Optimising Olive Leaf Phenolic Compounds: Cultivar and Temporal Interactions
Abstract
1. Introduction
2. Results
2.1. Leaf Phenolic Content
2.2. Olive Leaf Nutrient Concentrations
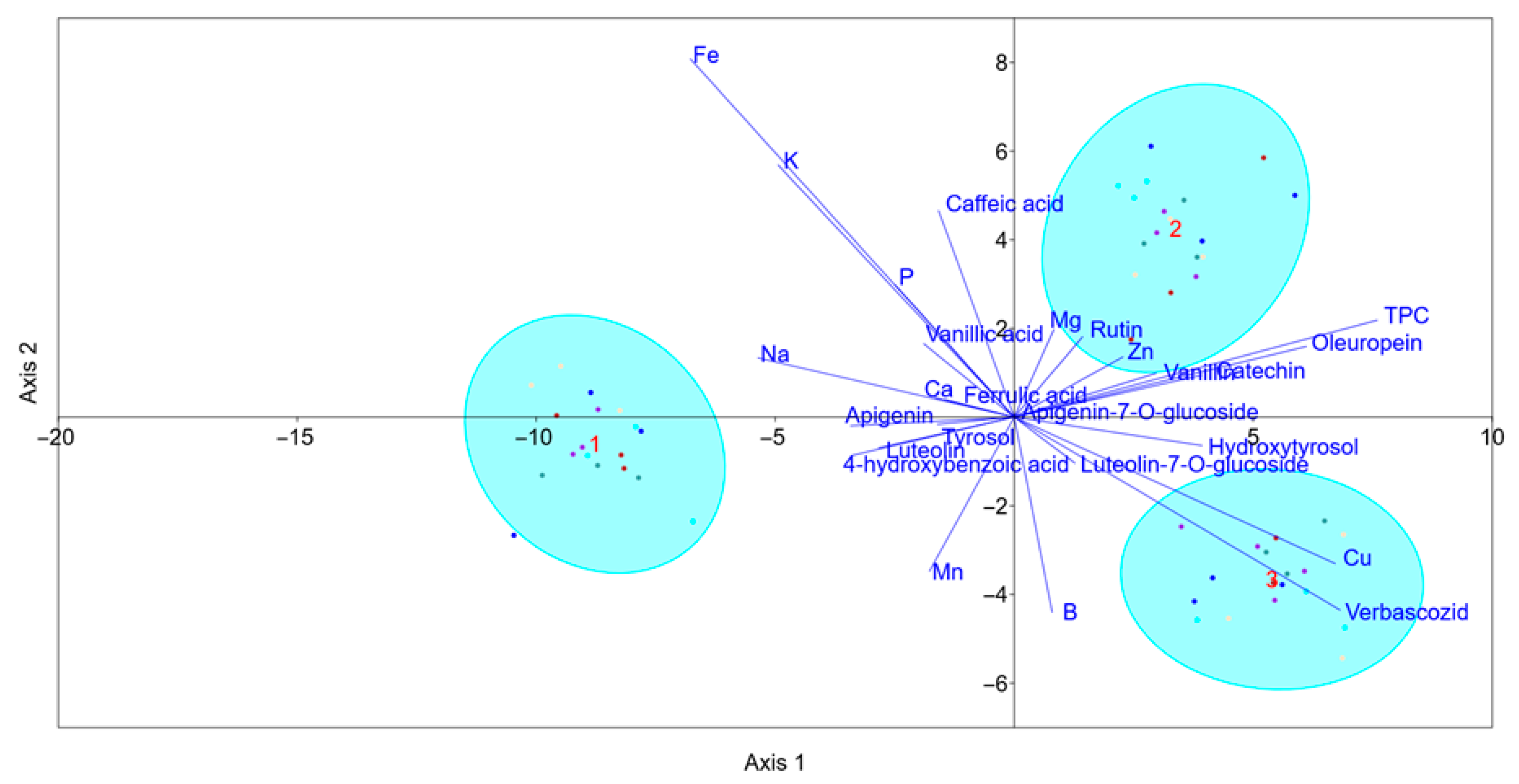
2.3. Separation of Cultivars and Sampling Periods Based on Linear Discriminant Analysis (LDA)
2.4. Analysis of Potential Links Between Phenolic Compounds and Nutrients Using Pearson’s Correlation Matrix
3. Discussion
4. Materials and Methods
4.1. Olive Leaf Sampling
4.2. Chemicals and Standards
4.3. High-Performance Liquid Chromatography (HPLC) and Elemental Analysis
4.4. Statistical Analysis
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Elkelish, A.; Hussein, S.; Warrad, M.; et al. Valorizing the usage of olive leaves, bioactive compounds, biological activities, and food applications: A comprehensive review. Front. Nutr. 2022, 9, 1008349. [Google Scholar] [CrossRef]
- Abaza, L.; Ben Youssef, N.; Manai, H.; Mahjoub Haddada, F.; Methenni, K.; Zarrouk, M. Chétoui olive leaf extracts: Influence of the solvent type on phenolics and antioxidant activities. Grasas Aceites 2011, 62, 96–104. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Moreno, R.; García-Creus, M. Seasonal changes of mineral nutrients in olive leaves during the alternate-bearing cycle. Sci. Hortic. 1999, 82, 25–45. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Cukrov, M.; Ninković, V.; Maslov Bandić, L.; Marcelić, Š.; Palčić, I.; Franić, M.; Žurga, M.; Majetić Germek, V.; Lukić, I.; Lemić, D.; et al. Silicon-mediated modulation of olive leaf phytochemistry: Genotype-specific and stress-dependent responses. Plants 2025, 14, 1282. [Google Scholar] [CrossRef] [PubMed]
- Kubes, J.; Hnilicka, F.; Vachova, P.; Kudrna, J.; Tunklova, B.; Mrkacek, M.; Rygl, T. Changes in Secondary Metabolites Content and Antioxidant Enzymes Activity in Leaves of Two Prunus avium L. Genotypes During Various Phenological Phases. Life 2024, 14, 1567. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, X. A Comprehensive Review of Phenolic Compounds in Horticultural Plants. Int. J. Mol. Sci. 2025, 26, 5767. [Google Scholar] [CrossRef]
- Borghini, F.; Tamasi, G.; Loiselle, S.A.; Baglioni, M.; Ferrari, S.; Bisozzi, F.; Costantini, S.; Tozzi, C.; Riccaboni, A.; Rossi, C. Phenolic profiles in olive leaves from different cultivars in Tuscany and their use as a marker of varietal and geographical origin on a small scale. Molecules 2024, 29, 3617. [Google Scholar] [CrossRef]
- Gagour, J.; Ibourki, M.; El Antari, A.; Sakar, E.H.; Aissa, R.; Giuffrè, A.M.; Laknifli, A.; Gharby, S. Leaf mineral profiling and its correlation with oil physicochemical traits from four olive (Olea europaea L.) cultivars grown in Morocco as affected by olive ripening stages. Eur. Food Res. Technol. 2024, 250, 1443–1456. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Trivandrum, India, 2006; pp. 23–67. [Google Scholar]
- Del Río, J.A.; Báidez, A.G.; Botía, J.M.; Ortuno, A. Enhancement of phenolic compounds in olive plants (Olea europaea L.) and their influence on resistance against Phytophthora sp. Food Chem. 2003, 83, 75–78. [Google Scholar] [CrossRef]
- Pasković, I.; Soldo, B.; Talhaoui, N.; Palčić, I.; Brkljača, M.; Koprivnjak, O.; Germek, V.M.; Ban, D.; Klanjac, J.; Franić, M.; et al. Boron foliar application enhances oleuropein level and modulates volatile compound composition in olive leaves. Sci. Hortic. 2019, 257, 108688. [Google Scholar] [CrossRef]
- Vizzarri, V.; Ienco, A.; Benincasa, C.; Perri, E.; Pucci, N.; Cesari, E.; Novellis, C.; Rizzo, P.; Pellegrino, M.; Zaffina, F.; et al. Phenolic Extract from Olive Leaves as a Promising Endotherapeutic Treatment against Xylella fastidiosa in Naturally Infected Olea europaea (var. europaea) Trees. Biology 2023, 12, 1141. [Google Scholar] [CrossRef]
- Báidez, A.G.; Gómez, P.; Del Río, J.A.; Ortuño, A. Antifungal capacity of major phenolic compounds of Olea europaea L. against Phytophthora megasperma Drechsler and Cylindrocarpon destructans (Zinssm.) Scholten. Physiol. Mol. Plant Pathol. 2006, 69, 224–229. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, J.A.; Moya-Laraño, J.; Poveda, J.; Lino-Neto, T.; Baptista, P. Deciphering plant health status: The link between secondary metabolites, fungal community and disease incidence in olive tree. Front. Plant Sci. 2023, 14, 1048762. [Google Scholar] [CrossRef]
- Ministry of Agriculture of the Republic of Croatia. Calculations for Measure 11: Organic Farming. 2022. Available online: https://ruralnirazvoj.hr/files/Kalkulacije_Mjera11_24032022_final.pdf (accessed on 29 March 2025).
- Kostenidou, E.; Kaltsonoudis, C.; Tsiflikiotou, M.; Louvaris, E.; Russell, L.M.; Pandis, S.N. Burning of olive tree branches: A major organic aerosol source in the Mediterranean. Atmos. Chem. Phys. 2013, 13, 8797–8811. [Google Scholar] [CrossRef]
- Treutter, D. Managing phenol contents in crop plants by phytochemical farming and breeding—Visions and constraints. Int. J. Mol. Sci. 2010, 11, 807–857. [Google Scholar] [CrossRef]
- Anttonen, M.J.; Hoppula, K.I.; Nestby, R.; Verheul, M.J.; Karjalainen, R.O. Influence of fertilization, mulch color, early forcing, fruit order, planting date, shading, growing environment, and genotype on the contents of selected phenolics in strawberry (Fragaria × ananassa Duch.) fruits. J. Agric. Food Chem. 2006, 54, 2614–2620. [Google Scholar] [CrossRef]
- Pasković, I.; Herak Ćustić, M.; Pecina, M.; Bronić, J.; Ban, D.; Radić, T.; Pošćić, F.; Jukić Špika, M.; Soldo, B.; Palčić, I.; et al. Manganese soil and foliar fertilization of olive plantlets: The effect on leaf mineral and phenolic content and root mycorrhizal colonization. J. Sci. Food Agric. 2019, 99, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Pasković, I.; Franić, M.; Polić Pasković, M.; Talhaoui, N.; Marcelić, Š.; Lukić, I.; Fredotović, Ž.; Žurga, P.; Major, N.; Goreta Ban, S.; et al. Silicon Foliar Fertilisation Ameliorates Olive Leaves Polyphenolic Compounds Levels and Elevates Its Potential towards Different Cancer Cells. Appl. Sci. 2024, 14, 4669. [Google Scholar] [CrossRef]
- Polić Pasković, M.; Vidović, N.; Lukić, I.; Žurga, M.; Majetić Germek, V.; Goreta Ban, S.; Kos, T.; Čoga, L.; Tomljanović, T.; Simonić-Kocijan, S.; et al. Phenolic potential of olive leaves from different Istrian cultivars in Croatia. Horticulturae 2023, 9, 594. [Google Scholar] [CrossRef]
- Polić Pasković, M.; Herak Ćustić, M.; Lukić, I.; Marcelić, Š.; Žurga, M.; Vidović, N.; Major, N.; Goreta Ban, S.; Pecina, M.; Ražov, J.; et al. Foliar nutrition strategies for enhancing phenolic and amino acid content in olive leaves. Plants 2024, 13, 3514. [Google Scholar] [CrossRef]
- Chaji, S.; Zenasni, W.; Ouaabou, R.; Ajal, E.A.; Lahlali, R.; Fauconnier, M.-L.; Hanine, H.; Černe, M.; Pasković, I.; Merah, O.; et al. Nutrient and Bioactive Fraction Content of Olea europaea L. Leaves: Assessing the Impact of Drying Methods in a Comprehensive Study of Prominent Cultivars in Morocco. Plants 2024, 13, 1961. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz-Düzyaman, H.; Medina-Alonso, M.G.; Sanz, C.; Pérez, A.G.; de la Rosa, R.; León, L. Influence of genotype and environment on fruit phenolic composition of olive. Horticulturae 2023, 9, 1087. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, C.; Liu, L.; Xu, Z.; Chen, T.; Zhou, L.; Yuan, M.; Li, T.; Ding, C. Comparison of phenolic compounds in olive leaves by different drying and storage methods. Separations 2021, 8, 156. [Google Scholar] [CrossRef]
- Pasković, I.; Lukić, I.; Žurga, M.; Majetić Germek, V.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D. Temporal variation of phenolic and mineral composition in olive leaves is cultivar dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Ivanauskas, L.; Uminska, K.; Gudžinskas, Z.; Heinrich, M.; Georgiyants, V.; Kozurak, A.; Mykhailenko, O. Phenological Variations in the Content of Polyphenols and Triterpenoids in Epilobium angustifolium Herb Originating from Ukraine. Plants 2024, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, N.M.; Lopes, L.; Chéu, M.H.; Soares, E.; Meireles, D.; Machado, J. Updated mineral composition and potential therapeutic properties of different varieties of olive leaves from Olea europaea. Plants 2023, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Chatzistathis, T.; Therios, I.; Alifragis, D. Differential uptake, distribution within tissues, and use efficiency of manganese, iron, and zinc by olive cultivars Kothreiki and Koroneiki. HortScience 2009, 44, 1994–1999. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Therios, I.; Alifragis, D.; Dimassi, K. Effect of sampling time and soil type on Mn, Fe, Zn, Ca, Mg, K and P concentrations of olive (Olea europaea L., cv. ‘Koroneiki’) leaves. Sci. Hortic. 2010, 126, 291–296. [Google Scholar] [CrossRef]
- Manolikaki, I.; Digalaki, N.; Psarras, G.; Tzerakis, C.; Sergentani, C.; Papamanolioudaki, A.; Tul, S.; Koubouris, G. Seasonal variation of leaf Ca, Fe, and Mn concentration in six olive varieties. Int. J. Plant Biol. 2022, 13, 95–105. [Google Scholar] [CrossRef]
- Diab, M.A.; Ibrahim, A.K.; Hadad, G.M.; Elkhoudary, M.M. Seasonal variations in antioxidant components of Olea europaea in leaves of different cultivars, seasons, and oil products in Sinai. Food Anal. Methods 2021, 14, 773–783. [Google Scholar] [CrossRef]
- Kaygisiz, F.; Kaya, E.; Yilmaz, M.A. Nizip Yaglık olive leaves (Olea europaea L.) collected at different seasons and altitudes: Enzyme inhibition, antioxidant activities and phenolic compound profiles. Food Biosci. 2024, 62, 105524. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Therios, I. How soil nutrient availability influences plant biomass and how biomass stimulation alleviates heavy metal toxicity in soils: The cases of nutrient use efficient genotypes and phytoremediators, respectively. In Biomass Now—Cultivation and Utilization; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Cardoni, M.; Mercado-Blanco, J.; Villar, R. Functional traits of olive varieties and their relationship with the tolerance level towards verticillium wilt. Plants 2021, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- Alhaithloul, H.A.; Awad, N.S.; Qari, S.H.; El-Homosy, R.F.; Qaoud, E.S.M.; Alqahtani, M.M.; Ghanem, K.Z.; Alasmari, A.; Alzuaibr, F.M.; Ghazzawy, H.S.; et al. Genetic diversity, chemical constituents and anatomical analysis of eight popular olive (Olea europaea L.) cultivars in Al-Jouf region, Saudi Arabia. Sci. Rep. 2024, 14, 14688. [Google Scholar] [CrossRef]
- Vidović, N.; Pasković, I.; Lukić, I.; Žurga, M.; Majetić Germek, V.; Grozić, K.; Cukrov, M.; Marcelić, Š.; Ban, D.; Talhaoui, N.; et al. Biophenolic profile modulations in olive tissues as affected by manganese nutrition. Plants 2021, 10, 1724. [Google Scholar] [CrossRef]
- Fernández-Escobar, R. Trends in olive nutrition. Acta Hortic. 2018, 1199, 215–223. [Google Scholar] [CrossRef]
- Restrepo-Díaz, H.; Benlloch, M.; Fernández-Escobar, R. Plant water stress and K+ starvation reduce absorption of foliar applied K+ by olive leaves. Sci. Hortic. 2008, 116, 409–413. [Google Scholar] [CrossRef]
- Stateras, D.C.; Moustakas, N.K. Seasonal changes of macro- and micro-nutrients concentration in olive leaves. J. Plant Nutr. 2018, 41, 186–196. [Google Scholar] [CrossRef]
- Lukić, I.; Pasković, I.; Žurga, M.; Majetić Germek, V.; Brkljača, M.; Marcelić, Š.; Ban, D.; Grozić, K.; Lukić, M.; Užila, Z.; et al. Determination of the variability of biophenols and mineral nutrients in olive leaves with respect to cultivar, collection period and geographical location for their targeted and well-timed exploitation. Plants 2020, 9, 1667. [Google Scholar] [CrossRef]
- Zeng, J.; Quan, X.; He, X.; Cai, S.; Ye, Z.; Chen, G.; Zhang, G. Root and leaf metabolite profiles analysis reveals the adaptive strategies to low potassium stress in barley. BMC Plant Biol. 2018, 18, 187. [Google Scholar] [CrossRef]
- Fahmy, I.; Nasrallah, S. Changes in macro-nutrient elements of Souri olive leaves in alternate bearing years. Proc. Am. Soc. Hortic. Sci. 1959, 74, 372–377. [Google Scholar]
- Mughal, A.; Jabeen, N.; Ashraf, K.; Sultan, K.; Farhan, M.; Hussain, M.I.; Deng, G.; Alsudays, I.M.; Saleh, M.A.; Tariq, S.; et al. Exploring the role of caffeic acid in mitigating abiotic stresses in plants: A review. Plant Stress 2024, 12, 100487. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book 2021, 9, e0152. [Google Scholar] [CrossRef]
- Sotiropoulos, S.; Chatzissavvidis, C.; Papadakis, I.; Kavvadias, V.; Paschalidis, C.; Antonopoulou, C.; Koriki, A. Inorganic and organic foliar fertilization in olives. Hortic. Sci. 2023, 50, 1–11. [Google Scholar] [CrossRef]
- Franić, M.; Pasković, I.; Marcelić, Š.; Lukić, I.; Major, N.; Palčić, I.; Goreta Ban, S.; Polić Pasković, M. Discrimination of farming practices through olive leaf phenolic and mineral analysis. Horticulturae 2024, 10, 1334. [Google Scholar] [CrossRef]
- Mydy, L.S.; Chigumba, D.N.; Kersten, R.D. Plant copper metalloenzymes as prospects for new metabolism involving aromatic compounds. Front. Plant Sci. 2021, 12, 692108. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Ali, S.; Liu, S.; Zhou, J.; Tang, Y. Characterization of plant laccase genes and their functions. Gene 2023, 852, 147060. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, G.; Karabourniotis, G. Boron deficiency and concentrations and composition of phenolic compounds in Olea europaea leaves: A combined growth chamber and field study. Tree Physiol. 2005, 25, 307–315. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of ‘Sikitita’ olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents ‘Arbequina’ and ‘Picual’ olive leaves. LWT–Food Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Romero, C.; Medina, E.; Mateo, M.A.; Brenes, M. Quantification of bioactive compounds in Picual and Arbequina olive leaves and fruit. J. Sci. Food Agric. 2017, 97, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Ortega-García, F.; Peragón, J. The response of phenylalanine ammonia-lyase, polyphenol oxidase and phenols to cold stress in the olive tree (Olea europaea L. cv. Picual). J. Sci. Food Agric. 2009, 89, 1565–1573. [Google Scholar] [CrossRef]
- Martínez-Navarro, M.E.; Cebrián-Tarancón, C.; Salinas, M.R.; Alonso, G.L. Evolution of Oleuropein and Other Bioactive Compounds in Arbequina Olive Leaves under Different Agronomic Conditions. Horticulturae 2022, 8, 530. [Google Scholar] [CrossRef]
- Ugolini, T.; Esposto, S.; Taticchi, A.; Servili, M.; Selvaggini, R. Seasonal and Cultivar-Dependent Phenolic Dynamics in Tuscan Olive Leaves: A Two-Year Study by HPLC-DAD-MS for Food By-Product Valorization. Separations 2025, 12, 192. [Google Scholar] [CrossRef]
- Dias, M.; Figueiredo, C.; Pinto, D.; Freitas, H.; Santos, C.; Silva, A. Heat shock and UV-B episodes modulate olive leaves lipophilic and phenolic metabolite profiles. Ind. Crops Prod. 2019, 133, 269–275. [Google Scholar] [CrossRef]
- Croatian Meteorological and Hydrological Service. Available online: https://meteo.hr/index_en.php (accessed on 15 February 2025).
- Arias-Sibillotte, M.; Considine, M.J.; Signorelli, S. Reinterpreting olive bud dormancy. J. Exp. Bot. 2024, 75, 6017–6021. [Google Scholar] [CrossRef]
- Noronha, H.; Garcia, V.; Silva, A.; Delrot, S.; Gallusci, P.; Gerós, H. Molecular reprogramming in grapevine woody tissues at bud burst. Plant Sci. 2021, 311, 110984. [Google Scholar] [CrossRef] [PubMed]
- Lorini, A.; Aranha, B.C.; Antunes, B.D.F.; Otero, D.M.; Jacques, A.C.; Zambiazi, R.C. Metabolic profile of olive leaves of different cultivars and collection times. Food Chem. 2021, 345, 128758. [Google Scholar] [CrossRef]
- Arslan, D.; Karabekir, Y.; Schreiner, M. Variations of phenolic compounds, fatty acids and some qualitative characteristics of Sariulak olive oil as induced by growing area. Food Res. Int. 2013, 54, 1897–1906. [Google Scholar] [CrossRef]
- Alderotti, F.; Piccolo, E.L.; Brunetti, C.; Stefano, G.; Ugolini, T.; Cecchi, L.; Beccaluva, M.; Renna, L.; Detti, C.; Ferrini, F.; et al. Cultivar-Specific Responses of Young Olive Trees to Water Deficit: Impacts on Physiology, Leaf Anatomy, and Fruit Quality in ‘Arbequina’, ‘Leccio del Corno’ and ‘Maurino’. Plant Physiol. Biochem. 2025, 213, 110331. [Google Scholar] [CrossRef]
- Mert, C.; Barut, E.; Pek, A. Quantitative seasonal changes in the leaf phenolic content related to the alternate-bearing patterns of olive (Olea europaea L. cv. Gemlik). J. Agric. Sci. Technol. 2013, 15, 995–1006. [Google Scholar]
- Zakraoui, M.; Hannachi, H.; Pasković, I.; Vidović, N.; Polić Pasković, M.; Palčić, I.; Major, N.; Goreta Ban, S.; Hamrouni, L. Effect of geographical location on the phenolic and mineral composition of Chetoui olive leaves. Foods 2023, 12, 2565. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Urbani, E.; Simonetti, M.S.; Chiesi, C.; Cossignani, L. Seasonal variations in antioxidant compounds of Olea europaea leaves collected from different Italian cultivars. J. Appl. Bot. Food Qual. 2016, 89, 202–207. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Feng, S.; Chen, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Seasonal variations in the chemical composition of Liangshan olive leaves and their antioxidant and anticancer activities. Foods 2019, 8, 657. [Google Scholar] [CrossRef]
- Meirinhos, J.; Silva, B.M.; Valentão, P.; Seabra, R.M.; Pereira, J.A.; Dias, A.; Andrade, P.B.; Ferreres, F. Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Nat. Prod. Res. 2005, 19, 189–195. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; García-García, R.; Granados, M.; Sentellas, S.; Saurina, J. Exploring polyphenol content in olive leaf waste: Effects of geographical, seasonal and varietal differences. Microchem. J. 2025, 215, 114347. [Google Scholar] [CrossRef]
- Orak, H.H.; Karamać, M.; Amarowicz, R.; Orak, A.; Penkacik, K. Genotype-related differences in the phenolic compound profile and antioxidant activity of extracts from olive (Olea europaea L.) leaves. Molecules 2019, 24, 1130. [Google Scholar] [CrossRef] [PubMed]
- Mykhailenko, O.; Jalil, B.; Gudžinskas, Z.; Uminska, K.; Ivanauskas, L.; Heinrich, M. The Phenology of Epilobium hirsutum L.: Assessing Marker Compounds Variability of a Pharmaceutically Important Plant Remedy. Front. Pharmacol. 2025, 16, 1602819. [Google Scholar] [CrossRef] [PubMed]
- Godena, S.; Damijanić, K.; Milotić, A. Morfološke karakteristike masline sorte Rosinjola u Istri. Pomol. Croat. Glas. Hrvat. Agron. Društva 2009, 15, 27–36. [Google Scholar]
- Klepo, T.; Benčić, Đ.; Liber, Z.; Belaj, A.; Strikić, F.; Kević, N.; Šatović, Z. Revealing the diversity and complex relationships of Croatian olive germplasm. Int. J. Mol. Sci. 2024, 25, 3170. [Google Scholar] [CrossRef]
- Ercişli, S.; Benčić, D.; Liber, Z. Genetic relationships among olive (Olea europaea L.) cultivars native to Croatia and Turkey. J. Appl. Bot. Food Qual. 2012, 85, 144–149. [Google Scholar]
- Podgornik, M.; Vesel, V.; Bandelj, D.; Butinar, B.; Bonin, E.; Brajnik, J.; Bešter, E.; Fantinič, J.; Fičur, K.; Juretič, V.; et al. Poročilo 2020 o Izvajanju Letnega Programa dela Javnih Služb v Oljkarstvu; Znanstveno-Raziskovalno Središče Koper, Annales ZRS: Koper, Slovenia, 2021. [Google Scholar]
- Mark, J.; Fantke, P.; Soheilifard, F.; Alcon, F.; Contreras, J.; Abrantes, N.; Campos, I.; Baldi, I.; Bureau, M.; Alaoui, A.; et al. Selected farm-level crop protection practices in Europe and Argentina: Opportunities for moving toward sustainable use of pesticides. J. Clean Prod. 2024, 477, 143577. [Google Scholar] [CrossRef]
- Hein, J.A.; Sherrard, M.E.; Manfredi, K.P.; Abebe, T. The fifth leaf and spike organs of barley (Hordeum vulgare L.) display different physiological and metabolic responses to drought stress. BMC Plant Biol. 2016, 16, 248. [Google Scholar] [CrossRef] [PubMed]
- Siamandoura, P.; Tzia, C. Comparative Study of Novel Methods for Olive Leaf Phenolic Compound Extraction Using NADES as Solvents. Molecules 2023, 28, 353. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Quirós, P.; Mir-Cerdà, A.; Granados, M.; Sentellas, S.; Saurina, J. From Waste to Resource: Exploring Green Approaches for Phenolics Recovery from Olive Leaves. Antioxidants 2025, 14, 136. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 16 August 2025).
- RStudio Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2024; Available online: https://posit.co/ (accessed on 16 August 2025).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Hammer, Ø.; Ryan, P.D.; Harper, D.A.T. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 178. [Google Scholar]
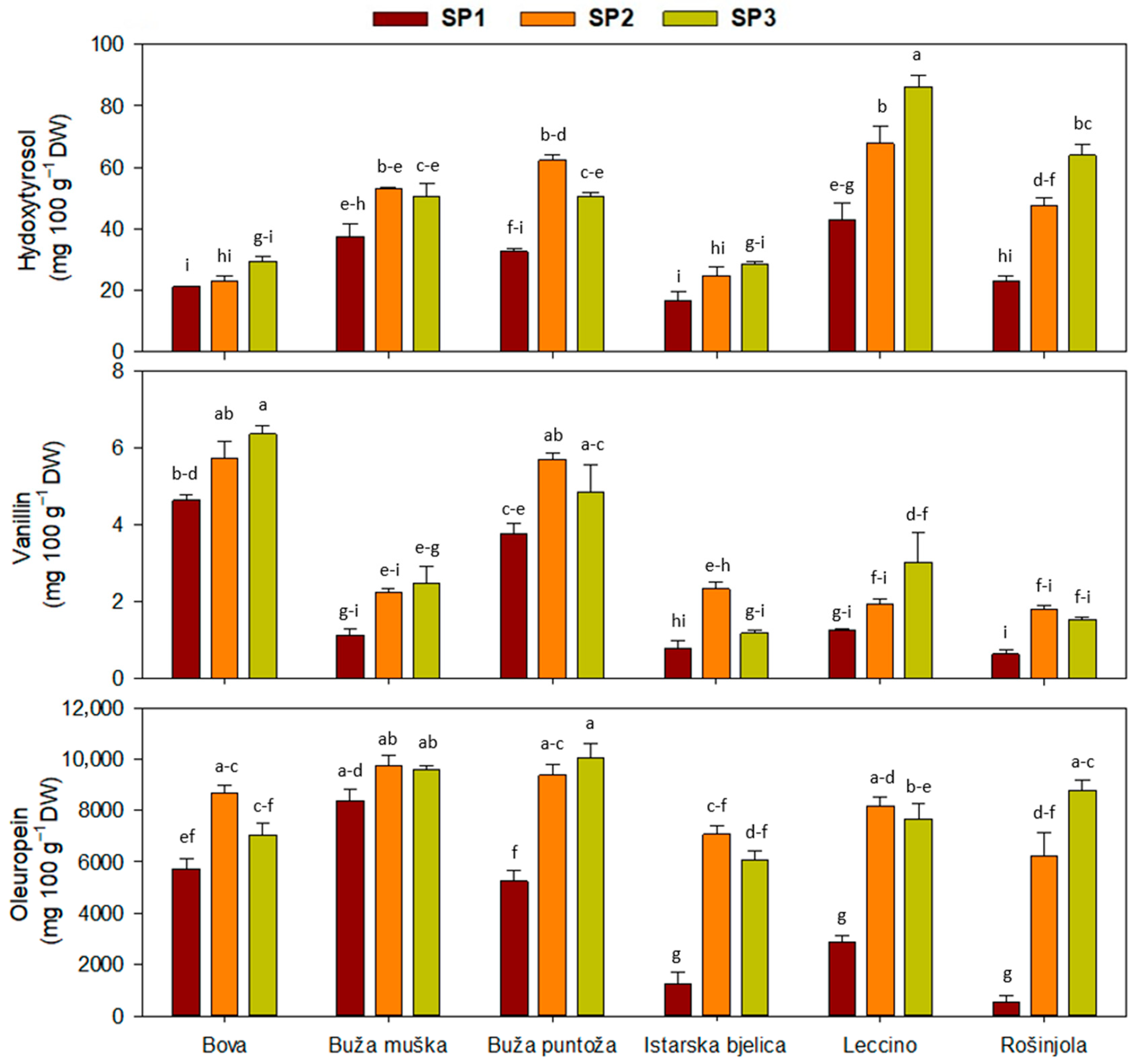
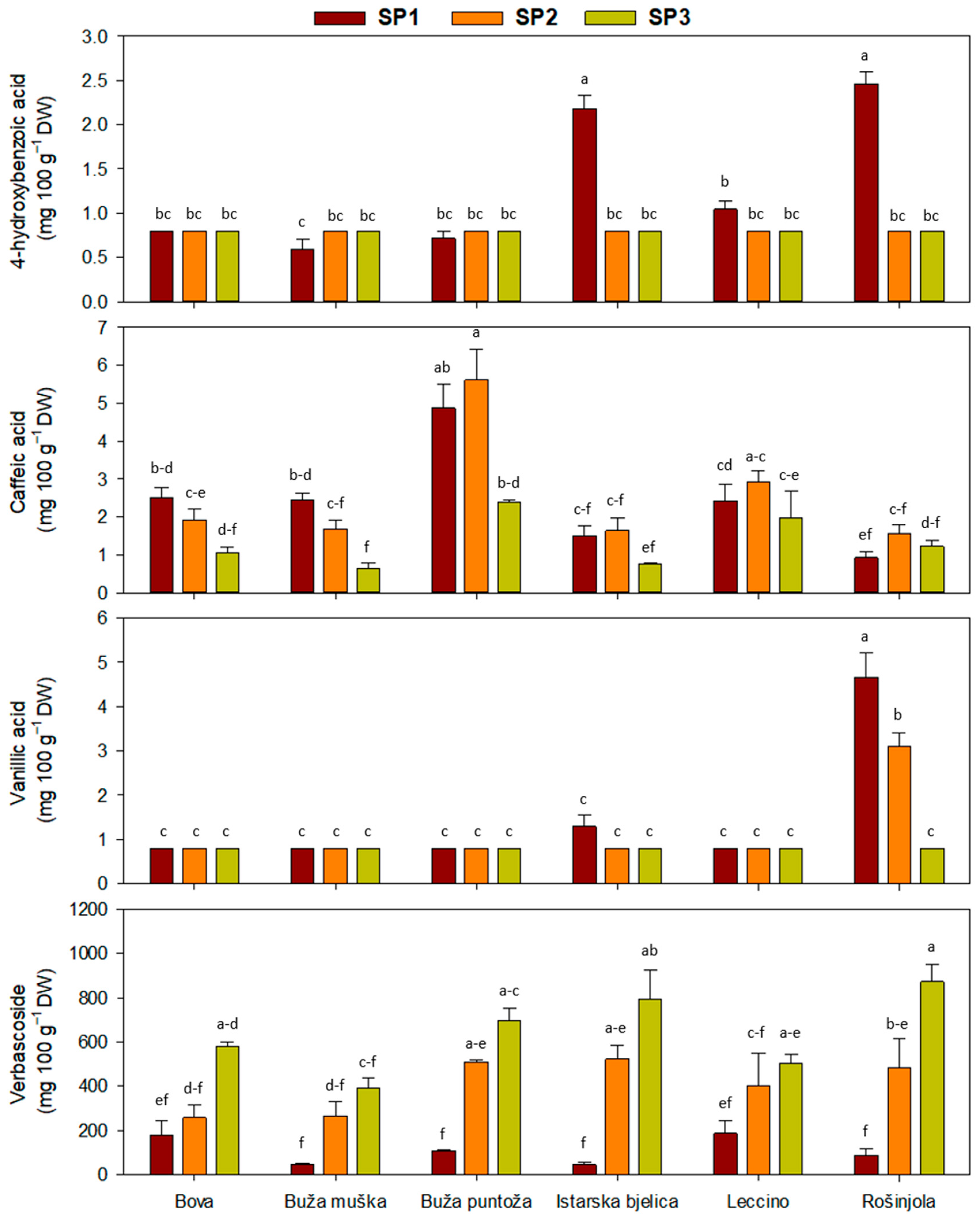
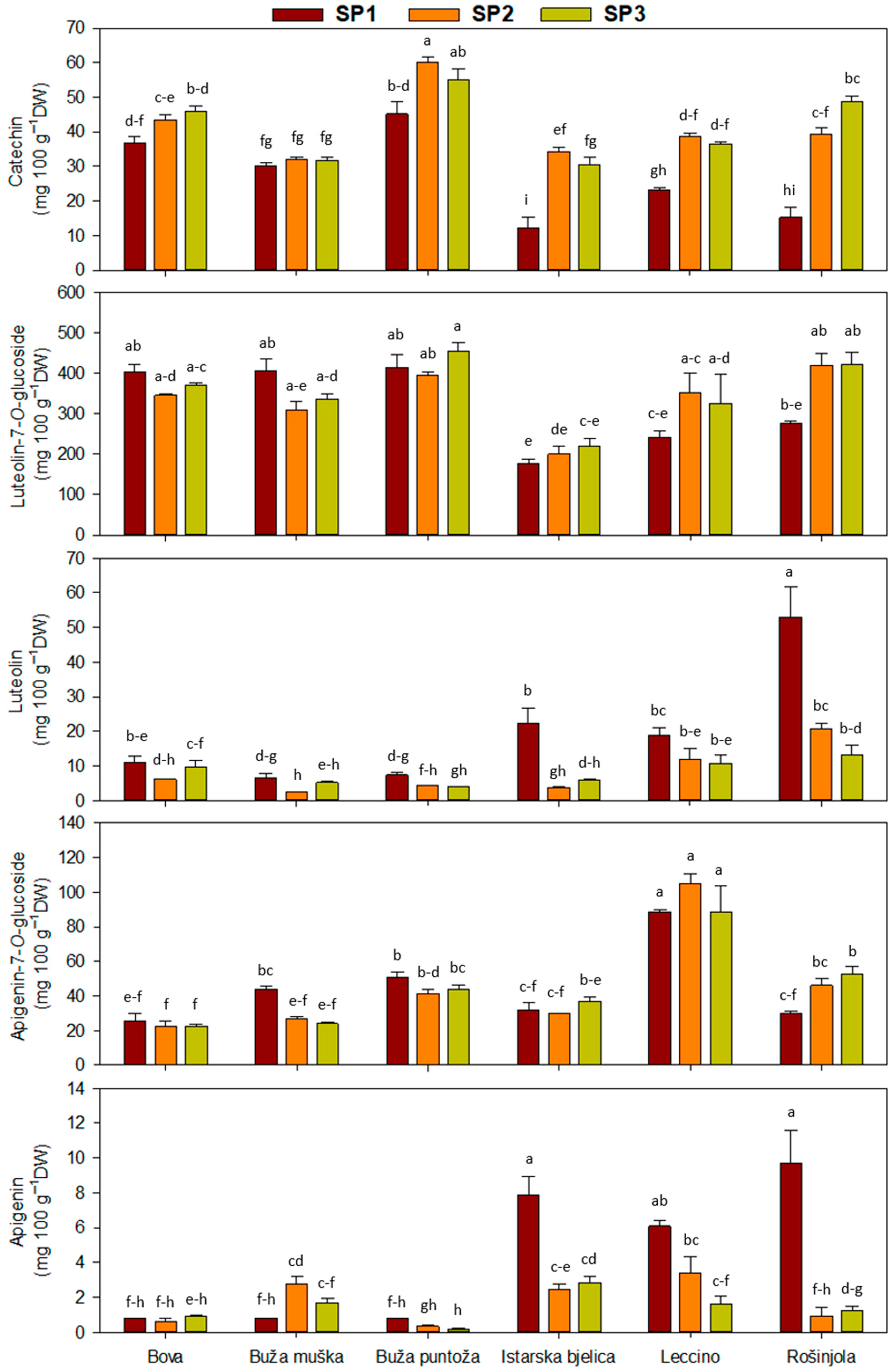
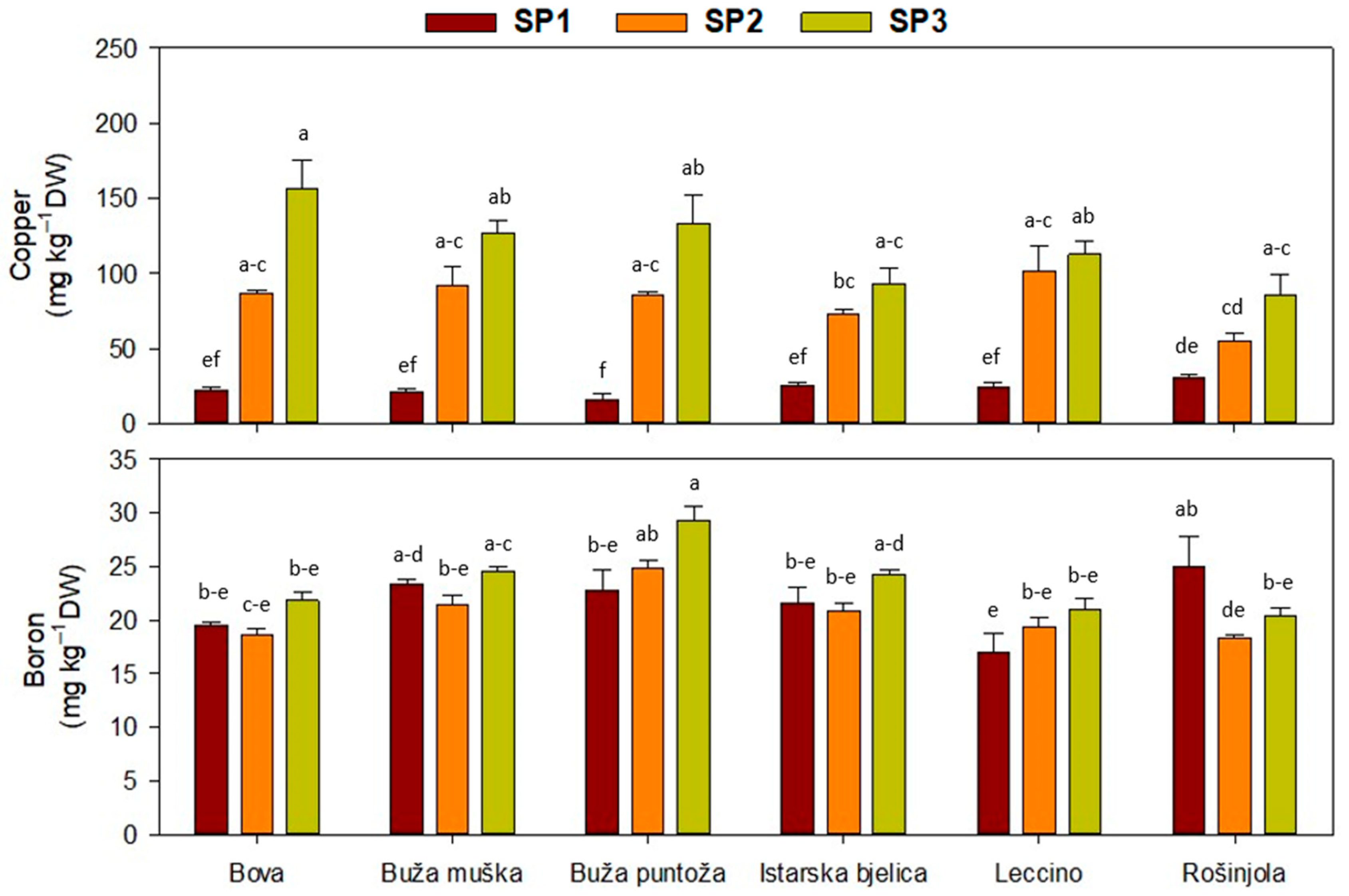

| Main Effect | Simple Phenols | Secoiridoids | |||
|---|---|---|---|---|---|
| Hydroxytyrosol | Tyrosol | Vanillin | Oleuropein | Total Phenol Concentration | |
| Cultivar (Cv.) | |||||
| ‘Bova’ | 25 ± 2 c | 5.3 ± 0.8 c | 5.6 ± 0.3 a | 7162 ± 463 bc | 4247 ± 158 ab |
| ‘Buža muška’ | 47 ± 3 b | 8.2 ± 0.5 b | 1.9 ± 0.3 b | 9218 ± 282 a | 4460 ± 304 a |
| ‘Buža puntoža’ | 484 ± 4 b | 6.7 ± 0.5 bc | 4.8 ± 0.4 a | 8218 ± 784 ab | 4630 ± 380 a |
| ‘Istarska bjelica’ | 23 ± 2 c | 6.7 ± 0.6 bc | 1.4 ± 0.2 b | 4801 ± 923 e | 3878 ± 297 b |
| ‘Leccino’ | 66 ± 7 a | 12.2 ± 0.5 a | 2.1 ± 0.3 b | 6239 ± 869 cd | 4284 ± 368 ab |
| ‘Rošinjola’ | 45 ± 6 b | 7.1 ± 0.3 bc | 1.3 ± 0.2 b | 5179 ± 1250 de | 4626 ± 315 a |
| Sampling period (SP) | |||||
| SP1 | 28.9 ± 10.8 c | 8.5 ± 0.6 | 2.0 ± 0.4 b | 4005 ± 673 b | 3230 ± 103 b |
| SP2 | 46.4 ± 18.0 b | 7.4 ± 0.8 | 3.3 ± 0.4 a | 8209 ± 345 a | 4933 ± 111 a |
| SP3 | 51.4 ± 20.9 a | 7.2 ± 0.6 | 3.2 ± 0.5 a | 8194 ± 369 a | 4899 ± 123 a |
| Cv. | *** | *** | *** | *** | ** |
| SP | *** | * | *** | *** | *** |
| Cv. × SP | *** | ns | ** | *** | ns |
| Main Effect | Phenolic Acid | ||||
|---|---|---|---|---|---|
| 4-Hydroxybenzoic Acid | Caffeic Acid | Vanillic Acid | Ferulic Acid | Verbascoside | |
| Cultivar (Cv.) | |||||
| ‘Bova’ | 0.8 ± 0 b | 1.8 ± 0.2 bc | 0.8 ± 0 b | 0.6 ± 0.1 | 339 ± 66 ab |
| ‘Buža muška’ | 0.7 ± 0 b | 1.6 ± 0.3 c | 0.8 ± 0 b | 0.9 ± 0.1 | 233 ± 55 b |
| ‘Buža puntoža’ | 0.8 ± 0 b | 4.3 ± 0.6 a | 0.8 ± 0 b | 0.8 ± 0.2 | 438 ± 88 a |
| ‘Istarska bjelica’ | 1.3 ± 0.2 a | 1.3 ± 0.2 c | 1.0 ± 0.1 b | 0.9 ± 0.1 | 454 ± 117 a |
| ‘Leccino’ | 0.9 ± 0 b | 2.4 ± 0.3 b | 0.8 ± 0 b | 0.9 ± 0.1 | 364 ± 67 ab |
| ‘Rošinjola’ | 1.4 ± 0.3 a | 1.2 ± 0.1 c | 2.9 ± 0.6 a | 0.9 ± 0.2 | 482 ± 121 a |
| Sampling period (SP) | |||||
| SP1 | 1.3 ± 0.2 a | 2.4 ± 0.3 a | 1.5 ± 0.4 a | 1.0 ± 0.1 | 108 ± 19 c |
| SP2 | 0.8 ± 0 b | 2.6 ± 0.4 a | 1.2 ± 0.2 b | 0.8 ± 0.1 | 406 ± 41 b |
| SP3 | 0.8 ± 0 b | 1.3 ± 0.2 b | 0.8 ± 0 c | 0.7 ± 0.1 | 639 ± 47 a |
| Cv. | *** | *** | *** | ns | ** |
| SP | *** | *** | *** | ns | *** |
| Cv. × SP | *** | * | *** | ** | * |
| Main Effect | Flavonoids | |||||
|---|---|---|---|---|---|---|
| Catechin | Luteolin-7-O- Glucoside | Luteolin | Rutin | Apigenin-7-O- Glucoside | Apigenin | |
| Cultivar (Cv.) | ||||||
| ‘Bova’ | 42 ± 2 b | 374 ± 10 ab | 8.9 ± 1.1 c | 52 ± 2 ab | 23.6 ± 4.87 d | 0.8 ± 0.1 d |
| ‘Buža muška’ | 31 ± 1 c | 351 ± 18 b | 4.6 ± 0.7 d | 38 ± 3 c | 31.5 ± 9.46 c | 1.7 ± 0.3 c |
| ‘Buža puntoža’ | 53 ± 3 a | 422 ± 15 a | 5.2 ± 0.6 d | 32 ± 3 c | 45.5 ± 5.64 b | 0.4 ± 0.1 d |
| ‘Istarska bjelica’ | 26 ± 4 d | 199 ± 10 c | 10.6 ± 3.2 c | 50 ± 3 ab | 32.9 ± 5.46 c | 4.4 ± 0.9 a |
| ‘Leccino’ | 33 ± 2 c | 307 ± 31 b | 13.9 ± 1.8 b | 63 ± 12 a | 94.1 ± 16.2 a | 3.7 ± 0.7 ab |
| ‘Rošinjola’ | 34 ± 5 c | 373 ± 27 ab | 28.9 ± 6.7 a | 36 ± 5 c | 42.9 ± 11.5 b | 4.0 ± 1.5 b |
| Sampling period (SP) | ||||||
| SP1 | 27 ± 3 b | 320 ± 24 | 19.8 ± 4.1 a | 37.8 ± 14.2 b | 45 ± 5 | 4.3 ± 0.9 a |
| SP2 | 41 ± 2 a | 338 ± 19 | 8.2 ± 1.6 b | 51.9 ± 20.8 a | 45 ± 71 | 1.8 ± 0.3 b |
| SP3 | 41 ± 2 a | 355 ± 22 | 8.1± 1.0 b | 45.8 ± 21.9 ab | 45 ± 6 | 1.4 ± 0.2 b |
| Cv. | *** | *** | *** | *** | *** | *** |
| SP | *** | ns | *** | * | ns | *** |
| Cv. × SP | *** | ** | *** | ns | *** | *** |
| Main Effect | Macronutrients | Micronutrients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | Na | Fe | Zn | Mn | Cu | B | |
| Cultivar (Cv.) | ||||||||||
| ‘Bova’ | 1.5 ± 0.0 c | 5.3 ± 0.4 d | 23 ± 1 a | 1.5 ± 0.1 bc | 0.4 ± 0.0 | 84 ± 5 ab | 22 ± 0 b | 32 ± 3 ab | 88 ± 20 | 20 ± 1 cd |
| ‘Buža muška’ | 1.1 ± 0.0 b | 7.2 ± 0.4 ab | 14 ± 1 c | 1.2 ± 0.1 cd | 0.5 ± 0.0 | 87 ± 4 a | 24 ± 1 ab | 30 ± 2 ab | 80 ± 16 | 23 ± 1 ab |
| ‘Buža puntoža’ | 2.5 ± 0.1 a | 8.0 ± 0.7 a | 12 ± 1 c | 1.0 ± 0.0 d | 0.4 ± 0.0 | 83 ± 6 ab | 27 ± 1 a | 28 ± 1 b | 78 ± 18 | 26 ± 1 a |
| ‘Istarska bjelica’ | 1.5 ± 0.1 c | 6.1 ± 0.6 c | 18 ± 1 b | 1.6 ± 0.1 b | 0.4 ± 0.1 | 79 ± 5 ab | 22 ± 1 b | 27 ± 2 b | 64 ± 10 | 22 ± 1 bc |
| ‘Leccino’ | 1.9 ± 0.1 b | 6.8 ± 0.5 bc | 22 ± 1 a | 1.1 ± 0.1 d | 0.5 ± 0.0 | 74 ± 5 b | 20 ± 1 b | 37 ± 2 a | 80 ± 15 | 19 ± 1 d |
| ‘Rošinjola’ | 1.6 ± 0.1 c | 5.5 ± 0.6 d | 24± 1 a | 2.1 ± 0.1 a | 0.4 ± 0.0 | 83 ± 5 ab | 26 ± 1 a | 39 ± 3 a | 57 ± 19 | 21 ± 1 bcd |
| Sampling period (SP) | ||||||||||
| SP1 | 2.0 ± 0.1 a | 7.9 ± 0.3 a | 20 ± 2 | 1.3 ± 0.1 | 0.5 ± 0.0 a | 96 ± 2 a | 22 ± 1 b | 35 ± 2 a | 23. ± 1 c | 22 ± 1 b |
| SP2 | 1.8 ± 0.1 b | 6.7 ± 0.3 b | 19 ± 1 | 1.5 ± 0.1 | 0.4 ± 0.0 b | 85 ± 2 b | 24 ± 1 a | 29 ± 2 b | 82 ± 5 b | 21 ± 1 b |
| SP3 | 1.6 ± 0.1 c | 4.7 ± 0.3 c | 18 ± 1 | 1.4 ± 0.1 | 0.4 ± 0.0 b | 65 ± 2 c | 24 ± 1 ab | 33 ± 2 ab | 118 ± 8 a | 24 ± 1 a |
| Cv. | *** | *** | *** | *** | * | ** | *** | *** | ns | *** |
| SP | *** | *** | ns | ns | *** | *** | ** | * | *** | *** |
| Cv. × SP | ns | ns | ns | ns | ns | ns | ns | ns | ** | ** |
| Phenolic Compounds/ Nutrients | P | K | Cu | B |
|---|---|---|---|---|
| Hydroxytyrosol | 0.18 | −0.13 | 0.44 | 0.01 |
| Tyrosol | 0.23 | 0.35 | −0.24 | −0.37 |
| Vanillin | 0.19 | −0.14 | 0.45 | 0.14 |
| Oleuropein | 0.00 | −0.30 | 0.65 ** | 0.21 |
| TPC | −0.15 | −0.51 * | 0.79 ** | 0.20 |
| 4-Hydroxybenzoic acid | 0.00 | 0.18 | −0.37 | 0.12 |
| Caffeic acid | 0.84 ** | 0.67 ** | −0.26 | 0.07 |
| Vanillic acid | −0.07 | 0.09 | −0.32 | 0.06 |
| Ferrulic acid | 0.29 | 0.33 | −0.28 | 0.10 |
| Verbascoside | −0.36 | −0.75 ** | 0.70 ** | 0.21 |
| Catechin | 0.26 | −0.21 | 0.49 * | 0.21 |
| Luteolin-7-O-glucoside | 0.25 | −0.04 | 0.17 | 0.18 |
| Luteolin | −0.07 | 0.11 | −0.42 | −0.06 |
| Rutin | −0.36 | −0.25 | 0.27 | −0.45 |
| Apigenin-7-O-glucoside | 0.24 | 0.15 | −0.03 | −0.32 |
| Apigenin | −0.02 | 0.24 | −0.41 | −0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasković, I.; Franić, M.; Chatzistathis, T.; Pongrac, P.; Žurga, P.; Majetić Germek, V.; Palčić, I.; Goreta Ban, S.; Zakraoui, M.; Marcelić, Š.; et al. Optimising Olive Leaf Phenolic Compounds: Cultivar and Temporal Interactions. Plants 2025, 14, 2789. https://doi.org/10.3390/plants14172789
Pasković I, Franić M, Chatzistathis T, Pongrac P, Žurga P, Majetić Germek V, Palčić I, Goreta Ban S, Zakraoui M, Marcelić Š, et al. Optimising Olive Leaf Phenolic Compounds: Cultivar and Temporal Interactions. Plants. 2025; 14(17):2789. https://doi.org/10.3390/plants14172789
Chicago/Turabian StylePasković, Igor, Mario Franić, Theocharis Chatzistathis, Paula Pongrac, Paula Žurga, Valerija Majetić Germek, Igor Palčić, Smiljana Goreta Ban, Mariem Zakraoui, Šime Marcelić, and et al. 2025. "Optimising Olive Leaf Phenolic Compounds: Cultivar and Temporal Interactions" Plants 14, no. 17: 2789. https://doi.org/10.3390/plants14172789
APA StylePasković, I., Franić, M., Chatzistathis, T., Pongrac, P., Žurga, P., Majetić Germek, V., Palčić, I., Goreta Ban, S., Zakraoui, M., Marcelić, Š., Mravlje, J., Kaliterna, J., & Polić Pasković, M. (2025). Optimising Olive Leaf Phenolic Compounds: Cultivar and Temporal Interactions. Plants, 14(17), 2789. https://doi.org/10.3390/plants14172789












