Perovskia atriplicifolia Benth (Russian Sage), a Source of Diterpenes Exerting Antioxidant Activity in Caco-2 Cells
Abstract
1. Introduction
2. Results
2.1. Chemical Investigation of the P. atriplicifolia Aerial Parts EtOH Extract
2.2. Isolation and Identification of Compounds 1–19
2.3. Evaluation of the Antioxidant Activity of EtOH Extract of P. atriplicifolia and Pure Compounds
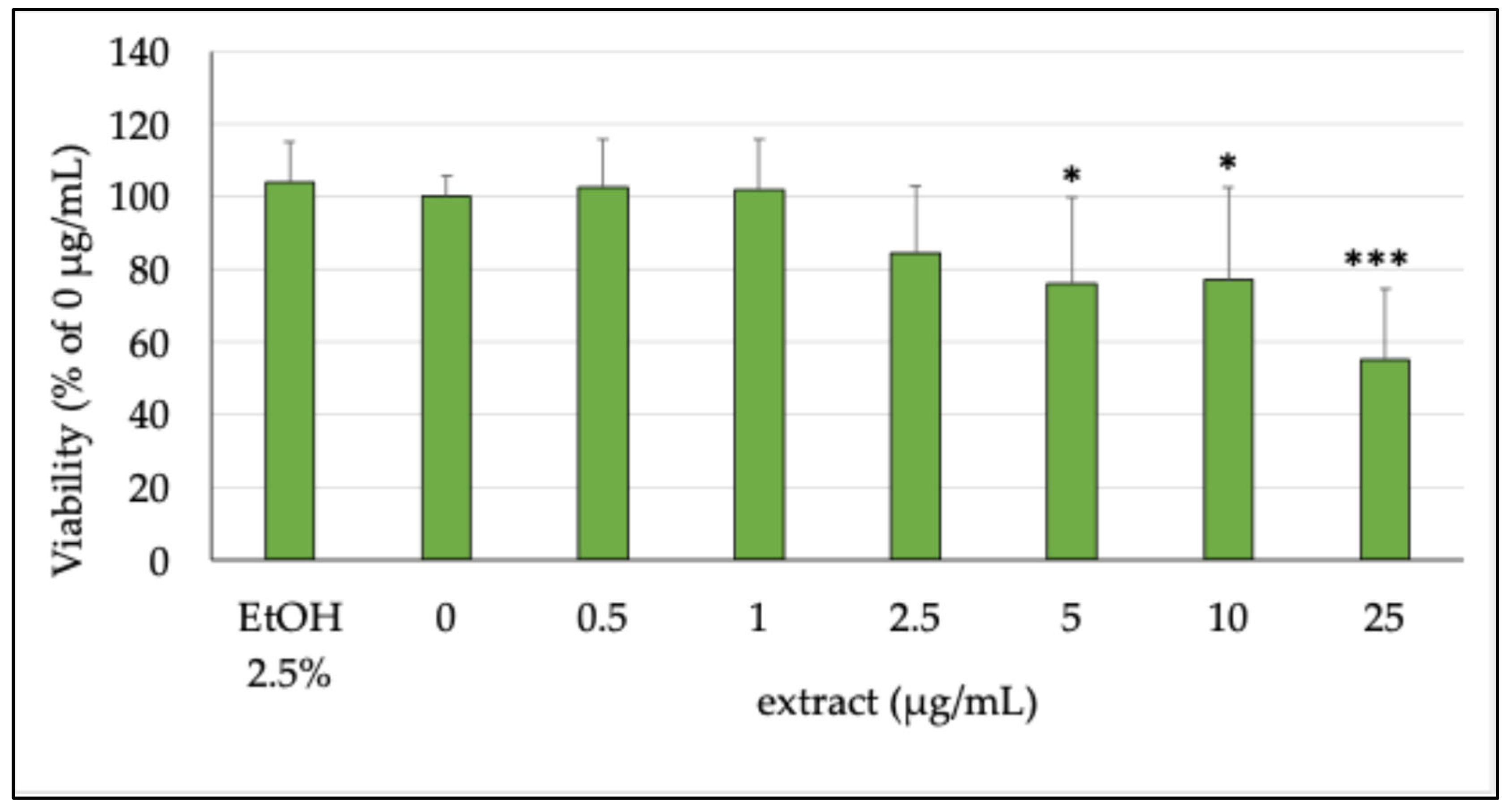
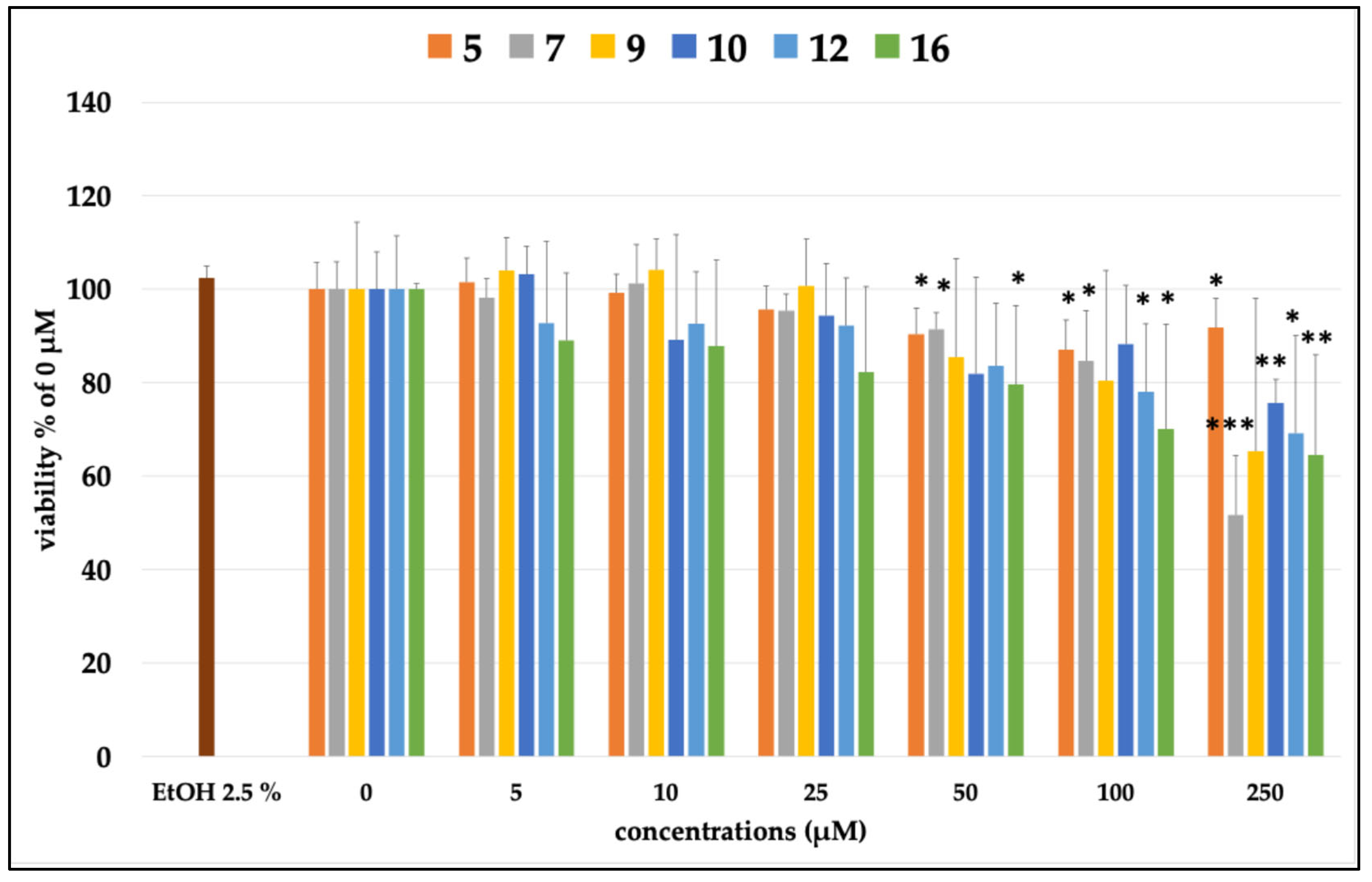
2.3.1. Cell Viability
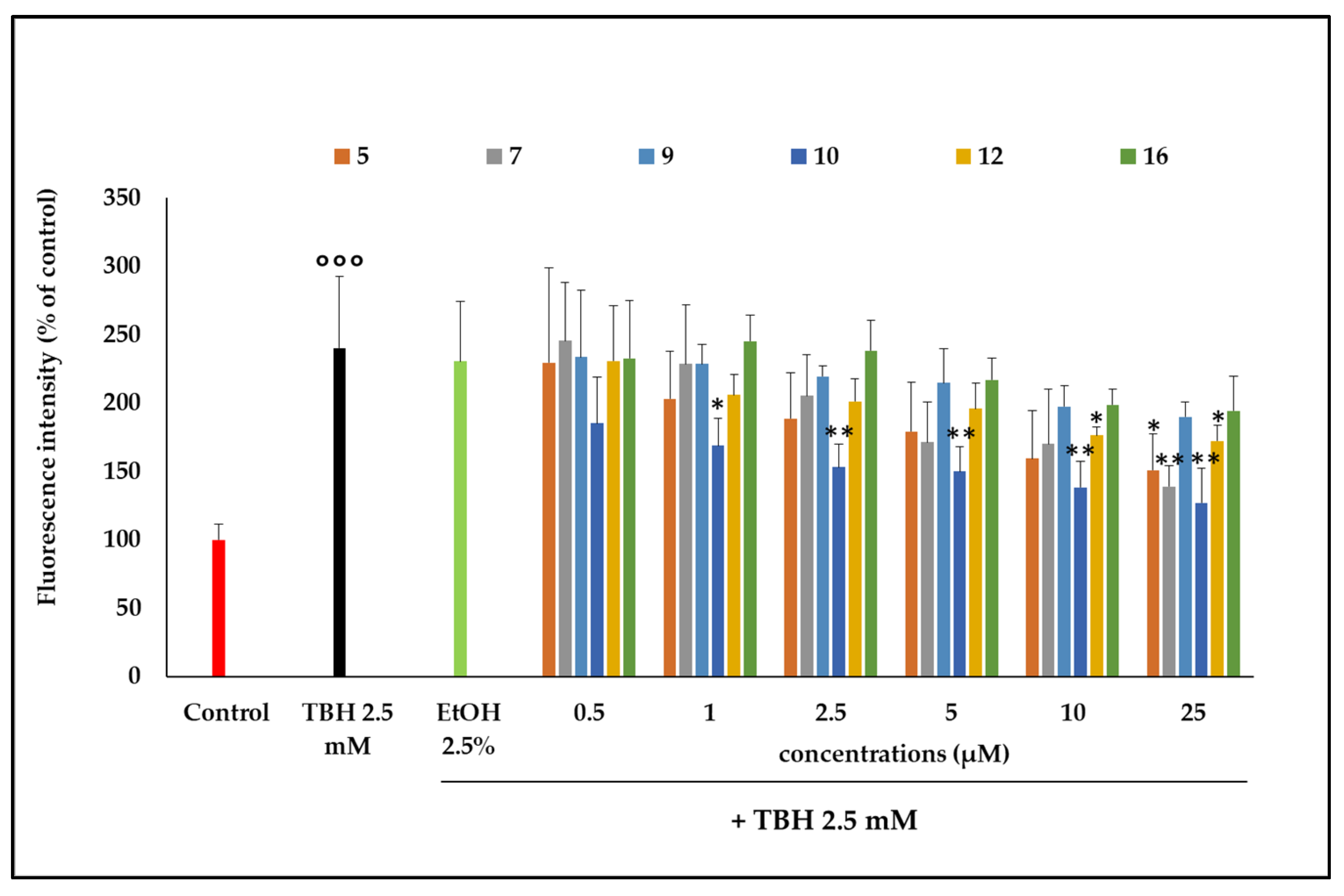
2.3.2. Inhibition of ROSs Production
2.4. Quantitative Analysis of the Constituents of P. atriplicifolia EtOH Extract
3. Discussion
3.1. Quantitative Analysis of Carnosol (12) and Carnosic Acid (16)
3.2. Bioactivity of Carnosol (12) and Carnosic Acid (16)
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. LC-(-)ESI/QExactive/MS/MS Analysis
4.3. Isolation Procedure
4.4. Cell Culture
4.5. MTT Viability Test
4.6. Determination of Intracellular Reactive Oxygen Species (ROS) Production
4.7. Statistical Analyses
4.8. Quantitative Analysis of Main Diterpenoid Derivatives (5, 7, 9, 10, 12, 16) by LC-(-)ESI/QExactive/MS/MS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hashemifar, Z.; Rahimmalek, M. Genetic structure and variation in Perovskia abrotanoides Karel and P. atriplicifolia as revealed by molecular and morphological markers. Sci. Hortic. 2018, 230, 169–177. [Google Scholar] [CrossRef]
- Tabefam, M.; Farimani, M.M.; Danton, O.; Ramseyer, J.; Kaiser, M.; Ebrahimi, S.N.; Salehi, P.; Batooli, H.; Potterat, O.; Hamburger, M. Antiprotozoal diterpenes from Perovskia abrotanoides. Planta Med. 2018, 84, 913–919. [Google Scholar] [CrossRef]
- Ahmad, I.; Fozia; Waheed, A.; Tahir, N.B.; Rais, A.K. Anti-inflammatory constituents from Perovskia atriplicifolia. Pharm. Biol. 2015, 53, 1628–1631. [Google Scholar] [CrossRef]
- Pourhosseini, S.H.; Hadian, J.; Sonboli, A.; Nejad Ebrahimi, S.; Mirjalili, M.H. Genetic and Chemical Diversity in Perovskia abrotanoides Kar. (Lamiaceae) Populations Based on ISSRs Markers and Essential Oils Profile. Chem. Biodivers. 2018, 15, e1700508. [Google Scholar] [CrossRef]
- Duke, J.; Bogenschuts, J.; Cellier, D. Handbook of Medicinal Herbs; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Tareen, R.B.; Bibi, T.; Khan, M.A.; Ahmad, M.; Zafar, M.; Hina, S. Indigenous knowledge of folk medicine by the women of Kalat and Khuzdar regions of Balochistan, Pakistan. Pak. J. Bot. 2010, 42, 1465–1485. [Google Scholar]
- Alizadeh, Z.; Farimani, M.M.; Parisi, V.; Marzocco, S.; Ebrahimi, S.N.; De Tommasi, N. Nor-abietane Diterpenoids from Perovskia abrotanoides Roots with Anti-inflammatory Potential. J. Nat. Prod. 2021, 84, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Afshari, M.; Rahimmalek, M.; Sabzalian, M.R.; Bielecka, M.; Matkowski, A.; Talebi, M. Changes in physiological, phytochemical traits and gene expression of two Perovskia species in response to water deficit. Sci. Hortic. 2022, 293, 110747. [Google Scholar] [CrossRef]
- Zaker, A.; Sykora, C.; Gössnitzer, F.; Abrishamchi, P.; Asili, J.; Mousavi, S.H.; Wawrosch, C. Effects of some elicitors on tanshinone production in adventitious root cultures of Perovskia abrotanoides Karel. Ind. Crop. Prod. 2015, 67, 97–102. [Google Scholar] [CrossRef]
- Miroliaei, M.; Aminjafari, A.; Slusarczyk, S.; Nawrot-Hadzik, I.; Rahimmalek, M.; Matkowski, A. Inhibition of Glycation-induced Cytotoxicity, Protein Glycation, and Activity of Proteolytic Enzymes by Extract from Perovskia atriplicifolia Roots. Pharmacogn. Mag. 2017, 13, s676–s683. [Google Scholar] [CrossRef] [PubMed]
- Ciobotaru, G.V.; Goje, I.-D.; Dehelean, C.A.; Danciu, C.; Magyari-Pavel, I.Z.; Moacă, E.-A.; Muntean, D.; Imbrea, I.M.; Sărățeanu, V.; Pop, G. Analysis of the Antioxidant and Antimicrobial Activity, Cytotoxic, and Anti-Migratory Properties of the Essential Oils Obtained from Cultivated Medicinal Lamiaceae Species. Plants 2025, 14, 846. [Google Scholar] [CrossRef]
- Johnson, J.J. Carnosol: A promising anti-cancer and anti-inflammatory agent. Cancer Lett. 2011, 305, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, M.; Sefidkon, F. Analysis of the essential oil from aerial parts of Perovskia atriplicifolia Benth. at different stages of plant growth. Flavour Fragr. J. 2001, 16, 435–438. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Masullo, M.; Cerulli, A.; Nazzaro, F.; Farimani, M.; Piacente, S. Terpenoid Constituents of Perovskia artemisioides Aerial Parts with Inhibitory Effects on Bacterial Biofilm Growth. J. Nat. Prod. 2020, 84, 26–36. [Google Scholar] [CrossRef]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act through Different Mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Masullo, M.; Cerulli, A.; Martucciello, S.; Ayyari, M.; Pizza, C.; Piacente, S. Antiproliferative Cardenolides from the Aerial Parts of Pergularia tomentosa. J. Nat. Prod. 2019, 82, 74–79. [Google Scholar] [CrossRef]
- Ali, M.S.; Saleem, M.; Erian, A.W. A new acylated steroid glucoside from Perovskia atriplicifolia. Fitoterapia 2001, 72, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Rahmani Samani, M.; D’Urso, G.; Nazzaro, F.; Fratianni, F.; Masullo, M.; Piacente, S. Phytochemical Investigation and Biofilm-Inhibitory Activity of Bachtiari Savory (Satureja bachtiarica Bunge) Aerial Parts. Plants 2024, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Piccolella, S.; Lettieri, A.; Nocera, P.; Bollino, F.; Catauro, M. A metabolic profiling approach to an Italian sage leaf extract (SoA541) defines its antioxidant and anti-acetylcholinesterase properties. J. Funct. Foods. 2017, 29, 1–9. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Cerulli, A.; Marzocco, S.; Moridi Farimani, M.; Masullo, M.; Piacente, S. Anti-inflammatory Activity of Tanshinone-Related Diterpenes from Perovskia artemisioides Roots. J. Nat. Prod. 2023, 86, 812–821. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A.; Fawzy, G.; Ibrahim, T.; Malik, A.; Khan, A. Cholinesterase inhibitory triterpenes from Perovskia atriplicifolia. Asian J. Chem. 2014, 26, 6163. [Google Scholar] [CrossRef]
- Khaliq, S.; Volk, F.-J.; Frahm, A. Phytochemical Investigation of Perovskia abrotanoides. Planta Med. 2007, 73, 77–83. [Google Scholar] [CrossRef]
- Tarawneh, A.; León, F.; Pettaway, S.; Elokely, K.M.; Klein, M.L.; Lambert, J.; Mansoor, A.; Cutler, S.J. Flavonoids from Perovskia atriplicifolia and Their in Vitro Displacement of the Respective Radioligands for Human Opioid and Cannabinoid Receptors. J. Nat. Prod. 2015, 78, 1461–1465. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Sultana, I.; Begum, S. Triterpenoidal constituents from Eucalyptus camaldulensis var. obtusa leaves. Phytochemistry 2000, 54, 861–865. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Li, Z.-Q.; Huang, C.-G.; Zhou, J.; Hu, Q.-F.; Liu, W.-X.; Huang, X.-Z.; Wang, W.; Zhang, L.-Z.; Xia, F.-T. Abietane Diterpenoids from Perovskia atriplicifolia and Their Anti-HBV Activities. Bull. Korean Chem. Soc. 2015, 36, 623–627. [Google Scholar] [CrossRef]
- Marrero, J.G.; Moujir, L.; Andrés, L.S.; Montaño, N.P.; Araujo, L.; Luis, J.G. Semisynthesis and Biological Evaluation of Abietane-Type Diterpenes. Revision of the Structure of Rosmaquinone. J. Nat. Prod. 2009, 72, 1385–1389. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Zhou, J.; Huang, C.-G.; Hu, Q.-F.; Huang, X.-Z.; Wang, W.; Zhang, L.-Z.; Li, G.-P.; Xia, F.-T. Two novel antiviral terpenoids from the cultured Perovskia atriplicifolia. Tetrahedron 2015, 71, 3844–3849. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Al-Shihry, S.S.; Son, B.W. Diterpenoid quinones from Rosemary (Rosmarinus officinalis L.). Phytochemistry 2005, 66, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Hongcheng, W.; Fujimotot, Y. Triterpene esters from Eucalyptus tereticornis. Phytochemistry 1993, 33, 151–153. [Google Scholar] [CrossRef]
- Liang, H.; Guan, M.; Li, T.; Li, S.; Ma, C.; Li, C. Critical review on biological effect and mechanisms of diterpenoids in Rosmarinus officinalis. Food Med. Homol. 2025, 2, 9420021. [Google Scholar] [CrossRef]
- Santoru, M.L.; Piras, C.; Murgia, F.; Spada, M.; Tronci, L.; Leoni, V.P.; Serreli, G.; Deiana, M.; Atzori, L. Modulatory Effect of Nicotinic Acid on the Metabolism of Caco-2 Cells Exposed to IL-1beta and LPS. Metabolites 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Irtegun Kandemir, S.; Fidan, H.S.; Yener, I.; Mete, N.; Ertas, A.; Topcu, G.; Kolak, U. Investigation of cytotoxic and apoptotic effects of 63 compounds obtained from Salvia species: Promising anticancer agents. J. Food Biochem. 2022, 46, e14226. [Google Scholar] [CrossRef]
- Rauniyar, N. Parallel Reaction Monitoring: A Targeted Experiment Performed Using High Resolution and High Mass Accuracy Mass Spectrometry. Int. J. Mol. Sci. 2015, 16, 28566–28581. [Google Scholar] [CrossRef]
- Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Phytochemical composition of fractions isolated from ten Salvia species by supercritical carbon dioxide and pressurized liquid extraction methods. Food Chem. 2017, 224, 37–47. [Google Scholar] [CrossRef]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Effect of the phenological stage on the chemical composition, and antimicrobial and antioxidant properties of Rosmarinus officinalis L. essential oil and its polyphenolic extract. Ind. Crop. Prod. 2013, 48, 144–152. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, L.; Hou, L.; Zhou, Z.; Wang, C. Mechanism of protective effect of carnosol on pig intestinal epithelial cells. Int. J. Clin. Exp. Pathol. 2020, 13, 447–455. [Google Scholar] [PubMed]
- Zhang, J.; Shen, M.; Yin, Y.; Chen, Y.; Deng, X.; Mo, J.; Zhou, X.; Lin, J.; Chen, X.; Xie, X.; et al. Carnosic acid reduces lipid content, enhances gut health, and modulates microbiota composition and metabolism in diet-induced obese mice. Food Funct. 2025, 16, 1888–1902. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Li, Z.; Zhang, J.; Yin, N.; Tang, L.; Li, J.; Sun, J.; Yu, X.; Chen, W.; Xiao, H.; et al. The protective effect of carnosic acid on dextran sulfate sodium-induced colitis based on metabolomics and gut microbiota analysis. Food Sci. Hum. Well. 2023, 12, 1212–1223. [Google Scholar] [CrossRef]
- Wan, S.S.; Li, X.Y.; Liu, S.R.; Tang, S. The function of carnosic acid in lipopolysaccharides-induced hepatic and intestinal inflammation in poultry. Poult. Sci. 2024, 103, 103415. [Google Scholar] [CrossRef]
- Mirza, F.; Zahid, S.; Holsinger, R.M.D. Neuroprotective Effects of Carnosic Acid: Insight into Its Mechanisms of Action. Molecules 2023, 28, 2306. [Google Scholar] [CrossRef]
- Hou, C.-W.; Lin, Y.-T.; Chen, Y.-L.; Wang, Y.-H.; Chou, J.-L.; Ping, L.-Y.; Jeng, K.-C. Neuroprotective effects of carnosic acid on neuronal cells under ischemic and hypoxic stress. Nutr. Neurosci. 2012, 15, 257–263. [Google Scholar] [CrossRef]
- Poeckel, D.; Greiner, C.; Verhoff, M.; Rau, O.; Tausch, L.; Hörnig, C.; Steinhilber, D.; Schubert-Zsilavecz, M.; Werz, O. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem. Pharmacol. 2008, 76, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Hong, H.-L.; Kim, G.M.; Leem, J.; Kwon, H.H. Protective Effects of Carnosic Acid on Lipopolysaccharide-Induced Acute Kidney Injury in Mice. Molecules 2021, 26, 7589. [Google Scholar] [CrossRef] [PubMed]
- Rechinger, K.H.W. Flora Iranica; Akademic Druck-u. Verlagsanstalt: Graz, Austria, 1982; Volume 150, pp. 749–781. [Google Scholar]
- Spada, M.; Piras, C.; Diana, G.; Leoni, V.P.; Frau, D.V.; Serreli, G.; Simbula, G.; Loi, R.; Noto, A.; Murgia, F.; et al. Glutamine Starvation Affects Cell Cycle, Oxidative Homeostasis and Metabolism in Colorectal Cancer Cells. Antioxidants 2023, 12, 683. [Google Scholar] [CrossRef] [PubMed]
- Gil, K.A.; Nowicka, P.; Wojdylo, A.; Serreli, G.; Deiana, M.; Tuberoso, C.I.G. Antioxidant Activity and Inhibition of Digestive Enzymes of New Strawberry Tree Fruit/Apple Smoothies. Antioxidants 2023, 12, 805. [Google Scholar] [CrossRef]
- Montoro, P.; Molfetta, I.; Maldini, M.; Ceccarini, L.; Piacente, S.; Pizza, C.; Macchia, M. Determination of six steviol glycosides of Stevia rebaudiana (Bertoni) from different geographical origin by LC-ESI-MS/MS. Food Chem. 2013, 141, 745–753. [Google Scholar] [CrossRef]
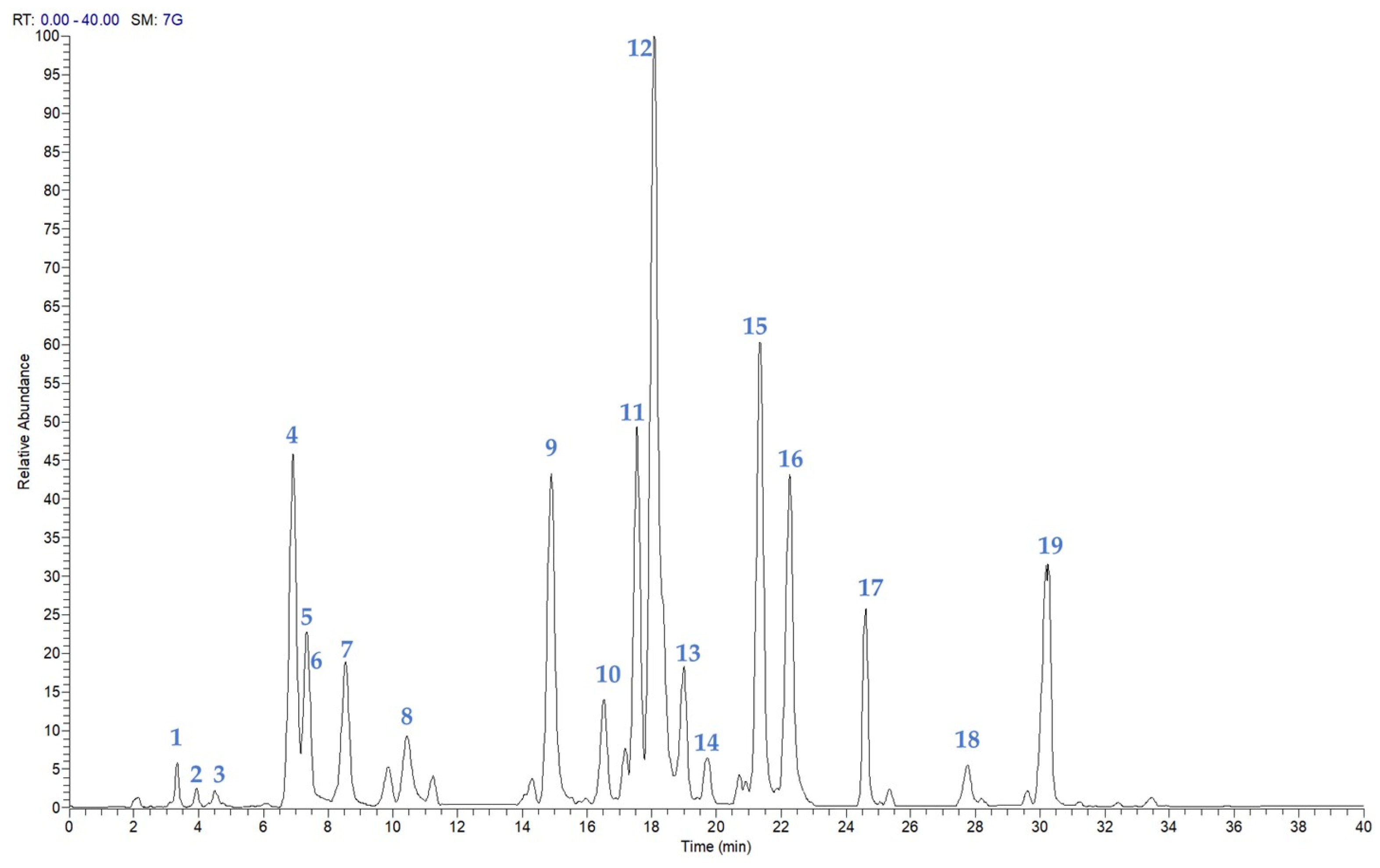
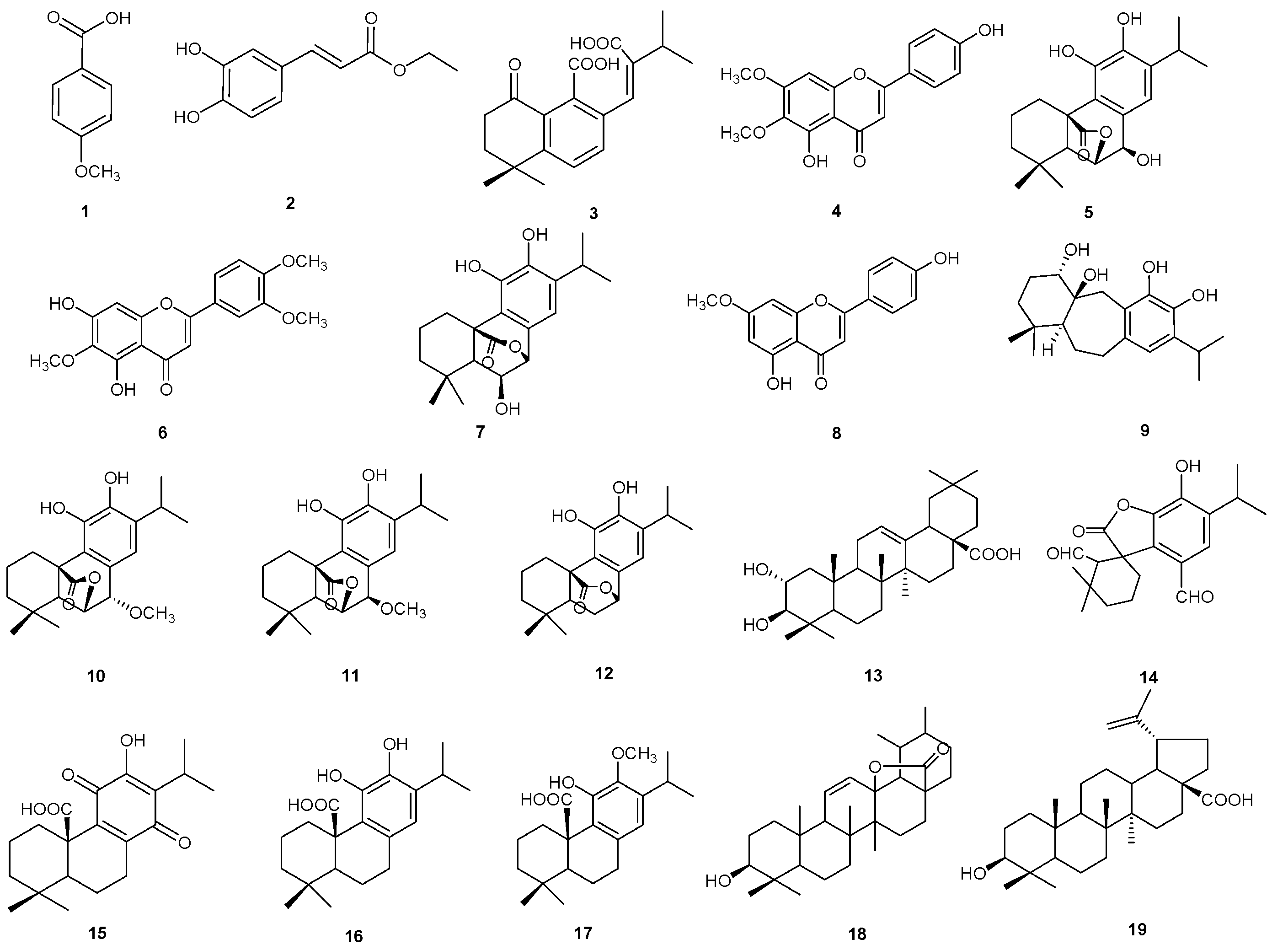

| N° | Rt | [M-H]− | Molecular Formula | Δ ppm | MS/MS | Compound |
|---|---|---|---|---|---|---|
| 1 | 3.42 | 151.0392 | C8H8O3 | 1.32 | 136.01 | p-methoxybenzoic acid |
| 2 | 3.96 | 207.0659 | C11H12O4 | 3.30 | 162.98, 179.03, 135.04 | caffeic acid ethyl ester |
| 3 | 4.79 | 329.1398 | C19H22O5 | 4.50 | 242.15, 285.14 | perovskin A |
| 4 | 6.93 | 313.0718 | C17H14O6 | 3.56 | 283.02, 298.05 | scrophulein |
| 5 | 7.33 | 345.1708 | C20H26O5 | 3.27 | 283.17, 301.18 | epirosmanol |
| 6 | 7.52 | 343.0830 | C18H16O7 | 5.19 | 328.05 | eupatilin |
| 7 | 8.53 | 345.1708 | C20H26O5 | 3.36 | 283.17, 301.18 | epiisorosmanol |
| 8 | 10.42 | 283.0613 | C16H12O5 | 4.38 | 268.04 | genkwanin |
| 9 | 14.89 | 333.2073 | C20H30O4 | 3.91 | 179.11, 191.11, 315.20 | 1α-hydroxydemethylsalvicanol |
| 10 | 16.47 | 359.1868 | C21H28O5 | 4.09 | 283.17, 329.18, 315.16 | 7-O-methylrosmanol |
| 11 | 17.54 | 359.1867 | C21H28O5 | 3.84 | 283.17, 329.18, 315.16 | 7-epi-7-O-methylrosmanol |
| 12 | 18.07 | 329.1760 | C20H26O4 | 3.93 | 285.19 | carnasol |
| 13 | 18.99 | 471.3486 | C30H48O4 | 3.64 | 453.34 | maslinic acid |
| 14 | 19.70 | 343.1555 | C20H24O5 | 4.43 | 219.06, 299.16, 315.16, 325.14 | rosmadial |
| 15 | 21.33 | 345.1710 | C20H26O5 | 3.88 | 286.19, 301.22 | royleanonic acid |
| 16 | 22.28 | 331.1916 | C20H28O4 | 3.82 | 287.20 | carnosic acid |
| 17 | 24.61 | 345.2072 | C21H30O4 | 3.31 | 283.17, 286.19, 301.18 | 12-O-methylcarnosic acid |
| 18 | 27.73 | 453.3377 | C30H46O3 | 3.13 | 407.33 | urs-11-en-28,13β-olide |
| 19 | 30.21 | 455.3534 | C30H48O3 | 3.05 | 189.14, 206.99 | betulinic acid |
| Compound | mg/100 g Dry Plant ± SD |
|---|---|
| 5 | 20.73 ± 0.14 |
| 7 | 11.74 ± 0.39 |
| 9 | 91.73 ± 1.87 |
| 10 | 1.73 ± 0.40 |
| 12 | 520.21 ± 23.29 |
| 16 | 88.16 ± 7.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samani, M.R.; Cerulli, A.; Serreli, G.; Melis, M.P.; Deiana, M.; Masullo, M.; Piacente, S. Perovskia atriplicifolia Benth (Russian Sage), a Source of Diterpenes Exerting Antioxidant Activity in Caco-2 Cells. Plants 2025, 14, 2795. https://doi.org/10.3390/plants14172795
Samani MR, Cerulli A, Serreli G, Melis MP, Deiana M, Masullo M, Piacente S. Perovskia atriplicifolia Benth (Russian Sage), a Source of Diterpenes Exerting Antioxidant Activity in Caco-2 Cells. Plants. 2025; 14(17):2795. https://doi.org/10.3390/plants14172795
Chicago/Turabian StyleSamani, Marzieh Rahmani, Antonietta Cerulli, Gabriele Serreli, Maria Paola Melis, Monica Deiana, Milena Masullo, and Sonia Piacente. 2025. "Perovskia atriplicifolia Benth (Russian Sage), a Source of Diterpenes Exerting Antioxidant Activity in Caco-2 Cells" Plants 14, no. 17: 2795. https://doi.org/10.3390/plants14172795
APA StyleSamani, M. R., Cerulli, A., Serreli, G., Melis, M. P., Deiana, M., Masullo, M., & Piacente, S. (2025). Perovskia atriplicifolia Benth (Russian Sage), a Source of Diterpenes Exerting Antioxidant Activity in Caco-2 Cells. Plants, 14(17), 2795. https://doi.org/10.3390/plants14172795









