Ethnobotany in a Modern City: The Persistence in the Use of Medicinal Plants in Guadalajara, Mexico
Abstract
1. Introduction
2. Results and Discussion
2.1. Principal Coordinate Analysis
2.2. Ethnopharmacology
2.2.1. Acanthaceae
2.2.2. Anacardiaceae
2.2.3. Asteraceae
2.2.4. Caprifoliaceae
2.2.5. Cucurbitaceae
2.2.6. Equisetaceae
2.2.7. Fabaceae
2.2.8. Lamiaceae
2.2.9. Lauraceae
2.2.10. Tiliaceae
2.2.11. Verbenaceae
2.2.12. Vitaceae
2.2.13. Zingiberaceae
2.2.14. Zygophyllaceae
3. Materials and Methods
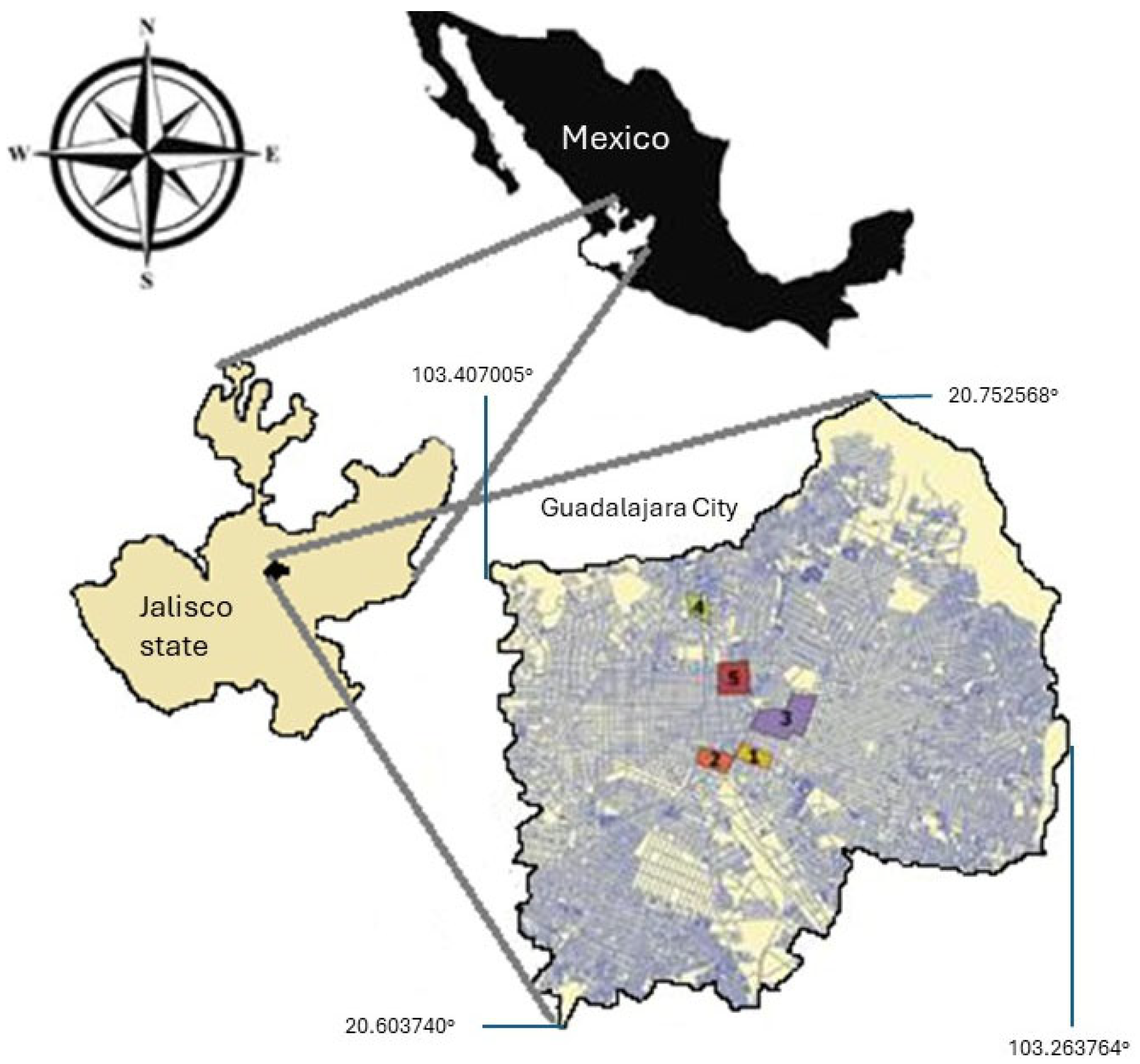
3.1. Study Area
3.2. Field Work and Interviews
3.3. Systematic and Literature Review
3.4. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Gusain, P.; Sharifi-Rad, J.; et al. Bioactive compounds and health benefits of edible Rumex species—A review. Cell. Mol. Biol. 2018, 64, 27–34. [Google Scholar] [CrossRef]
- Govea-Salas, M.; Morlett-Chavez, J.; Rodriguez-Herrera, R.; Ascencio-Valdes, J. Some mexican plants used in tradicional medicine. In Aromatic and Medicinal Plants—Back to the Nature; El-shemy, H.A., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Roberts, T.H.; Matthews, K.R.; Bezerra, C.F.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Sharopov, F.; Salehi, B.; Yousaf, Z.; Sharifi-Rad, M.; et al. Ethnobotany of the genus Taraxacum—Phytochemicals and antimicrobial activity. Phytother. Res. 2018, 32, 2131–2145. [Google Scholar] [CrossRef]
- Kopp, B. Herbal medicinal products and phytotherapy—A thematic issue. Wien. Med. Wochenschr. 2017, 167, 145–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlaepfer, L.; Mendoza-Espinoza, J.A. Las plantas medicinales en la lucha contra el cáncer, relevancia para México. Rev. Mex. De Cienc. Farm. 2010, 41, 18–27. [Google Scholar][Green Version]
- Barrera-Vázquez, O.S.; Montenegro-Herrera, S.A.; Martínez-Enríquez, M.E.; Escobar-Ramírez, J.L.; Magos-Guerrero, G.A. Selection of Mexican Medicinal Plants by Identification of Potential Phytochemicals with Anti-Aging, Anti-Inflammatory, and AntiOxidant Properties through Network Analysis and Chemoinformatic Screening. Biomolecules 2023, 13, 1673. [Google Scholar] [CrossRef]
- Huerta-Martínez, F.M. Traditional use of biodiversity for nutrition and health in the Americas: A case study from Mexico. In Ethnic Knowledge and Perspectives of Medicinal Plants. Curative Properties and Treatment Strategies; Östürk, M., Sridhar, K.R., Sarwat, M., Altay, V., Huerta-Martínez, F.M., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2024; Volume 1, pp. 3–52. [Google Scholar][Green Version]
- Villaseñor, J.L.; Meave, J.A. Floristics in Mexico today: Insights into a better understanding of biodiversity in a megadiverse country. Bot. Sci. 2022, 100, S14–S33. [Google Scholar] [CrossRef]
- INPI Atlas de los Pueblos Indígenas de México. Gobierno de México. Available online: http://atlas.inpi.gob.mx (accessed on 20 November 2024).
- Hernandez-Xolocotzi, E.; Vargas, A.; Gomez, T.J.; Brauer, F. Consideraciones etnobotanicas de los mercados en Mexico. Rev. Geogr. Agric. 1983, 4, 13–28. [Google Scholar]
- White-Olascoaga, L.; Juan-Perez, J.I.; Chavez-Mejia, C.; Gutierrez-Cedillo, J.G. Flora medicinal en San Nicolás, Municipio de Malinalco, Estado de Mexico. Polibotanica 2013, 35, 173–206. [Google Scholar]
- Lira, R.; Casas, A.; Blancas, J. (Eds.) Etnobotany of Mexico: Interactions of People and Plants in Mesoamerica; Springer: New York, NY, USA, 2016. [Google Scholar]
- Palma-Tenango, M.; San Miguel-Chavez, R.; Soto-Hernandez, R.M. Aromatic and Medicinal Plants in Mexico. In Aromatic and Medicinal Plants—Back to Nature; El-Shemy, H.A., Ed.; InTech: London, UK, 2017; pp. 133–147. [Google Scholar] [CrossRef]
- Hernandez-Delgado, T.; Villagomez-Guzman, A.K.; Arreaga-Gonzalez, H.M. Plantas medicinales mexicanas: Extraordinarios laboratorios para el desarrollo terapeutico. Rev. Digit. Univ. 2025, 26, 3. [Google Scholar] [CrossRef]
- Narvaez-Elizondo, R.E.; Gonzalez-Elizondo, M.; Castro-Castro, A.; Gonzalez-Elizondo, M.S.; Tena-Flores, J.; Chairez-Hernandez, I. Comparison of traditional knowledge about edible plants among young Southern Tepehuans of Durango, Mexico. Bot. Sci. 2021, 99, 834–849. [Google Scholar] [CrossRef]
- Vilaboa Arroniz, J.; Platas Rosado, D.E.; Zetina Córdoba, P.; Gasperín García, E.M.; Velázquez Muñoz, C.A.; Santiago Hernández, J.L. Conocimiento y uso de plantas medicinales en Calpan, Puebla, México: Percepción de varios sectores sociales. Bol. Latinoam. Y Caribe Plantas Med. Y Aromat. 2023, 22, 676–688. [Google Scholar] [CrossRef]
- Cruz-Pérez, A.L.; Barrera-Ramos, J.; Bernal-Ramírez, L.A.; Bravo-Avilez, D.; Rendón-Aguilar, B. Actualized inventory of medicinal plants used in traditional medicine in Oaxaca, Mexico. J. Ethnobiol. Ethnomed. 2021, 17, 7. [Google Scholar] [CrossRef]
- Martinez-Lopez, G.; Palacios-Rangel, M.I.; Guizar-Nolazco, E.; Villanueva-Morales, A. Local uses and tradition: Ethnobotanical study of useful plants in San Pablo Cuatro Venados (Valles centrales, Oaxaca). Polibotanica 2021, 52, 193–212. [Google Scholar] [CrossRef]
- De La Cruz-Jiménez, L.; Hernández-Torres, M.A.; Monroy-García, I.N.; Rivas-Morales, C.; Verde-Star, M.J.; Gonzalez-Villasana, V.; Viveros-Valdez, E. Biological Activities of Seven Medicinal Plants Used in Chiapas, Mexico. Plants 2022, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Castillon, E.; Villarreal-Quintanilla, J.A.; Cuellar-Rodriguez, L.G.; March-Salas, M.; Encina-Domínguez, J.A.; Himmeslbach, W.; Salinas-Rodríguez, M.M.; Guerra, J.; Cotera-Correa, M.; Scott-Morales, L.M.; et al. Ethnobotany in Iturbide, Nuevo León: The Traditional Knowledge on Plants Used in the Semiarid Mountains of Northeastern Mexico. Sustainability 2022, 14, 12751. [Google Scholar] [CrossRef]
- Bermudez, A.; Oliveira-Miranda, M.A.; Velazquez, D. La investigación etnobotanica sobre plantas medicinales: Una revisión de sus objetivos y enfoques actuales. Interciencia 2005, 30, 453–459. [Google Scholar]
- Martínez-González, R.E.; Huerta-Martínez, F.M.; Neri-Luna, C.; Barrientos-Ramírez, L. Etnobotánica de los barrios antiguos de Guadalajara, Jalisco, México: El uso de plantas medicinales. Boletín Latinoam. Y Caribe Plantas Med. Y Aromáticas 2024, 23, 75–110. [Google Scholar] [CrossRef]
- Emery, M.R.; Hurley, P.T. Ethnobiology in the City: Embracing the Urban Ecological Moment. J. Ethnobiol. 2016, 36, 807–819. [Google Scholar] [CrossRef]
- Albuquerque, U.P.; Ladio, A.; Duarte, A.E.; Vandebroek, I.; Pulido, S.M.T.; Stern da Fonseca-Kruel, V. Exploring biocultural diversity in urban ecosystems: An ethnobiological perspective. Ethnobiol. Conserv. 2023, 12, 10. [Google Scholar] [CrossRef]
- Dutta, T.; Anand, U.; Saha, S.C.; Mane, A.B.; Prasanth, D.A.; Kandimalla, R.; Prockow, J.; Dey, A. Advancing urban ethnopharmacology: A modern concept of sustainability, conservation and cross-cultural adaptations of medicinal plant lore in the urban environment. Conserv. Physiol. 2021, 9, coab073. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moreno, D.; Valdez-Eleuterio, G.; Basurto-Peña, F.; Andres-Hernandez, A.R.; Rodriguez-Ramirez, T.; Figueroa-Castillo, A. Plantas medicinales de los mercados de Izúcar de Matamoros y Acatlán de Osorio, Puebla. Polibotanica 2016, 41, 153–178. [Google Scholar] [CrossRef]
- Juarez-Perez, J.C.; Cabrera-Luna, J.A. Plantas para afecciones respiratorias comercializadas en tres mercados de la ciudad de Santiago de Querétaro. Polibotanica 2019, 47, 167–178. [Google Scholar] [CrossRef]
- Villanueva-Solis, I.; Arreguin-Sanchez, M.L.; Quiroz-Garcia, D.L.; Fernandez-Nava, R. Plantas medicinales que se comercializan en el mercado 8 de julio y uno tradicional, ambos localizados en en Centro de Actopan, Hidalgo, Mexico. Polibotanica 2020, 50, 209–243. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; León-Becerril, E.; García-Depraect, O. Beyond the Exploration of Muicle (Justicia spicigera): Reviewing Its Biological Properties, Bioactive Molecules and Materials Chemistry. Processes 2022, 10, 1035. [Google Scholar] [CrossRef]
- Márquez, C.; Ochoa, L.; Rodríguez, E.; Essayag, M. Plantas Medicinales de México Composición, Usos Y Actividad Biológica; Universidad Nacional Autónoma de México: México City, Mexico, 1999. [Google Scholar]
- Weng, J.R.; Ko, H.H.; Yeh, T.L.; Lin, H.C.; Lin, C.N. Two New Arylnaphthalide Lignans and Antiplatelet Constituents from Justicia procumbens. Arch. Pharm. 2004, 337, 207–212. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P.; Flores, J.M.M.; Gonzalez, A.M.N. Anti-inflammatory effect of procumbenoside B from Justicia spicigera on lipopolysaccharide-stimulated RAW264.7 macrophages and zebrafish model. Pharmacogn. Res. 2018, 10, 218–224. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia officinalis L. and Schinus molle L. essential oils: Their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef]
- Salazar-Aranda, R.; Pérez-López, L.A.; Lopez-Arroyo, J.; Alanís-Garza, B.A.; Waksman de Torres, N. Antimicrobial and antioxidant activities of plants from northeast of Mexico. Evid.-Based Complement. Altern. Med. 2011, 2011, 536139. [Google Scholar] [CrossRef] [PubMed]
- Do Prado, A.C.; Garces, H.G.; Bagagli, E.; Rall, V.L.M.; Furlanetto, A.; Fernandes, J.A.; Furtado, F.B. Schinus molle essential oil as a potential source of bioactive compounds: Antifungal and antibacterial properties. J. Appl. Microbiol. 2019, 126, 516–522. [Google Scholar] [CrossRef]
- Guala, M.S.; Lapissonde, M.O.; Elder, H.V.; van Baren, C.M.; Bandoni, A.L.; Dellacassa, E. Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 689–695. [Google Scholar]
- Machado, C.D.; Raman, V.; Rehman, J.U.; Maia, B.H.L.N.S.; Meneghetti, E.K.; Almeida, V.P.; Silva, R.Z.; Farago, P.V.; Khan, I.A.; Budel, J.M. Schinus molle: Anatomy of leaves and stems, chemical composition and insecticidal activities of volatile oil against bed bug (Cimex lectularius). Rev. Bras. Farmacogn. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Ferrero, A.A.; Sánchez-Chopa, C.; Werdin-Gonzalez, J.O.; Alzogaray, R.A. Repellence and toxicity of Schinus molle extracts on Blattlla germanica. Fitoterapia 2007, 78, 311–314. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; Zaitoun, A.A.; Farag, M.A.; Gayed, S.H.E.; Harraz, F.M.H. Chemical composition, insecticidal and insect repellent activity of Schinus molle L. leaf and fruit essential oils against Trogoderma granarium and Tribolium castaneum. Nat. Prod. Res. 2010, 24, 226–235. [Google Scholar] [CrossRef]
- Pino, J.A.; Baya, F.; Marbot, R.; Agüero, J. Essential oil of Chamomilla recutita (L.) Rausch. From Iran. J. Essent. Oil Res. 2002, 14, 407–408. [Google Scholar] [CrossRef]
- Pirzad, A.; Alyari, H.; Shakiba, M.R.; Zehtab-Salmasi, S.; Moammadi, A. Essential oil content and composition of German chamomile (Matricaria chamomilla L.) at different irrigation regimes. J. Agron. 2006, 5, 451–455. [Google Scholar] [CrossRef]
- Mann, C.; Staba, E.J. The chemistry, pharmacology and commercial formulations of chamomile. In Herbs, Spices and Medicinal Plants-Recent Advances in Botany, Horticulture and Pharmacology; Craker, L.E., Simon, J.E., Eds.; Haworth Press Inc.: Binghamton, NY, USA, 2002; pp. 235–280. [Google Scholar]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- Hajaji, S.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Jiménez, I.A.; Bazzocchi, I.L.; Valladares, B.; Akkari, H.; Lorenzo-Morales, J.; Piñero, J.E. Leishmanicidal activity of α-bisabolol from Tunisian chamomile essential oil. Parasitol. Res. 2018, 117, 2855–2867. [Google Scholar] [CrossRef]
- Alamri, Z.Z. Apigenin attenuates indomethacin-induced gastric ulcer in rats: Emphasis on antioxidant, anti-inflammatory, anti-apoptotic, and TGF-β1 enhancing activities. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 8803–8814. [Google Scholar] [CrossRef] [PubMed]
- Murti, K.; Kaushik, M.; Sangwan, Y.; Kaushik, A. Pharmacological properties of Valeriana officinalis—A review. Pharmacol. Online 2011, 3, 641–646. [Google Scholar]
- Ratnam, N.; Naijibullah, M.; Ibrahim, M.D. A review on Cucurbita pepo. Int. J. Pharmacol. Phytochem. Res. 2017, 9, 1190–1194. [Google Scholar] [CrossRef]
- Murkovic, M.; Mülleder, U.; Neunteufl, H. Carotenoid Content in Different Varieties of Pumpkins. J. Food Compos. Anal. 2002, 15, 633–638. [Google Scholar] [CrossRef]
- Caili, F.U.; Huan, S.H.I.; Quanhong, L.I. A Review on Pharmacological Activities and Utilization Technologies of Pumpkin. Plant Foods Hum. Nutr. 2006, 61, 70–77. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The pharmacology of Equisetum arvense—A review. IOSR J. Pharm. 2017, 7, 31–42. [Google Scholar] [CrossRef]
- Uslu, M.E.; Erdogan, I.; Oguzbayraktar, O.; Ates, M. Optimization of extraction conditions for active components in Equisetum arvense extract. Rom. Biotechnol. Lett. 2013, 18, 8115–8131. [Google Scholar]
- Sureshkumar, J.; Jenipher, C.; Sriramavaratharajan, V.; Gurav, S.S.; Rajiv Gandhi, G.; Ravichandran, K.; Ayyanar, M. Genus Equisetum L: Taxonomy, toxicology, phytochemistry and pharmacology. J. Ethnopharmacol. 2023, 314, 116630. [Google Scholar] [CrossRef] [PubMed]
- Eghlima, G.; Esmaeili, H.; Frzaneh, M.; Mirjalili, M.H. Multivariate analysis of Equisetum arvense L. ecotypes based on silicon content, phytochemical and morphological characterization. Silicon 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Meczarska, K.; Cyboran-Mikołajczyk, S.; Solarska-Sciuk, K.; Oszmianski, J.; Siejak, K.; Bonarska-Kujawa, D. Protective Effect of Field Horsetail Polyphenolic Extract on Erythrocytes and Their Membranes. Int. J. Mol. Sci. 2025, 26, 3213. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Kim, D.H.; Cho, J.H.; Kim, Y.C. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. J. Ethnopharmacol. 2004, 95, 421–424. [Google Scholar] [CrossRef]
- Camargo-Ricalde, S.L. Descripción, distribución, anatomía, composición química y usos de Mimosa tenuiflora (Fabaceae-Mimosoideae) en México. Rev. Biol. Trop. 2000, 48, 939–954. [Google Scholar]
- Rivera-Arce, E.; Gattuso, M.; Alvarado, R.; Zarate, E.; Agüero, J.; Feria, I.; Lozoya, X. Pharmacognostical studies of the plant drug Mimosae tenuiflorae cortex. J. Ethnopharmacol. 2007, 113, 400–408. [Google Scholar] [CrossRef]
- Souza, R.S.O.; Albuquerque, U.P.; Monteiro, J.M.; Cavalcanti de Amorim, E.L. Jurema-Preta (Mimosa tenuiflora [Willd.] Poir.): A Review of its Traditional Use, Phytochemistry and Pharmacology. Braz. Arch. Biol. Technol. 2008, 51, 937–947. [Google Scholar] [CrossRef]
- Rivera-Arce, E.; Chavez-Soto, M.A.; Herrera-Arellano, A.; Arzate, S.; Agüero, J.; Feria-Romero, I.A.; Cruz-Guzman, A.; Lozoya, X. Therapeutic effectiveness of a Mimosa tenuiflora cortex extract in venous leg ulceration treatment. J. Ethnopharmacol. 2007, 109, 523–528. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive profile of various Salvia officinalis L. preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef]
- Miraj, S.; Kiani, S. A review study of therapeutic effects of Salvia officinalis L. Scholars Research Library. Pharm. Lett. 2016, 8, 299–303. [Google Scholar]
- Baricevic, D.; Sosa, S.; Della Loggia, R.; Tubaro, A.; Simonovska, B.; Krasna, A.; Zupancic, A. Topical anti-inflammatory activity of Salvia officinalis L. leaves: The relevance of ursolic acid. J. Ethnopharmacol. 2001, 75, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid.-Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Ugarte, G.A.; López-Malo, A.; Sosa-Morales, M.E. Cinnamon (Cinnamomum zeylanicum) Essential Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preddy, V.R., Ed.; Academic Press: London, UK, 2016. [Google Scholar]
- Avula, B.; Smillie, T.J.; Wang, Y.H.; Zweigenbaum, J.; Khan, I.A. Authentication of true cinnamon (Cinnamon verum) utilising direct analysis in real time (DART)-QToF-MS. Food Addit. Contam. Part A 2015, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sánchez, K.; Cruz-Sosa, F.; Zamilpa-Alvarez, A.; Nicasio-Torres, P. Active compounds and anti-inflammatory activity of the methanolic extracts of the leaves and callus from Tilia americana var. mexicana propagated plants. Plant Cell Tissue Organ Cult. 2019, 137, 55–64. [Google Scholar] [CrossRef]
- Pavón, N.P.; Rico, G.V. An endangered and potentially economic tree of Mexico: Tilia mexicana (Tiliaceae). Econ. Bot. 2000, 54, 113–114. [Google Scholar]
- Monroy-Ortiz, C.; Castillo-España, P. Plantas Medicinales Utilizadas en el Estado de Morelos; Editorial Universidad Autónoma del Estado de Morelos: Morelos, Mexico, 2007. [Google Scholar]
- Cardenas-Rodriguez, N.; Gonzalez-Trujano, M.E.; Aguirre-Hernandez, E.; Ruiz-Garcia, M.; Sampieri, A.; Coballase-Urrutia, E.; Carmona-Aparicio, L. Anticonvulsant and antioxidant effects of Tilia americana var. mexicana and flavonoids constituents in the pentylenetetrazole-induced seizures. Oxidative Med. Cell. Longev. 2014, 2014, 329172. [Google Scholar] [CrossRef]
- Carmona-Aparicio, L.; Ortega-Cuellar, D.; González-Trujano, M.E.; Rodríguez-Chávez, J.L.; Aguirre-Hernández, E.; Sampieri, A.; Floriano-Sánchez, E.; Coballase-Urrutia, E.; Cárdenas-Rodríguez, N. Ethyl acetate extract of Tilia americana var. mexicana, a new cytotoxicity and antioxidant agent. J. Food Agric. Environ. 2014, 12, 78–81. [Google Scholar]
- Coballase-Urrutia, E.; Cárdenas-Rodríguez, N.; González-García, M.C.; Núñez-Ramírez, E.; Floriano-Sánchez, E.; González-Trujano, M.E.; Fernández-Rojas, B.; Pedraza-Chaverrí, J.; Montesinos-Correa, H.; Rivera-Espinosa, L.; et al. Biochemical and molecular modulation of CCl4-induced peripheral and central damage by Tilia americana var. mexicana extracts. Saudi Pharm. J. 2017, 25, 319–331. [Google Scholar] [CrossRef]
- Aguirre-Hernández, E.; González-Trujano, M.E.; Terrazas, T.; Herrera-Santoyo, J.; Guevara-Fefer, P. Anxiolytic and sedative-like effects of flavonoids from Tilia americana var. mexicana: GABAergic and serotonergic participation. Salud Ment. 2016, 39, 37–46. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia citrodora Paláu (Lemon verbena): A review of phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Compounds. Phytother. Res. 2009, 23, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Hosseinzadeh, H. Grapes (Vitis vinifera) as a Potential Candidate for the Therapy of the Metabolic Syndrome. Phytother. Res. 2016, 30, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Chan, S.W. A review of the pharmacological effects of piceatannol on cardiovascular diseases. Phytother. Res. 2014, 28, 1581–1588. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Chen, Y.; Agarwal, R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from Grape Seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidin B5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis 1999, 20, 1737–1745. [Google Scholar] [CrossRef]
- Ali, B.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef]
- Lantz, R.C.; Chen, G.J.; Sarihan, M.; Sólyom, A.M.; Jolad, S.D.; Timmermann, B.N. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine 2007, 14, 123–128. [Google Scholar] [CrossRef]
- Iwaskai, Y.; Morita, A.; Iwasawa, T.; Kobata, K.; Sekiwa, Y.; Morimitsu, Y.; Kubota, K.; Watanabe, T. A nonpungent component of steamed ginger— (10) shogaol—Increases adrenaline secretion via the activation of TRPV1. Nutr. Neurosci. 2006, 9, 169–178. [Google Scholar]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Valladares-Carranza, B.; Madariaga-Navarrete, A.; Higuera-Piedrahita, R.I.; Delgadillo-Ruiz, L.; Bañuelos-Valenzuela, R.; Zaragoza-Bastida, A. Phytochemical Compounds and Pharmacological Properties of Larrea tridentata. Molecules 2022, 27, 5393. [Google Scholar] [CrossRef]
- Alcaraz-Padilla, J.C. La Socialización Vecinal, un Requerimiento de Integración Entre los Macroproyectos con los Barrios Tradicionales Y Parques Históricos. Caso de Estudio: El Parque Morelos y el barrio El Retiro, en Guadalajara, Jalisco; Editorial Universitat Politècnica de València: Guadalajara, México, 2019. [Google Scholar]
- Verma, A.K.; Kumar, M.; Bussmann, R.W. Medicinal plants in an urban environment: The medicinal flora of Banares Hindu University, Varanasi, Uttar Pradesh. J. Ethnobiol. Ethnomed. 2007, 3, 35. [Google Scholar] [CrossRef]
- Morett-Saucedo, G.; Calleja-Ávila, L.; Luna-Ríos, M.C. El Santuario de Guadalajara: De la pérdida de la tradición a la recuperación del barrio. Proyecto de Aplicación Profesional (PAP). In Programa de Construcción de Opinión Pública e Incidencia en los Medios; ITESO: Guadalajara, Mexico, 2017; 50p. [Google Scholar]
- GDLAHORA, Breve Historia Jardín Botánico. 2015. Available online: https://gdlahora.wordpress.com/2015/06/24/breve-historia-jardin-botanico/#respond (accessed on 12 August 2025).
- El Informador, Entregan Obras Alrededor del Hospital Civil. 2017. Available online: https://www.informador.mx/jalisco/Entregan-obras-alrededor-del-Hospital-Civil-20171217-0046.html (accessed on 12 August 2025).
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Serie Libros, Number 6; Instituto de Geografía Universidad Autónoma de México: México city, Mexico, 2004; pp. 21–50. [Google Scholar]
- Martin, G.J. Ethnobotany, 1st ed.; Chapman & Hall: London, UK, 1995. [Google Scholar]
- International Society of Ethnobiology (ISE). ISE Code of Ethics. 2006. Available online: http://ethnobiology.net/code-of-ethics/ (accessed on 15 October 2022).
- Albuquerque, U.P.; Cunha, L.; Lucena, R.; Alves, R.R.N. Methods and Techniques in Ethnobiology and Ethnoecology, 1st ed.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Tropicos.org. Missouri Botanical Garden. Available online: https://tropicos.org (accessed on 2 February 2024).
- IPNI, International Plant Names Index. The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Herbarium. Available online: http://www.ipni.org (accessed on 17 June 2025).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software package for education and data analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Desing: Gleneden Beach, OR, USA, 2002; 300p. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology and Belongs to a Series Named Developments in Environmental Modelling 20, 2nd ed.; Elsevier Science, B.V.: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Carneiro, M.R.B.; Sallum, L.O.; Martins, J.L.R.; de Castro Peixoto, J.; Napolitano, H.B.; Rosseto, L.P. Overview of the Justicia Genus: Insights into Its Chemical Diversity and Biological Potential. Molecules 2023, 28, 1190. [Google Scholar] [CrossRef] [PubMed]
- Vega-Avila, E.; Tapia-Aguilar, R.; Reyes-Chilpa, R.; Guzmán-Gutiérrez, S.L.; Pérez-Flores, J.; Velasco-Lezama, R. Actividad antibacteriana y antifúngica de Justicia spicigera. Rev. Latinoam. Química 2012, 40, 75–82. [Google Scholar]
- Awad, N.E.; Abdelkawy, M.A.; Hamed, M.A.; Souleman, A.M.A.; Abdelrahman, E.H.; Ramadan, N.S. Anti-oxidant and hepatoprotective effects of Justicia spicigera ethyl acetate fraction and characterization of its anthocyanin content. Int. J. Pharm. Pharm. Sci. 2015, 7, 91–96. [Google Scholar]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Baqueiro-Peña, I.; Guerrero-Beltrán, J. Physicochemical and antioxidant characterization of Justicia spicigera. Food Chem. 2017, 218, 305–312. [Google Scholar] [CrossRef]
- García-Ríos, R.I.; Mora-Pérez, A.; González-Torres, D.; Carpio-Reyes, R.J.; Soria-Fregozo, C. Anxiolytic-like effect of the aqueous extract of Justicia spicigera leaves on female rats: A comparison to diazepam. Phytomedicine 2019, 55, 9–13. [Google Scholar] [CrossRef]
- Belhoussaine, O.; El Kourchi, C.; Harhar, H.; Bouyahya, A.; El Yadini, A.; Fozia, F.; Alotaibi, A.; Ullah, R.; Tabyaoui, M. Chemical Composition, Antioxidant, Insecticidal Activity, and Comparative Analysis of Essential Oils of Leaves and Fruits of Schinus molle and Schinus terebinthifolius. Evid.-Based Complement. Altern. Med. 2022, 2022, 4288890. [Google Scholar] [CrossRef]
- Santos, A.C.A.; Rossato, M.; Agostini, F.; Serafini, L.A.; Santos, P.L.; Molon, R.; Dellacassa, E.; Moyna, P. Chemical composition of the essential oils from leaves and fruits of Schinus molle L. and Schinus terebinthifolius Raddi from Southern Brazil. J. Essent. Oil Bear. Plants 2009, 12, 16–25. [Google Scholar] [CrossRef]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius. J. Food Sci. 2010, 75, 466–472. [Google Scholar] [CrossRef]
- Deveci, O.; Sukan, A.; Tuzun, N.; Kocabas, E.E.H. Chemical composition, repellent and antimicrobial activity of Schinus molle L. J. Med. Plants Res. 2010, 4, 2211–2216. [Google Scholar]
- Tolouee, M.; Alinezhad, S.; Saberi, R.; Eslamifar, A.; Zad, S.J.; Jaimand, K.; Taeb, J.; Rezaee, M.B.; Kawachi, M.; Shams-Ghahfarokhi, M.; et al. Effect of Matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Aspergillus niger van Tieghem. Int. J. Food Microbiol. 2010, 139, 127–133. [Google Scholar] [CrossRef]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G.A. Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef]
- Sepp, J.; Koshovyi, O.; Jakstas, V.; Zvikas, V.; Botsula, I.; Kireyev, I.; Tsemenko, K.; Kukhtenko, O.; Kogermann, K.; Heinamaki, J.; et al. Phytochemical, Technological, and Pharmacological Study on the Galenic Dry Extracts Prepared from German Chamomile (Matricaria chamomilla L.) Flowers. Plants 2024, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Khanavi, M.; Shahsavari, K.; Manayi, A. Matricaria chamomilla: An updated review on biological activities of the plant and constituents. Res. J. Pharmacogn. 2024, 11, 109–136. [Google Scholar] [CrossRef]
- Funke, E.D.; Friedrich, H. Valepotriate in oberirdischen organen einiger arten der valerianaceen. Phytochemistry 1974, 13, 2023–2024. [Google Scholar] [CrossRef]
- Becker, H.; Schrall, R. Valepotriates in tissue cultures of nine different Valerianaceae species in comparison to literature data of the intact plants. J. Natl. Prod. 1980, 43, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Perez Gutierrez, R.M. Review of Cucurbita pepo (Pumpkin) its Phytochemistry and Pharmacology. Med. Chem. 2016, 6, 12–21. [Google Scholar] [CrossRef]
- Adnan, M.; Gul, S.; Batool, S.; Fatima, B.; Rehman, A.; Yaqoob, S.; Shabir, H.; Yousaf, T.; Mussarat, S.; Ali, N.; et al. A review on the ethnobotany, phytochemistry, pharmacology and nutritional composition of Cucurbita pepo L. J. Phytopharm. 2017, 6, 133–139. [Google Scholar] [CrossRef]
- Phillips, K.M.; Ruggio, D.M.; Ashraf-Khorassani, M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J. Agric. Food Chem. 2005, 53, 9436–9445. [Google Scholar] [CrossRef] [PubMed]
- Sabudak, T. Fatty acid composition of seed and leaf oils of pumpkin, walnut, almond, maize, sunflower and melon. Chem. Nat. Compd. 2007, 43, 465–467. [Google Scholar] [CrossRef]
- Stevenson, D.; Eller, F.J.; Wang, L.; Jay-Lin, J.; Wang, T.; Inglett, G.E. Oil and Tocopherol Content and Composition of Pumpkin Seed Oil in 12 Cultivars. J. Agric. Food Chem. 2007, 55, 4005–4013. [Google Scholar] [CrossRef]
- Rabrenovi, B.B.; Dimic, E.B.; Novakovic, M.M.; Tesevic, V.V.; Basic, Z.N. The most important bioactive components of cold pressed oil from different pumpkin (Cucurbita pepo L.) seeds. LWT-Food Sci. Technol. 2014, 55, 521–527. [Google Scholar] [CrossRef]
- Obi, R.K.; Nwanebu, F.C.; Ndubuisi, U.U.; Orji, N.M. Antibacterial qualities and phytochemical screening of the oils of Curcubita pepo and Brassica nigra. J. Med. Plants Res. 2009, 3, 429–432. [Google Scholar]
- Sandhu, N.S.; Kaur, S.; Chopra, D. Equisetum arvense: Pharmacology and phytochemistry—A review. Asian J. Pharm. Clin. Res. 2010, 3, 146–150. [Google Scholar]
- Carneiro, D.M.; Jardim, T.V.; Araújo, Y.C.L.; Arantes, A.C.; de Sousa, A.C.; Barroso, W.K.S.; Sousa, A.L.L.; da Cunha, L.C.; Cirilo, H.N.C.; Bara, M.T.F.; et al. Equisetum arvense: New Evidence Supports Medical use in Daily Clinic. Pharmacog. Rev. 2019, 13, 50–58. [Google Scholar] [CrossRef]
- Economics, M. PDR for Herbal Medicines; Medical Economics Company, Inc.: At Montvale, NJ, USA, 2000; p. 409. [Google Scholar]
- Do Monte, F.H.M.; Do Santos, J.G., Jr.; Russi, M.; Lanziotti, V.M.N.B.; Leal, L.K.A.M.; Cunha, G.M.A. Antinociceptive and anti-inflammatory properties of the hydroalcoholic extract of stems from Equisetum arvense L. in mice. Pharmacol. Res. 2004, 49, 239–243. [Google Scholar] [CrossRef]
- Holzhueter, G.; Narayanan, K.; Gerber, T. Structure of silica in Equisetum arvense. Anal. Bioanal. Chem. 2003, 376, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Pinto de Sousa, R.; Freitas de Oliveira, C.M.; de Lima Sousa Rde, C.; Lima Leite, L.L.; Ozório Oliveira, A.L.; Pereira Ferreira, J.V.B.; de Freitas Pessoa, A.M.; Silva Oliveira, J.L.; Silva Meireles Vde, J.; Lustosa Barros, E.M.; et al. Unraveling the metabolomic profile and acute toxicity of ethanolic extract from Mimosa tenuiflora (Willd.) Poir. root bark. Toxicon 2024, 249, 108076. [Google Scholar] [CrossRef]
- Cruz, M.P.; Andrade, C.M.F.; Silva, K.O.; de Souza, E.P.; Yatsuda, R.; Marques, L.M.; David, J.P.; David, J.M.; Napimoga, M.H.; Clemente-Napimoga, J.T. Antinoceptive and Anti-inflammatory Activities of the Ethanolic Extract, Fractions and Flavones Isolated from Mimosa tenuiflora (Willd.) Poir (Leguminosae). PLoS ONE 2016, 11, e0150839. [Google Scholar] [CrossRef]
- Lima Bezerra, J.J.; Vieira Pinheiro, A.A.; Barbosa Lucena, R. Phytochemistry and teratogenic potential of Mimosa tenuiflora (willd.) poir. (Fabaceae) in ruminants: A systematic review. Toxicon 2021, 195, 78–85. [Google Scholar] [CrossRef]
- Rizwan, K.; Majeed, I.; Bilal, M.; Rasheed, T.; Shakeel, A.; Iqbal, S. Phytochemistry and Diverse Pharmacology of Genus Mimosa: A Review. Biomolecules 2022, 12, 83. [Google Scholar] [CrossRef]
- Longaray Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Zhumaliyeva, G.; Zhussupova, A.; Zhusupova, G.E.; Błonska-Sikora, E.; Cerreto, A.; Omirbekova, N.; Zhunusbayeva, Z.; Gemejiyeva, N.; Ramazanova, M.; Wrzosek, M.; et al. Natural Compounds of Salvia L. Genus and Molecular Mechanism of Their Biological Activity. Biomedicines 2023, 11, 3151. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; Garcia-Moura, L.A.; Ramos de Melo, N.; Sanches-Silva, A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169. [Google Scholar] [CrossRef]
- Singh, N.; Rao, A.S.; Nandal, A.; Kumar, S.; Yadav, S.S.; Ganaie, S.A.; Narasimhan, B. Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chem. 2021, 338, 127773. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Sharma, H. A Review on Medicinal Uses of Cinnamomum verum (Cinnamon). J. Drug Deliv. Ther. 2021, 11, 161–166. [Google Scholar] [CrossRef]
- Juárez-Ramírez, V.A.; Jiménez-Beltrán, M.I.; Zamilpa, A.; Herrera-Ruiz, M.; Abarca-Vargas, R.; Lombardo-Earl, G.; Tortoriello, J.; Jiménez-Ferrer, E. Pharmacokinetic Study of Biotransformation Products from an Anxiolytic Fraction of Tilia americana. Molecules 2017, 22, 1260. [Google Scholar] [CrossRef]
- Szucs, Z.; Cziáky, Z.; Volánszki, L.; Máthé, C.; Vasas, G.; Gonda, S. Production of Polyphenolic Natural Products by Bract-Derived Tissue Cultures of Three Medicinal Tilia spp.: A Comparative Untargeted Metabolomics Study. Plants 2024, 13, 1288. [Google Scholar] [CrossRef]
- Noguerón-Merino, M.C.; Jiménez-Ferrer, E.; Román-Ramos, R.; Zamilpa, A.; Tortoriello, J.; Herrera-Ruiz, M. Interactions of a standardized flavonoid fraction from Tilia americana with Serotoninergic drugs in elevated plus maze. J. Ethnopharmacol. 2015, 164, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Pimenta, A.I.; Calhelha, R.C.; Antonio, A.L.; Barros, L.; Santos-Buelga, C.; Verde, S.C.; Ferreira, C.F.R. Infusions of gamma irradiated Aloysia citrodora L. and Mentha x piperita L: Effects on phenolic composition, cytotoxicity, antibacterial and virucidal activities. Ind. Crops Prod. 2017, 97, 582–590. [Google Scholar] [CrossRef]
- Ragone, M.I.; Sella, M.; Conforti, P.; Volonte, M.G.; Consolini, A.E. The spasmolytic effect of Aloysia citriodora, Palau (South American cedron) is partially due to its vitexin but not isovitexin on rat duodenums. J. Ethnopharmacol. 2007, 113, 258–266. [Google Scholar] [CrossRef]
- Abuhamdah, S.; Abuhamdah, R.; Howes, M.R.; Al-Olimat, S.; Ennaceur, A.; Chazot, P.L. Pharmacological and neuroprotective profile of an essential oil derived from leaves of Aloysia citrodora Palau. J. Pharm. Pharmacol. 2015, 67, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Sprea, R.M.; Fernandes, L.H.M.; Pires, T.C.S.P.; Calhelha, R.C.; Rodrigues, P.J.; Amaral, J.S. Volatile Compounds and Biological Activity of the Essential Oil of Aloysia citrodora Paláu: Comparison of Hydrodistillation and Microwave-Assisted Hydrodistillation. Molecules 2023, 28, 4528. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Salama, Y.; Al-Hajj, N.; Jaradat, N.; Jobran, N.T.; Warad, I.; Hamdan, L.; Alrob, M.A.; Sawafta, A.; Hidmi, A. Chemical composition, anticancer, antimicrobial activity of Aloysia citriodora Palau essential oils from four different locations in Palestine. BMC Complement. Med. Ther. 2024, 24, 94. [Google Scholar] [CrossRef]
- Amico, V.; Barresi, V.; Chillemi, R.; Condorelli, D.F.; Sciuto, S.; Spatafora, C.; Tringali, C. Bioassay-guided isolation of antiproliferative compounds from grape (Vitis vinifera) stems. Nat. Prod. Commun. 2009, 4, 1934578X0900400108. [Google Scholar] [CrossRef]
- Weidner, S.; Rybarczyk, A.; Karamać, M.; Król, A.; Mostek, A.; Grębosz, J.; Amarowicz, R. Differences in the phenolic composition and antioxidant properties between Vitis coignetiae and Vitis vinifera seeds extracts. Molecules 2013, 18, 3410–3426. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, A.; Pragi Kumar, V. Pharmacological effects of Vitis vinifera subsp. vinifera: A review. Int. J. Pharm. Sci. Res. 2023, 14, 2670–2677. [Google Scholar] [CrossRef]
- Dissanayake, K.G.C.; Waliwita, W.A.L.C.; Liyanage, R.P.A. Review on Medicinal Uses of Zingiber officinale (Ginger). Int. J. Health Sci. Res. 2020, 10, 142–148. [Google Scholar]
- Dhanik, J.; Arya, N.; Nand, V. A Review on Zingiber officinale. J. Pharmacogn. Phytochem. 2017, 6, 174–184. [Google Scholar]
- Zammel, N.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Rebai, T.; Kausar, M.A.; Adnan, M.; Siddiqui, A.J.; Badraoui, R. Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study. Foods 2021, 10, 1383. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Tarfaoui, K.; Brhadda, N.; Ziri, R.; Oubihi, A.; Imtara, H.; Haida, S.; Al Kamaly, O.M.; Saleh, A.; Parvez, M.K.; Fettach, S.; et al. Chemical Profile, Antibacterial and Antioxidant Potential of Zingiber officinale Roscoe and Elettaria cardamomum (L.) Maton Essential Oils and Extracts. Plants 2022, 11, 1487. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabiana, M.H.; Suna, W.; Chenga, Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019, 69, 546–556. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.; Lu, F.; Peng, W.; Wu, C. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytother. Res. 2021, 35, 711–742. [Google Scholar] [CrossRef]
- Skouta, R.; Morán-Santibañez, K.; Valenzuela, C.A.; Vasquez, A.H.; Fenelon, K. Assessing the Antioxidant Properties of Larrea tridentata Extract as a Potential Molecular Therapy against Oxidative Stress. Molecules 2018, 23, 1826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vassão, D.G.; Kim, S.-J.; Milhollan, J.K.; Eichinger, D.; Davin, L.B.; Lewis, N.G. A pinoresinol–lariciresinol reductase homologue from the creosote bush (Larrea tridentata) catalyzes the efficient in vitro conversion of p-coumaryl/coniferyl alcohol esters into the allylphenols chavicol/eugenol, but not the propenylphenols p-anol/isoeugenol, Arch. Biochem. Biophys. 2007, 465, 209–218. [Google Scholar] [CrossRef]
- Núñez-Mojica, G.; Vázquez-Ramírez, A.L.; García, A.; Rivas-Galindo, V.M.; Garza-González, E.; Cuevas González-Bravo, G.E.; Toscano, R.A.; Moo-Puc, R.E.; Villanueva-Toledo, J.R.; Marchand, P.; et al. New cyclolignans of Larrea tridentata and their antibacterial and cytotoxic activities. Phytochem. Lett. 2021, 43, 212–218. [Google Scholar] [CrossRef]
- Arteaga, S.; Andrade-Cetto, A.; Cardenas, R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J. Ethnopharmacol. 2005, 98, 231–239. [Google Scholar] [CrossRef] [PubMed]
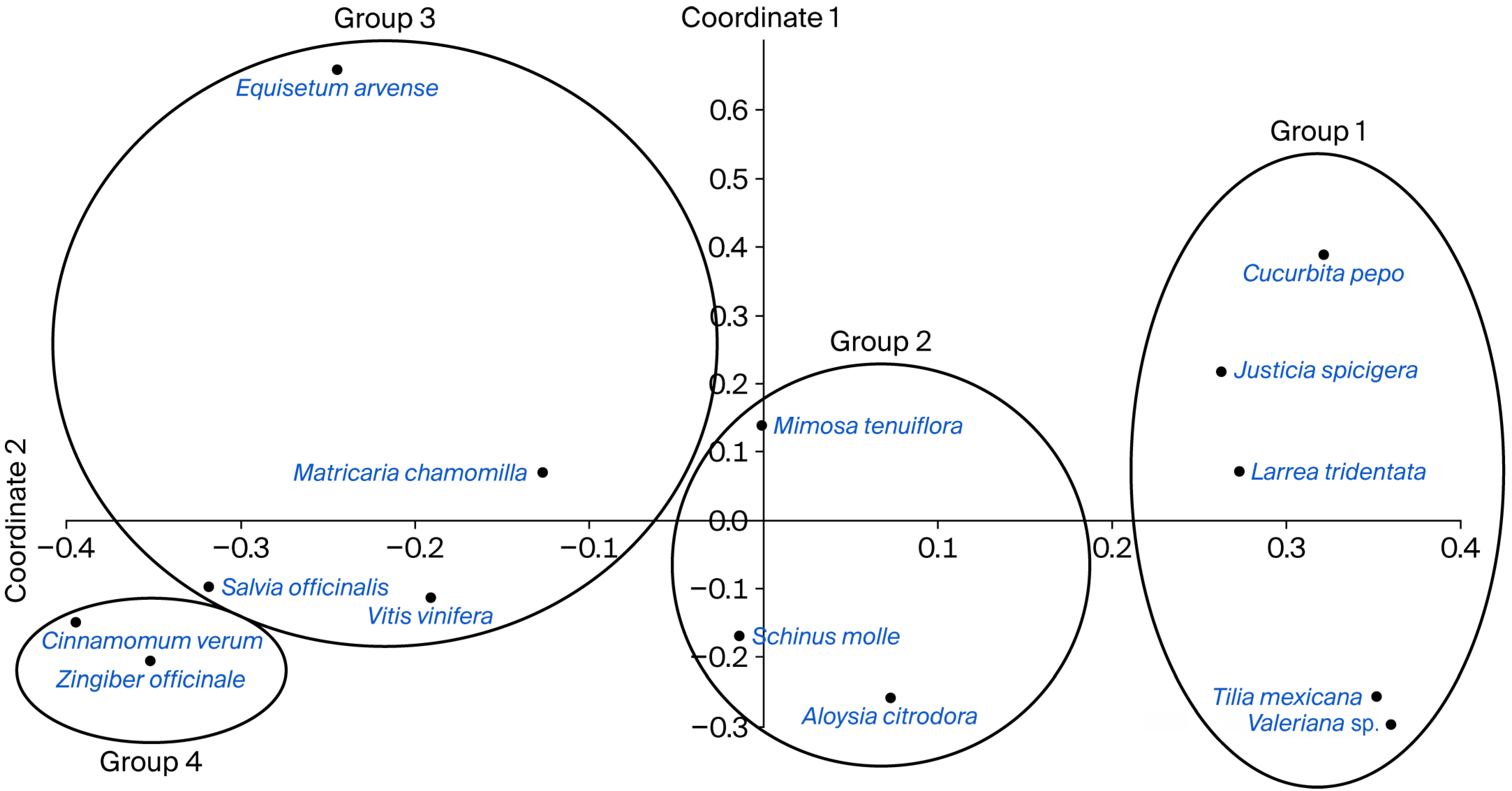
| Explained Variance: | Axis 1 | Axis 2 | Axis 3 |
|---|---|---|---|
| Extracted | 42.99% | 12.76% | 10.13% |
| Cumulative | 29.861% | 55.75% | 65.88% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-González, R.E.; Huerta-Martínez, F.M.; Neri-Luna, C.; Barrientos-Ramírez, L.; Muñoz-Urias, A. Ethnobotany in a Modern City: The Persistence in the Use of Medicinal Plants in Guadalajara, Mexico. Plants 2025, 14, 2788. https://doi.org/10.3390/plants14172788
Martínez-González RE, Huerta-Martínez FM, Neri-Luna C, Barrientos-Ramírez L, Muñoz-Urias A. Ethnobotany in a Modern City: The Persistence in the Use of Medicinal Plants in Guadalajara, Mexico. Plants. 2025; 14(17):2788. https://doi.org/10.3390/plants14172788
Chicago/Turabian StyleMartínez-González, Rosa Elena, Francisco Martín Huerta-Martínez, Cecilia Neri-Luna, Lucía Barrientos-Ramírez, and Alejandro Muñoz-Urias. 2025. "Ethnobotany in a Modern City: The Persistence in the Use of Medicinal Plants in Guadalajara, Mexico" Plants 14, no. 17: 2788. https://doi.org/10.3390/plants14172788
APA StyleMartínez-González, R. E., Huerta-Martínez, F. M., Neri-Luna, C., Barrientos-Ramírez, L., & Muñoz-Urias, A. (2025). Ethnobotany in a Modern City: The Persistence in the Use of Medicinal Plants in Guadalajara, Mexico. Plants, 14(17), 2788. https://doi.org/10.3390/plants14172788








