduced invasive and introduced noninvasive plant species within two closely related clades. Am. J. Bot. 2006, 93, 188–196. [Google Scholar] [CrossRef]
- Kaur, A.; Sharma, A.; Kaur, S.; Siddiqui, M.H.; Alamri, S.; Ahmad, M.; Kohli, R.K.; Singh, H.P.; Batish, D.R. Role of plant functional traits in the invasion success: Analysis of nine species of Asteraceae. BMC Plant Biol. 2024, 24, 784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hastings, A.; Grosholz, E.D.; Zhai, L. The comparison of dispersal rate between invasive and native species varied by plant life form and functional traits. Mov. Ecol. 2023, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Hibit, J.; Daehler, C.C. Plant functional, biogeographical and phylogenetic diversity are related to native and non-native plant abundance in invaded Hawaiian forests. Biol. Invasions 2024, 26, 705–717. [Google Scholar] [CrossRef]
- Dawson, W. Release from belowground enemies and shifts in root traits as interrelated drivers of alien plant invasion success: A hypothesis. Ecol. Evol. 2015, 5, 4505–4516. [Google Scholar] [CrossRef]
- Nunez-Mir, G.C.; McCary, M.A. Invasive plants and their root traits are linked to the homogenization of soil microbial communities across the United States. Proc. Natl. Acad. Sci. USA 2024, 121, e2418632121. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, D.J.; Ding, J. Remove or retain: Ecosystem effects of woody encroachment and removal are linked to plant structural and functional traits. New Phytol. 2020, 229, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, X.; Iqbal, B.; Zhao, X.; Liu, J.; Javed, Q.; Du, D. The effect of soil microplastics on Oryza sativa L. root growth traits under alien plant invasion. Front. Ecol. Evol. 2023, 11, 1172093. [Google Scholar] [CrossRef]
- Fort, F. Grounding trait-based root functional ecology. Funct. Ecol. 2023, 37, 2159–2169. [Google Scholar] [CrossRef]
- Weber, E. Morphological variation of the introduced perennial Solidago canadensis L. sensu lato (Asteraceae) in Europe. Bot. J. Linn. Soc. 1997, 123, 197–210. [Google Scholar] [CrossRef]
- Lu, J.Z. Potential distribution of Solidago canadensis in China. Acta Phytotaxon. Sin. 2007, 45, 670–674. [Google Scholar] [CrossRef]
- Karpavičienė, B.; Radušienė, J.; Viltrakytė, J. Distribution of two invasive goldenrod species Solidago canadensis and S. gigantea in lithuania. Bot. Lith. 2015, 21, 125–132. [Google Scholar] [CrossRef][Green Version]
- Liao, M.; Xie, X.M.; Peng, Y.; Chai, J.J.; Chen, N. Characteristics of soil microbial community functional and structure diversity with coverage of Solidago canadensis L. J. Cent. S. Univ. 2013, 20, 749–756. [Google Scholar] [CrossRef]
- Ye, X.Q.; Wu, M.; Shao, X.X.; Jiang, Y.P.; Wang, M. Capabilities of native herbosa inhibiting Solidago canadensis at Early stage of its invasion. J. Ecol. Rural Environ. 2014, 30, 608–613. [Google Scholar]
- Chen, G.Q.; Zhang, C.B.; Ma, L.; Qiang, S.; Silander, J.A.; Qi, L.L. Biotic homogenization caused by the invasion of Solidago canadensis in China. J. Integr. Agric. 2013, 12, 835–845. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zhu, Y.; Li, L.; Zhang, Y.; Li, J.; Song, X.; Qiang, S. Biological control of Solidago canadensis using a bioherbicide isolate of Sclerotium rolfsii SC64 increased the biodiversity in invaded habitats. Biol. Control 2019, 139, 104093. [Google Scholar] [CrossRef]
- Ye, X.; Meng, J.; Ma, R.; Wu, M. Effects of clipping an invasive plant species on the growth of planted plants of two co-occurring species in a greenhouse study. Biology 2023, 12, 1282. [Google Scholar] [CrossRef]
- Li, Y.; Long, Y.; Liu, T.; Zhang, D.; He, M.; Xie, Q.; Zhang, Z. The role of soil microorganisms and physicochemical properties in determining the germinate of invasive Solidago canadensis L. Plant Soil 2024, 507, 897–914. [Google Scholar] [CrossRef]
- Możdżeń, K.; Barabasz-Krasny, B.; Zandi, P.; Kliszcz, A.; Puła, J. Effect of aqueous extracts from Solidago canadensis L. leaves on germination and early growth stages of three cultivars of Raphanus sativus L. Var. Radicula Pers. Plants 2020, 9, 1549. [Google Scholar] [CrossRef]
- Baranová, B.; Troščáková-Kerpčárová, E.; Gruľová, D. Survey of the Solidago canadensis L. morphological traits and essential oil production: Aboveground biomass growth and abundance of the invasive goldenrod appears to be reciprocally enhanced within the invaded stands. Plants 2022, 11, 535. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Guo, S.L. Study on reproductive biology of the invasive plant Solidago canadensis. ACTA Ecol. Sin. 2005, 25, 3–11. [Google Scholar]
- Czortek, P.; Królak, E.; Borkowska, L.; Bielecka, A. Impacts of soil properties and functional diversity on the performance of invasive plant species Solidago canadensis L. on post-agricultural wastelands. Sci. Total Environ. 2020, 729, 139077. [Google Scholar] [CrossRef]
- Schultheis, E.H.; MacGuigan, D.J. Competitive ability, not tolerance, may explain success of invasive plants over natives. Biol. Invasions 2018, 20, 2793–2806. [Google Scholar] [CrossRef]
- Zhang, J.; Ning, Y.; Li, J.; Shi, Z.; Zhang, Q.; Li, L.; Kang, B.; Du, Z.; Luo, J.; He, M.; et al. Invasion stage and competition intensity co-drive reproductive strategies of native and invasive saltmarsh plants: Evidence from field data. Sci. Total Environ. 2024, 954, 176383. [Google Scholar] [CrossRef]
- Gong, W.; Wang, Y.; Chen, C.; Xiong, Y.; Zhou, Y.; Xiao, F.; Li, B.; Wang, Y. The rapid evolution of an invasive plant due to increased selection pressures throughout its invasive history. Ecotoxicol. Environ. Saf. 2022, 233, 113322. [Google Scholar] [CrossRef]
- Moles, A.T.; Ackerly, D.D.; Webb, C.O.; Tweddle, J.C.; Dickie, J.B.; Westoby, M. A brief history of seed size. Science 2005, 307, 576–580. [Google Scholar] [CrossRef]
- Schwinning, S.; Weiner, J. Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia 1998, 113, 447–455. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Shao, H.; Tang, S. Selected Aspects of Invasive Solidago canadensis with an Emphasis on Its Allelopathic Abilities: A Review. Chem. Biodivers. 2022, 19, e202200728. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.A.; Brown, B.J.; Reich, P.B. Ecophysiology of exotic and native shrubs in Southern Wisconsin. Oecologia 1989, 80, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Fridley, J.D. Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature 2012, 485, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Wilsey, B.J.; Martin, L.M.; Kaul, A.D.; Firn, J. Phenology differences between native and novel exotic-dominated grasslands rival the effects of climate change. J. Appl. Ecol. 2017, 55, 863–873. [Google Scholar] [CrossRef]
- McEwan, R.W.; Birchfield, M.K.; Schoergendorfer, A.; Arthur, M.A. Leaf phenology and freeze tolerance of the invasive shrub Amur honeysuckle and potential native competitors. J. Torrey Bot. Soc. 2009, 136, 212–220. [Google Scholar] [CrossRef]
- Ye, X.; Meng, J.; Ma, R.; Liang, J.; Wu, M.; Man, R.; Yu, F. Winter leaf phenology differences facilitate selective control of an invasive plant species by herbicide. NeoBiota 2024, 96, 67–87. [Google Scholar] [CrossRef]
- Grange, M.C.; Munoz, F.; Moretti, M.; Varona-Y. -Varona, S.; Renaud, J.; Colace, M.-P.; Gueguen, M.; Arnoldi, C.; Bernard, L.; Gallien, L. Avoid, tolerate, or escape? Native vegetation responses to invasion vary between functional groups. Biol. Invasions 2022, 25, 1387–1401. [Google Scholar] [CrossRef]
- Friedman, J. The evolution of annual and perennial plant life histories: Ecological correlates and genetic mechanisms. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 461–481. [Google Scholar] [CrossRef]
- Liu, J.; Xia, T.; Wang, Y.; He, Y.; Wu, C.; Shen, K.; Tan, Q.; Kang, L.; Guo, Y.; Wu, B.; et al. An invasive plant experiences greater benefits of root morphology from enhancing nutrient competition associated with arbuscular mycorrhizae in karst soil than a native plant. PLoS ONE 2020, 15, e0234410. [Google Scholar] [CrossRef]
- Erktan, A.; Roumet, C.; Munoz, F. Dissecting fine root diameter distribution at the community level captures root morphological diversity. Oikos 2022, 2023, e08907. [Google Scholar] [CrossRef]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A starting guide to root ecology: Strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef]
- Shao, X.; Wang, L.; Zhang, Q.; Liu, Y.; Yang, X. Future direction of searching for root economics spectrum: Focusing on the fibrous roots “absorptive unit”. Ecosphere 2019, 10, e02716. [Google Scholar] [CrossRef]
- Bukovsky-Reyes, S.; Isaac, M.E.; Blesh, J. Effects of intercropping and soil properties on root functional traits of cover crops. Agr. Ecosyst. Environ. 2019, 285, 106614. [Google Scholar] [CrossRef]
- Comas, L.H.; Eissenstat, D.M. Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Funct. Ecol. 2004, 18, 388–397. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef]
- Isaac, M.E.; Martin, A.R.; de Melo Virginio Filho, E.; Rapidel, B.; Roupsard, O.; Van den Meersche, K. Intraspecific trait variation and coordination: Root and leaf economics spectra in coffee across environmental gradients. Front. Plant Sci. 2017, 8, 1196. [Google Scholar] [CrossRef]
- Gaudet, C.L.; Keddy, P.A. A comparative approach to predicting competitive ability from plant traits. Nature 1988, 334, 242–243. [Google Scholar] [CrossRef]
- Ye, Z.-L.; Zang, Q.-L.; Cheng, D.-X.; Li, X.-Y.; Qi, L.W.; Li, W.F. Over-expression of larch DAL1 accelerates life-cycle progression in Arabidopsis. Forests 2022, 13, 953. [Google Scholar] [CrossRef]
- Kettenring, K.M.; Adams, C.R. Lessons learned from invasive plant control experiments: A systematic review and meta-analysis. J. Appl. Ecol. 2011, 48, 970–979. [Google Scholar] [CrossRef]
- Li, W.; Luo, J.; Tian, X.; Soon Chow, W.; Sun, Z.; Zhang, T.; Peng, S.; Peng, C. A new strategy for controlling invasive weeds: Selecting valuable native plants to defeat them. Sci. Rep. 2015, 5, 11004. [Google Scholar] [CrossRef]
- Tang, W.; Kuang, J.; Qiang, S. Biological control of the invasive alien weed Solidago canadensis: Combining an indigenous fungal isolate of Sclerotium rolfsii SC64 with mechanical control. Biocontrol. Sci. Technol. 2013, 23, 1123–1136. [Google Scholar] [CrossRef]
- Guo, S.L.; Jiang, H.W.; Fang, F.; Chen, G.Q. Influences of herbicides, uprooting and use as cut flowers on sexual reproduction of Solidago canadensis. Weed Res. 2009, 49, 291–299. [Google Scholar] [CrossRef]
- van Kleunen, M.; Ramponi, G.; Schmid, B. Effects of herbivory simulated by clipping and jasmonic acid on Solidago canadensis. Basic Appl. Ecol. 2004, 5, 173–181. [Google Scholar] [CrossRef]
- Xu, M.Q.; Gao, Y.J.; Zhang, Z.H.; Zeng, F.J. Adaptation of the main functional trait of Alhagi sparsifolia leaves and roots to soil water stress. Pratacultural Sci. 2021, 38, 1559–1569. [Google Scholar] [CrossRef]
- Vila, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarosik, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pysek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Moyo, B.; Ravhuhali, K.E. Abandoned croplands: Drivers and secondary succession trajectories under livestock grazing in communal areas of south africa. Sustainability 2022, 14, 6168. [Google Scholar] [CrossRef]
- Guillerme, S.; Barcet, H.; de Munnik, N.; Maire, E.; Marais-Sicre, C. Evolution of traditional agroforestry landscapes and development of invasive species: Lessons from the Pyrenees (France). Sustain. Sci. 2020, 15, 1285–1299. [Google Scholar] [CrossRef]
- Kopp, E.B.; Anten, N.P.R.; Niklaus, P.A.; Wuest, S.E. Belowground competition increases root allocation in agreement with game-theoretical predictions, but only when plants simultaneously compete aboveground. bioRxiv 2025. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Luque, M.T. Changes in plant interactions along a gradient of environmental stress. Oikos 2001, 93, 42–49. [Google Scholar] [CrossRef]
- Petruzzella, A.; Manschot, J.; van Leeuwen, C.H.A.; Grutters, B.M.C.; Bakker, E.S. Mechanisms of Invasion Resistance of Aquatic Plant Communities. Front. Plant Sci. 2018, 9, 134. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Burns, J.H.; Liao, Z.Y.; Li, Y.P.; Yang, J.; Chen, Y.J.; Zhang, J.L.; Zheng, Y.G. Species composition, functional and phylogenetic distances correlate with success of invasive Chromolaena odorata in an experimental test. Ecol. Lett. 2018, 21, 1211–1220. [Google Scholar] [CrossRef]
- Liu, Y.J.; Huang, W.; Yang, Q.; Zheng, Y.L.; Li, S.P.; Wu, H.; Ju, R.T.; Sun, Y.; Ding, J.Q. Research advances of plant invasion ecology over the past 10 years. Biodivers. Sci. 2022, 30, 276–292. [Google Scholar] [CrossRef]
- Ren, G.Q.; Yang, B.; Cui, M.M.; Yu, H.C.; Fan, X.; Dai, Z.C.; Sun, J.F.; Li, G.L.; Zhang, H.Y.; Du, D.L. Additive effects of warming and nitrogen addition on the performance and competitiveness of invasive Solidago canadensis L. Front. Plant Sci. 2022, 13, 1017554. [Google Scholar] [CrossRef]
- Haeuser, E.; Dawson, W.; van Kleunen, M. Introduced garden plants are strong competitors of native and alien residents under simulated climate change. Ecology 2019, 107, 1328–1342. [Google Scholar] [CrossRef]
- Snaydon, R.W. Replacement or additive designs for competition studies? J. Appl. Ecol. 1991, 28, 930–946. [Google Scholar] [CrossRef]
- Grace, J.B. On the Measurement of Plant Competition Intensity. Ecology 1995, 76, 305–308. [Google Scholar] [CrossRef]
- King, J.S.; Albaugh, T.J.; Allen, H.L.; Buford, M.; Strain, B.R.; Dougherty, P. Below-ground carbon input to soil is controlled by nutrient availability and fine root dynamics in loblolly pine. New Phytol. 2002, 154, 389–398. [Google Scholar] [CrossRef]


| Functional Traits | CRCI-s | CRCI-n | ||
|---|---|---|---|---|
| Correlation with Functional Traits of Native Plants Species | Correlation with Functional Traits of S. canadensis | |||
| Annual | Perennial | Annual | Perennial | |
| LA | 0.320 * | 0.298 * | 0.143 | 0.184 |
| RL | 0.468 * | 0.228 | 0.233 * | 0.018 |
| RSA | 0.479 * | 0.184 | 0.220 * | −0.024 |
| RD | 0.296 * | −0.168 | 0.032 | −0.085 |
| RT | 0.435 * | 0.297 * | 0.272 * | 0.007 |
| Mroot | 0.498 * | 0.255 | 0.144 | 0.032 |
| Mstem | 0.474 * | 0.397 * | 0.105 | 0.104 |
| Mleaf | 0.355 * | 0.314 * | 0.141 | 0.089 |
| M | 0.492 * | 0.387 * | 0.144 | 0.088 |
| Mag | 0.487 * | 0.397 * | 0.139 | 0.105 |
| H10 | 0.177 | −0.094 | 0.028 | −0.019 |
| H20 | 0.147 | 0.494 * | 0.034 | 0.028 |
| H30 | 0.274 * | 0.460 * | −0.041 | −0.023 |
| H40 | 0.365 * | 0.510 * | 0.123 | −0.085 |
| H50 | 0.420 * | 0.389 * | 0.115 | −0.084 |
| H60 | 0.397 * | 0.546 * | 0.07 | 0.155 |
| H70 | 0.436 * | 0.539 * | 0.095 | 0.17 |
| RSR | 0.139 | −0.027 | 0.088 | −0.136 |
| SLA | −0.048 | −0.199 | −0.028 | 0.305 * |
| SRL | 0.015 | −0.198 | 0.091 | −0.053 |
| Dependent Variable | Native Plant Type | Adjusted R2 | F | p |
|---|---|---|---|---|
| CRCI of S. canadensis | Annual | 0.38 | 13.142 | p < 0.05 |
| Perennial | 0.387 | 10.521 | p < 0.05 | |
| CRCI of native plants | Annual | 0.056 | 6.886 | p < 0.05 |
| Perennial | 0.062 | 4.582 | p < 0.05 |
| Latin Name | Family | Life Form (Annual/Perennial) |
|---|---|---|
| Achyranthes bidentata | Amaranthaceae | Perennial |
| Ageratum houstonianum | Asteraceae | Annual |
| Artemisia lactiflora | Asteraceae | Perennial |
| Artemisia lavandulifolia | Asteraceae | Perennial |
| Astragalus sinicus | Fabaceae | Perennial |
| Brassica juncea | Brassicaceae | Annual |
| Carpesium abrotanoides | Asteraceae | Perennial |
| Crotalaria pallida | Fabaceae | Perennial |
| Cynanchum hemsleyanum | Apocynaceae | Perennial |
| Festuca elata | Poaceae | Perennial |
| Glycine soja | Fabaceae | Perennial |
| Hemisteptia lyrata | Asteraceae | Annual |
| Imperata cylindrica | Poaceae | Perennial |
| Ipomoea nil | Convolvulaceae | Annual |
| Kummerowia striata | Fabaceae | Perennial |
| Lactuca indica | Asteraceae | Annual |
| Ludwigia prostrata | Onagraceae | Perennial |
| Melilotus albus | Fabaceae | Perennial |
| Miscanthus sacchariflorus | Poaceae | Perennial |
| Persicaria lapathifolia | Polygonaceae | Annual |
| Persicaria maculosa | Polygonaceae | Annual |
| Phragmites australis | Poaceae | Perennial |
| Physalis angulata | Solanaceae | Annual |
| Rottboellia cochinchinensis | Poaceae | Annual |
| Rudbeckia hirta | Asteraceae | Annual |
| Rumex japonicus | Polygonaceae | Perennial |
| Sesbania cannabina | Fabaceae | Annual |
| Setaria pumila | Poaceae | Annual |
| Setaria viridis | Poaceae | Annual |
| Trifolium repens | Fabaceae | Perennial |
| Vicia hirsuta | Fabaceae | Perennial |
| Youngia japonica | Asteraceae | Annual |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Liang, J.; Zuo, K.; Wu, M.; Ye, X. Functional Traits of Native Plant Species That Inhibit the Seedling Growth of the Exotic Invader Solidago canadensis. Plants 2025, 14, 2806. https://doi.org/10.3390/plants14172806
Ma R, Liang J, Zuo K, Wu M, Ye X. Functional Traits of Native Plant Species That Inhibit the Seedling Growth of the Exotic Invader Solidago canadensis. Plants. 2025; 14(17):2806. https://doi.org/10.3390/plants14172806
Chicago/Turabian StyleMa, Ruixiang, Jili Liang, Keyi Zuo, Ming Wu, and Xiaoqi Ye. 2025. "Functional Traits of Native Plant Species That Inhibit the Seedling Growth of the Exotic Invader Solidago canadensis" Plants 14, no. 17: 2806. https://doi.org/10.3390/plants14172806
APA StyleMa, R., Liang, J., Zuo, K., Wu, M., & Ye, X. (2025). Functional Traits of Native Plant Species That Inhibit the Seedling Growth of the Exotic Invader Solidago canadensis. Plants, 14(17), 2806. https://doi.org/10.3390/plants14172806






