Habitat Suitability and Driving Factors of Cycas panzhihuaensis in the Hengduan Mountains
Abstract
1. Introduction
2. Results
2.1. MaxEnt Performance and Environmental Contributions
2.2. Spatial Patterns of Suitable Habitats for C. panzhihuaensis
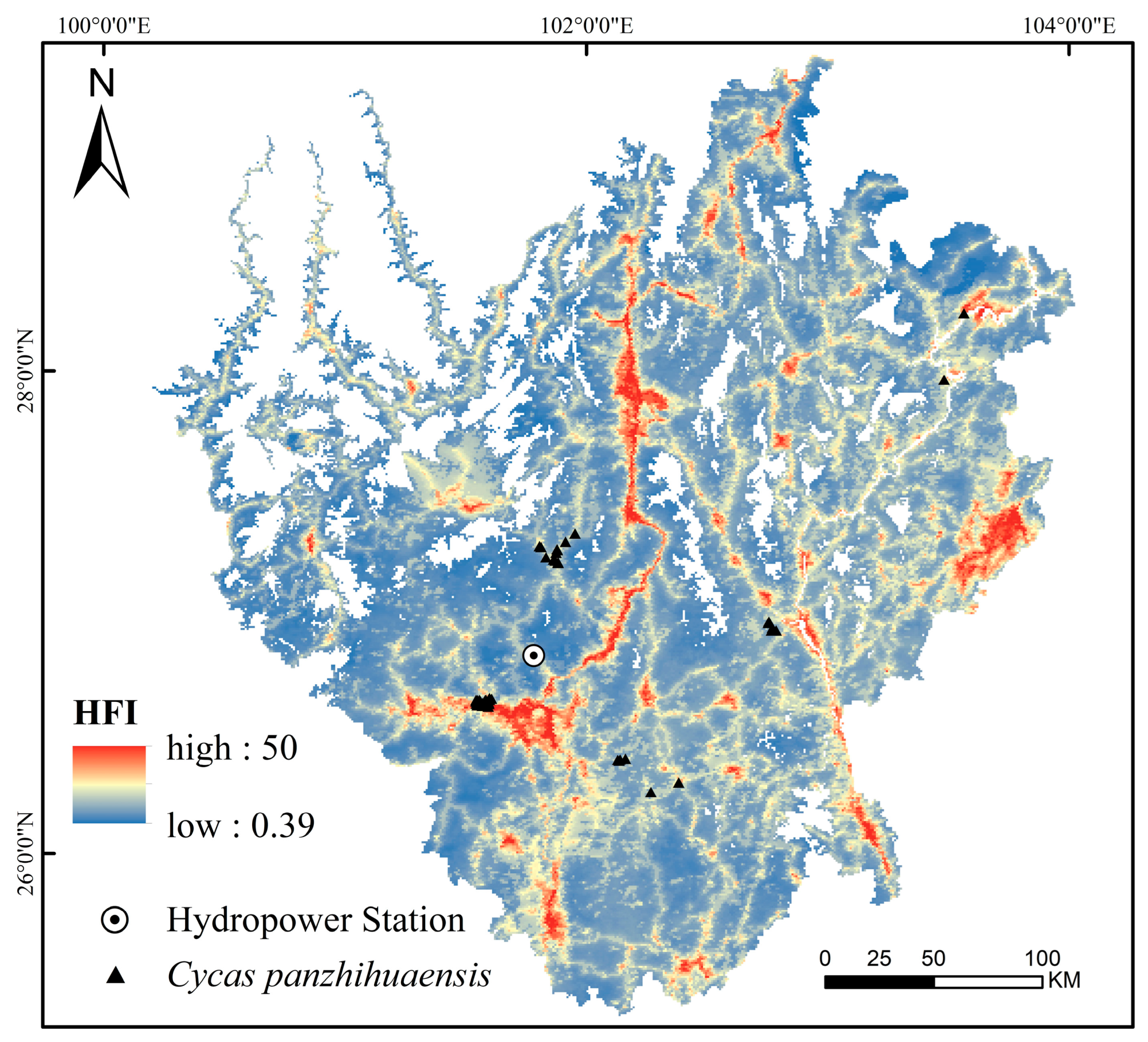
2.2.1. Current Potential Distribution
2.2.2. Future Potential Distribution Under Climate Change
2.3. Identification of Driving Factors
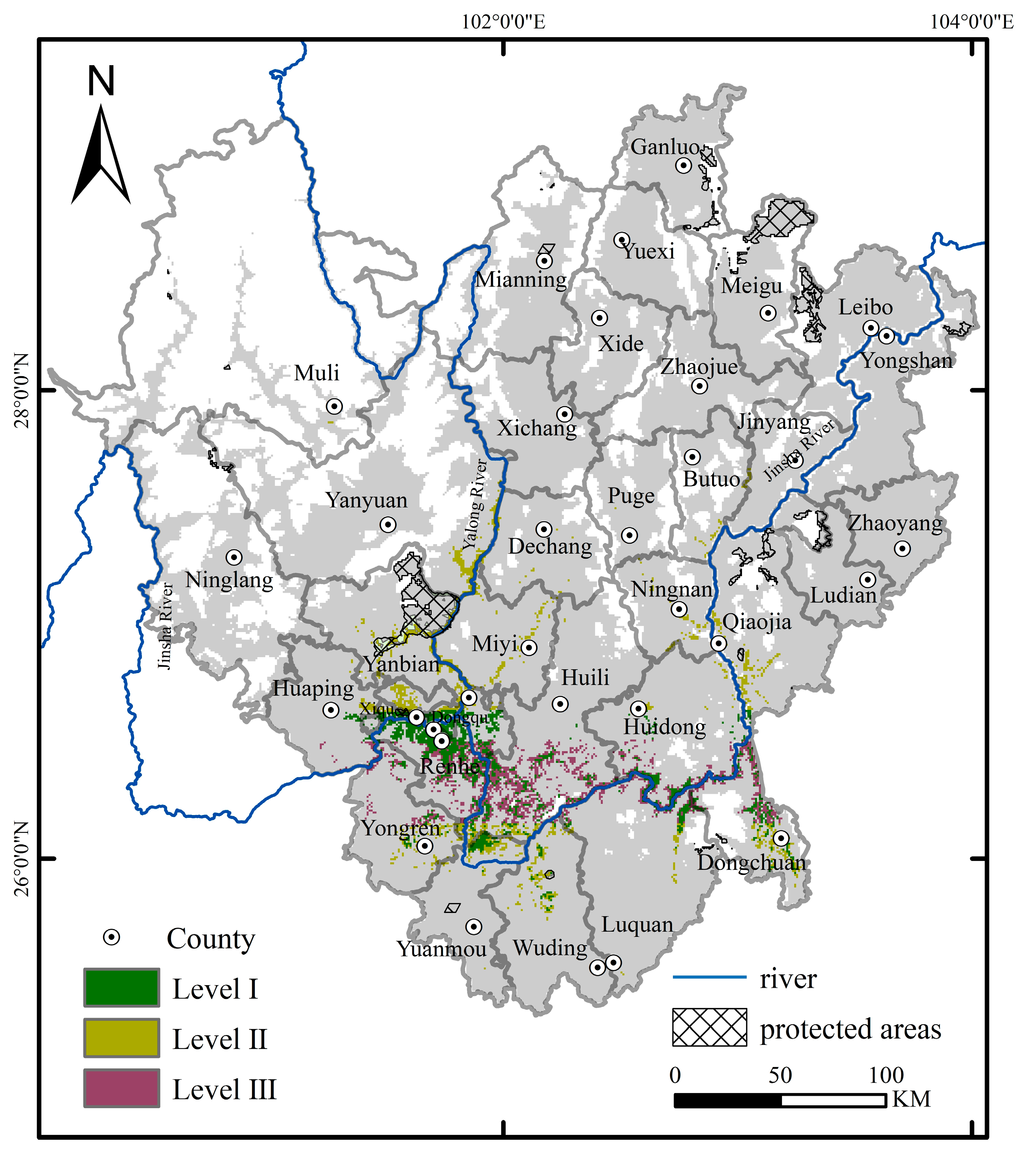
2.4. Determination of Conservation Prioritization
3. Discussion
3.1. Fragmented Habitat and Conservation Challenges of C. panzhihuaensis
3.2. Dominance of Elevation and Multi-Factor Synergy
3.3. Gaps in Exiting Protected Area Network
3.4. Implications for Conservation Strategies of Rare Plants in the HDM
3.5. Limitations
4. Materials and Methods
4.1. Study Area
4.2. Occurrence Records
4.3. Data Sources and Pre-Processing of Environmental Variables
4.3.1. Climatic Variables
4.3.2. Topographic Variables
4.3.3. Soil Variables
4.3.4. Anthropogenic Variable
4.4. Habitat Suitability Modeling Using MaxEnt
4.5. Quantifying Driving Factors with Geodetector
4.6. Identification of Spatial Conservation Prioritization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- López-Pujol, J.; Zhang, F.; Sun, H.; Ying, T.S.; Ge, S. Centres of Plant Endemism in China: Places for Survival or for Speciation? J. Biogeogr. 2011, 38, 1267–1280. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, P.; Liu, X.; Zhou, Y. Forest Fragmentation Trends and Modes in China: Implications for Conservation and Restoration. Int. J. Appl. Earth Obs. Geoinf. 2024, 133, 104094. [Google Scholar] [CrossRef]
- Liu, J.; Coomes, D.A.; Gibson, L.; Hu, G.; Liu, J.; Luo, Y.; Wu, C.; Yu, M. Forest Fragmentation in China and Its Effect on Biodiversity. Biol. Rev. 2019, 94, 1636–1657. [Google Scholar] [CrossRef] [PubMed]
- Sloan, S.; Jenkins, C.N.; Joppa, L.N.; Gaveau, D.L.A.; Laurance, W.F. Remaining Natural Vegetation in the Global Biodiversity Hotspots. Biol. Conserv. 2014, 177, 12–24. [Google Scholar] [CrossRef]
- Ye, X.; Liu, G.; Li, Z.; Wang, H.; Zeng, Y. Assessing Local and Surrounding Threats to the Protected Area Network in a Biodiversity Hotspot: The Hengduan Mountains of Southwest China. PLoS ONE 2015, 10, e0138533. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xiong, Q.; Yu, L.; Yan, W.; Qu, X. Impact of Climate Change on Potential Distribution Patterns of Alpine Vegetation in the Hengduan Mountains Region, China. Mt. Res. Dev. 2020, 40, R48–R54. [Google Scholar] [CrossRef]
- Hu, Z.; Guo, K.; Jin, S.; Pan, H. The Influence of Climatic Changes on Distribution Pattern of Six Typical Kobresia Species in Tibetan Plateau Based on MaxEnt Model and Geographic Information System. Theor. Appl. Climatol. 2019, 135, 375–390. [Google Scholar] [CrossRef]
- Norstog, K.J.; Nicholls, T.J. The Biology of the Cycads; Comstock Publishing Associates: Ithaca, NY, USA, 1997. [Google Scholar]
- Liao, H.; Zhong, L.; He, Y.; He, J.; Wu, Y.; Guo, Y.; Mei, L.; Wang, G.; Cao, F.; Fu, F.; et al. Construction of Ancestral Chromosomes in Gymnosperms and the Application in Comparative Genomic Analysis. Plants 2025, 14, 2361. [Google Scholar] [CrossRef]
- Hill, K. The Genus Cycas (Cycadaceae) in China. Telopea 2008, 12, 71–118. [Google Scholar] [CrossRef]
- Xi, H.; Wang, Y.; Pan, Y.; Xu, T.; Zhan, Q.; Liu, J.; Feng, X.; Gong, X. Resources and Protection of Cycas Plants in China. Biodivers. Sci. 2022, 30, 21495. [Google Scholar] [CrossRef]
- He, Y.; Li, C. The ecological geographic distribution, spatial pattern and collecting history of Cycas panzhihuaensis populations. Chin. J. Plant Ecol. 1999, 23, 23–30. [Google Scholar]
- Yang, Y.; Huang, B.; Yu, Z.; Liao, P. Inferences of Demographic History and Fine-Scale Landscape Genetics in Cycas panzhihuaensis and Implications for Its Conservation. Tree Genet. Genomes 2015, 11, 78. [Google Scholar] [CrossRef]
- Bösenberg, J.D. Cycas panzhihuaensis. The IUCN Red List of Threatened Species 2023. Available online: https://www.iucnredlist.org/en (accessed on 23 July 2025).
- Zheng, Y.; Yang, Y.; Wang, M.; Hu, S.; Wu, J.; Yu, Z. Differences in Lipid Homeostasis and Membrane Lipid Unsaturation Confer Differential Tolerance to Low Temperatures in Two Cycas Species. BMC Plant Biol. 2021, 21, 377. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, Y.; Zheng, Y. Effects of Heat Shock on Photosynthesis-Related Characteristics and Lipid Profile of Cycas multipinnata and C. panzhihuaensis. BMC Plant Biol. 2022, 22, 442. [Google Scholar] [CrossRef]
- Xiao, S.; Ji, Y.; Liu, J.; Gong, X. Genetic Characterization of the Entire Range of Cycas panzhihuaensis (Cycadaceae). Plant Divers. 2020, 42, 7–18. [Google Scholar] [CrossRef]
- Chen, N.; Yuan, L.; Wu, H.; Xue, J. Climate Change’s Influence on Chelydra serpentina’s Global and Chinese Distribution and Invasion: A MaxEnt Model-Based Prediction. Glob. Ecol. Conserv. 2024, 54, e03137. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Jiang, X.; Chen, H.; Liu, M.; Wang, R. Potential Geographical Distribution of the Edangred Plant Isoetes under Human Activities Using MaxEnt and GARP. Glob. Ecol. Conserv. 2022, 38, e02186. [Google Scholar] [CrossRef]
- Meza-Mori, G.; Nematollahi, S.; Amasifuen Guerra, C.A.; Oliva-Cruz, M.; Coronel-Castro, E.; Guzmán, C.T.; Darvishi, A. Integrating MaxEnt and InVEST Modeling Methods to Identify Priority Areas for the Conservation of Emblematic and Endemic Wildlife in the Peruvian Tropical Andes. Glob. Ecol. Conserv. 2025, 62, e03626. [Google Scholar] [CrossRef]
- Sun, X.; Long, Z.; Jia, J. A Multi-Scale Maxent Approach to Model Habitat Suitability for the Giant Pandas in the Qionglai Mountain, China. Glob. Ecol. Conserv. 2021, 30, e01766. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Fu, B. A Measure of Spatial Stratified Heterogeneity. Ecol. Indic. 2016, 67, 250–256. [Google Scholar] [CrossRef]
- Han, Y.; Dong, S.; Wu, X.; Liu, S.; Su, X.; Zhang, Y.; Zhao, H.; Zhang, X.; Swift, D. Integrated Modeling to Identify Priority Areas for the Conservation of the Endangered Plant Species in Headwater Areas of Asia. Ecol. Indic. 2019, 105, 47–56. [Google Scholar] [CrossRef]
- Sala, E.; Mayorga, J.; Bradley, D.; Cabral, R.B.; Atwood, T.B.; Auber, A.; Cheung, W.; Costello, C.; Ferretti, F.; Friedlander, A.M.; et al. Protecting the Global Ocean for Biodiversity, Food and Climate. Nature 2021, 592, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Huan-Cheng, M.; McConchie, J.A. The Dry-Hot Valleys and Forestation in Southwest China. J. For. Res. 2001, 12, 35–39. [Google Scholar] [CrossRef]
- Wei, L.; Chen, X.; Yang, H.; Sun, W.; Pan, Y.; Wang, G. Predicting Suitable Habitat for China’s Endangered Plant Cycas segmentifida Using Maxent under Climate Change. Pak. J. Bot. 2024, 56, 1881–1888. [Google Scholar] [CrossRef]
- Xie, C.; Li, M.; Jim, C.Y.; Chen, R. Distribution Pattern of Endangered Cycas taiwaniana Carruth. in China under Climate-Change Scenarios Using the MaxEnt Model. Plants 2025, 14, 1600. [Google Scholar] [CrossRef]
- Qiu, Y.; Fu, C.; Comes, H.P. Plant Molecular Phylogeography in China and Adjacent Regions: Tracing the Genetic Imprints of Quaternary Climate and Environmental Change in the World’s Most Diverse Temperate Flora. Mol. Phylogenet. Evol. 2011, 59, 225–244. [Google Scholar] [CrossRef]
- Xiao, L.; Ge, X.; Gong, X.; Hao, G.; Zheng, S. ISSR Variation in the Endemic and Endangered Plant Cycas guizhouensis (Cycadaceae). Ann. Bot. 2004, 94, 133–138. [Google Scholar] [CrossRef]
- Dupont-Nivet, G.; Krijgsman, W.; Langereis, C.G.; Abels, H.A.; Dai, S.; Fang, X. Tibetan Plateau Aridification Linked to Global Cooling at the Eocene–Oligocene Transition. Nature 2007, 445, 635–638. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Qiao, D.; Su, L. Increasing Precipitation during First Half of Growing Season Enhances Ecosystem Water Use Efficiency in a Semiarid Grassland. Front. Plant Sci. 2023, 14, 1119101. [Google Scholar] [CrossRef]
- Daramola, M.T.; Li, R.; Xu, M. Increased diurnal temperature range in global drylands in more recent decades. Int. J. Climatol. 2024, 44, 521–533. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, L.; Spalink, D.; Sun, L.; Chen, J.; Sun, H. Spatial Phylogenetics of Two Topographic Extremes of the Hengduan Mountains in Southwestern China and Its Implications for Biodiversity Conservation. Plant Divers. 2021, 43, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Xue, H.; Dong, G.; Liu, D.; Li, Z. Vegetation Changes and Driving Factors in the Qilian Mountains during 1982-2022. Chin. J. Ecol. 2024, 43, 1576. [Google Scholar] [CrossRef]
- Elsen, P.R.; Monahan, W.B.; Merenlender, A.M. Topography and Human Pressure in Mountain Ranges Alter Expected Species Responses to Climate Change. Nat. Commun. 2020, 11, 1974. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Z.; Hu, J.; Han, J.; Yang, J.; Ying, L. Protection and utilization of plant biodiversity resources in dry valleys of Southwest China. Biodivers. Sci. 2016, 24, 475–488. [Google Scholar] [CrossRef]
- Luo, Y.; Shi, C.; Yang, S.; Liu, Y.; Zhao, S.; Zhang, C. Characteristics of Soil Calcium Content Distribution in Karst Dry-Hot Valley and Its Influencing Factors. Water 2023, 15, 1119. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Kongsurakan, P.; Yuttitham, M.; Hatano, R. Variations of soil properties and soil surface loss after fire in rotational shifting cultivation in Northern Thailand. Front. Environ. Sci. 2023, 11, 1213181. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.; Zheng, Y. Effect of different elevation on soil physical chemical properties and erodibility in dry-hot valley. Bull. Soil Water Conserv. 2011, 31, 103–107. [Google Scholar] [CrossRef]
- Joppa, L.N.; Pfaff, A. Global Protected Area Impacts. Proc. R. Soc. B Biol. Sci. 2011, 278, 1633–1638. [Google Scholar] [CrossRef]
- Cobb, G.; Nalau, J.; Chauvenet, A.L.M. Global Trends in Geospatial Conservation Planning: A Review of Priorities and Missing Dimensions. Front. Ecol. Evol. 2024, 11, 1209620. [Google Scholar] [CrossRef]
- Ranius, T.; Widenfalk, L.A.; Seedre, M.; Lindman, L.; Felton, A.; Hämäläinen, A.; Filyushkina, A.; Öckinger, E. Protected Area Designation and Management in a World of Climate Change: A Review of Recommendations. Ambio 2023, 52, 68–80. [Google Scholar] [CrossRef]
- Fos, S.; Laguna, E.; Jiménez, J.; Gómez-Serrano, M.Á. Plant Micro-Reserves in Valencia (E. Spain): A Model to Preserve Threatened Flora in China? Plant Divers. 2017, 39, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Laguna, E.; Deltoro, V.I.; Pèrez-Botella, J.; Pèrez-Rovira, P.; Serra, L.; Olivares, A.; Fabregat, C. The Role of Small Reserves in Plant Conservation in a Region of High Diversity in Eastern Spain. Biol. Conserv. 2004, 119, 421–426. [Google Scholar] [CrossRef]
- Liu, J.; Lindstrom, A.J.; Gong, Y.; Dong, S.; Liu, Y.C.; Zhang, S.; Gong, X. Eco-Evolutionary Evidence for the Global Diversity Pattern of Cycas (Cycadaceae). J. Integr. Plant Biol. 2024, 66, 1170–1191. [Google Scholar] [CrossRef]
- Perrigo, A.; Hoorn, C.; Antonelli, A. Why Mountains Matter for Biodiversity. J. Biogeogr. 2020, 47, 315–325. [Google Scholar] [CrossRef]
- Liang, G.; Niu, H.; Li, Y. A Multi-Species Approach for Protected Areas Ecological Network Construction Based on Landscape Connectivity. Glob. Ecol. Conserv. 2023, 46, e02569. [Google Scholar] [CrossRef]
- Liu, F.; Li, H.; Sun, Y.; Tang, G.; Zhang, C. Effects of Climate on Vegetaion Recovery in Dry-Hot-Valleys of Hengduan Mountainous Region in Southwest China. Resour. Environ. Yangtze Basin 2010, 19, 1386–1391. [Google Scholar]
- NASA/METI/AIST/Japan Spacesystems; U.S./Japan ASTER Science Team. ASTER Global Digital Elevation Model V003; NASA Land Processes Distributed Active Archive Center: Sioux Falls, SD, USA, 2019. [CrossRef]
- Global Biodiversity Information Facility (GBIF). Available online: https://doi.org/10.15468/dl.qx3exa (accessed on 11 November 2024).
- Chinese Virtual Herbarium (CVH). Available online: https://www.cvh.ac.cn/ (accessed on 11 November 2024).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Shangguan, W.; Dai, Y.; Liu, B.; Zhu, A.; Duan, Q.; Wu, L.; Ji, D.; Ye, A.; Yuan, H.; Zhang, Q.; et al. A China Data Set of Soil Properties for Land Surface Modeling. J. Adv. Model. Earth Syst. 2013, 5, 212–224. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar] [CrossRef]
- Mu, H.; Li, X.; Wen, Y.; Huang, J.; Du, P.; Su, W.; Miao, S.; Geng, M. A Global Record of Annual Terrestrial Human Footprint Dataset from 2000 to 2018. Sci. Data 2022, 9, 176. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Ge, Y.; Xu, C. An Optimal Parameters-Based Geographical Detector Model Enhances Geographic Characteristics of Explanatory Variables for Spatial Heterogeneity Analysis: Cases with Different Types of Spatial Data. GISci. Remote Sens. 2020, 57, 593–610. [Google Scholar] [CrossRef]
- Wang, J.; Haining, R.; Zhang, T.; Xu, C.; Hu, M.; Yin, Q.; Li, L.; Zhou, C.; Li, G.; Chen, H. Statistical Modeling of Spatially Stratified Heterogeneous Data. Ann. Am. Assoc. Geogr. 2024, 114, 499–519. [Google Scholar] [CrossRef]
- Lehtomäki, J.; Moilanen, A. Methods and Workflow for Spatial Conservation Prioritization Using Zonation. Environ. Model. Softw. 2013, 47, 128–137. [Google Scholar] [CrossRef]
- Moilanen, A. Landscape Zonation, Benefit Functions and Target-Based Planning: Unifying Reserve Selection Strategies. Biol. Conserv. 2007, 134, 571–579. [Google Scholar] [CrossRef]
- Convention on Biological Diversity (CBD). Kunming-Montreal Global Biodiversity Framework. Available online: https://www.cbd.int/gbf (accessed on 23 July 2025).
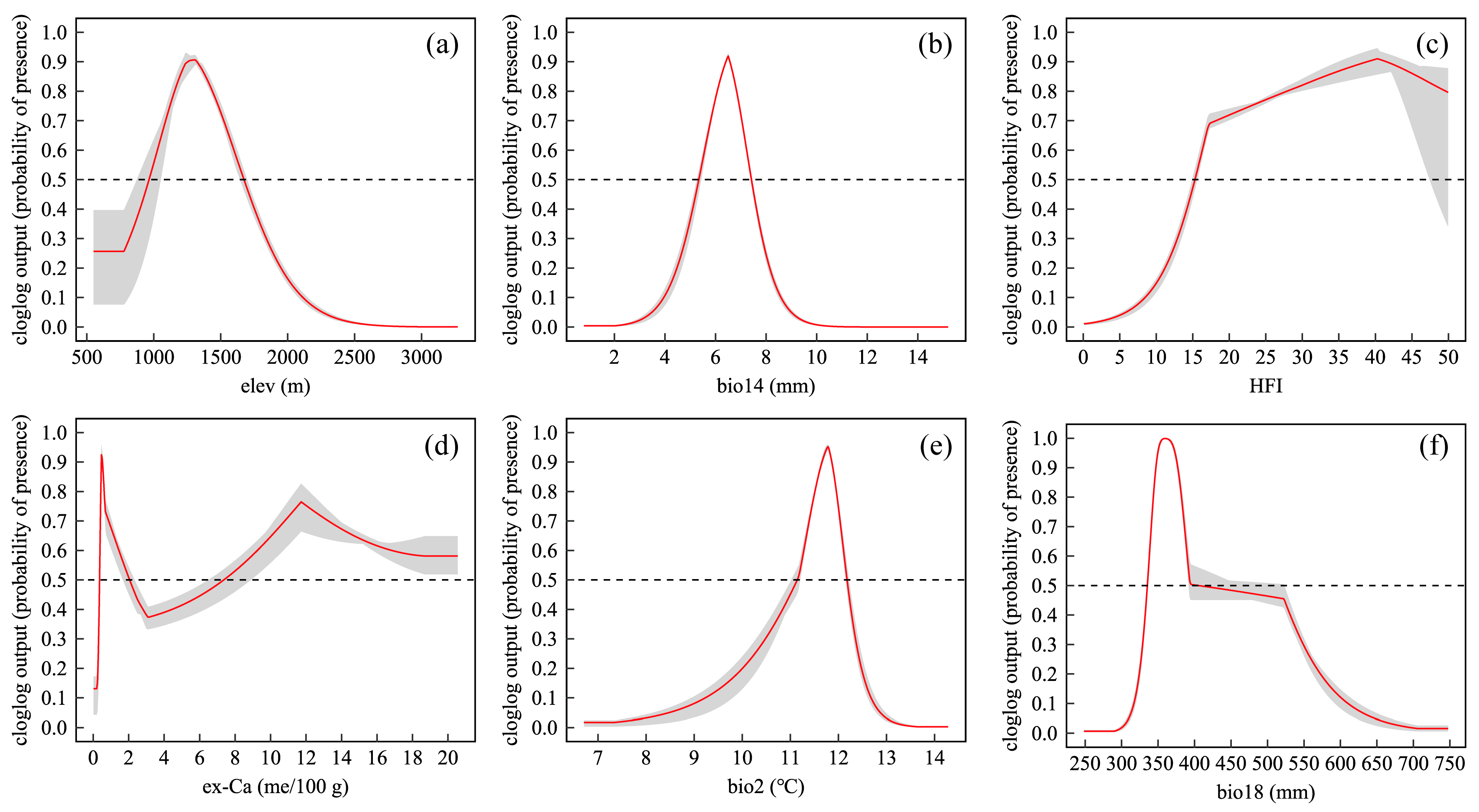

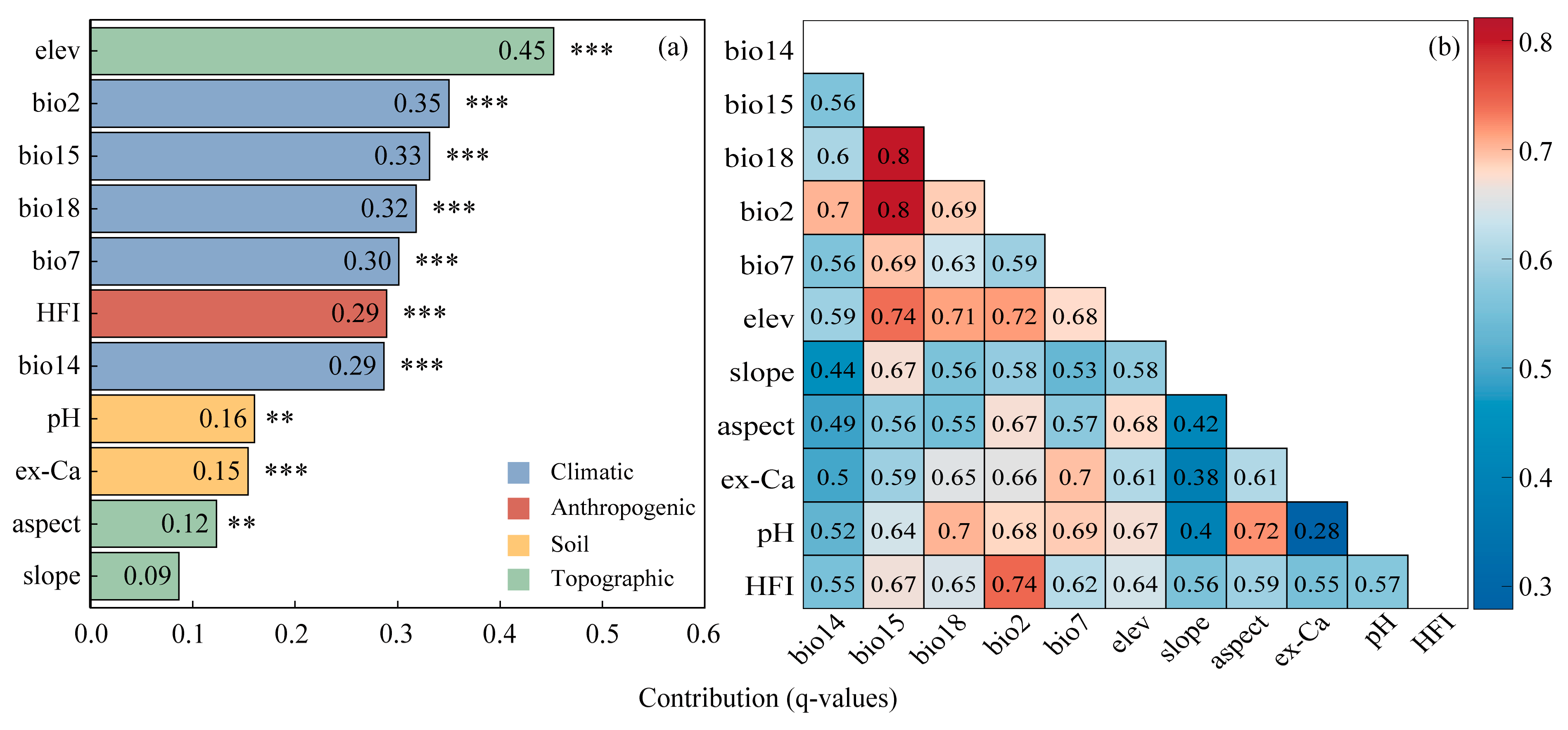

| Variables | Description and Unit | Percent Contribution (%) | Permutation Importance (%) |
|---|---|---|---|
| elev | Elevation (m) | 43.1 | 48.7 |
| bio14 | Precipitation of Driest Month (mm) | 16.9 | 11.7 |
| HFI | Human Footprint Index | 8.8 | 6.4 |
| ex-Ca | Exchangeable calcium (me/100 g) | 7.0 | 5.3 |
| bio2 | Mean Diurnal Range (°C) | 6.8 | 10.7 |
| bio18 | Precipitation of Warmest Quarter (mm) | 5.8 | 6.4 |
| slope | Slope (°) | 4.9 | 4.7 |
| bio15 | Precipitation Seasonality (mm) | 3.2 | 0.4 |
| pH | Soil pH value (H2O) | 1.5 | 1.5 |
| aspect | Aspect (°) | 1.2 | 0.5 |
| bio7 | Temperature Annual Range (°C) | 0.6 | 3.6 |
| Period | Current | 2021–2040 | 2041–2060 | ||||
|---|---|---|---|---|---|---|---|
| SSP126 | SSP245 | SSP585 | SSP126 | SSP245 | SSP585 | ||
| Low suitability (km2) | 6935.82 | 3385.11 | 2693.4 | 3099.86 | 3878.37 | 1812.5 | 2097.20 |
| Medium suitability (km2) | 3549.03 | 1692.83 | 816.13 | 854.99 | 1887.5 | 395.75 | 363.30 |
| High suitability (km2) | 2056.92 | 752.19 | 202.89 | 165.73 | 749.88 | 68.02 | 48.60 |
| Total suitable area (km2) | 12,541.77 | 5830.13 | 3712.42 | 4120.58 | 6515.75 | 2276.27 | 2509.10 |
| Priority | County | Total Area (km2) | Area Covered by Existing Protected Areas (km2) | Coverage (%) |
|---|---|---|---|---|
| Level I | Renhe, Yanbian, Wuding, Huidong, Luquan, Dongchuan, Yongren, Yuanmou, Huaping, Xiqu, Dongqu, Huili, Qiaojia | 1246.77 | 8.91 | 0.71 |
| Level II | Renhe, Yanbian, Wuding, Huidong, Luquan, Dongchuan, Yongren, Yuanmou, Huaping, Xiqu, Dongqu, Butuo, Jinyang, Ningnan, Puge, Dechang, Qiaojia, Miyi, Yanyuan, Huili | 1200.32 | 65.52 | 5.46 |
| Level III | Renhe, Yanbian, Wuding, Huidong, Luquan, Dongchuan, Yongren, Huaping, Huili | 1227.81 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Yang, Y.; Peng, X.; Wang, J.; Wu, M.; Zhang, Y.; Liu, X.; Peng, P. Habitat Suitability and Driving Factors of Cycas panzhihuaensis in the Hengduan Mountains. Plants 2025, 14, 2797. https://doi.org/10.3390/plants14172797
Ding Y, Yang Y, Peng X, Wang J, Wu M, Zhang Y, Liu X, Peng P. Habitat Suitability and Driving Factors of Cycas panzhihuaensis in the Hengduan Mountains. Plants. 2025; 14(17):2797. https://doi.org/10.3390/plants14172797
Chicago/Turabian StyleDing, Yuting, Yuanfeng Yang, Xuefeng Peng, Juan Wang, Mengjie Wu, Ying Zhang, Xing Liu, and Peihao Peng. 2025. "Habitat Suitability and Driving Factors of Cycas panzhihuaensis in the Hengduan Mountains" Plants 14, no. 17: 2797. https://doi.org/10.3390/plants14172797
APA StyleDing, Y., Yang, Y., Peng, X., Wang, J., Wu, M., Zhang, Y., Liu, X., & Peng, P. (2025). Habitat Suitability and Driving Factors of Cycas panzhihuaensis in the Hengduan Mountains. Plants, 14(17), 2797. https://doi.org/10.3390/plants14172797





