Integrating Artificial Intelligence and Biotechnology to Enhance Cold Stress Resilience in Legumes
Abstract
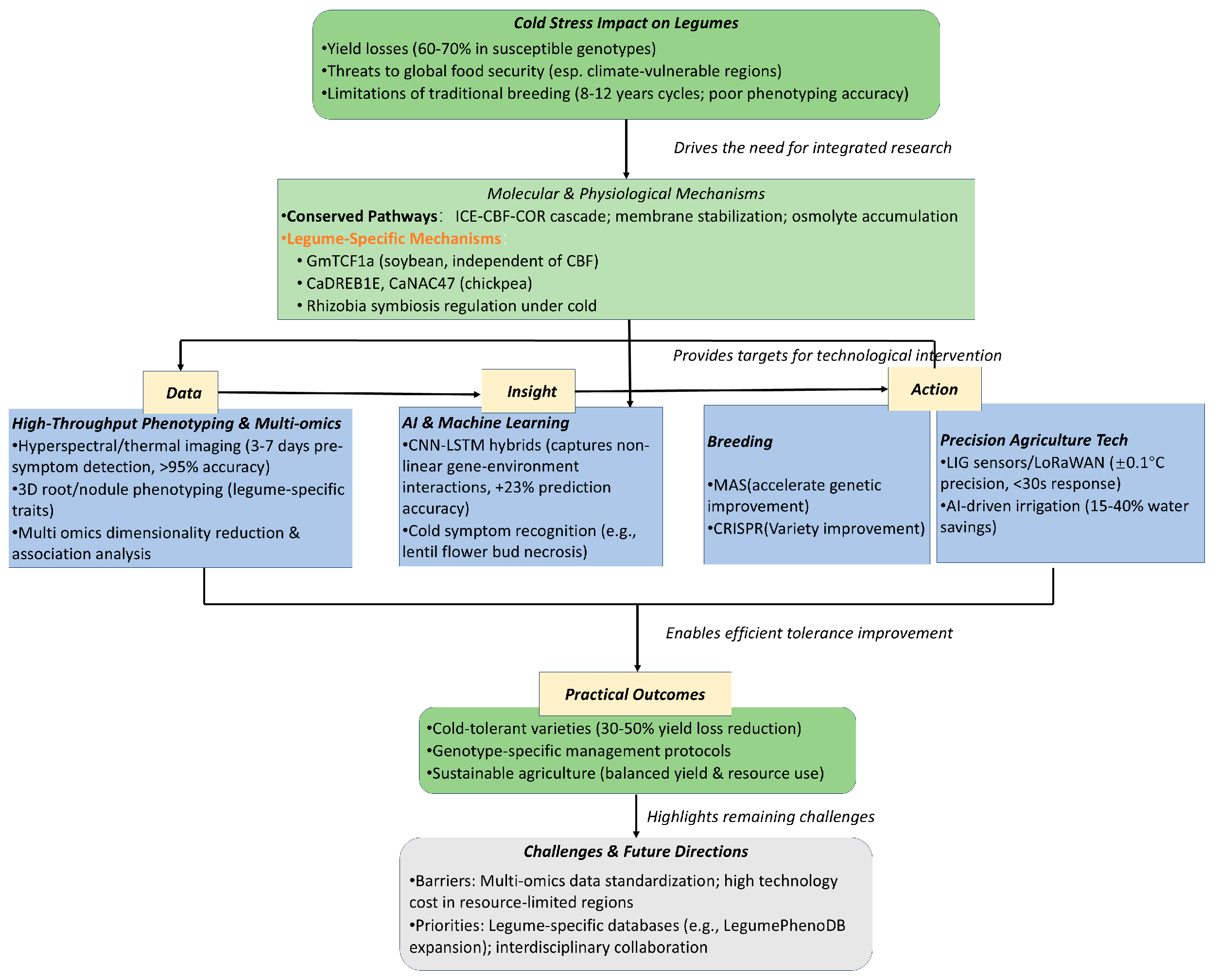
1. Introduction
2. Molecular Foundations of Cold Tolerance in Leguminous Plants
2.1. Core Regulatory Pathways and Mechanisms
2.2. Genomic Architecture and QTL Mapping
2.3. Physiological Adaptations and Cellular Protection
3. AI Applications in Cold-Tolerance Research
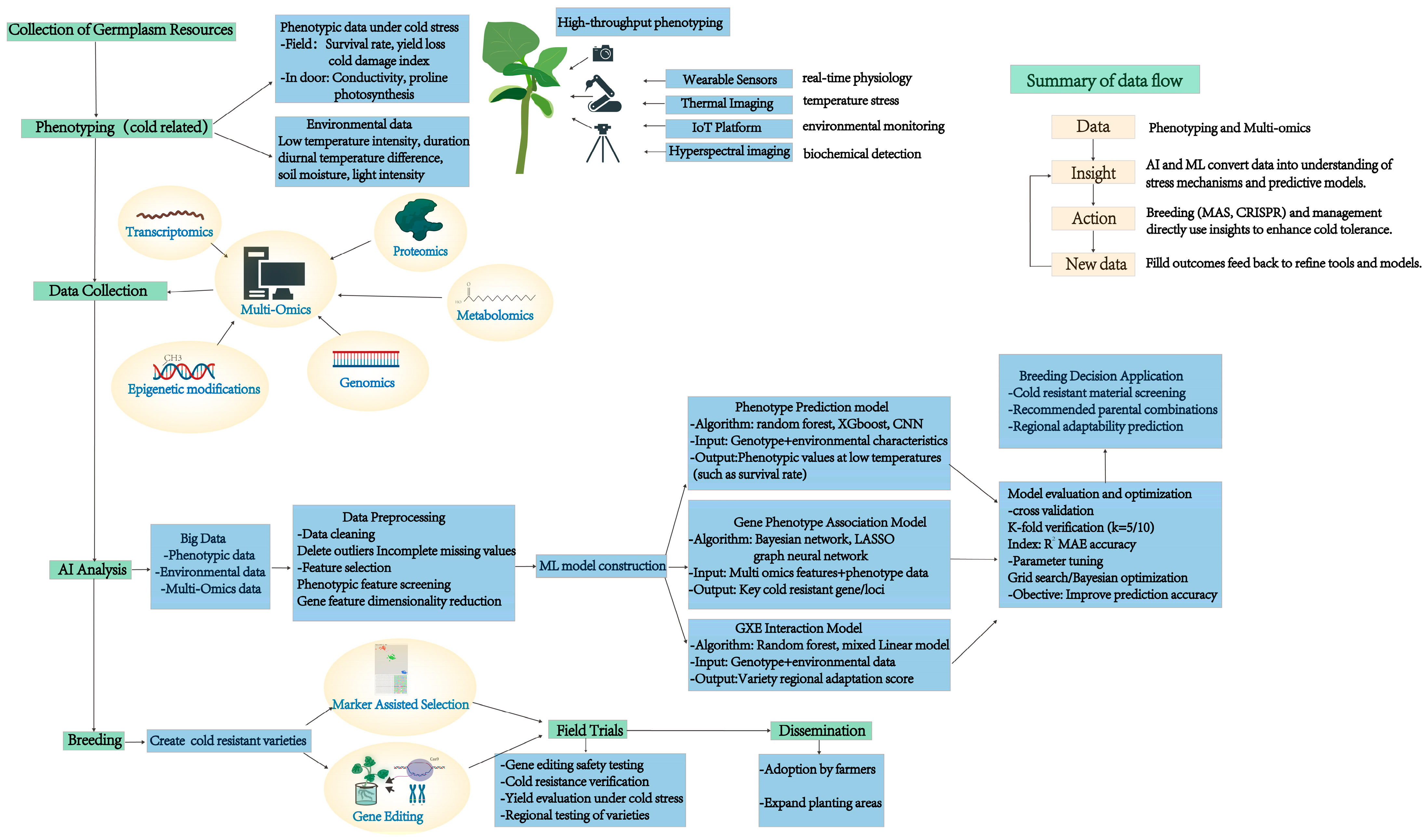
3.1. Computer Vision and Deep Learning for Advanced Plant Phenotyping
3.2. Advanced High-Throughput Phenotyping Platforms
3.3. Hyperspectral and Multispectral Imaging Applications
3.4. Thermal Imaging and Temperature-Based Stress Assessment
3.5. Segmentation Techniques and Morphometric Analysis
4. Machine Learning Models for Stress Prediction and Analysis
4.1. Deep Learning Architectures for Temporal Stress Dynamics
4.2. Classical Machine Learning Approaches
4.3. Explainable AI and Feature Analysis
5. Advanced Sensor Technologies and IoT Integration for Stress Monitoring
5.1. Next-Generation Sensing Platforms
5.2. Multi-Modal Hyperspectral and Thermal Integration
5.3. IoT Platform Integration and Edge Computing
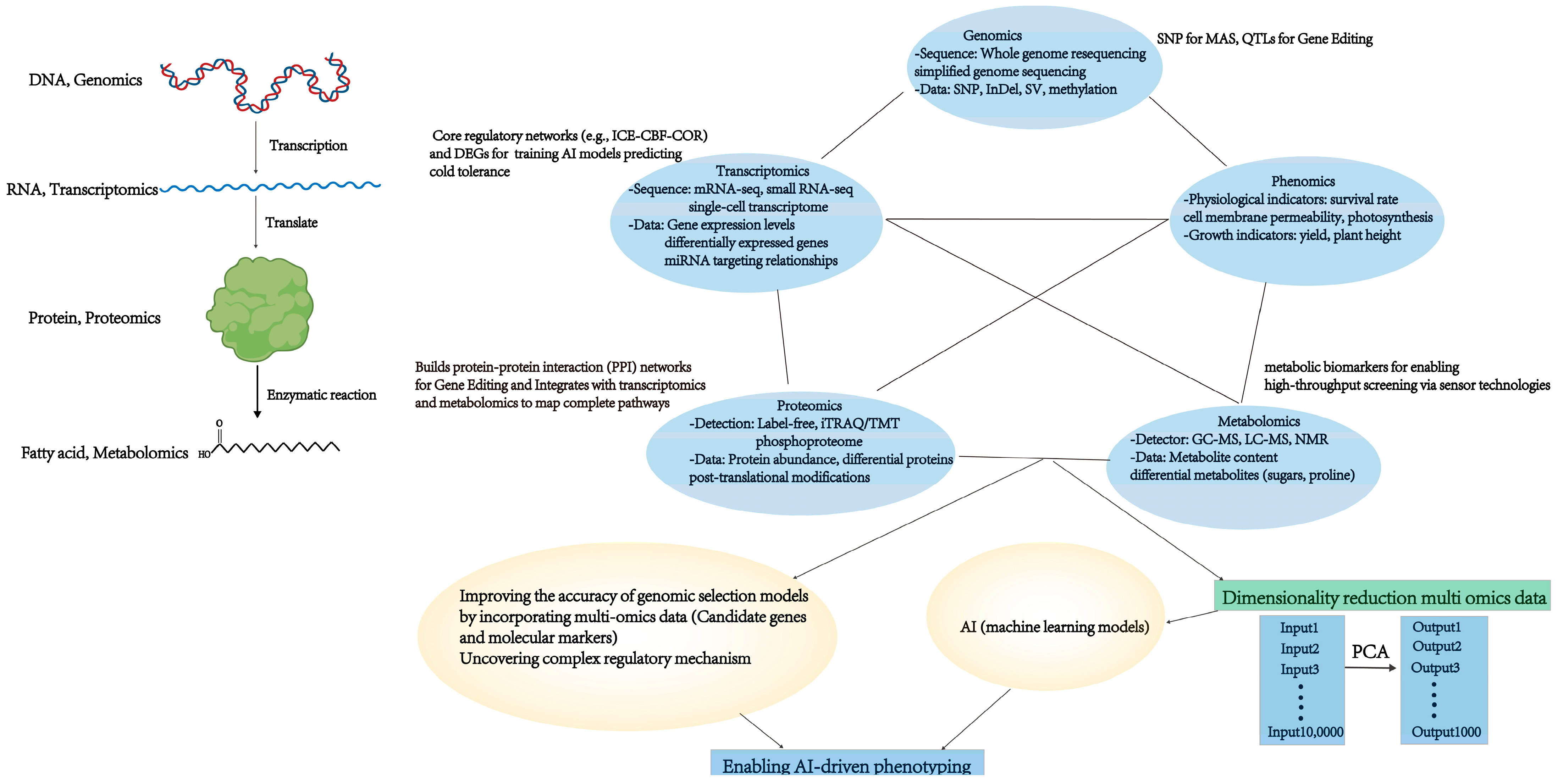
6. Big Data Applications in Legume Breeding and Management
6.1. Genomic Big Data Analytics
6.2. High-Throughput Data Analytics and Machine Learning for Legume-Specific Innovations
6.3. Precision Agriculture Implementation for Legume-Centric Optimization
7. Genomic Selection and AI Integration for Cold Tolerance
7.1. Multi-Trait Genomic Selection Models
7.2. Systems Biology and Multi-Omics Integration
7.3. CRISPR and Gene Editing Applications
8. Conclusions and Future Research Directions
Implementation Roadmap
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| HTP | High-throughput phenotyping |
| GWAS | Genome-wide association studies |
| IoT | Internet of Things |
| QTL | Quantitative trait locus |
| CNNs | Convolutional neural networks |
| LASSO | Least absolute shrinkage and selection operator |
| PLSR | Partial least squares regression |
| CWSI | Crop water stress index |
| RNNs | Recurrent neural networks |
| LSTM | Long short-term memory |
| SVMs | Support vector machines |
| EAI | Explainable AI |
| LIG | Laser-induced graphene |
| LoRaWAN | Long-range wide-area network |
| FPGAs | Field-programmable gate arrays |
| GPUs | Graphics processing units |
| GS | Genomic selection |
| GEBVs | Genomic estimated breeding values |
| MAS | Marker-assisted selection |
References
- Akibode, S.; Maredia, M. Global and regional trends in production, trade and consumption of food legume crops. Food Secur. 2011, 3, 175–187. [Google Scholar]
- Foyer, C.H.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.; Nayyar, H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef]
- Varshney, R.K.; Pandey, M.K.; Bohra, A.; Singh, V.K.; Thudi, M.; Saxena, R.K. Toward the sequence-based breeding in legumes in the post-genome sequencing era. Theor. Appl. Genet. 2019, 132, 797–816. [Google Scholar] [CrossRef]
- Jan, N.; Hussain, M.; Andrabi, K.I. Cold stress and photosynthesis. In Photosynthesis: Molecular Approaches to Solar Energy Conversion; Springer: Berlin/Heidelberg, Germany, 2022; pp. 297–315. [Google Scholar]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Bhat, K.; Mahajan, R.; Pakhtoon, M.; Urwat, U.; Bashir, Z.; Shah, A.; Agrawal, A.; Bhat, B.; Sofi, P.; Masi, A.; et al. Low Temperature Stress Tolerance: An Insight Into the Omics Approaches for Legume Crops. Front. Plant Sci. 2022, 13, 888710. [Google Scholar] [CrossRef] [PubMed]
- Tsegaw, M.; Zegeye, W.A.; Jiang, B.; Sun, S.; Yuan, S.; Han, T.; Wu, T. Progress and Prospects of the Molecular Basis of Soybean Cold Tolerance. Plants 2023, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Hegde, V.; Pattanayak, A.; Singh, A.K.; Reddy, P.S. Genetic diversity and population structure analysis of Indian pigeonpea [Cajanus cajan (L.) Millsp.] landraces using genomic SSR markers. 3 Biotech 2021, 11, 1–14. [Google Scholar]
- Singh, A.; Jones, S.; Ganapathysubramanian, B.; Sarkar, S.; Mueller, D.; Sandhu, K.; Nagasubramanian, K. Challenges and opportunities in machine-augmented plant stress phenotyping. Trends Plant Sci. 2021, 26, 53–69. [Google Scholar] [CrossRef]
- UN DESA (United Nations, Department of Economic and Social Affairs, Population Division). World Population Prospects 2019: Highlights, ST/ESA/SER.A/423; UN DESA (United Nations, Department of Economic and Social Affairs, Population Division): New York, NY, USA, 2019. [Google Scholar]
- Elferink, M.; Schierhorn, F. Global demand for food is rising. Can we meet it? Harv. Bus. Rev. 2016, 7, 2016. [Google Scholar]
- Sita, K.; Sehgal, A.; HanumanthaRao, B.; Nair, R.M.; Vara Prasad, P.V.; Kumar, S.; Gaur, P.M.; Farooq, M.; Siddique, K.H.; Varshney, R.K.; et al. Food legumes and rising temperatures: Effects, adaptive functional mechanisms specific to reproductive growth stage and strategies to improve heat tolerance. Front. Plant Sci. 2017, 8, 1658. [Google Scholar] [CrossRef]
- Suzuki, K.; Takeda, H.; Tsukaguchi, T.; Egawa, Y. Ultrastructural study on degeneration of tapetum in anther of snap bean (Phaseolus vulgaris L.) under heat stress. Sex. Plant Reprod. 2001, 13, 293–299. [Google Scholar] [CrossRef]
- Smýkal, P.; Coyne, C.J.; Ambrose, M.J.; Maxted, N.; Schaefer, H.; Blair, M.W.; Berger, J.; Greene, S.L.; Nelson, M.N.; Besharat, N.; et al. Legume crops phylogeny and genetic diversity for crop improvement. Crit. Rev. Plant Sci. 2015, 34, 43–104. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M. Domestication of Plants in the Old World: The Origin and Spread of Cultivated Plants in West Asia, Europe and the Nile Valley; Oxford University Press: Oxford, UK, 2000. [Google Scholar] [CrossRef]
- Varshney, R.K.; Mohan, S.M.; Gaur, P.M.; Gangarao, N.V.; Pandey, M.K.; Bohra, A.; Sawargaonkar, S.L.; Chitikineni, A.; Kimurto, P.K.; Janila, P.; et al. Achievements and prospects of genomics-assisted breeding in three legume crops of the semi-arid tropics. Biotechnol. Adv. 2013, 31, 1120–1134. [Google Scholar] [CrossRef]
- Gaur, P.M.; Samineni, S.; Krishnamurthy, L.; Kumar, S.; Ghanem, M.E.; Beebe, S.; Rao, I.; Chaturvedi, S.K.; Basu, P.S.; Nayyar, H.; et al. Heat tolerance in grain legumes. Plant Breed. 2015, 134, 379–391. [Google Scholar]
- Nayyar, H.; Chander, S.; Kumar, S.; Bains, T. Glycine betaine mitigates cold stress damage in chickpea. Agron. Sustain. Dev. 2005, 25, 381–388. [Google Scholar] [CrossRef]
- Sanghera, G.S.; Wani, S.H.; Hussain, W.; Singh, N.B. Engineering cold stress tolerance in crop plants. Curr. Genom. 2011, 12, 30–43. [Google Scholar] [CrossRef]
- Thudi, M.; Upadhyaya, H.D.; Rathore, A.; Gaur, P.M.; Krishnamurthy, L.; Roorkiwal, M.; Nayak, S.N.; Chaturvedi, S.K.; Basu, P.S.; Gangarao, N.V.; et al. Genetic diversity and population structure of chickpea (Cicer arietinum L.). PLoS ONE 2014, 9, e96292. [Google Scholar]
- Roorkiwal, M.; Bharadwaj, C.; Barmukh, R.; Dixit, G.P.; Thudi, M.; Gaur, P.M.; Chaturvedi, S.K.; Fikre, A.; Hamwieh, A.; Kumar, S.; et al. Integrating genomics for chickpea improvement: Achievements and opportunities. Theor. Appl. Genet. 2020, 133, 1703–1720. [Google Scholar] [CrossRef]
- Liakos, K.G.; Busato, P.; Moshou, D.; Pearson, S.; Bochtis, D. Machine learning in agriculture: A review. Sensors 2018, 18, 2674. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sarkar, S.; Ganapathysubramanian, B.; Singh, A.K. Deep learning for plant stress phenotyping: Trends and future perspectives. Trends Plant Sci. 2024, 29, 290–305. [Google Scholar] [CrossRef]
- Crossa, J.; Pérez-Rodríguez, P.; Cuevas, J.; Montesinos-López, O.; Jarquín, D.; de los Campos, G.; Burgueño, J.; González-Camacho, J.M.; Pérez-Elizalde, S.; Beyene, Y.; et al. Genomic selection in plant breeding: Methods, models, and perspectives. Trends Plant Sci. 2017, 22, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Camacho, J.M.; de Los Campos, G.; Pérez, P.; Gianola, D.; Cairns, J.E.; Mahuku, G.; Babu, R.; Crossa, J. Genome-enabled prediction of genetic values for yield in the CIMMYT maize breeding program. Plant Genome 2012, 5, 55–66. [Google Scholar]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop phenomics and high-throughput phenotyping: Past decades, current challenges, and future perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef]
- Furbank, R.T.; Jimenez-Berni, J.A.; George-Jaeggli, B.; Potgieter, A.B.; Deery, D.M. Field crop phenomics: Enabling breeding for radiation use efficiency and biomass in cereal crops. New Phytol. 2019, 223, 1714–1727. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Barbedo, J.G.A. Deep learning approaches for plant disease detection and diagnosis: A comprehensive review. Comput. Electron. Agric. 2019, 164, 104924. [Google Scholar]
- Kamilaris, A.; Prenafeta-Boldú, F.X. Deep learning in agriculture: A survey. Comput. Electron. Agric. 2018, 147, 70–90. [Google Scholar] [CrossRef]
- Varshney, R.K.; Roorkiwal, M.; Sorrells, M.E. Genomic selection for crop improvement in the post-genomic era. Theor. Appl. Genet. 2021, 134, 297–319. [Google Scholar]
- Hayes, B.J.; Bowman, P.J.; Chamberlain, A.C.; Verbyla, K.; Goddard, M.E. Accuracy of genomic breeding values in multi-breed dairy cattle populations. Genet. Sel. Evol. 2009, 41, 51. [Google Scholar] [CrossRef]
- Taneja, M.; Byabazaire, J.; Jalodia, N.; Davy, A.; Olariu, C.; Malone, P. Machine learning based fog computing assisted data-driven approach for early lameness detection in dairy cattle. Comput. Electron. Agric. 2019, 171, 105286. [Google Scholar] [CrossRef]
- Villa-Henriksen, A.; Edwards, G.T.; Pesonen, L.A.; Green, O.; Sørensen, C.A.G. Internet of Things in arable farming: Implementation, applications, challenges, and potential. Biosyst. Eng. 2020, 191, 60–84. [Google Scholar] [CrossRef]
- Ojha, T.; Misra, S.; Raghuwanshi, N.S. Wireless sensor networks for agriculture: The state-of-the-art in practice and future challenges. Comput. Electron. Agric. 2015, 118, 66–84. [Google Scholar] [CrossRef]
- Vellidis, G.; Tucker, M.; Perry, C.; Kvien, C.; Bednarz, C. A real-time wireless smart sensor array for scheduling irrigation. Comput. Electron. Agric. 2008, 61, 44–50. [Google Scholar] [CrossRef]
- Shi, W.; Cao, J.; Zhang, Q.; Li, Y.; Xu, L. Edge computing: Vision and challenges. IEEE Internet Things J. 2016, 3, 637–646. [Google Scholar] [CrossRef]
- Satyanarayanan, M. The emergence of edge computing. Computer 2017, 50, 30–39. [Google Scholar] [CrossRef]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef]
- Scheben, A.; Batley, J.; Edwards, D. Genotyping-by-sequencing approaches to characterize crop genomes: Choosing the right tool for the right application. Plant Biotechnol. J. 2017, 15, 149–161. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.R.; Zaman-Allah, M.; Sreenivasulu, N.; Trethowan, R.; Varshney, R.K. Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 2012, 125, 625–645. [Google Scholar] [CrossRef] [PubMed]
- Crain, J.; Mondal, S.; Rutkoski, J.; Singh, R.P.; Poland, J. Combining high-throughput phenotyping and genomic information to increase prediction and selection accuracy in wheat breeding. Plant Genome 2018, 11, 170043. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.H.; Hong, X.; Agarwal, M.; Zhu, J.K. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, Y.; Yang, S. Cold signal transduction and its interplay with phytohormones during cold acclimation. Plant Cell Physiol. 2015, 56, 7–15. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Jin, Y.N.; Tang, R.J.; Wang, H.H.; Jiang, C.M.; Bao, Y.; Yang, Y.; Liang, M.X.; Ma, D.F.; Lv, J.; Chen, S.P.; et al. Identification of genes from the ICE-CBF-COR pathway under cold stress in Aegilops-Triticum composite group and the evolution analysis with those from Triticeae. Physiol. Mol. Biol. Plants 2018, 24, 211–229. [Google Scholar] [CrossRef]
- Akbari, M.; Mahna, N.; Ramesh, S.V.; Kappachery, S.; Venkatesh, J.; Yu, J.W.; Park, S.W. Genome-wide transcriptional profiling provides clues to molecular mechanisms underlying cold tolerance in chickpea. Sci. Rep. 2023, 13, 6080. [Google Scholar] [CrossRef]
- Ahmad, S.; Abbas, G.; Ahmed, M.; Fatima, Z.; Anjum, M.A.; Rasul, G.; Khan, M.A.; Hoogenboom, G.; Siddique, K.H.M. Climate change and chickpea: Problems and solutions. Crop Pasture Sci. 2023, 74, 520–536. [Google Scholar]
- Dong, Z.; Wang, H.; Li, X.; Ji, H. Enhancement of plant cold tolerance by soybean RCC1 family gene GmTCF1a. BMC Plant Biol. 2021, 21, 369. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3-and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642. [Google Scholar] [CrossRef]
- Miura, K.; Jin, J.B.; Lee, J.; Yoo, C.Y.; Stirm, V.; Miura, T.; Ashworth, E.N.; Bressan, R.A.; Yun, D.J.; Hasegawa, P.M. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell 2007, 19, 1403–1414. [Google Scholar] [CrossRef]
- Jähne, F.; Hahn, V.; Würschum, T.; Leiser, W.L. Cold stress tolerance of soybeans during flowering: QTL mapping and efficient selection strategies under controlled conditions. Plant Breed. 2019, 138, 708–721. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Zhang, Q.; Song, C.; Zhong, C.; Zhang, X.; Zhao, X.; Wang, H.; Huang, L.; Zhou, W.; et al. Rapid identification of molecular markers for cold tolerance at the germination stage in soybean. Plant Breed. 2022, 141, 488–498. [Google Scholar]
- Lakhiar, I.A.; Yan, H.; Zhang, C.; Wang, G.; He, B.; Hao, B.; Han, Y.; Wang, B.; Bao, R.; Syed, T.N.; et al. A review of precision irrigation water-saving technology under changing climate for enhancing water use efficiency, crop yield, and environmental footprints. Agriculture 2024, 14, 1141. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Kaplan, F.; Guy, C.L. β-Amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 2004, 135, 1674–1684. [Google Scholar] [CrossRef]
- Steponkus, P.L. Role of the plasma membrane in freezing injury and cold acclimation. Ann. Rev. Plant Physiol. 1984, 35, 543–584. [Google Scholar] [CrossRef]
- Yousefi, V.; Ahmadi, J.; Sadeghzadeh-Ahari, D.; Esfandiari, E. Influence of long-term cold stress on enzymatic antioxidative defense system in chickpea (Cicer arietinum L.). Pol. Bot. Soc. 2018, 71, 1752. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Singh, A.; Ganapathysubramanian, B.; Singh, A.K.; Sarkar, S. Machine learning for high-throughput stress phenotyping in plants. Trends Plant Sci. 2016, 21, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Archana, U.; Khan, A.; Sudarshanam, A.; Sathya, C.; Koshariya, A.K.; Krishnamoorthy, R. Plant disease detection using ResNet. In Proceedings of the 2023 International Conference on Inventive Computation Technologies (ICICT), Lalitpur, Nepal, 26–28 April 2023; pp. 1–6. [Google Scholar]
- Saleem, M.H.; Potgieter, J.; Arif, K.M. Plant disease detection and classification by deep learning. Plants 2019, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Lim, K.M.; Song, Y.X.; Alqahtani, A. Plant-CNN-ViT: Plant Classification with Ensemble of Convolutional Neural Networks and Vision Transformer. Plants 2023, 12, 2642. [Google Scholar] [CrossRef]
- Möller, B.; Poeschl, Y.; Plötner, R.; Bürstenbinder, K. PaCeQuant: A Tool for High-Throughput Quantification of Pavement Cell Shape Characteristics. Plant Physiol. 2017, 175, 998–1017. [Google Scholar] [CrossRef]
- Latif, G.; Abdelhamid, S.E.; Mallouhy, R.E.; Alghazo, J.; Kazimi, Z.A. Deep Learning Utilization in Agriculture: Detection of Rice Plant Diseases Using an Improved CNN Model. Plants 2022, 11, 2230. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Le, Q. EfficientNet: Rethinking model scaling for convolutional neural networks. In International Conference on Machine Learning; PMLR: Westminster, UK, 2019; pp. 6105–6114. [Google Scholar] [CrossRef]
- Ubbens, J.R.; Stavness, I. Deep plant phenomics: A deep learning platform for complex plant phenotyping tasks. Fron. Plant Sci. 2017, 8, 1190. [Google Scholar] [CrossRef]
- Banerjee, B.P.; Joshi, S.; Thoday-Kennedy, E.; Pasam, R.K.; Tibbits, J.; Hayden, M.; Spangenberg, G.; Kant, S. High-throughput phenotyping using digital and hyperspectral imaging-derived biomarkers for genotypic nitrogen response. J. Exp. Bot. 2020, 71, 4604–4615. [Google Scholar] [CrossRef]
- Pineda, M.; Barón, M.; Pérez-Bueno, M.L. Thermal imaging for plant stress detection and phenotyping. Remote Sens. 2021, 13, 68. [Google Scholar] [CrossRef]
- Ghosal, S.; Blystone, D.; Singh, A.K.; Ganapathysubramanian, B.; Singh, A.; Sarkar, S. An explainable deep machine vision framework for plant stress phenotyping. Proc. Natl. Acad. Sci. USA 2018, 115, 4613–4618. [Google Scholar] [CrossRef] [PubMed]
- Millan-Almaraz, J.R.; Romero-Troncoso, R.d.J.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Carrillo-Serrano, R.V.; Osornio-Rios, R.A.; Duarte-Galvan, C.; Rios-Alcaraz, M.A.; Torres-Pacheco, I. FPGA-based Fused Smart Sensor for Real-Time Plant-Transpiration Dynamic Estimation. Sensors 2010, 10, 8316–8331. [Google Scholar] [CrossRef]
- Thomas, S.; Kuska, M.T.; Bohnenkamp, D.; Brugger, A.; Alisaac, E.; Wahabzada, M.; Behmann, J.; Mahlein, A.K. Benefits of hyperspectral imaging for plant disease detection and plant protection: A technical perspective. J. Plant Dis. Prot. 2018, 125, 5–20. [Google Scholar] [CrossRef]
- Zou, Y.; Zhong, M.; Li, S.; Qing, Z.; Xing, X.; Gong, G.; Yan, R.; Qin, W.; Shen, J.; Zhang, H.; et al. Flexible wearable strain sensors based on laser-induced graphene for monitoring human physiological signals. Polymers 2023, 15, 3553. [Google Scholar] [CrossRef] [PubMed]
- Atanda, S.A.; Steffes, J.; Lan, Y.; Al Bari, A.; Kim, J.; Morales, M.; Johnson, J.P.; Saludares, R.; Worral, H.; Piche, L.; et al. Multi-trait genomic prediction improves selection accuracy for enhancing seed mineral concentrations in pea. Plant Genome 2022, 15, e20260. [Google Scholar] [CrossRef]
- Voss-Fels, K.P.; Cooper, M.; Hayes, B.J. Accelerating crop genetic gains with genomic selection. Theor. Appl. Genet. 2019, 132, 669–686. [Google Scholar] [CrossRef]
- Tyumentseva, M.; Tyumentsev, A.; Akimkin, V. CRISPR/Cas9 Landscape: Current State and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 16077. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant–environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Mahlein, A.K. Plant disease detection by imaging sensors–parallels and specific demands for precision agriculture and plant phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef]
- El-Manawy, A.I.; Huang, Y.; Zhao, K.; Lv, L.; Huang, M.; Hu, K.; Fletcher, A.; Xu, K.; Li, Y. HSI-PP: A flexible open-source software for hyperspectral imaging-based plant phenotyping. Comput. Electron. Agric. 2022, 197, 106988. [Google Scholar] [CrossRef]
- Sytar, O.; Brestic, M.; Zivcak, M.; Olsovska, K.; Kovar, M.; Shao, H.; He, X. Applying hyperspectral imaging to explore natural plant diversity towards improving salt stress tolerance. Sci. Total Environ. 2017, 578, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Behmann, J.; Steinrücken, J.; Plümer, L. Detection of early plant stress responses in hyperspectral images. ISPRS J. Photogramm. 2014, 93, 98–111. [Google Scholar] [CrossRef]
- Jones, H.G. Application of thermal imaging and infrared sensing in plant physiology and ecophysiology. Adv. Bot. Res. 2004, 41, 107–163. [Google Scholar] [CrossRef]
- Costa, J.M.; Egipto, R.; Sánchez-Virosta, A.; Lopes, C.M.; Chaves, M.M. Canopy and soil thermal patterns to support water and heat stress management in vineyards. Agric. Water Manag. 2019, 216, 484–496. [Google Scholar] [CrossRef]
- Jones, H.G. Thermal imaging and infrared sensing in plant ecophysiology. In Advances in Plant Ecophysiology Techniques; Springer: Berlin/Heidelberg, Germany, 2018; pp. 135–151. [Google Scholar] [CrossRef]
- Chaerle, L.; Van Der Straeten, D. Imaging techniques and the early detection of plant stress. Trends Plant Sci. 2000, 5, 495–501. [Google Scholar] [CrossRef]
- Zubler, A.V.; Yoon, J.-Y. Proximal Methods for Plant Stress Detection Using Optical Sensors and Machine Learning. Biosensors 2020, 10, 193. [Google Scholar] [CrossRef]
- Ghazouani, H.; Rallo, G.; Mguidiche, A.; Latrech, B.; Douh, B.; Boujelben, A.; Provenzano, G. Assessing Hydrus-2D Model to Investigate the Effects of Different On-Farm Irrigation Strategies on Potato Crop under Subsurface Drip Irrigation. Water 2019, 11, 540. [Google Scholar] [CrossRef]
- Elmetwalli, A.H.; El-Hendawy, S.; Al-Suhaibani, N.; Alotaibi, M.; Tahir, M.U.; Mubushar, M.; Hassan, W.M.; Elsayed, S. Potential of Hyperspectral and Thermal Proximal Sensing for Estimating Growth Performance and Yield of Soybean Exposed to Different Drip Irrigation Regimes Under Arid Conditions. Sensors 2020, 20, 6569. [Google Scholar] [CrossRef] [PubMed]
- Paulus, S.; Behmann, J.; Mahlein, A.K.; Plümer, L.; Kuhlmann, H. Low-cost 3D systems: Suitable tools for plant phenotyping. Sensors 2014, 14, 3001–3018. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, D.; Dee, H.; Pridmore, T. Imaging methods for phenotyping of plant traits. In Phenomics in Crop Plants: Trends, Options and Limitations; Springer: Berlin/Heidelberg, Germany, 2013; pp. 61–74. [Google Scholar]
- Khaki, S.; Wang, L. A CNN-RNN framework for crop yield prediction. Front. Plant Sci. 2020, 11, 1750. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, B.-Y.; Kim, M.-J.; Sang, W.; Seo, M.C.; Baek, J.-K.; Yang, J.E.; Mo, C. Development of a soil moisture prediction model based on recurrent neural network long short-term memory (RNN-LSTM) in soybean cultivation. Sensors 2023, 23, 1976. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, M.; Mohammadi, K.; Song, D.; Bi, J.; Wang, G. Machine learning crop yield models based on meteorological features and comparison with a process-based model. Artif. Intell. Earth Syst. 2022, 1, e22000. [Google Scholar] [CrossRef]
- Jácome Galarza, L.; Realpe, M.; Viñán-Ludeña, M.S.; Calderón, M.F.; Jaramillo, S. AgriTransformer: A Transformer-Based Model with Attention Mechanisms for Enhanced Multimodal Crop Yield Prediction. Electronics 2025, 14, 2466. [Google Scholar] [CrossRef]
- Shi, J.; Wang, S.; Qu, P.; Shao, J. Time series prediction model using LSTM-Transformer neural network for mine water inflow. Sci. Rep. 2024, 14, 19085. [Google Scholar] [CrossRef]
- Ham, Y.-G.; Kim, J.-H.; Luo, J.-J. A self-attention–based neural network for three-dimensional multivariate modeling and its skillful ENSO predictions. Sci. Adv. 2023, 9, eadf2827. [Google Scholar] [CrossRef]
- Prechsl, U.E.; Mejia-Aguilar, A.; Cullinan, C.B. In vivo spectroscopy and machine learning for the early detection and classification of different stresses in apple trees. Sci. Rep. 2023, 13, 15857. [Google Scholar] [CrossRef]
- Naik, H.S.; Zhang, J.; Lofquist, A.; Assefa, T.; Sarkar, S.; Ackerman, D.; Singh, A.; Singh, A.K.; Ganapathysubramanian, B. A real-time phenotyping framework using machine learning for plant stress severity rating in soybean. Plant Methods 2017, 13, 23. [Google Scholar] [CrossRef]
- Garg, D.; Singh, H.; Shacham-Diamand, Y. AdapTree: Data-driven approach to assessing plant stress through the AI-sensor synergy. Sensors 2025, 25, 3149. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.; Gill, S.K.; Saini, D.K.; Chopra, Y.; de Koff, J.P.; Sandhu, K.S. A comprehensive review of high throughput phenotyping and machine learning for plant stress phenotyping. Phenomics 2022, 2, 156–183. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Schmid, L.; Walter, A.; Nieto, J.; Liebisch, F. A spatio temporal spectral framework for plant stress phenotyping. Plant Methods 2019, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Thokala, B.; Doraikannan, S. Detection and classification of plant stress using hybrid deep convolution neural networks: A multi-scale vision transformer approach. Trait. Signal 2023, 40, 6. [Google Scholar] [CrossRef]
- Duhan, S.; Gulia, P.; Gill, N.S.; Shukla, P.K.; Khan, S.B.; Almusharraf, A.; Alkhaldi, N. Investigating attention mechanisms for plant disease identification in challenging environments. Front. Plant Sci. 2024, 15, 1396835. [Google Scholar] [CrossRef]
- Wang, T.; Qiu, Z.; Li, H.; Lu, H.; Gu, Y.; Zhu, S.; Liu, G.; Yang, B. High sensitivity, wide linear-range strain sensor based on MXene/AgNW composite film with hierarchical microcrack. Small 2023, 19, 2304033. [Google Scholar] [CrossRef]
- Tang, R.; Aridas, N.K.; Abu Talip, M.S. Design of wireless sensor network for agricultural greenhouse based on improved Zigbee protocol. Agriculture 2023, 13, 1518. [Google Scholar] [CrossRef]
- Guerri, M.F.; Distante, C.; Spagnolo, P.; Bougourzi, F.; Taleb-Ahmed, A. Deep learning techniques for hyperspectral image analysis in agriculture: A review. ISPRS Open J. Photogramm. Remote Sens. 2024, 12, 100062. [Google Scholar] [CrossRef]
- Kaur, S.; Kakani, V.G.; Carver, B.; Jarquin, D.; Singh, A. Hyperspectral imaging combined with machine learning for high-throughput phenotyping in winter wheat. Plant Phenome J. 2024, 7, e20111. [Google Scholar] [CrossRef]
- Caba, J.; Díaz, M.; Barba, J.; Guerra, R.; de la Torre, J.A.; López, S. FPGA-based on-board hyperspectral imaging compression: Benchmarking performance and energy efficiency against GPU implementations. Remote Sens. 2020, 12, 3741. [Google Scholar]
- Shafi, U.; Mumtaz, R.; García-Nieto, J.; Hassan, S.A.; Zaidi, S.A.R.; Iqbal, N. Precision Agriculture Techniques and Practices: From Considerations to Applications. Sensors 2019, 19, 3796. [Google Scholar] [CrossRef]
- Baba, A.; Bonny, T. FPGA-based parallel implementation to classify hyperspectral images by using a convolutional neural network. J. Real-Time Image Process. 2023, 20, 107. [Google Scholar] [CrossRef]
- Li, C.; Peng, Y.; Su, M.; Jiang, T. GPU parallel implementation for real-time feature extraction of hyperspectral images. Appl. Sci. 2020, 10, 6680. [Google Scholar] [CrossRef]
- Haq, I.U.; Mumtaz, R.; Talha, M.; Shafaq, Z.; Owais, M. Wheat Rust Disease Classification using Edge-AI. In Proceedings of the 2022 2nd International Conference on Artificial Intelligence (ICAI), Islamabad, Pakistan, 30–31 March 2022; pp. 58–63. [Google Scholar] [CrossRef]
- Rico-Chávez, A.K.; Franco, J.A.; Fernandez-Jaramillo, A.A.; Contreras-Medina, L.M.; Guevara-González, R.G.; Hernandez-Escobedo, Q. Machine Learning for Plant Stress Modeling: A Perspective towards Hormesis Management. Plants 2022, 11, 970. [Google Scholar] [CrossRef]
- Sinha, D.; Maurya, A.K.; Abdi, G.; Majeed, M.; Agarwal, R.; Mukherjee, R.; Ganguly, S.; Aziz, R.; Bhatia, M.; Majgaonkar, A.; et al. Integrated genomic selection for accelerating breeding programs of climate-smart cereals. Genes 2023, 14, 1484. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Botkin, J.; Curtin, S.J.; Zsögön, A. Ideotype breeding and genome engineering for legume crop improvement. Curr. Opin. Biotechnol. 2023, 82, 102961. [Google Scholar] [CrossRef] [PubMed]
- Mousavi-Derazmahalleh, M.; Bayer, P.E.; Hane, J.K.; Valliyodan, B.; Nguyen, H.T.; Nelson, M.N.; Erskine, W.; Varshney, R.K.; Papa, R.; Edwards, D. Adapting legume crops to climate change using genomic approaches. Plant Cell Environ. 2019, 42, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Jubery, T.Z.; Carley, C.N.; Singh, A.; Sarkar, S.; Ganapathysubramanian, B.; Singh, A.K. Using Machine Learning to Develop a Fully Automated Soybean Nodule Acquisition Pipeline (SNAP). Plant Phenomics 2021, 28, 9834746. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, J.; Shi, X.; Bai, M.; Yuan, C.; Cai, C.; Wang, N.; Zhu, X.; Kuang, H.; Wang, X.; et al. Genetically optimizing soybean nodulation improves yield and protein content. Nat. Plants 2024, 10, 736–742. [Google Scholar] [CrossRef]
- Montesinos-López, O.A.; Montesinos-López, A.; Pérez-Rodríguez, P.; Barrón-López, J.A.; Martini, J.W.R.; Fajardo-Flores, S.B.; Gaytan-Lugo, L.S.; Santana-Mancilla, P.C.; Crossa, J. A review of deep learning applications for genomic selection. BMC Genom. 2021, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Beji, S.; Fontaine, V.; Devaux, R.; Thomas, M.; Negro, S.S.; Bahrman, N.; Siol, M.; Aubert, G.; Burstin, J.; Hilbert, J.L.; et al. Genome-wide association study identifies favorable SNP alleles and candidate genes for frost tolerance in pea. BMC Genom. 2020, 21, 536. [Google Scholar] [CrossRef] [PubMed]
- Revilla, P.; Rodríguez, V.M.; Ordás, A.; Rincent, R.; Charcosset, A.; Giauffret, C.; Melchinger, A.E.; Schön, C.-C.; Bauer, E.; Altmann, T.; et al. Association mapping for cold tolerance in two large maize inbred panels. BMC Plant Biol. 2016, 16, 127. [Google Scholar] [CrossRef]
- Zeng, Y.; Wen, J.; Zhao, W.; Wang, Q.; Huang, W. Rational improvement of rice yield and cold tolerance by editing the three genes OsPIN5b, GS3, and OsMYB30 with the CRISPR—Cas9 system. Front. Plant Sci. 2019, 10, 1663. [Google Scholar] [CrossRef] [PubMed]
- Barbier, E.B.; Hochard, J.P. Does Land Degradation Increase Poverty in Developing Countries? PLoS ONE 2016, 11, e0152973. [Google Scholar] [CrossRef]
- Tyczewska, A.; Twardowski, T.; Woźniak-Gientka, E. Agricultural biotechnology for sustainable food security. Trends Biotechnol. 2023, 41, 331–341. [Google Scholar] [CrossRef]
- Garland, S.; Curry, H.A. Turning promise into practice: Crop biotechnology for increasing genetic diversity and climate resilience. PLoS Biol. 2022, 20, e3001716. [Google Scholar] [CrossRef]
- Obe, M.M.; Kpadé, C.P.; Singbo, A. Identifying and overcoming barriers to climate change adaptation innovations among smallholder farmers in developing countries: A literature review and meta-analysis. Clim. Change 2025, 178, 49. [Google Scholar] [CrossRef]
- Li, Y.; Hallerman, E.M.; Wu, K.; Peng, Y. Insect-Resistant Genetically Engineered Crops in China: Development, Application, and Prospects for Use. Annu. Rev. Entomol. 2020, 65, 273–292. [Google Scholar] [CrossRef] [PubMed]

| Technology | Application | Advantages | Limitations |
|---|---|---|---|
| Convolutional Neural Networks [24] | Plant health monitoring and disaster assessment | Powerful feature extraction capability, scalability, and flexibility | Dependency on large-scale annotated data, high consumption of computing resources |
| High-throughput phenotyping [70] | Obtain observable traits | Non-invasive, high spatiotemporal resolution, and large-scale data processing capability | High cost, environmental and technological limitations, and complex data analysis |
| Thermal Imaging [71] | Monitors canopy temperature | Indicates water stress | Limited to specific conditions |
| Machine Learning [23] | Analyzes complex datasets | High accuracy (95–100%) | Requires large training data |
| Explainable AI [72] | Crop health monitoring and yield prediction | Enhances trust, reduces risks and biases, and assists in decision-making | The trade-off between performance and interpretability, and explain complexity |
| IoT Platform [56] | Monitoring the growth and health status of plants | Promotes the digital transformation of agriculture | Data redundancy, high cost |
| Edge Computing [73] | The agricultural production environment is complex, such as remote farmland and greenhouses | Real-time performance and low latency, low power consumption, and stability | Low precision and high maintenance costs |
| Hyperspectral imaging [74] | Chlorophyll, moisture content, and biomass estimation | Information-rich, non-destructive testing | High cost, highly affected by the environment |
| Wearable Sensors [75] | Real-time physiological monitoring | Early stress detection | Battery life, scalability |
| GWAS [76] | Analyzing the genetic basis of traits | No need to build a group of human work diagrams, higher efficiency | Relying on large sample sizes and high-precision phenotype data |
| Genomic selection [77] | Crop improvement and breeding | Accelerate the breeding process and improve breeding efficiency | Relying on high-quality genomic markers and reference populations |
| Multi-Omics [20] | Complex trait analysis | Systematically analyze complex traits | Large amount of data and high integration difficulty |
| CRISPR/Cas9 [78] | Crop improvement | High precision and shortened breeding cycle | Off-target effects, delivery efficiency |
| Algorithm | Core Features | Key Applications in Legume Cold Stress Research | Pros | Cons |
|---|---|---|---|---|
| Random Forest | Ensemble of decision trees; aggregates predictions via majority voting | Predicting cold tolerance from genomic data; classifying stress phenotypes (e.g., leaf damage) | Robust to overfitting; handles mixed data types (numerical/categorical); provides feature importance scores | Computationally intensive with large datasets; may overfit to noisy sensor data |
| Support Vector Machines (SVM) | Finds optimal hyperplane to separate classes; uses kernel functions for non-linear data | Classifying legume genotypes to cold based on gene expression or metabolite profiles | Effective with high-dimensional data; performs well with small datasets | Less efficient with very large datasets; sensitive to kernel and parameter selection |
| XGBoost/LightGBM | Gradient boosting framework: sequential weak learners correct prior errors | Identifying QTLs linked to cold tolerance; predicting yield loss under cold stress using multi-omics data | High accuracy; handles imbalanced data; fast training (LightGBM) | Prone to overfitting without proper regularization; requires careful parameter tuning |
| Convolutional Neural Networks (CNNs) | Deep learning with convolutional layers for spatial feature extraction | Analyzing hyperspectral/thermal images to detect cold stress symptoms (e.g., chlorosis, canopy temperature) | Automatically learns complex spatial patterns; high accuracy with image data | Requires large labeled datasets; computationally expensive; “black-box” interpretability |
| Long Short-Term Memory (LSTMs) | Recurrent neural network with memory cells for time-series data | Modeling dynamic cold stress responses (e.g., gene expression changes over time; photosynthetic rate fluctuations) | Captures temporal dependencies; handles variable-length time series | Prone to overfitting on short time series; slow training with large sequences |
| Technology | Frequency Bands | Legal Compliance | Scientific Validation |
|---|---|---|---|
| 5G | Sub-6 GHz (3.3–5.0 GHz), mmWave (24–40 GHz) | 3GPP standards, regional spectrum policies (e.g., China’s 3.5 GHz allocation) | <10 ms latency, 99.999% reliability, real-time hyperspectral processing |
| LoRaWAN | 470–510 MHz (China), 863–870 MHz (Europe) | China’s MIIT regulations, LoRa Alliance certification | 99% uptime, 15 km range, 2–10 year battery life |
| ZigBee | 2.4 GHz (global), 868/915 MHz (regional) | China’s RFID regulations, EU duty-cycle limits | EMP-ZBR protocol reduces latency by 30%, and 99% network reliability in greenhouses |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Xia, L.; Yang, X.; Du, C.; Tang, T.; Yang, Z.; Ma, S.; Wan, X.; Guan, F.; Shi, B.; et al. Integrating Artificial Intelligence and Biotechnology to Enhance Cold Stress Resilience in Legumes. Plants 2025, 14, 2784. https://doi.org/10.3390/plants14172784
Wang K, Xia L, Yang X, Du C, Tang T, Yang Z, Ma S, Wan X, Guan F, Shi B, et al. Integrating Artificial Intelligence and Biotechnology to Enhance Cold Stress Resilience in Legumes. Plants. 2025; 14(17):2784. https://doi.org/10.3390/plants14172784
Chicago/Turabian StyleWang, Kai, Lei Xia, Xuetong Yang, Chang Du, Tong Tang, Zheng Yang, Shijie Ma, Xinjian Wan, Feng Guan, Bo Shi, and et al. 2025. "Integrating Artificial Intelligence and Biotechnology to Enhance Cold Stress Resilience in Legumes" Plants 14, no. 17: 2784. https://doi.org/10.3390/plants14172784
APA StyleWang, K., Xia, L., Yang, X., Du, C., Tang, T., Yang, Z., Ma, S., Wan, X., Guan, F., Shi, B., Xie, Y., & Zhang, J. (2025). Integrating Artificial Intelligence and Biotechnology to Enhance Cold Stress Resilience in Legumes. Plants, 14(17), 2784. https://doi.org/10.3390/plants14172784






