Genetic Polymorphisms of Invasive Ambrosia artemisiifolia L. in Localities of Slovakia Accessed by Bet v 1 Homologs Differ in Discrimination of Accessions and Show Their Outcrossing in This Area
Abstract
1. Introduction
2. Results
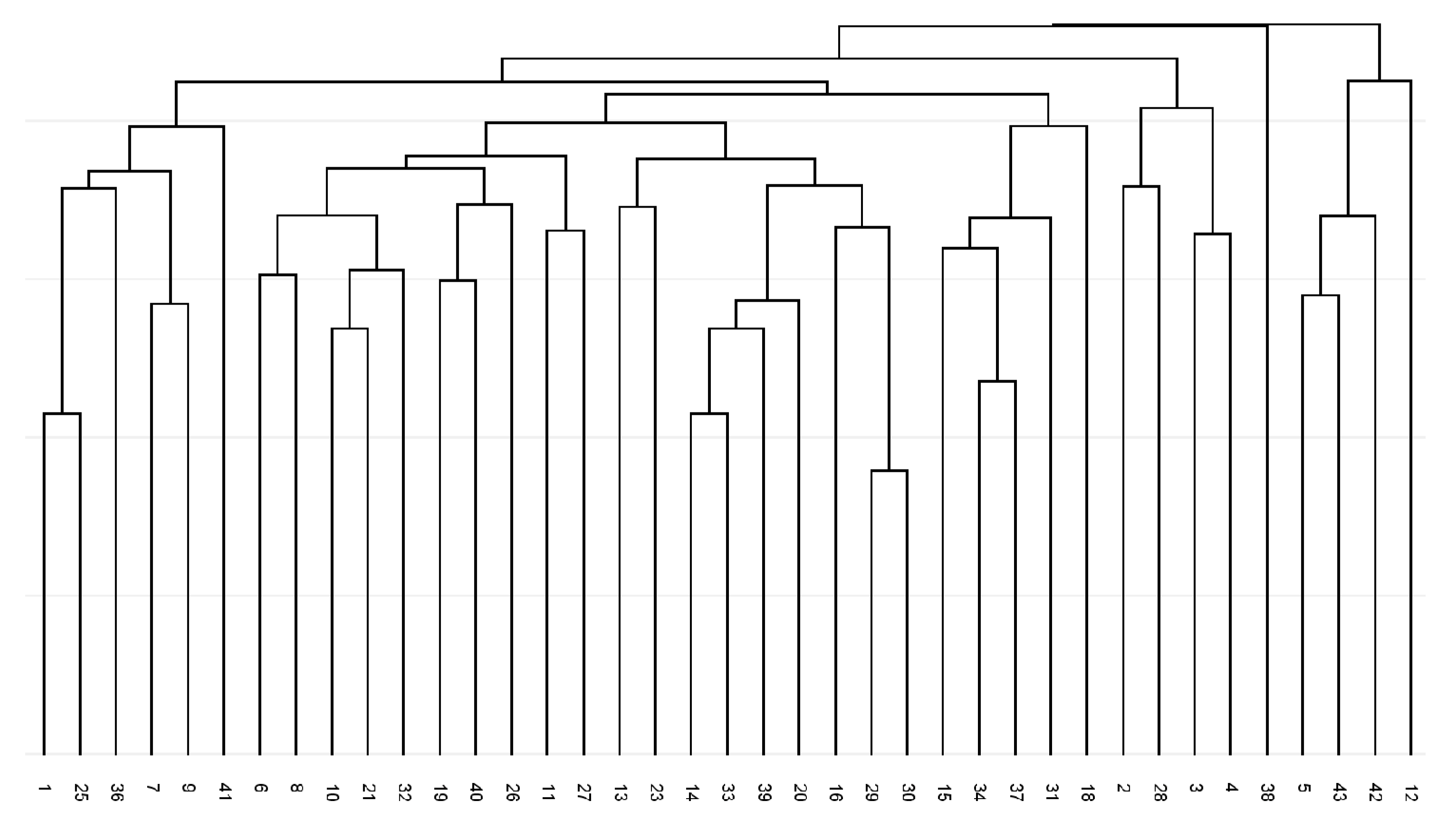
2.1. BBAP Fingerprint Diversity Using the Degenerated Forward Primer
2.2. BBAP Fingerprint Diversity Using the Non-Degenerated Variants of Forward Primer
2.3. Comparison of Obtained BBAP Fingerprint Profiles for Individual Primer Combinations
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. DNA Extraction
4.3. PCR Amplifications and Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AN | Average number of amplified alleles per accession |
| Amb a 1 | Ambrosia artemisiifolia 1 main pollen allergen |
| BBAP | Bet v 1-based amplified polymorphism |
| Bet v 1 | Betula verrucosa 1 main pollen allergen |
| CI | Confidence interval |
| D | Discriminating power |
| DNA | Deoxyribonucleic acid |
| IgE | Immunoglobulin E |
| MI | Marker index |
| PCR | Polymerase chain reaction |
| PIC | Polymorphism information content |
| PR-10 | Pathogen-related protein class 10 |
| SSR | Single sequence repeats |
| UPGMA | Unweighted Pair Group Method with Arithmetic Mean |
References
- Govaerts, R. The World Checklist of Vascular Plants (WCVP). Royal Botanic Gardens, Kew. Checklist Dataset. Available online: https://doi.org/10.15468/6h8ucr (accessed on 15 March 2025).
- Allard, H.A. The North American ragweed and their occurrence in other parts of the world. Science 1943, 98, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Jehlík, V.; Hejný, S.; Kropáč, Z.; Lhotská, M.; Kopecký, K.; Slavík, B.; Svobodová, Z. Cizí Expanzivní Plevele České Republiky a Slovenské Republiky/The Foreign Expansive Weeds of the Czech Republic and the Slovak Republic, 1st ed.; Academia: Praha, Czech Republic, 1998; pp. 165–179. [Google Scholar]
- Umankal, B.; Girod, C.; Fried, G.; Bretagnolle, F.; Chauvel, B. Can the large ecological amplitude of Ambrosia artemisiifolia explain its invasive success in France? Weed Res. 2008, 48, 349–359. [Google Scholar] [CrossRef]
- Iamonico, D. Ambrosia Artemisiifolia (Common Ragweed). Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.4691 (accessed on 24 June 2025).
- Schaffner, U.; Steinbach, S.; Sun, Y.; Skjøth, C.A.; de Weger, L.A.; Lommen, S.T.; Augustinus, B.A.; Bonini, M.; Karrer, G.; Šikoparija, B.; et al. Biological weed control to relieve millions from Ambrosia allergies in Europe. Nat. Commun. 2020, 11, e1745. [Google Scholar] [CrossRef]
- Hrabovský, M.; Kubalová, S.; Kanka, R. The impact of changing climate on the spread of the widely expanding species Ambrosia artemisiifolia in Slovakia. Theor. Appl. Clim. 2024, 155, 6137–6150. [Google Scholar] [CrossRef]
- Vitousek, P.M.; D’Antonio, C.; Loope, L.L.; Rejmánek, M.; Westbrooks, R. Introduced species: A significant component of human-caused global change. N. Z. J. Ecol. 1997, 21, 1–16. [Google Scholar]
- Lambdon, P.W.; Pyšek, P.; Basnou, C.; Hejda, M.; Arianoutson, M.; Essel, F.; Jarošík, V.; Pergl, J.; Winter, M.; Anastasiu, P.; et al. Alien flora of Europe: Species diversity, temporal trends, geographical patterns and research needs. Preslia 2008, 80, 101–149. [Google Scholar]
- Dickerson, J.C.T.; Sweet, R.D. Common ragweed ecotypes. Weed Sci. 1971, 19, 64–69. [Google Scholar] [CrossRef]
- Gadermaier, G.; Dedic, A.; Obermeyer, G.; Frank, S.; Himly, M.; Ferreirova, F. Biology of weed pollen allergens. Curr. Allergy Asthma Rep. 2004, 4, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Basset, I.J.; Crompton, C.W. The biology of Canadian weeds, Ambrosia artemisiifolia L. and A. psilostachya DC. J. Plant Sci. 1975, 55, 463–476. [Google Scholar] [CrossRef]
- Fernández-Llamazares, Á.; Belmonte, J.; Alarcón, M.; López-Pacheco, M. Ambrosia L. in catalonia (NE Spain): Expansion and aerobiology of a new bioinvader. Aerobiologia 2012, 28, 435–451. [Google Scholar] [CrossRef]
- Brandes, D.; Nitzsche, J. Verbreitung, Ökologie und Soziologie von Ambrosia artemisiifolia L. in Mitteleuropa. Tuexenia 2007, 27, 167–194. [Google Scholar]
- Jäger, S.; Litschauer, R. Ragweed (Ambrosia) in Austria. In Ragweed in Europe, Proceedings of the 6th International Congress on Aerobiology, Satellite Symposium, Perugia, Italy, 31 August–5 September 1998; Spieksma, F.T.H., Ed.; Alk-Abelló A/S: Horsholm, Denmark, 1998; pp. 6–8. [Google Scholar]
- Cvitanović, S.; Znaor, L.; Kanceljak-Macan, B.; Macan, J.; Gudelj, I.; Grbić, D. Allergic rhinitis and asthma in Southern Croatia: Impact of sensitization to Ambrosia elatior. Croat. Med. J. 2007, 48, 68–75. [Google Scholar]
- Csontos, P.; Vitalos, M.; Barina, Z.; Kiss, L. Early distribution and spread of Ambrosia artemisiifolia in Central and Eastern Europe. Bot. Helv. 2010, 120, 75–78. [Google Scholar] [CrossRef]
- Jäger, S. Ragweed (Ambrosia) sensitisation rates correlate with the amount of inhaled airborne pollen. A 14-year study in Vienna, Austria. Aerobiologia 2000, 16, 149–153. [Google Scholar] [CrossRef]
- Makra, L.; Juhász, M.; Béczi, R.; Borsos, E. The history and impacts of airborne Ambrosia (Asteraceae) pollen in Hungary. Grana 2005, 44, 57–64. [Google Scholar] [CrossRef]
- Chauvel, B.; Dessaint, F.; Cardinal-Legrand, C.; Bretagnolle, F. The historical spread of Ambrosia artemisiifolia L. in France from herbarium records. J. Biogeogr. 2006, 33, 665–673. [Google Scholar] [CrossRef]
- Smith, M.; Cecchi, L.; Skjøth, C.A.; Karrer, G.; Šikoparija, B. Common ragweed: A threat to environmental health in Europe. Environ. Int. 2013, 61, 115–126. [Google Scholar] [CrossRef]
- White, J.F.; Bernstein, D.I. Key pollen allergens in North America. Ann. Allergy Asthma Immiunol. 2003, 91, 425–435. [Google Scholar] [CrossRef]
- Chan-Yeung, M.; Anthonisen, N.R.; Becklake, M.R.; Browie, D.; Sonia Buist, A.; Dimich-Ward, H.; Ernst, P.; Sears, M.R.; Siersted, H.C.; Sweet, L.; et al. Geographical variations in the prevalence of atopic sensitization in six study sites across Canada. Allergy 2010, 65, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Burbach, G.J.; Heinzerling, L.M.; Rohnelt, C.; Bergmann, K.C.; Behrendt, H.; Zuberbier, T. Ragweed sensitization in Europe—GA(2)LEN study suggests increasing prevalence. Allergy 2009, 64, 664–665. [Google Scholar] [CrossRef] [PubMed]
- Pablos, I.; Egger, M.; Vejvar, E.; Reichl, V.; Briza, P.; Zennaro, D.; Rafaiani, C.; Pickl, W.; Bohle, B.; Mari, A.; et al. Similar Allergenicity to Different Artemisia Species Is a Consequence of Highly Cross-Reactive Art v 1-Like Molecules. Medicina 2019, 55, 504. [Google Scholar] [CrossRef] [PubMed]
- Hrubiško, M. Polinóza—Aktuálny problem aj v XXI. Storočí. Časť III: Poradie a skrížené reactivity alergénnych druhov stromov, tráv a bylín podľa ich klinického významu/Pollinosis—Actual problem also in XXI. Century. Part III: Sequence and cross reactivity of the tree, grass and plant allergen species by their clinical significance. Clin. Immunol. Allergol. 1998, 2, 9–17. [Google Scholar]
- D’Amato, G.; Cecchi, L.; Bonini, S.; Nunes, C.; Annesi-Maesano, I.; Behrendt, H.; Liccardi, G.; Popov, T.; Van Cauwenberge, P. Allergenic pollen and pollen allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef]
- Šaulienė, I.; Veriankaitė, L.; Šaulys, A. Biometrical assessment of ragweed (Ambrosia artemisiifolia L.). Zemdirbystė 2012, 99, 319–326. [Google Scholar]
- Bergmann, K.C.; Werchan, D.; Zuberbier, M.M. The threshold value of Ambrosia pollen inducing acute nasal reactions is very low. Allergo J. 2008, 17, 375–376. [Google Scholar]
- Laaidi, K.; Laaidi, M. Airborne pollen of Ambrosia in Burgundy (France) 1996–1997. Aerobiologia 1999, 15, 65–69. [Google Scholar] [CrossRef]
- Ihler, F.; Canis, M. Ragweed-induced allergic rhinoconjunctivitis: Current and emerging treatment options. J. Asthma Allergy 2015, 8, 15–24. [Google Scholar] [CrossRef]
- Sikoparija, B.; Skjøth, C.A.; Celenk, S.; Testoni, C.; Abramidze, T.; Alm Kübler, K.; Belmonte, J.; Berger, U.; Bonini, M.; Charalampopoulos, A.; et al. Spatial and Temporal variations in airborne Ambrosia pollen in Europe. Aerobiologia 2017, 33, 181–189. [Google Scholar] [CrossRef]
- Adolphson, C.; Goodfriend, L.; Gleich, G.J. Reactivity of ragweed allergens with IgE antibodies. Analyses by leukocyte Histamine release and the Radioallergosorbent test and determination of cross-reactivity. J. Allergy Clin. Immunol. 1978, 62, 197–210. [Google Scholar] [CrossRef]
- Wopfner, N.; Gadermaier, G.; Egger, M.; Asero, R.; Ebner, C.; Jahn-Schmid, B.; Ferreira, F. The spectrum of allergens in ragweed and mugwort pollen. Int. Arch. Allergy Immunol. 2005, 138, 337–346. [Google Scholar] [CrossRef]
- Oberhuber, C.; Ma, Y.; Wopfner, N.; Gadermaier, G.; Dedic, A.; Niggemann, B.; Maderegger, B.; Gruber, P.; Ferreira, F.; Scheiner, O.; et al. Prevalence of IgE-binding to Art V 1, Art V 4 and amb a 1 in mugwort-allergic patients. Int. Arch. Allergy Immunol. 2008, 145, 94–101. [Google Scholar] [CrossRef]
- Weber, R.W. Patterns of pollen cross-allergenicity. J. Allergy Clin. Immunol. 2003, 112, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Singh, R.P.; Kushwaha, G.S.; Iqbal, N.; Singh, A.; Kaushik, S.; Singh, T.P. Current overview of allergens of plant pathogenesis related protein families. Sci. World J. 2014, 2014, 543195. [Google Scholar] [CrossRef]
- Führer, S.; Kamenik, A.S.; Zeindl, R.; Nothegger, B.; Hofer, F.; Reider, N.; Tollinger, M. Inverse relation between structural flexibility and IgE reactivity of Cor a 1 hazelnut allergens. Sci. Rep. 2021, 11, 4173. [Google Scholar] [CrossRef]
- Žiarovská, J.; Urbanová, L.; Montero-Torres, J.; Kováčik, A.; Klongová, L.; Bharati, R.; Romero-Ortega, S.; Fernández-Cusimamani, E.; Leuner, O. Polymorphism of Bolivian accessions of Arachis hypogaea L. revealed by allergen coding DNA markers. Plant Soil Environ. 2023, 69, 615–627. [Google Scholar] [CrossRef]
- Žiarovská, J.; Zeleňáková, L. Application of genomic data for PCR Screening of BET v 1 conserved sequence in clinically relevant plant species. In Systems Biology; Vlachakis, D., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Žiarovská, J.; Urbanová, L. Utilization of Bet v 1 homologs based amplified profile (BBAP) variability in allergenic plants fingerprinting. Biologia 2022, 77, 517–523. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Vilà, M.; Hulme, P. (Eds.) Impact of Biological Invasions on Ecosystem Services; Springer: Cham, Switzerland, 2017; Volume 12. [Google Scholar]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Bradshaw, C.J.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.-M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- Schindler, S.; Staska, B.; Adam, M.; Rabitsch, W.; Essl, F. Alien species and public health impacts in Europe: A literature review. NeoBiota 2015, 27, 1–23. [Google Scholar] [CrossRef]
- Essl, F.; Biró, K.; Brandes, D.; Broennimann, O.; Bullock, J.M.; Chapman, D.S.; Chauvel, B.; Dullinger, S.; Fumanal, B.; Guisan, A.; et al. Biological flora of the British Isles: Ambrosia artemisiifolia. J. Ecol. 2015, 103, 1069–1098. [Google Scholar] [CrossRef]
- Hamaoui-Laguel, L.; Vautard, R.; Liu, L.; Solmon, F.; Viovy, N.; Khvorostyanov, D.; Essl, F.; Chuine, I.; Colette, A.; Semenov, M.A.; et al. Effects of climate change and seed dispersal on airborne ragweed pollen loads in Europe. Nat. Clim. Change 2015, 5, 766–771. [Google Scholar] [CrossRef]
- Lake, I.R.; Jones, N.R.; Agnew, M.; Goodess, C.M.; Giorgi, F.; Hamaoui-Laguel, L.; Semenov, M.A.; Solomon, F.; Storkey, J.; Vautard, R.; et al. Climate change and future pollen allergy in Europe. Environ. Health Persp. 2017, 125, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Brönnimann, O.; Roderick, G.K.; Poltavsky, A.; Lommen, S.T.; Müller‐Schärer, H. Climatic suitability ranking of biological control candidates: A biogeographic approach for ragweed management. Eur. Ecosphere 2017, 8, e01731. [Google Scholar] [CrossRef]
- Genton, B.J.; Shykoff, J.A.; Giraud, T. High genetic diversity in French invasive populations of common ragweed, Ambrosia artemisiifolia, as a result of multiple sources of introduction. Mol. Ecol. 2005, 14, 4275–4285. [Google Scholar] [CrossRef]
- Chun, Y.J.; Fumanal, B.; Laitung, B.; Bretagnolle, F. Gene flow and population admixture as the primary post-invasion processes in common ragweed (Ambrosia artemisiifolia) populations in France. New Phytol. 2010, 185, 1100–1107. [Google Scholar] [CrossRef]
- Meyer, L.; Causse, R.; Pernin, F.; Scalone, R.; Bailly, G.; Chauvel, B.; Délye, C.; Le Corre, V. New gSSR and EST-SSR markers reveal high genetic diversity in the invasive plant Ambrosia artemisiifolia L. and can be transferred to other invasive Ambrosia species. PLoS ONE 2017, 10, e0176197. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Schierenbeck, K. Hybridization as a stimulus for the evolution of invasiveness in plants? Proc. Natl. Acad. Sci. USA 2000, 97, 7043–7050. [Google Scholar] [CrossRef]
- Chun, Y.J.; Corre, V.; Bretagnolle, F. Adaptive divergence for a fitness-related trait among invasive Ambrosia artemisiifolia populations in France. Mol. Ecol. 2011, 20, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Battlay, P.; Wilson, J.; Bieker, V.C.; Lee, C.; Prapas, D.; Petersen, B.; Craig, S.; van Boheemen, L.; Scalone, R.; de Silva, N.P.; et al. Large haploblocks underlie rapid adaptation in the invasive weed Ambrosia artemisiifolia. Nat. Commun. 2023, 14, 1717. [Google Scholar] [CrossRef] [PubMed]
- Hrabovský, M.; Kubalová, S.; Mičieta, K.; Ščevková, J. Environmental impacts on intraspecific variation in Ambrosia artemisiifolia genome size in Slovakia, Central Europe. Environ. Sci. Pollut. Res. Int. 2024, 31, 33960–33974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Breiteneder, H.; Ebner, C. Atopic allergens of plant foods. Curr. Opin. Allergy Clin. Immunol. 2001, 1, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.; Sato, K.; Abe, Y.; Katagiri, M. Sequential IgE epitope analysis of a birch pollen allergen (Bet v1) and an apple allergen (Mal d 1). Allergol. Int. 2001, 50, 57–62. [Google Scholar] [CrossRef]
- King, T.P.; Norman, P.S.; Connell, J.T. Isolation and characterization of allergens from ragweed pollen. Biochem. 1964, 3, 458–468. [Google Scholar] [CrossRef]
- Gadermaier, G.; Wopfner, N.; Wallner, M.; Egger, M.; Didierlaurent, A.; Regl, G.; Aberger, F.; Lang, R.; Ferreira, F.; Hawranek, T. Array-based profiling of ragweed and mugwort pollen allergens. Allergy 2008, 63, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.B.; Heslop-Harrison, J. Pollen-wall proteins: Localization of antigenic and allergenic proteins in the pollen-grains walls of Ambrosia spp. (ragweeds). Cytobios 1971, 4, 49–54. [Google Scholar]
- Tegart, L.J.; Johnston, F.H.; Borchers Arriagada, N.; Workman, A.; Dickinson, J.L.; Green, B.J.; Jones, P.J. Pollen potency’: The relationship between atmospheric pollen counts and allergen exposure. Aerobiologia 2021, 37, 825–841. [Google Scholar] [CrossRef]
- Speváková, I.; Urbanová, L.; Kyseľ, M.; Bilčíková, J.; Farkasová, S.; Žiarovská, J. BBAP amplification profiles of apple varieties. Sci. Technol. Innov. 2021, 13, 1–6. [Google Scholar] [CrossRef]
- Urbanová, L.; Farkasová, S.; Speváková, I.; Kyseľ, M.; Šimora, V.; Kačániová, M.; Žiarovská, J. DNA-Based Variability of Length Polymorphism of Plant Allergens Coding Genes Homologs in Selected Lamiaceae Herbs. OBM Genet. 2024, 8, 263. [Google Scholar] [CrossRef]
- Moravčíková, D.; Žiarovská, J. Variability of Genomic Profile of ypr-10 Gene in Citrus sinensis L. Osbeck. Biol. Life Sci. Forum. 2024, 30, 2. [Google Scholar] [CrossRef]
- Saqib, S.; Ullah, F.; Omollo, W.O.; Liu, Y.; Tao, H.Y.; Zaman, W.; Temur, A.; Liu, B.; Lai, Y.; Chen, Z.; et al. Identifying hotspots and climate drivers of alien plant species for conservation prioritization across the Pan-Himalaya. Biol. Conserv. 2025, 302, 110994. [Google Scholar] [CrossRef]
- Yurukova-Grancharova, P.; Yankova-Tsvetkova, E.; Baldjiev, G.; Vladimirov, V. Reproductive characteristics of Ambrosia artemisiifolia and Iva xantifolia—Two invasive alien species in Bulgaria. Comptes Rendus L’académie Bulg. Sci. 2015, 68, 853–862. [Google Scholar]
- Fu, L.; Cherayil, B.; Shi, H.; Wang, Y.; Zhu, Y. Species and structure of food allergens: Epitopes and cross-reactivity. In Food Allergy: From Molecular Mechanisms to Control Strategies; Fu, L., Cherayil, B., Shi, H., Wang, Y., Zhu, Y., Eds.; Springer: Singapore, 2019; pp. 13–39. ISBN 978-981-13-6927-8. [Google Scholar]
- Urbanová, L.; Žiarovská, J. Variability of DNA based amplicon profiles generated by Bet v 1 homologous among different vegetable species. Acta Fytotech. Zootech. 2021, 24, 1–6. [Google Scholar] [CrossRef]
- Heras, J.; Domínguez, C.; Mata, E.; Pascual, V.; Lozano, C.; Torres, C.; Zarazaga, M. GelJ—A tool for analyzing DNA fingerprint gel images. BMC Bioinform. 2015, 16, 270. [Google Scholar] [CrossRef] [PubMed]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. iMEC: Online Marker Efficiency Calculator. Appl. Plant Sci. 2018, 24, e01159. [Google Scholar] [CrossRef] [PubMed]










| Forward Primer | PIC | AN | MI | D |
|---|---|---|---|---|
| F degenerate | 0.184 | 6.6 | 0.00056 | 0.99 |
| F1 non-degenerate | 0.25 | 9.67 | 0.0013 | 0.97 |
| F2 non-degenerate | 0.26 | 10.28 | 0.299 | 0.24 |
| F3 non-degenerate | 0.37 | 8.81 | 0.41 | 0.38 |
| F4 non-degenerate | 0.16 | 3.95 | 0.0004 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klongová, L.; Kováčik, A.; Štefúnová, V.; Tóthová, M.; Žiarovská, J. Genetic Polymorphisms of Invasive Ambrosia artemisiifolia L. in Localities of Slovakia Accessed by Bet v 1 Homologs Differ in Discrimination of Accessions and Show Their Outcrossing in This Area. Plants 2025, 14, 2790. https://doi.org/10.3390/plants14172790
Klongová L, Kováčik A, Štefúnová V, Tóthová M, Žiarovská J. Genetic Polymorphisms of Invasive Ambrosia artemisiifolia L. in Localities of Slovakia Accessed by Bet v 1 Homologs Differ in Discrimination of Accessions and Show Their Outcrossing in This Area. Plants. 2025; 14(17):2790. https://doi.org/10.3390/plants14172790
Chicago/Turabian StyleKlongová, Lucia, Adam Kováčik, Veronika Štefúnová, Monika Tóthová, and Jana Žiarovská. 2025. "Genetic Polymorphisms of Invasive Ambrosia artemisiifolia L. in Localities of Slovakia Accessed by Bet v 1 Homologs Differ in Discrimination of Accessions and Show Their Outcrossing in This Area" Plants 14, no. 17: 2790. https://doi.org/10.3390/plants14172790
APA StyleKlongová, L., Kováčik, A., Štefúnová, V., Tóthová, M., & Žiarovská, J. (2025). Genetic Polymorphisms of Invasive Ambrosia artemisiifolia L. in Localities of Slovakia Accessed by Bet v 1 Homologs Differ in Discrimination of Accessions and Show Their Outcrossing in This Area. Plants, 14(17), 2790. https://doi.org/10.3390/plants14172790







