Transcriptome-Based Identification of Novel Transcription Factors Regulating Seed Storage Proteins in Rice
Abstract
1. Introduction
2. Results
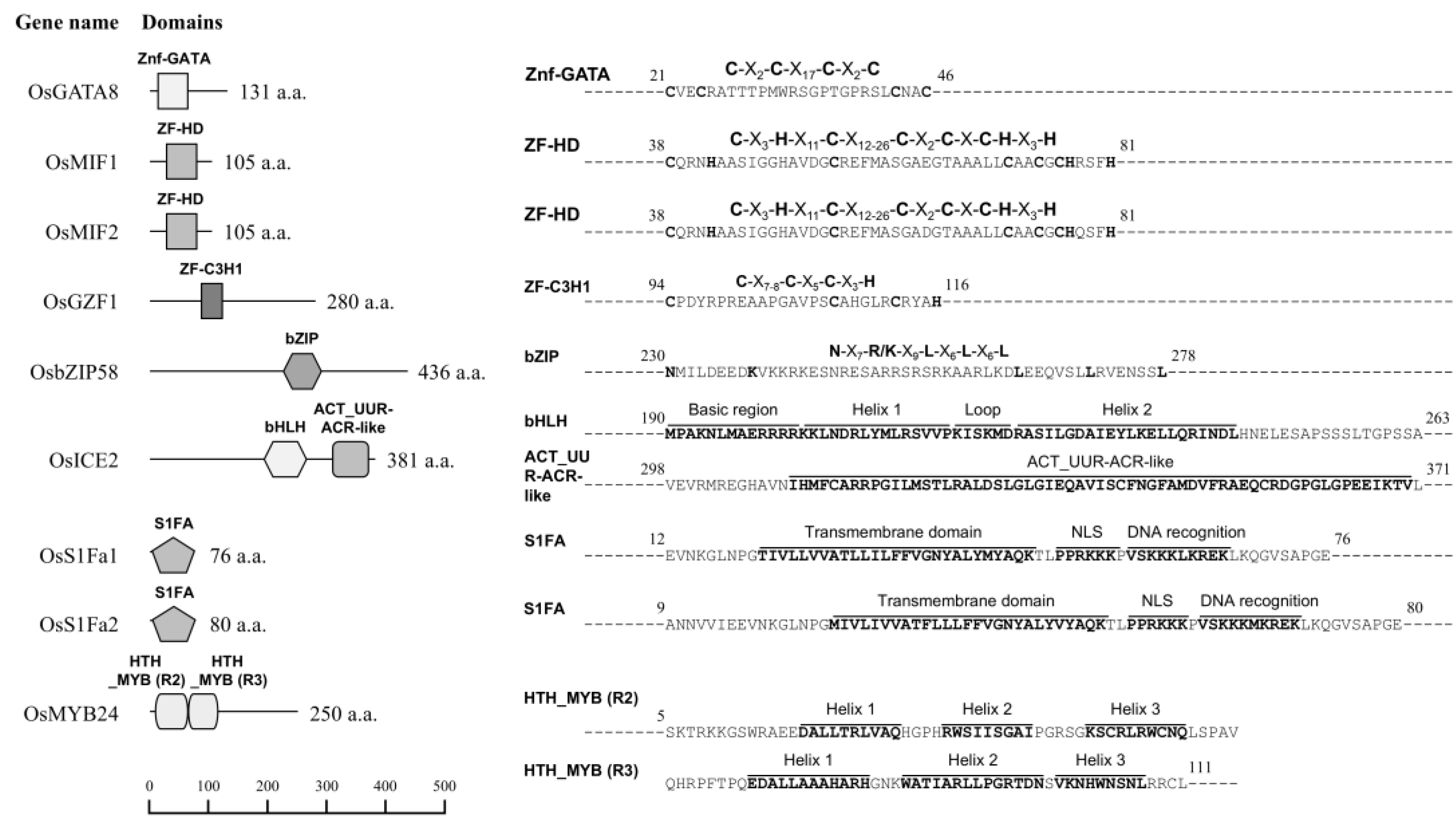
2.1. Identification of Novel Transcription Factors Correlated with Seed Storage Protein Genes
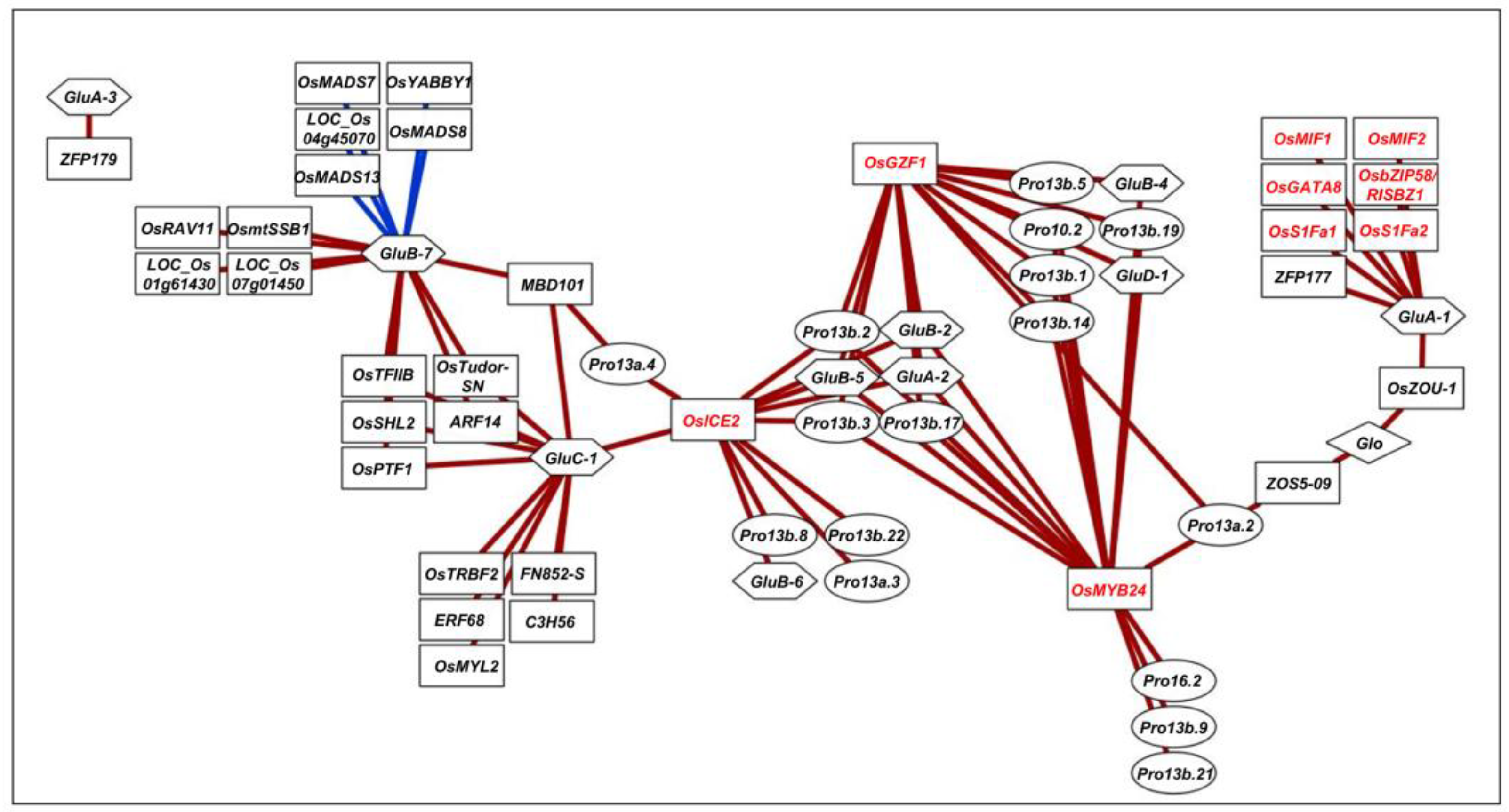
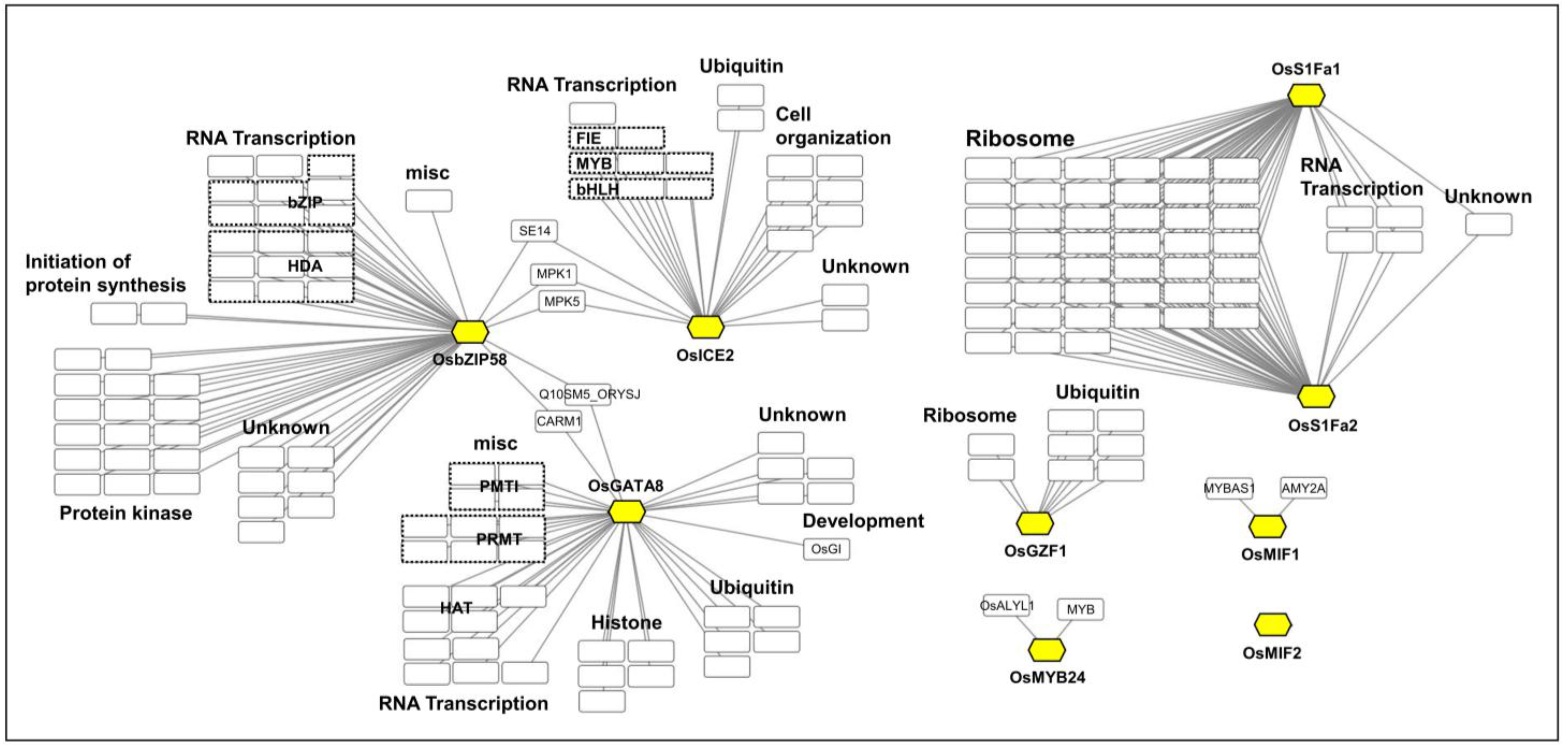
2.2. Protein–Protein Interaction (PPI) Network Analysis of Candidate TFs
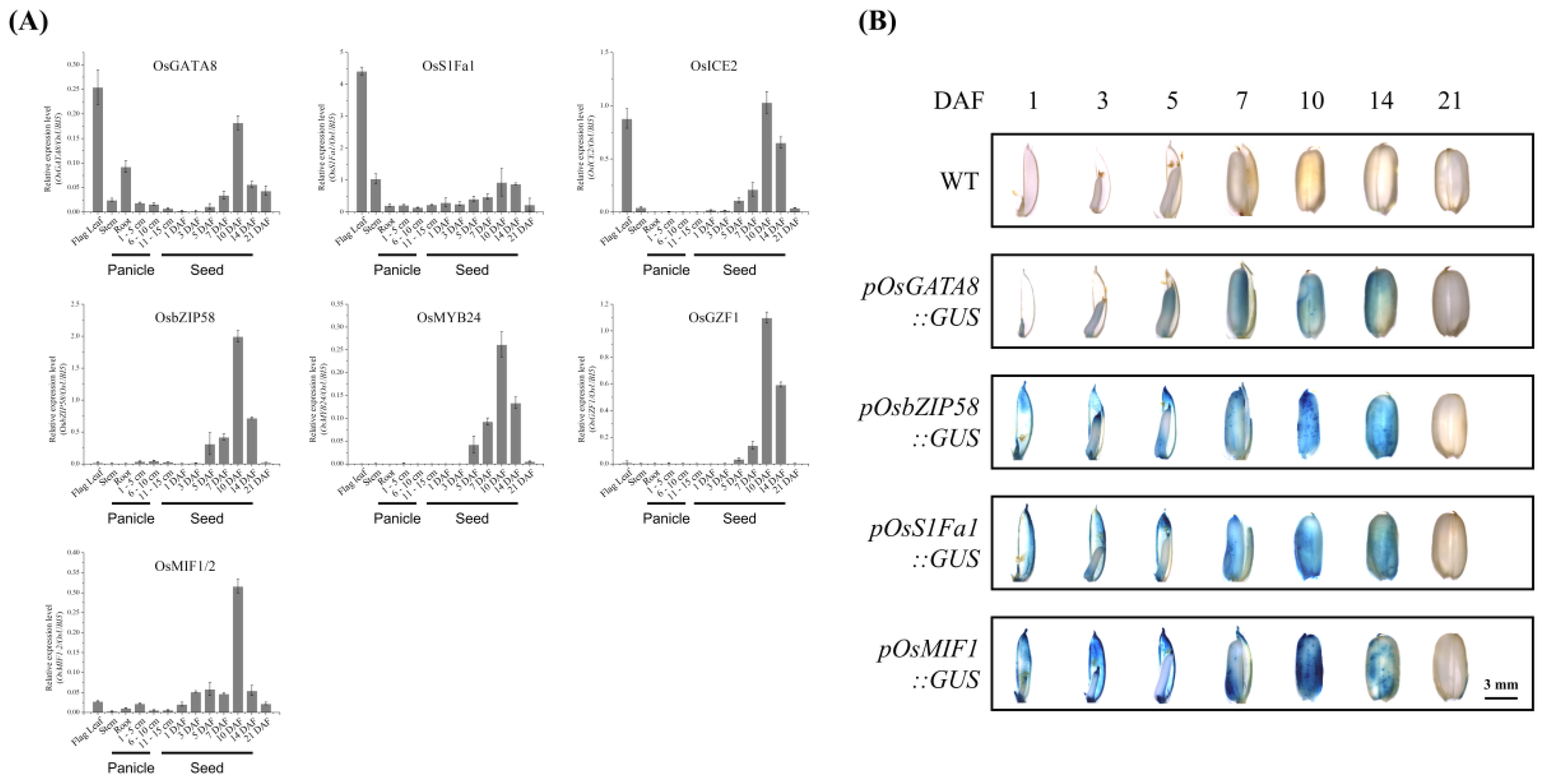
2.3. Expression Profiles of TFs During Seed Development
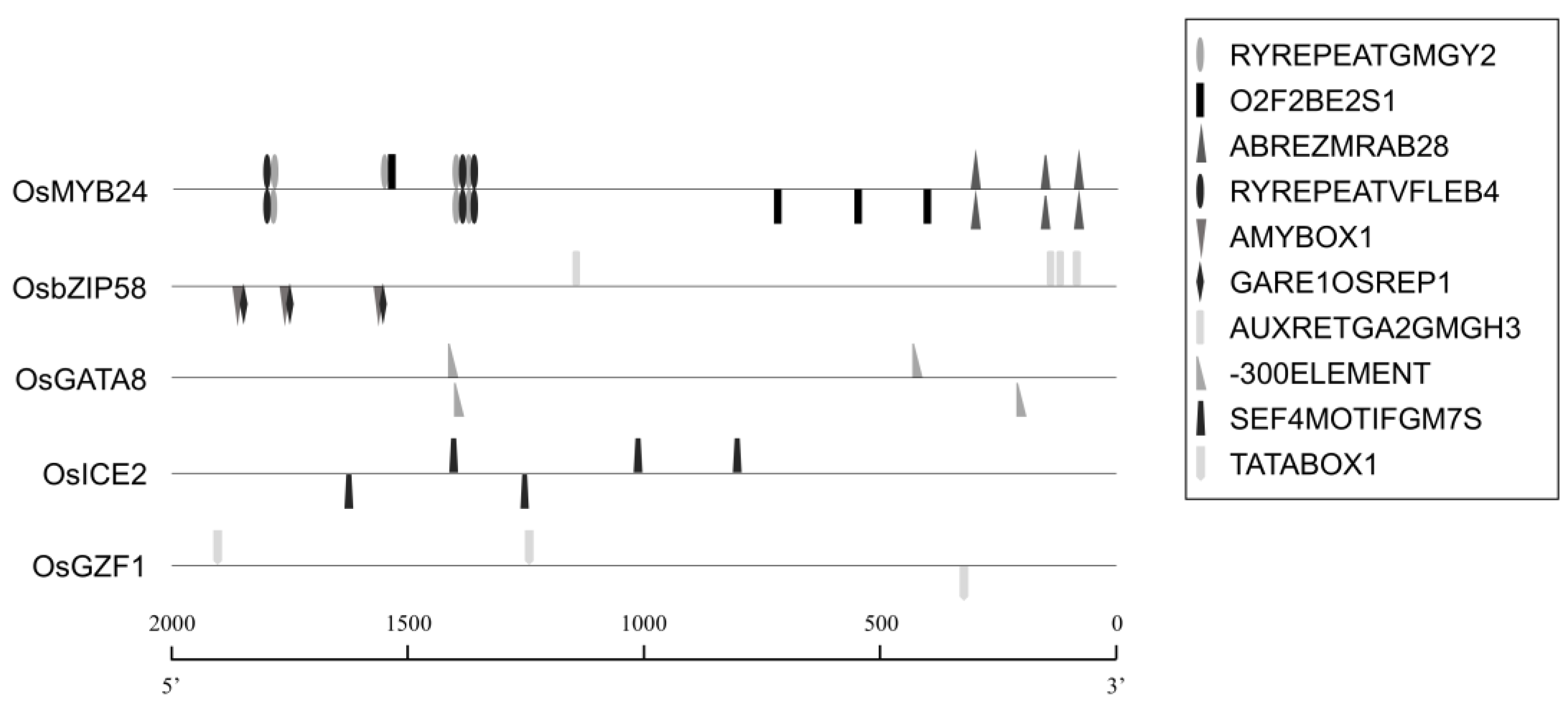
2.4. Genome-Wide Enrichment Analysis of Cis-Regulatory Elements (CREs)
3. Discussion
4. Materials and Methods
4.1. Correlation Analysis of Transcription Factor and Seed Storage Protein Genes Using Rice Seed Transcriptome
4.2. Information on Selected Transcription Factors
4.3. Protein–Protein Interaction (PPI) Network Analysis
4.4. Plant Materials and Growth Conditions
4.5. Rice Protoplast Transient Expression
4.6. RNA Extraction and Quantitative RT-PCR
4.7. GUS Staining Assay
4.8. Yeast Two-Hybrid Assay
4.9. Cis-Regulatory Element (CRE) Frequency Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| bHLH | Basic helix–loop–helix |
| bZIP | Basic leucine zipper |
| CRE | Cis-regulatory element |
| CRISPR-Cas9 | Clustered regularly interspaced short palindromic repeats–CRISPR associated protein 9 |
| DAF | Days after flowering |
| FIMO | Find Individual Motif Occurrence |
| GATA | GATA-type zinc finger protein |
| GEO | Gene Expression Omnibus |
| GUS | β-glucuronidase |
| MIF | Mini zinc finger protein |
| MSU | Michigan State University |
| MYB | Myeloblastosis-related transcription factor |
| PPI | Protein–protein interaction |
| PLACE | Plant cis-acting regulatory DNA elements database |
| RAP-DB | Rice Annotation Project Database |
| RGAP | Rice Genome Annotation Project |
| RNAi | RNA interference |
| RPBF | Rice Prolamin Box Binding Factor |
| S1F | Site 1 Factor |
| SSP | Seed storage proteins |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| TF | Transcription factor |
| Y2H | Yeast two-hybrid |
| ZF-HD | Zinc finger homeodomain |
References
- Li, R.; Li, M.; Ashraf, U.; Liu, S.; Zhang, J. Exploring the Relationships Between Yield and Yield-Related Traits for Rice Varieties Released in China From 1978 to 2017. Front. Plant Sci. 2019, 10, 543. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.-H.; Lu, J.; Zhang, C.-Q.; Liu, Q.-Q.; Li, Q.-F. Genes and Their Molecular Functions Determining Seed Structure, Components, and Quality of Rice. Rice 2022, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Abro, T.F.; Kabir, M.S.; Latif, M.A. Rice Quality: Biochemical Composition, Eating Quality, and Cooking Quality. In The Future of Rice Demand: Quality Beyond Productivity; Costa de Oliveira, A., Pegoraro, C., Ebeling Viana, V., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–24. [Google Scholar]
- Chen, P.; Shen, Z.; Ming, L.; Li, Y.; Dan, W.; Lou, G.; Peng, B.; Wu, B.; Li, Y.; Zhao, D.; et al. Genetic Basis of Variation in Rice Seed Storage Protein (Albumin, Globulin, Prolamin, and Glutelin) Content Revealed by Genome-Wide Association Analysis. Front. Plant Sci. 2018, 9, 612. [Google Scholar] [CrossRef]
- Long, X.; Guan, C.; Wang, L.; Jia, L.; Fu, X.; Lin, Q.; Huang, Z.; Lin, C. Rice Storage Proteins: Focus on Composition, Distribution, Genetic Improvement and Effects on Rice Quality. Rice Sci. 2023, 30, 207–221. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Qing, T.; Shu, X.-L.; Liu, J.-X. Unfolded protein response and storage product accumulation in rice grains. Seed Biol. 2022, 1, 4. [Google Scholar] [CrossRef]
- Cho, K.; Lee, H.-J.; Jo, Y.-M.; Lim, S.-H.; Rakwal, R.; Lee, J.-Y.; Kim, Y.-M. RNA Interference-Mediated Simultaneous Suppression of Seed Storage Proteins in Rice Grains. Front. Plant Sci. 2016, 7, 1624. [Google Scholar] [CrossRef]
- AlHusnain, L.; AlKahtani, M.D.F.; Attia, K.A.; Sanaullah, T.; Elsharnoby, D.E. Application of CRISPR/Cas9 system to knock out GluB gene for developing low glutelin rice mutant. Bot. Stud. 2024, 65, 27. [Google Scholar] [CrossRef]
- Chandra, D.; Cho, K.; Pham, H.A.; Lee, J.-Y.; Han, O. Down-Regulation of Rice Glutelin by CRISPR-Cas9 Gene Editing Decreases Carbohydrate Content and Grain Weight and Modulates Synthesis of Seed Storage Proteins during Seed Maturation. Int. J. Mol. Sci. 2023, 24, 16941. [Google Scholar] [CrossRef]
- Chen, Z.; Du, H.; Tao, Y.; Xu, Y.; Wang, F.; Li, B.; Zhu, Q.-H.; Niu, H.; Yang, J. Efficient breeding of low glutelin content rice germplasm by simultaneous editing multiple glutelin genes via CRISPR/Cas9. Plant Sci. 2022, 324, 111449. [Google Scholar] [CrossRef]
- Pham, H.A.; Cho, K.; Tran, A.D.; Chandra, D.; So, J.; Nguyen, H.T.T.; Sang, H.; Lee, J.-Y.; Han, O. Compensatory Modulation of Seed Storage Protein Synthesis and Alteration of Starch Accumulation by Selective Editing of 13 kDa Prolamin Genes by CRISPR-Cas9 in Rice. Int. J. Mol. Sci. 2024, 25, 6579. [Google Scholar] [CrossRef]
- Lee, H.-J.; Jo, Y.-M.; Lee, J.-Y.; Lim, S.-H.; Kim, Y.-M. Lack of Globulin Synthesis during Seed Development Alters Accumulation of Seed Storage Proteins in Rice. Int. J. Mol. Sci. 2015, 16, 14717–14736. [Google Scholar] [CrossRef]
- Xiong, Y.; Ren, Y.; Li, W.; Wu, F.; Yang, W.; Huang, X.; Yao, J. NF-YC12 is a key multi-functional regulator of accumulation of seed storage substances in rice. J. Exp. Bot. 2019, 70, 3765–3780. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, L.; Yang, Y.; Zhang, W.; Chen, Z.; Li, X.; Qian, Q.; Kong, F.; Li, Y.; Liu, X.; et al. The gibberellin signaling negative regulator RGA-LIKE3 promotes seed storage protein accumulation. Plant Physiol. 2021, 185, 1697–1707. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Yamamoto, M.P.; Touno, S.M.; Yasuda, H.; Takaiwa, F. Compensation and interaction between RISBZ1 and RPBF during grain filling in rice. Plant J. 2009, 59, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Onodera, Y.; Suzuki, A.; Wu, C.Y.; Washida, H.; Takaiwa, F. A rice functional transcriptional activator, RISBZ1, responsible for endosperm-specific expression of storage protein genes through GCN4 motif. J. Biol. Chem. 2001, 276, 14139–14152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, A.; Wang, M.; Zhu, Z.; Ouwerkerk, P.B.F. Functions of the CCCH type zinc finger protein OsGZF1 in regulation of the seed storage protein GluB-1 from rice. Plant Mol. Biol. 2014, 84, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Zhang, Q.; Meng, S.; Wei, C. The NAC Transcription Factors OsNAC20 and OsNAC26 Regulate Starch and Storage Protein Synthesis. Plant Physiol. 2020, 184, 1775–1791. [Google Scholar] [CrossRef]
- Kim, S.; Cho, K.; Lim, S.-H.; Goo, T.-W.; Lee, J.-Y. Transcriptome profiling of transgenic rice seeds lacking seed storage proteins (globulin, prolamin, and glutelin) by RNA-Seq analysis. Plant Biotechnol. Rep. 2021, 15, 77–93. [Google Scholar] [CrossRef]
- Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. The Saltol QTL-localized transcription factor OsGATA8 plays an important role in stress tolerance and seed development in Arabidopsis and rice. J. Exp. Bot. 2020, 71, 684–698. [Google Scholar] [CrossRef]
- Nakamura, J.; Yuasa, T.; Huong, T.T.; Harano, K.; Tanaka, S.; Iwata, T.; Phan, T.; Iwaya, M. Rice homologs of inducer of CBF expression (OsICE) are involved in cold acclimation. Plant Biotechnol. 2011, 28, 303–309. [Google Scholar] [CrossRef]
- Deng, C.; Ye, H.; Fan, M.; Pu, T.; Yan, J. The rice transcription factors OsICE confer enhanced cold tolerance in transgenic Arabidopsis. Plant Signal. Behav. 2017, 12, e1316442. [Google Scholar] [CrossRef]
- Kim, S.-I.; Lee, K.H.; Kwak, J.S.; Kwon, D.H.; Song, J.T.; Seo, H.S. Overexpression of Rice OsS1Fa1 Gene Confers Drought Tolerance in Arabidopsis. Plants 2021, 10, 2181. [Google Scholar] [CrossRef]
- Alonso-Blanco, C.; Gomez-Mena, C.; Llorente, F.; Koornneef, M.; Salinas, J.; Martínez-Zapater, J.M. Genetic and molecular analyses of natural variation indicate CBF2 as a candidate gene for underlying a freezing tolerance quantitative trait locus in Arabidopsis. Plant Physiol. 2005, 139, 1304–1312. [Google Scholar] [CrossRef]
- Vincentz, M.; Leite, A.; Neshich, G.; Vriend, G.; Mattar, C.; Barros, L.; Weinberg, D.; de Almeida, E.R.; de Carvalho, M.P.; Aragão, F.; et al. ACGT and vicilin core sequences in a promoter domain required for seed-specific expression of a 2S storage protein gene are recognized by the opaque-2 regulatory protein. Plant Mol. Biol. 1997, 34, 879–889. [Google Scholar] [CrossRef]
- Lelievre, J.M.; Oliveira, L.O.; Nielsen, N.C. 5′CATGCAT-3′ Elements Modulate the Expression of Glycinin Genes. Plant Physiol. 1992, 98, 387–391. [Google Scholar] [CrossRef]
- Curaba, J.; Moritz, T.; Blervaque, R.; Parcy, F.; Raz, V.; Herzog, M.; Vachon, G. AtGA3ox2, a key gene responsible for bioactive gibberellin biosynthesis, is regulated during embryogenesis by LEAFY COTYLEDON2 and FUSCA3 in Arabidopsis. Plant Physiol. 2004, 136, 3660–3669. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Sutliff, T.D.; Litts, J.C.; Rodriguez, R.L. Classification and characterization of the rice alpha-amylase multigene family. Plant Mol. Biol. 1990, 14, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.; Hagen, G.; Ulmasov, T.; Murfett, J. How does auxin turn on genes? Plant Physiol. 1998, 118, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Sutoh, K.; Yamauchi, D. Two cis-acting elements necessary and sufficient for gibberellin-upregulated proteinase expression in rice seeds. Plant J. 2003, 34, 635–645. [Google Scholar] [CrossRef]
- Thomas, M.S.; Flavell, R.B. Identification of an enhancer element for the endosperm-specific expression of high molecular weight glutenin. Plant Cell 1990, 2, 1171–1180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lessard, P.A.; Allen, R.D.; Bernier, F.; Crispino, J.D.; Fujiwara, T.; Beachy, R.N. Multiple nuclear factors interact with upstream sequences of differentially regulated beta-conglycinin genes. Plant Mol. Biol. 1991, 16, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.L.; Chandrasekharan, M.B.; Hall, T.C.; Crowe, A.J. Sequence and spacing of TATA box elements are critical for accurate initiation from the beta-phaseolin promoter. J. Biol. Chem. 2004, 279, 8102–8110. [Google Scholar] [CrossRef] [PubMed]
- Kawakatsu, T.; Hirose, S.; Yasuda, H.; Takaiwa, F. Reducing rice seed storage protein accumulation leads to changes in nutrient quality and storage organelle formation. Plant Physiol. 2010, 154, 1842–1854. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-Y.; Yoon, U.-H.; Lim, S.-H.; Kim, Y.-M. Effects of Reduced Prolamin on Seed Storage Protein Composition and the Nutritional Quality of Rice. Int. J. Mol. Sci. 2013, 14, 17073–17084. [Google Scholar] [CrossRef]
- Kaur, M.; Tak, Y.; Bhatia, S.; Asthir, B.; Lorenzo, J.M.; Amarowicz, R. Crosstalk during the Carbon–Nitrogen Cycle That Interlinks the Biosynthesis, Mobilization and Accumulation of Seed Storage Reserves. Int. J. Mol. Sci. 2021, 22, 12032. [Google Scholar] [CrossRef]
- Ellenberger, T. Getting a grip on DNA recognition: Structures of the basic region leucine zipper, and the basic region helix-loop-helix DNA-binding domains. Curr. Opin. Struct. Biol. 1994, 4, 12–21. [Google Scholar] [CrossRef]
- Nijhawan, A.; Jain, M.; Tyagi, A.K.; Khurana, J.P. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol. 2008, 146, 333–350. [Google Scholar] [CrossRef]
- Ding, J.; Chen, X.; Karim, H.; Carlos, G.; Harwood, W.; Tang, H.; Dong, H.; Xu, Q.; Zhang, Y.; Jiang, Y.; et al. Genome-wide identification of the basic leucine zipper transcription factor genes related to starch synthesis in wheat (Triticum aestivum L.). Plant Growth Regul. 2024, 103, 409–423. [Google Scholar] [CrossRef]
- Kim, J.S.; Chae, S.; Jun, K.M.; Pahk, Y.M.; Lee, T.H.; Chung, P.J.; Kim, Y.K.; Nahm, B.H. Genome-wide identification of grain filling genes regulated by the OsSMF1 transcription factor in rice. Rice 2017, 10, 16. [Google Scholar] [CrossRef]
- Wu, C.; Washida, H.; Onodera, Y.; Harada, K.; Takaiwa, F. Quantitative nature of the Prolamin-box, ACGT and AACA motifs in a rice glutelin gene promoter: Minimal cis-element requirements for endosperm-specific gene expression. Plant J. 2000, 23, 415–421. [Google Scholar] [CrossRef]
- Yamamoto, M.P.; Onodera, Y.; Touno, S.M.; Takaiwa, F. Synergism between RPBF Dof and RISBZ1 bZIP Activators in the Regulation of Rice Seed Expression Genes. Plant Physiol. 2006, 141, 1694–1707. [Google Scholar] [CrossRef]
- Massari, M.E.; Murre, C. Helix-loop-helix proteins: Regulators of transcription in eucaryotic organisms. Mol. Cell Biol. 2000, 20, 429–440. [Google Scholar] [CrossRef]
- Zuo, Z.-F.; Lee, H.-Y.; Kang, H.-G. Basic Helix-Loop-Helix Transcription Factors: Regulators for Plant Growth Development and Abiotic Stress Responses. Int. J. Mol. Sci. 2023, 24, 1419. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, L.; Yu, Q.; Zhou, W.; Gou, X.; Li, J.; Hou, S. Multiple transcriptional factors control stomata development in rice. New Phytol. 2019, 223, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zong, X.; Ren, P.; Qian, Y.; Fu, A. Basic Helix-Loop-Helix (bHLH) Transcription Factors Regulate a Wide Range of Functions in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 7152. [Google Scholar] [CrossRef]
- Reyes, J.C.; Muro-Pastor, M.I.; Florencio, F.J. The GATA Family of Transcription Factors in Arabidopsis and Rice. Plant Physiol. 2004, 134, 1718–1732. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. Abiotic Stresses Cause Differential Regulation of Alternative Splice Forms of GATA Transcription Factor in Rice. Front. Plant Sci. 2017, 8, 1944. [Google Scholar] [CrossRef]
- Wu, W.; Dong, X.; Chen, G.; Lin, Z.; Chi, W.; Tang, W.; Yu, J.; Wang, S.; Jiang, X.; Liu, X.; et al. The elite haplotype OsGATA8-H coordinates nitrogen uptake and productive tiller formation in rice. Nat. Genet. 2024, 56, 1516–1526. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Y.; Wu, C.; Yang, G.; Li, Y.; Zheng, C. Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genom. 2008, 9, 44. [Google Scholar] [CrossRef]
- Fauteux, F.; Strömvik, M.V. Seed storage protein gene promoters contain conserved DNA motifs in Brassicaceae, Fabaceae and Poaceae. BMC Plant Biol. 2009, 9, 126. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, J.; Xia, Y.; Zhang, L.; Du, H. Evolution and functional diversification of R2R3-MYB transcription factors in plants. Hortic. Res. 2022, 9, uhac058. [Google Scholar] [CrossRef]
- Zhang, H.C.; Gong, Y.H.; Tao, T.; Lu, S.; Zhou, W.Y.; Xia, H.; Zhang, X.Y.; Yang, Q.Q.; Zhang, M.Q.; Hong, L.M.; et al. Genome-wide identification of R2R3-MYB transcription factor subfamily genes involved in salt stress in rice (Oryza sativa L.). BMC Genom. 2024, 25, 797. [Google Scholar] [CrossRef]
- Zhou, D.X.; Li, Y.F.; Rocipon, M.; Mache, R. Sequence-specific interaction between S1F, a spinach nuclear factor, and a negative cis-element conserved in plastid-related genes. J. Biol. Chem. 1992, 267, 23515–23519. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.X.; Bisanz-Seyer, C.; Mache, R. Molecular cloning of a small DNA binding protein with specificity for a tissue-specific negative element within the rps1 promoter. Nucleic Acids Res. 1995, 23, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.S.; Lee, K.H.; Min, W.K.; Lee, S.J.; Song, J.T.; Seo, H.S. The transmembrane domain of the rice small protein OsS1Fa1 is responsible for subcellular localization and drought tolerance. Plant Biol. 2024, 26, 1079–1087. [Google Scholar] [CrossRef]
- Niu, H.; Xia, P.; Hu, Y.; Zhan, C.; Li, Y.; Gong, S.; Li, Y.; Ma, D. Genome-wide identification of ZF-HD gene family in Triticum aestivum: Molecular evolution mechanism and function analysis. PLoS ONE 2021, 16, e0256579. [Google Scholar] [CrossRef] [PubMed]
- Bollier, N.; Gonzalez, N.; Chevalier, C.; Hernould, M. Zinc Finger-Homeodomain and Mini Zinc Finger proteins are key players in plant growth and responses to environmental stresses. J. Exp. Bot. 2022, 73, 4662–4673. [Google Scholar] [CrossRef]
- Han, M.; Jin, X.; Yao, W.; Kong, L.; Huang, G.; Tao, Y.; Li, L.; Wang, X.; Wang, Y. A Mini Zinc-Finger Protein (MIF) from Gerbera hybrida Activates the GASA Protein Family Gene, GEG, to Inhibit Ray Petal Elongation. Front. Plant Sci. 2017, 8, 1649. [Google Scholar] [CrossRef]
- Bollier, N.; Sicard, A.; Leblond, J.; Latrasse, D.; Gonzalez, N.; Gévaudant, F.; Benhamed, M.; Raynaud, C.; Lenhard, M.; Chevalier, C.; et al. At-MINI ZINC FINGER2 and Sl-INHIBITOR OF MERISTEM ACTIVITY, a Conserved Missing Link in the Regulation of Floral Meristem Termination in Arabidopsis and Tomato. Plant Cell 2018, 30, 83–100. [Google Scholar] [CrossRef]
- Li, Z.; Trick, H.N. Rapid method for high-quality RNA isolation from seed endosperm containing high levels of starch. Biotechniques 2005, 38. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Nijhawan, A.; Tyagi, A.K.; Khurana, J.P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2006, 345, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Report. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, J.-Y.; Lee, T.; Lee, Y.-H.; Kim, S.-H.; Kang, S.-H.; Yoon, U.-H.; Ha, S.-H.; Lim, S.-H. The suppression of the glutelin storage protein gene in transgenic rice seeds results in a higher yield of recombinant protein. Plant Biotechnol. Rep. 2012, 6, 347–353. [Google Scholar] [CrossRef]
- Dedow, L.K.; Oren, E.; Braybrook, S.A. Fake news blues: A GUS staining protocol to reduce false-negative data. Plant Direct 2022, 6, e367. [Google Scholar] [CrossRef]
| Superclass | Class | Gene Name | MSU ID a | RAP Locus ID b | Functional Description | Reference |
|---|---|---|---|---|---|---|
| Zinc finger | GATA type | OsGATA8 | LOC_Os01g24070 | Os01g0343300 | Response salinity, drought stress, ABA | [21] |
| ZF-HD type | OsMIF1 | LOC_Os11g03420 | Os11g0128300 | |||
| OsMIF2 | LOC_Os12g03110 | Os12g0124500 | ||||
| C3H type | OsGZF1 | LOC_Os07g47240 | Os07g0668600 | Binding with the GluB-1 promoter and controls glutelin content | [18] | |
| Basic domains | Basic leucine zipper factors (bZIP) | OsbZIP58, RISBZ1 | LOC_Os07g08420 | Os07g0182000 | Regulate seed storage protein (SSP) and starch synthesis genes during grain filling stage | [16,17] |
| Basic helix–loop–helix factors (bHLH) | OsbHLH001, OsICE2 | LOC_Os01g70310 | Os01g0928000 | Response cold stress and regulate stomatal development | [22,23] | |
| S1Fa | S1Fa | OsS1Fa1 | LOC_Os04g33420 | Os04g0408900 | Response drought stress | [24] |
| OsS1Fa2 | LOC_Os04g33440 | Os04g0408700 | ||||
| MYB | R2R3 type | OsMYB24 | LOC_Os01g74590 | Os01g0977300 |
| NO. | Gene | Cis element | Consensus Sequence (5′-3′) | Description | Reference |
|---|---|---|---|---|---|
| 1 | OsMYB24 | ABREZMRAB28 | CCACGTGG | ABA and water–stress response element | [25] |
| 2 | OsMYB24 | O2F2BE2S1 | GCCACCTCAT | Seed-specific expression element | [26] |
| 3 | OsMYB24 | RYREPEATGMGY2 | CATGCAT | Seed-specific expression element | [27] |
| 4 | OsMYB24 | RYREPEATVFLEB4 | CATGCATG | ABA response and Seed storage protein expression element | [28] |
| 5 | OsbZIP58 | AMYBOX1 | TAACARA | Seed-specific expression element | [29] |
| 6 | OsbZIP58 | AUXRETGA2GMGH3 | TGACGTGGC | Auxin-responsive element | [30] |
| 7 | OsbZIP58 | GARE1OSREP1 | TAACAGA | Seed-specific expression and GA-responsive element | [31] |
| 8 | OsGATA8 | -300ELEMENT | TGHAAARK | Seed storage protein expression element | [32] |
| 9 | OsGZF1 | SEF4MOTIFGM7S | RTTTTTR | Seed storage protein expression element | [33] |
| 10 | OsICE2 | TATABOX1 | CTATAAATAC | TATA box-like and Seed-specific expression element | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, J.; Lee, J.-Y.; Cho, K.; Park, S.; Lee, K.; Kim, D.-K.; Han, O. Transcriptome-Based Identification of Novel Transcription Factors Regulating Seed Storage Proteins in Rice. Plants 2025, 14, 2791. https://doi.org/10.3390/plants14172791
So J, Lee J-Y, Cho K, Park S, Lee K, Kim D-K, Han O. Transcriptome-Based Identification of Novel Transcription Factors Regulating Seed Storage Proteins in Rice. Plants. 2025; 14(17):2791. https://doi.org/10.3390/plants14172791
Chicago/Turabian StyleSo, Jinpyo, Jong-Yeol Lee, Kyoungwon Cho, Suchan Park, Kyuhee Lee, Don-Kyu Kim, and Oksoo Han. 2025. "Transcriptome-Based Identification of Novel Transcription Factors Regulating Seed Storage Proteins in Rice" Plants 14, no. 17: 2791. https://doi.org/10.3390/plants14172791
APA StyleSo, J., Lee, J.-Y., Cho, K., Park, S., Lee, K., Kim, D.-K., & Han, O. (2025). Transcriptome-Based Identification of Novel Transcription Factors Regulating Seed Storage Proteins in Rice. Plants, 14(17), 2791. https://doi.org/10.3390/plants14172791








