Genetic Diversity and Disease Resistance Genes Profiling in Cultivated Coffea canephora Genotypes via Molecular Markers
Abstract
1. Introduction
2. Results
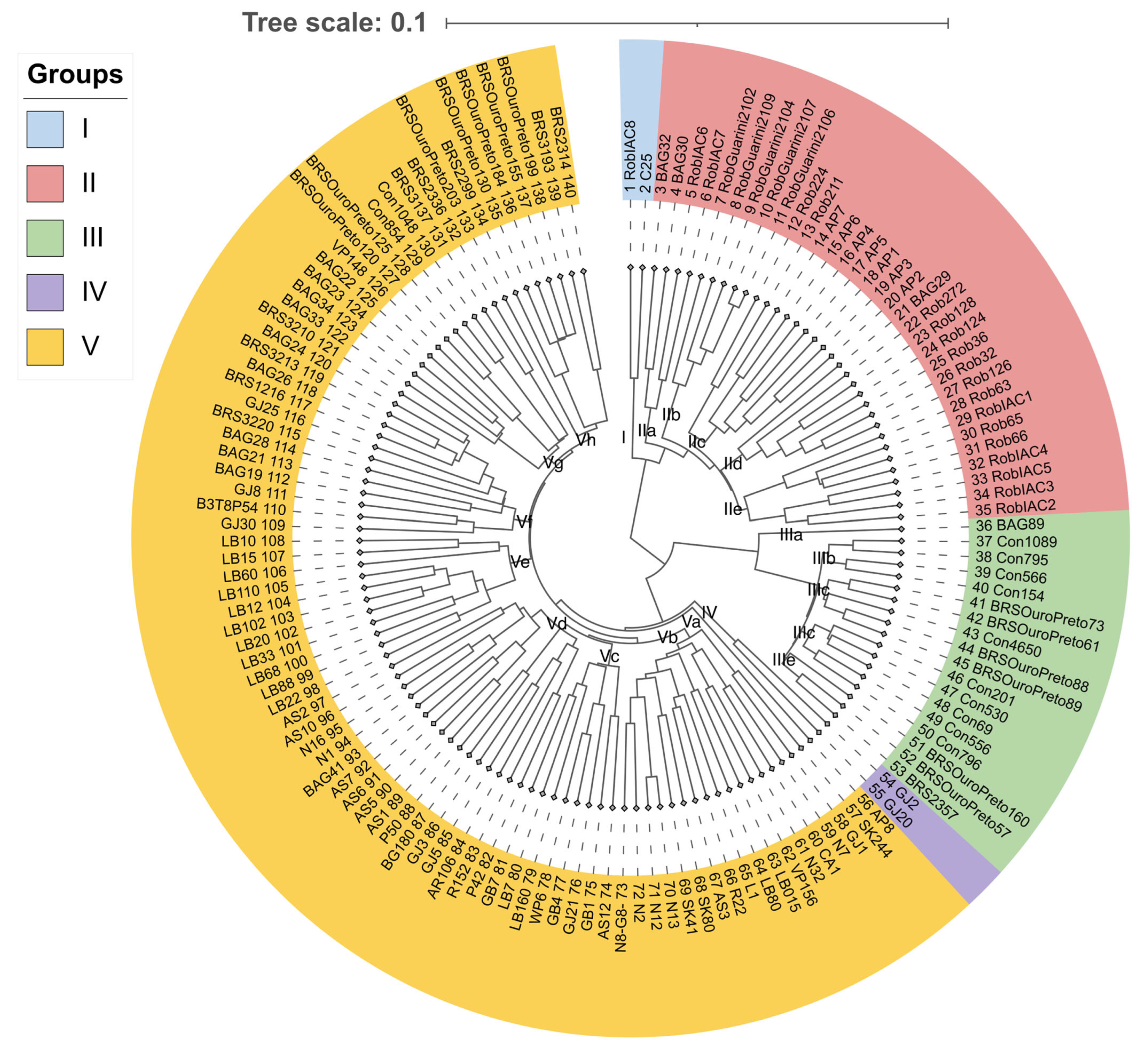
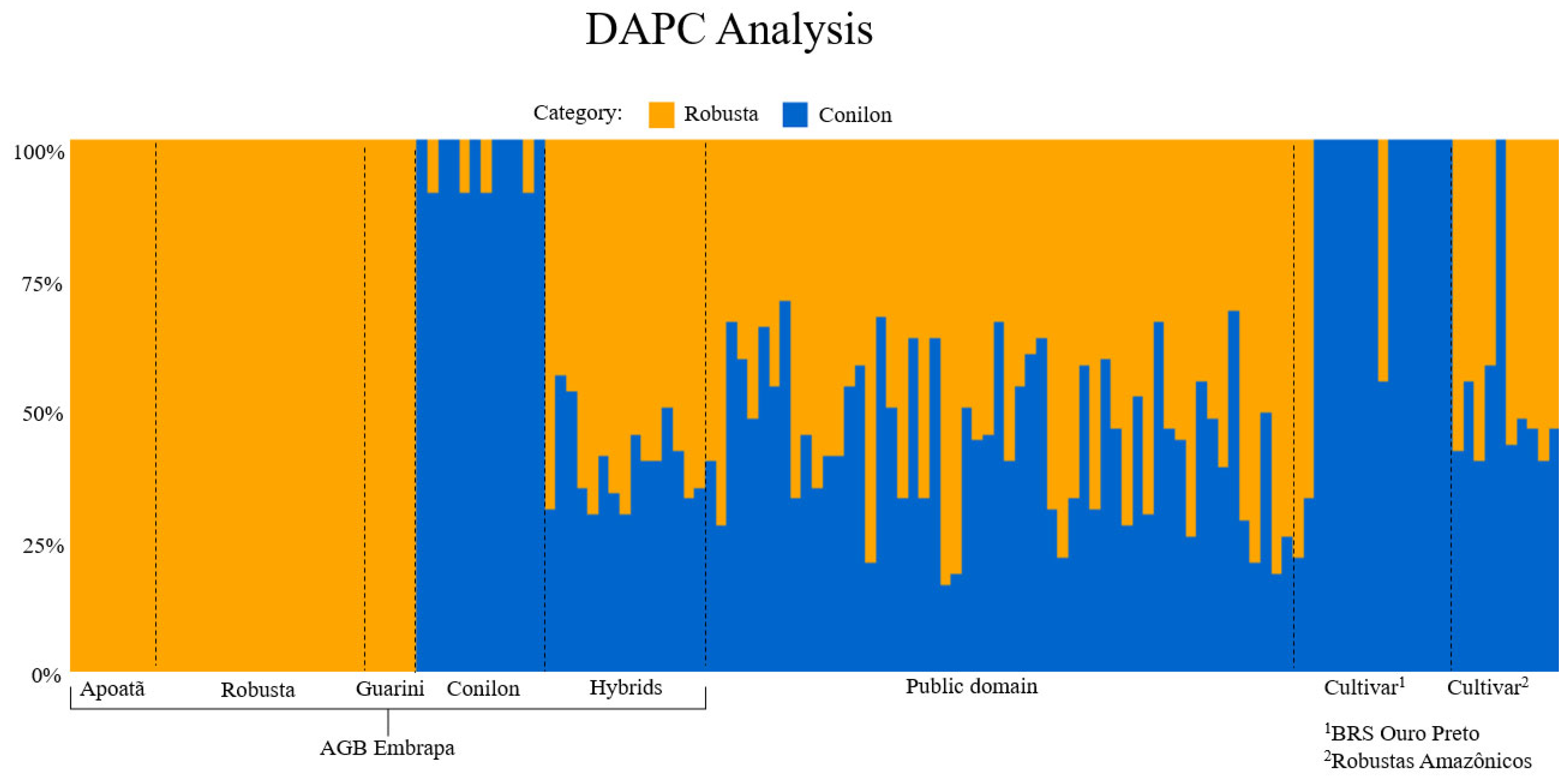
2.1. Genetic Diversity
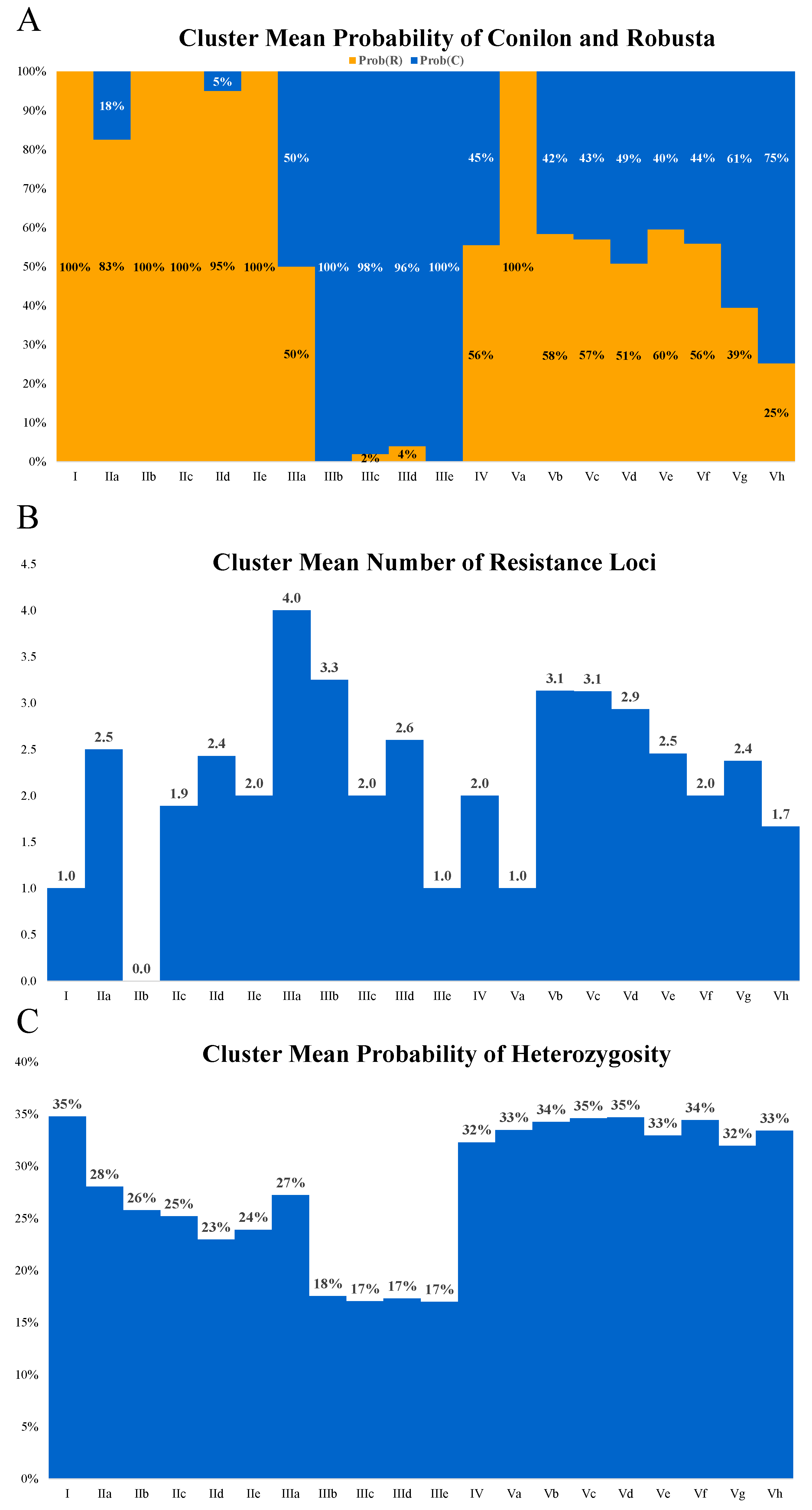
2.2. Segregation of Resistance Markers
3. Discussion
4. Materials and Methods
4.1. Genetic Materials
4.2. Quality Analysis of SNP Markers
4.3. Genetic Diversity Analysis
4.4. Molecular Markers Associated with Resistance to Rust and CBD
4.5. Markers SSR016 and CaRHv9
4.6. Marker CARF005
4.7. Marker RLK
4.8. Markers Sat207 and Sat235
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilen, C.; El Chami, D.; Mereu, V.; Trabucco, A.; Marras, S.; Spano, D. A Systematic Review on the Impacts of Climate Change on Coffee Agrosystems. Plants 2023, 12, 102. [Google Scholar] [CrossRef]
- Grüter, R.; Trachsel, T.; Laube, P.; Jaisli, I. Expected global suitability of coffee, cashew and avocado due to climate change. PLoS ONE 2022, 17, e0261976. [Google Scholar] [CrossRef]
- Jawo, T.O.; Kyereh, D.; Lojka, B. The Impact of Climate Change on Coffee Production of Small Farmers and Their Adaptation Strategies: A Review. Clim. Dev. 2023, 15, 93–109. [Google Scholar] [CrossRef]
- Davis, A.P. Psilanthus mannii, the Type Species of Psilanthus, Transferred to Coffea. Nord. J. Bot. 2011, 29, 471–472. [Google Scholar] [CrossRef]
- Maurin, O.; Davis, A.P.; Chester, M.; Mvungi, E.F.; Jaufeerally-Fakim, Y.; Fay, M.F. Towards a Phylogeny for Coffea (Rubiaceae): Identifying Well-Supported Lineages Based on Nuclear and Plastid DNA Sequences. Ann. Bot. 2007, 100, 1565–1583. [Google Scholar] [CrossRef]
- Espindula, M.C.; Dalazen, J.R.; Rocha, R.B.; Teixeira, A.L.; Diocleciano, J.M.; Dias, J.R.M.; Schmidt, R.; Lima, P.P.; Lima, G.M.; Gama, W. Robustas Amazônicos: Os Cafeeiros Cultivados em Rondônia, 1st ed.; Embrapa: Brasília, Brazil, 2022; 144p. [Google Scholar]
- Silva, A.N.R.; Rocha, R.B.; Teixeira, A.L.; Espindula, M.C.; Partelli, F.L.; Caixeta, E.T. Self-Incompatibility and Pollination Efficiency in Coffea canephora Using Fluorescence Microscopy. Agronomy 2024, 14, 1564. [Google Scholar] [CrossRef]
- Alkimim, E.R.; Caixeta, E.T.; Sousa, T.V.; Silva, F.L.; Sakiyama, N.S.; Zambolim, E.M.; Pereira, A.A.; Oliveira, A.C.B.; de Souza, F.F. Selective Efficiency of Genome-Wide Selection in Coffea canephora Breeding. Tree Genet. Genomes 2020, 16, 41. [Google Scholar] [CrossRef]
- Ferrão, L.F.V.; Caixeta, E.T.; Souza, F.D.F.; Zambolim, E.M.; Sakiyama, N.S.; Zambolim, L.; Cruz, C.D.; Pereira, A.A. Comparative Study of Different Molecular Markers for Classifying and Establishing Genetic Relationships in Coffea canephora. Plant Syst. Evol. 2013, 299, 225–238. [Google Scholar] [CrossRef]
- Rocha, R.B.; Teixeira, A.L.; Ramalho, A.R.; Espindula, M.C.; Lunz, A.M.P.; Souza, F.F. Coffea canephora Breeding: Estimated and Achieved Gains from Selection in the Western Amazon, Brazil. Ciênc. Rural 2021, 51, e20200713. [Google Scholar] [CrossRef]
- Silva, L.d.F.; Leichtweis, B.G.; Silva, A.C.A.; Rocha, R.B.; Teixeira, A.L.; Caixeta, E.T. Fingerprinting Amazonian coffees: Assessing diversity through molecular markers. Euphytica 2024, 220, 28. [Google Scholar] [CrossRef]
- Faria, L.S.; Alkimim, E.R.; Barreiro, P.R.R.M.; Caixeta, E.T.; Zambolim, E.M.; Oliveira, A.C.B.; Pereira, A.A.; Sakiyama, N.S.; de Souza, F.F. Genome-Wide Association Study of Plant Architecture and Disease Resistance in Coffea canephora. Euphytica 2022, 218, 92. [Google Scholar] [CrossRef]
- Oliveira, L.N.L.; Rocha, R.B.; Ferreira, F.M.; Spinelli, V.M.; Ramalho, A.R.; Teixeira, A.L. Selection of Coffea canephora Parents from the Botanical Varieties Conilon and Robusta for the Production of Intervarietal Hybrids. Ciênc. Rural 2018, 48, e20170444. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Souza, F.D.F.; Rocha, R.B.; Junior, J.R.V.; Torres, J.D.; Rodrigues, K.M.; Moraes, M.S.; Silva, C.A.; Oliveira, V.E.G.; Lourenço, J.L.R. Performance of Intraspecific Hybrids (Kouillou × Robusta) of Coffea canephora Pierre. Afr. J. Agric. Res. 2017, 12, 2675–2680. [Google Scholar]
- Alkimim, E.R.; Caixeta, E.T.; Sousa, T.V.; Silva, F.L.; Zambolim, E.M.; Pereira, A.A.; Oliveira, A.C.B.; Sakiyama, N.S.; de Souza, F.F. Designing the Best Breeding Strategy for Coffea canephora: Genetic Evaluation of Pure and Hybrid Individuals Aiming to Select for Productivity and Disease Resistance Traits. PLoS ONE 2021, 16, e0260997. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Rocha, R.B.; Espindula, M.C.; Ramalho, A.R.; Vieira Júnior, J.R.; Alves, E.A.; Lunz, A.M.P.; Souza, F.F.; Costa, J.N.M.; Fernandes, C.d.F. Amazonian Robustas: New Coffea canephora Coffee Cultivars for the Western Brazilian Amazon. Crop Breed. Appl. Biotechnol. 2020, 20, e323420318. [Google Scholar] [CrossRef]
- Leroy, T.; Marraccini, P.; Dufour, M.; Montagnon, C.; Lashermes, P.; Sabau, X.; Ferreira, L.P.; Jourdan, I.; Pot, D.; Andrade, A.C.; et al. Construction and Characterization of a Coffea canephora BAC Library to Study the Organization of Sucrose Biosynthesis Genes. Theor. Appl. Genet. 2005, 111, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, A.R.; Rocha, R.B.; Souza, F.F.; Veneziano, W.; Teixeira, A.L. Genetic Progress of Processed Coffee Yield with the Selection of Conilon Coffee Clones. Rev. Ciênc. Agron. 2016, 47, 516–523. [Google Scholar] [CrossRef]
- Viencz, T.; Acre, L.B.; Rocha, R.B.; Alves, E.A.; Ramalho, A.R.; Benassi, M.T. Caffeine, Trigonelline, Chlorogenic Acids, Melanoidins, and Diterpenes Contents of Coffea canephora coffees Produced in the Amazon. J. Food Compos. Anal. 2023, 117, 105140. [Google Scholar] [CrossRef]
- Velásquez, S.; Banchón, C. Influence of Pre- and Post-Harvest Factors on the Organoleptic and Physicochemical Quality of Coffee: A Short Review. J. Food Sci. Technol. 2022, 60, 2526–2538. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento (Conab). Acompanhamento da Safra Brasileira de Café—Terceiro Levantamento—Setembro 2024; Conab: Brasília, Brazil, 2024. [Google Scholar]
- Alkimim, E.R.; Caixeta, E.T.; Sousa, T.V.; da Silva, F.L.; Sakiyama, N.S.; Zambolim, L. High-throughput targeted genotyping using next-generation sequencing applied in Coffea canephora breeding. Euphytica 2018, 214, 50. [Google Scholar] [CrossRef]
- Alkimim, E.R.; Caixeta, E.T.; Sousa, T.V.; Pereira, A.A.; de Oliveira, A.C.B.; Zambolim, L.; Sakiyama, N.S. Marker-assisted selection provides arabica coffee with genes from other Coffea species targeting on multiple resistance to rust and coffee berry disease. Mol. Breed. 2017, 37, 6. [Google Scholar] [CrossRef]
- Almeida, D.P.; Caixeta, E.T.; Moreira, K.F.; Oliveira, A.C.B.; Zambolim, E.M.; Sakiyama, N.S.; Pereira, A.A. Marker-Assisted Pyramiding of Multiple Disease Resistance Genes in Coffee Genotypes (Coffea arabica). Agronomy 2021, 11, 1763. [Google Scholar] [CrossRef]
- Alvarenga, S.M.; Caixeta, E.T.; Hufnagel, B.; Maciel-Zambolim, E.; Zambolim, L.; Pereira, A.A.; Sakiyama, N.S. Marcadores Moleculares Derivados de Sequências Expressas do Genoma Café Potencialmente Envolvidas na Resistência à Ferrugem. Pesqui. Agropecu. Bras. 2011, 46, 890–898. [Google Scholar] [CrossRef]
- Combes, M.C.; Andrzejewski, S.; Anthony, F.; Bertrand, B.; Rovelli, P.; Lashermes, P. Characterization of Microsatellite Loci in Coffea arabica and Related Coffee Species. Mol. Ecol. 2000, 9, 1178–1180. [Google Scholar] [CrossRef]
- Caixeta, E.T.; Oliveira, A.C.B.; Brito, G.G.; Sakiyama, N.S.; Zambolim, E.M.; Pereira, A.A. Tipos de Marcadores Moleculares. In Marcadores Moleculares, 1st ed.; Borem, A.L., Caixeta, E.T., Eds.; Editora UFV: Viçosa, Brazil, 2016; p. 385. [Google Scholar]
- Barka, G.D.; Caixeta, E.T.; Ferreira, S.S.; Zambolim, E.M.; Pereira, A.A.; Oliveira, A.C.B.; Sakiyama, N.S. In Silico Guided Structural and Functional Analysis of Genes with Potential Involvement in Resistance to Coffee Leaf Rust: A Functional Marker Based Approach. PLoS ONE 2020, 15, e0222747. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.S.; Vieira, M.L.C. Genetic Maps in Plants. Bragantia 2002, 61, 89–100. [Google Scholar] [CrossRef]
- Ferrão, M.A.G.; da Fonseca, A.F.; Volpi, P.S.; de Souza, L.C.; Comério, M.; Filho, A.C.V.; Riva-Souza, E.M.; Munoz, P.R.; Ferrão, R.G.; Ferrão, L.F.V. Genomic-Assisted Breeding for Climate-Smart Coffee. Plant Genome 2023, 16, e20321. [Google Scholar] [CrossRef] [PubMed]
- Gichuru, E.K.; Agwanda, C.O.; Combes, M.C.; Mutitu, E.W.; Ngugi, E.C.K.; Bertrand, B.; Lashermes, P. Identification of Molecular Markers Linked to a Gene Conferring Resistance to Coffee Berry Disease (Colletotrichum kahawae) in Coffea arabica. Plant Pathol. 2008, 57, 1117–1124. [Google Scholar] [CrossRef]
- Gichuru, E.; Alwora, G.; Gimase, J.; Kathurima, C. Coffee Leaf Rust (Hemileia vastatrix) in Kenya—A Review. Agronomy 2021, 11, 2590. [Google Scholar] [CrossRef]
- Zambolim, L.; Caixeta, E.T. An Overview of Physiological Specialization of Coffee Leaf Rust—New Designation of Pathotypes. Int. J. Curr. Res. 2021, 13, 15479–15490. [Google Scholar] [CrossRef]
- Capucho, A.S.; Zambolim, E.M.; Freitas, R.L.; Oliveira, A.C.B.; Sakiyama, N.S.; Pereira, A.A. Identification of Race XXXIII of Hemileia vastatrix on Coffea arabica Catimor Derivatives in Brazil. Australas. Plant Dis. Notes 2012, 7, 189–191. [Google Scholar] [CrossRef]
- Diniz, L.E.; Sakiyama, N.S.; Lashermes, P.; Caixeta, E.T.; Oliveira, A.C.B.; Zambolim, E.M.; Pereira, A.A. Analysis of AFLP Markers Associated to the Mex-1 Resistance Locus in Icatu Progenies. Crop Breed. Appl. Biotechnol. 2005, 5, 387–393. [Google Scholar] [CrossRef]
- Pestana, K.N.; Capucho, A.S.; Caixeta, E.T.; Almeida, D.P.; Zambolim, E.M.; Cruz, C.D.; Zambolim, L.; Pereira, A.A.; Oliveira, A.C.B.; Sakiyama, N.S. Inheritance Study and Linkage Mapping of Resistance Loci to Hemileia vastatrix in Híbrido de Timor UFV 443-03. Tree Genet. Genomes 2015, 11, 72. [Google Scholar] [CrossRef]
- Zullo, J.; Pinto, H.S.; Assad, E.D.; Ávila, A.M.H. Potential for growing Arabica coffee in the extreme south of Brazil in a warmer world. Clim. Change 2011, 109, 535–548. [Google Scholar] [CrossRef]
- Hoque, A.; Fiedler, J.D.; Rahman, M. Genetic Diversity Analysis of a Flax (Linum usitatissimum L.) Global Collection. BMC Genom. 2020, 21, 557. [Google Scholar] [CrossRef]
- Sousa, T.V.; Caixeta, E.T.; Alkimim, E.R.; Oliveira, A.C.B.; Pereira, A.A.; Sakiyama, N.S.; Resende, M.D.V.; Zambolim, L. Population Structure and Genetic Diversity of Coffee Progenies Derived from Catuaí and Híbrido de Timor Revealed by Genome-Wide SNP Marker. Tree Genet. Genomes 2017, 13, 124. [Google Scholar] [CrossRef]
- Talhinhas, P.; Batista, D.; Diniz, I.; Vieira, A.; Silva, D.N.; Loureiro, A.; Tavares, S.; Pereira, A.P.; Azinheira, H.G.; Guerra-Guimarães, L.; et al. The Coffee Leaf Rust Pathogen Hemileia vastatrix: One and a Half Centuries Around the Tropics. Mol. Plant Pathol. 2017, 18, 1039–1051. [Google Scholar] [CrossRef]
- Vieira, A.; Diniz, I.; Loureiro, A.; Pereira, A.P.; Silva, M.C.; Várzea, V.; Batista, D. Aggressiveness Profiling of the Coffee Pathogen Colletotrichum kahawae. Plant Pathol. 2019, 68, 358–368. [Google Scholar] [CrossRef]
- Mahé, L.; Combes, M.C.; Várzea, V.M.P.; Guilhaumon, C.; Lashermes, P. Development of Sequence-Characterized DNA Markers Linked to Leaf Rust (Hemileia vastatrix) Resistance in Coffee (Coffea arabica L.). Mol. Breed. 2008, 21, 105–113. [Google Scholar] [CrossRef]
- Saavedra, L.M.; Caixeta, E.T.; Barka, G.D.; Borém, A.; Zambolim, L.; Nascimento, M.; Cruz, C.D.; Oliveira, A.C.B.d.; Pereira, A.A. Marker-Assisted Recurrent Selection for Pyramiding Leaf Rust and Coffee Berry Disease Resistance Alleles in Coffea arabica L. Genes 2023, 14, 189. [Google Scholar] [CrossRef]
- Silva, M.d.C.; Guerra-Guimarães, L.; Diniz, I.; Loureiro, A.; Azinheira, H.; Pereira, A.P.; Tavares, S.; Batista, D.; Várzea, V. An Overview of the Mechanisms Involved in Coffee-Hemileia vastatrix Interactions: Plant and Pathogen Perspectives. Agronomy 2022, 12, 326. [Google Scholar] [CrossRef]
- Denoeud, F.; Carretero-Paulet, L.; Dereeper, A.; Droc, G.; Guyot, R.; Pietrella, M.; Zheng, C.; Alberti, A.; Anthony, F.; Aprea, G.; et al. The coffee genome provides insight into the convergent evolution of caffeine biosynthesis. Nat. Genet. 2014, 46, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Zaidan, I.R.; Ferreira, A.; Noia, L.R.; Santos, J.G.; Arruda, V.C.; Couto, D.P.D.; Braz, R.A.; Senra, J.F.B.; Partelli, F.L.; Azevedo, C.F.; et al. Diversity and structure of Coffea canephora from old seminal crops in Espírito Santo, Brazil: Genetic resources for coffee breeding. Tree Genet. Genomes 2023, 19, 19. [Google Scholar] [CrossRef]
- Gnirke, A.; Melnikov, A.; Maguire, J.; Rogov, P.; LeProust, E.M.; Brockman, W.; Fennell, T.; Giannoukos, G.; Fisher, S.; Russ, C.; et al. Solution Hybrid Selection with Ultra-Long Oligonucleotides for Massively Parallel Targeted Sequencing. Nat. Biotechnol. 2009, 27, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Li, W.H. Mathematical Model for Studying Genetic Variation in Terms of Restriction Endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef]
- Rocha, R.B.; Suela, M.M.; Comério, M.; Souza, E.M.R.; Senra, J.F.B.; Ferrão, M.A.G.; Ferrão, R.G.; Fonseca, A.F.A.; Filho, A.C.V.; Volpi, P.S.; et al. Genomic-assisted selection to guide mate allocation and leverage hybrid vigor in Coffea canephora. Tree Genet. Genomes 2025, 21, 20. [Google Scholar] [CrossRef]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant Analysis of Principal Components: A New Method for the Analysis of Genetically Structured Populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef] [PubMed]
| Gen. | QTLGL2 | QTLGL10 | NB-ARC | RLK2 | Ck1-1 | Ck1-2 | R. loci a | Gen. | QTLGL2 | QTLGL10 | NB-ARC | RLK2 | Ck1-1 | Ck-1-2 | R. loci |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N12 | Aa | B_ | C_ | D_ | EE | FF | 5 | BRS2299 | Aa | B_ | cc | dd | EE | FF | 3 |
| N1 | AA | B_ | C_ | D_ | EE | FF | 5 | Rob211 | aa | bb | C_ | D_ | ee | ff | 2 |
| N16 | Aa | B_ | C_ | D_ | EE | FF | 5 | AP2 | Aa | bb | cc | dd | EE | FF | 2 |
| BAG30 | AA | B_ | C_ | D_ | ee | FF | 4 | Rob272 | AA | bb | C_ | dd | EE | ff | 2 |
| Rob36 | AA | B_ | C_ | D_ | ee | FF | 4 | Rob32 | Aa | bb | C_ | dd | EE | ff | 2 |
| BAG89 | Aa | B_ | C_ | D_ | ee | FF | 4 | Rob126 | Aa | bb | cc | D_ | EE | ff | 2 |
| Con154 | Aa | B_ | C_ | D_ | ee | ff | 4 | Rob63 | aa | bb | C_ | D_ | ee | FF | 2 |
| Con530 | AA | B_ | C_ | D_ | EE | ff | 4 | Rob65 | Aa | bb | cc | dd | EE | FF | 2 |
| Con69 | AA | B_ | C_ | D_ | ee | ff | 4 | Rob66 | AA | bb | C_ | dd | EE | ff | 2 |
| SK244 | Aa | B_ | C_ | D_ | ee | Ff | 4 | Con4650 | AA | bb | C_ | dd | ee | ff | 2 |
| SK41 | AA | B_ | C_ | dd | EE | FF | 4 | Con201 | aa | B_ | C_ | dd | ee | ff | 2 |
| N13 | Aa | B_ | C_ | D_ | ee | FF | 4 | Con556 | AA | B_ | cc | dd | ee | ff | 2 |
| N2 | Aa | B_ | C_ | dd | EE | FF | 4 | GJ1 | Aa | bb | C_ | dd | ee | ff | 2 |
| WP6 | AA | B_ | C_ | D_ | ee | FF | 4 | N32 | Aa | bb | C_ | dd | ee | ff | 2 |
| LB160 | AA | B_ | C_ | dd | Ee | Ff | 4 | LB80 | aa | B_ | C_ | dd | ee | ff | 2 |
| GB7 | Aa | B_ | C_ | D_ | EE | ff | 4 | L1 | AA | bb | C_ | dd | ee | FF | 2 |
| P42 | AA | B_ | C_ | D_ | EE | ff | 4 | BG180 | aa | bb | C_ | D_ | ee | ff | 2 |
| AR106 | Aa | B_ | C_ | D_ | ee | FF | 4 | P50 | Aa | B_ | cc | dd | EE | ff | 2 |
| GJ30 | AA | B_ | C_ | dd | EE | FF | 4 | AS1 | aa | bb | C_ | dd | EE | FF | 2 |
| LB10 | AA | B_ | C_ | D_ | ee | ff | 4 | AS7 | AA | bb | cc | D_ | EE | ff | 2 |
| BAG26 | Aa | B_ | C_ | D_ | ee | ff | 4 | BAG41 | AA | bb | C_ | dd | ee | FF | 2 |
| BAG34 | AA | B_ | C_ | D_ | ee | ff | 4 | AS10 | AA | bb | C_ | dd | ee | FF | 2 |
| Rob224 | Aa | bb | C_ | dd | EE | FF | 3 | GJ5 | Aa | bb | C_ | dd | ee | FF | 2 |
| AP7 | Aa | bb | C_ | dd | EE | FF | 3 | LB22 | AA | bb | C_ | dd | ee | Ff | 2 |
| AP6 | aa | bb | C_ | D_ | EE | FF | 3 | LB33 | AA | bb | C_ | dd | Ee | ff | 2 |
| AP4 | Aa | bb | C_ | D_ | EE | ff | 3 | LB20 | AA | bb | C_ | dd | ee | Ff | 2 |
| BAG29 | Aa | bb | C_ | D_ | EE | ff | 3 | LB102 | AA | bb | C_ | dd | ee | FF | 2 |
| Rob128 | AA | B_ | C_ | dd | ee | ff | 3 | LB12 | AA | bb | C_ | dd | ee | ff | 2 |
| Con1089 | AA | B_ | C_ | dd | ee | FF | 3 | BAG28 | aa | bb | C_ | dd | Ee | FF | 2 |
| Con795 | Aa | B_ | C_ | dd | ee | ff | 3 | BRS3213 | Aa | bb | C_ | dd | ee | FF | 2 |
| Con566 | AA | B_ | C_ | dd | ee | FF | 3 | BAG24 | Aa | bb | C_ | dd | ee | FF | 2 |
| GJ20 | aa | B_ | C_ | dd | EE | FF | 3 | BRS3210 | Aa | bb | C_ | dd | ee | ff | 2 |
| N7 | Aa | B_ | C_ | dd | ee | FF | 3 | BAG23 | AA | bb | C_ | dd | ee | FF | 2 |
| VP156 | Aa | B_ | C_ | dd | ee | Ff | 3 | Con1048 | Aa | B_ | cc | dd | ee | FF | 2 |
| R22 | AA | B_ | C_ | dd | EE | ff | 3 | RobIAC8 | aa | bb | C_ | dd | EE | ff | 1 |
| AS3 | aa | B_ | C_ | dd | EE | FF | 3 | BAG32 | AA | bb | cc | dd | EE | ff | 1 |
| SK80 | AA | B_ | C_ | dd | ee | FF | 3 | AP5 | aa | bb | cc | dd | EE | FF | 1 |
| N8(G8) | aa | B_ | cc | D_ | EE | FF | 3 | Rob124 | Aa | bb | cc | dd | EE | ff | 1 |
| GB1 | AA | bb | C_ | D_ | ee | ff | 3 | Con796 | AA | bb | cc | dd | ee | ff | 1 |
| GJ21 | AA | bb | C_ | dd | EE | FF | 3 | BRS2357 | aa | bb | cc | dd | EE | FF | 1 |
| GB4 | AA | B_ | C_ | dd | ee | FF | 3 | GJ2 | AA | bb | cc | dd | Ee | ff | 1 |
| LB7 | Aa | B_ | C_ | dd | ee | Ff | 3 | AP8 | aa | bb | cc | dd | EE | FF | 1 |
| AS5 | AA | B_ | C_ | dd | ee | FF | 3 | AS12 | aa | bb | C_ | dd | EE | ff | 1 |
| AS6 | Aa | B_ | C_ | dd | ee | FF | 3 | LB68 | aa | bb | C_ | dd | ee | ff | 1 |
| AS2 | Aa | B_ | cc | dd | EE | FF | 3 | GJ8 | AA | bb | cc | dd | ee | FF | 1 |
| R152 | Aa | B_ | C_ | dd | ee | FF | 3 | BAG19 | Aa | bb | cc | dd | ee | ff | 1 |
| LB88 | AA | B_ | C_ | dd | ee | ff | 3 | BAG21 | Aa | bb | cc | dd | ee | FF | 1 |
| LB110 | AA | B_ | C_ | dd | ee | Ff | 3 | BRS3220 | Aa | bb | cc | dd | ee | ff | 1 |
| LB60 | AA | B_ | cc | D_ | ee | ff | 3 | BRS3137 | aa | bb | C_ | dd | EE | ff | 1 |
| LB15 | AA | B_ | C_ | dd | EE | ff | 3 | BRS2336 | AA | bb | cc | dd | EE | ff | 1 |
| GJ30 | Aa | bb | cc | D_ | EE | Ff | 3 | BRS3193 | AA | bb | cc | dd | ee | FF | 1 |
| GJ25 | aa | B_ | C_ | D_ | ee | FF | 3 | BRS2314 | Aa | bb | cc | dd | ee | FF | 1 |
| BAG33 | AA | B_ | C_ | dd | ee | FF | 3 | AP1 | aa | bb | cc | dd | ee | ff | 0 |
| BAG22 | Aa | bb | C_ | D_ | ee | FF | 3 | AP3 | aa | bb | cc | dd | EE | ff | 0 |
| Con854 | AA | bb | C_ | D_ | ee | ff | 3 | BRS1216 | aa | bb | cc | dd | ee | ff | 0 |
| n | Genotype | Field Classification | Genealogy | Origin |
|---|---|---|---|---|
| 1 | RobIAC8 | Robusta | Open pollination | AGB Embrapa |
| 2 | C25 | Robusta | Open pollination | AGB Embrapa |
| 3 | BAG32 | Hybrid | Open pollination | AGB Embrapa |
| 4 | BAG30 | Hybrid | Open pollination | AGB Embrapa |
| 5 | RobIAC6 | Robusta | Open pollination | AGB Embrapa |
| 6 | RobIAC7 | Robusta | Open pollination | AGB Embrapa |
| 7 | RobGuarini2102 | Robusta | Open pollination | AGB Embrapa |
| 8 | RobGuarini2109 | Robusta | Open pollination | AGB Embrapa |
| 9 | RobGuarini2104 | Robusta | Open pollination | AGB Embrapa |
| 10 | RobGuarini2107 | Robusta | Open pollination | AGB Embrapa |
| 11 | RobGuarini2106 | Robusta | Open pollination | AGB Embrapa |
| 12 | Rob224 | Robusta | Open pollination | AGB Embrapa |
| 13 | Rob211 | Robusta | Open pollination | AGB Embrapa |
| 14 | AP7 | Robusta | Open pollination | AGB Embrapa |
| 15 | AP6 | Robusta | Open pollination | AGB Embrapa |
| 16 | AP4 | Robusta | Open pollination | AGB Embrapa |
| 17 | AP5 | Robusta | Open pollination | AGB Embrapa |
| 18 | AP1 | Robusta | Open pollination | AGB Embrapa |
| 19 | AP3 | Robusta | Open pollination | AGB Embrapa |
| 20 | AP2 | Robusta | Open pollination | AGB Embrapa |
| 21 | BAG29 | Hybrid | Open pollination | AGB Embrapa |
| 22 | Rob272 | Robusta | Open pollination | AGB Embrapa |
| 23 | Rob128 | Robusta | Open pollination | AGB Embrapa |
| 24 | Rob124 | Robusta | Open pollination | AGB Embrapa |
| 25 | Rob36 | Robusta | Open pollination | AGB Embrapa |
| 26 | Rob32 | Robusta | Open pollination | AGB Embrapa |
| 27 | Rob126 | Robusta | Open pollination | AGB Embrapa |
| 28 | Rob63 | Robusta | Open pollination | AGB Embrapa |
| 29 | RobIAC1 | Robusta | Open pollination | AGB Embrapa |
| 30 | Rob65 | Robusta | Open pollination | AGB Embrapa |
| 31 | Rob66 | Robusta | Open pollination | AGB Embrapa |
| 32 | RobIAC4 | Robusta | Open pollination | AGB Embrapa |
| 33 | RobIAC5 | Robusta | Open pollination | AGB Embrapa |
| 34 | RobIAC3 | Robusta | Open pollination | AGB Embrapa |
| 35 | RobIAC2 | Robusta | Open pollination | AGB Embrapa |
| 36 | BAG89 | Hybrid | Open pollination | AGB Embrapa |
| 37 | Con1089 | Conilon | Open pollination | AGB Embrapa |
| 38 | Con795 | Conilon | Open pollination | AGB Embrapa |
| 39 | Con566 | Conilon | Open pollination | AGB Embrapa |
| 40 | Con154 | Conilon | Open pollination | AGB Embrapa |
| 41 | BRSOuroPreto73 | Conilon | Open pollination | Cultivar |
| 42 | BRSOuroPreto61 | Conilon | Open pollination | Cultivar |
| 43 | Con4650 | Conilon | Open pollination | AGB Embrapa |
| 44 | BRSOuroPreto88 | Conilon | Open pollination | Cultivar |
| 45 | BRSOuroPreto89 | Conilon | Open pollination | Cultivar |
| 46 | Con201 | Conilon | Open pollination | AGB Embrapa |
| 47 | Con530 | Conilon | Open pollination | AGB Embrapa |
| 48 | Con69 | Conilon | Open pollination | AGB Embrapa |
| 49 | Con556 | Conilon | Open pollination | AGB Embrapa |
| 50 | Con796 | Conilon | Open pollination | AGB Embrapa |
| 51 | BRSOuroPreto160 | Conilon | Open pollination | Cultivar |
| 52 | BRSOuroPreto57 | Conilon | Open pollination | AGB Embrapa |
| 53 | BRS2357 | Conilon | Open pollination | Cultivar |
| 54 | GJ2 | Hybrid | Open pollination | Public domain 1 |
| 55 | GJ20 | Hybrid | Open pollination | Public domain 1 |
| 56 | AP8 | Robusta | Open pollination | AGB Embrapa |
| 57 | SK244 | Hybrid | Open pollination | Public domain 2 |
| 58 | GJ1 | Hybrid | Open pollination | Public domain 1 |
| 59 | N7 | Hybrid | Open pollination | Public domain 3 |
| 60 | CA1 | Hybrid | Open pollination | Public domain 4 |
| 61 | N32 | Hybrid | Open pollination | Public domain 3 |
| 62 | VP156 | Hybrid | Open pollination | Public domain 5 |
| 63 | LB015 | Hybrid | Open pollination | Public domain 6 |
| 64 | LB80 | Hybrid | Open pollination | Public domain 6 |
| 65 | L1 | Hybrid | Open pollination | Public domain 7 |
| 66 | R22 | Hybrid | Open pollination | Public domain 8 |
| 67 | AS3 | Hybrid | Open pollination | Public domain 10 |
| 68 | SK80 | Hybrid | Open pollination | Public domain 2 |
| 69 | SK41 | Hybrid | Open pollination | Public domain 2 |
| 70 | N13 | Hybrid | Open pollination | Public domain 3 |
| 71 | N12 | Hybrid | Open pollination | Public domain 3 |
| 72 | N2 | Hybrid | Open pollination | Public domain 3 |
| 73 | N8(G8) | Hybrid | Open pollination | Public domain 3 |
| 74 | AS12 | Hybrid | Open pollination | Public domain 10 |
| 75 | GB1 | Hybrid | Open pollination | Public domain 11 |
| 76 | GJ21 | Hybrid | Open pollination | Public domain 1 |
| 77 | GB4 | Hybrid | Open pollination | Public domain 11 |
| 78 | WP6 | Hybrid | Open pollination | Public domain 15 |
| 79 | LB160 | Hybrid | Open pollination | Public domain 6 |
| 80 | LB7 | Hybrid | Open pollination | Public domain 6 |
| 81 | GB7 | Hybrid | Open pollination | Public domain 11 |
| 82 | P42 | Hybrid | Open pollination | Public domain 13 |
| 83 | R152 | Hybrid | Open pollination | Public domain 9 |
| 84 | AR106 | Hybrid | Open pollination | Public domain 14 |
| 85 | GJ5 | Hybrid | Open pollination | Public domain 1 |
| 86 | GJ3 | Hybrid | Open pollination | Public domain 1 |
| 87 | BG180 | Hybrid | Open pollination | Public domain 12 |
| 88 | P50 | Hybrid | Open pollination | Public domain 5 |
| 89 | AS1 | Hybrid | Open pollination | Public domain 10 |
| 90 | AS5 | Hybrid | Open pollination | Public domain 10 |
| 91 | AS6 | Hybrid | Open pollination | Public domain 10 |
| 92 | AS7 | Hybrid | Open pollination | Public domain 10 |
| 93 | BAG41 | Hybrid | Open pollination | AGB Embrapa |
| 94 | N1 | Hybrid | Open pollination | Public domain 3 |
| 95 | N16 | Hybrid | Open pollination | Public domain 3 |
| 96 | AS10 | Hybrid | Open pollination | Public domain 10 |
| 97 | AS2 | Hybrid | Open pollination | Public domain 10 |
| 98 | LB22 | Hybrid | Open pollination | Public domain 6 |
| 99 | LB88 | Hybrid | Open pollination | Public domain 6 |
| 100 | LB68 | Hybrid | Open pollination | Public domain 6 |
| 101 | LB33 | Hybrid | Open pollination | Public domain 6 |
| 102 | LB20 | Hybrid | Open pollination | Public domain 6 |
| 103 | LB102 | Hybrid | Open pollination | Public domain 6 |
| 104 | LB12 | Hybrid | Open pollination | Public domain 6 |
| 105 | LB110 | Hybrid | Open pollination | Public domain 6 |
| 106 | LB60 | Hybrid | Open pollination | Public domain 6 |
| 107 | LB15 | Hybrid | Open pollination | Public domain 6 |
| 108 | LB10 | Hybrid | Open pollination | Public domain 6 |
| 109 | GJ30 | Hybrid | Open pollination | Public domain 1 |
| 110 | B3T8P54 | Hybrid | Emcapa03 × Robusta2258 | AGB Embrapa |
| 111 | GJ8 | Hybrid | Open pollination | Public domain 1 |
| 112 | BAG28 | Hybrid | Open pollination | AGB Embrapa |
| 113 | BAG19 | Hybrid | Emcapa03 × Robusta1675 | AGB Embrapa |
| 114 | BAG21 | Hybrid | Robusta1675 × Cpafro194 | AGB Embrapa |
| 115 | BRS3220 | Hybrid | Emcapa03 × Robusta1675 | Cultivar |
| 116 | GJ25 | Hybrid | Open pollination | Public domain 1 |
| 117 | BRS1216 | Hybrid | Emcapa03 × Robusta1675 | Cultivar |
| 118 | BAG26 | Hybrid | Emcapa03 × Robusta2258 | AGB Embrapa |
| 119 | BRS3213 | Hybrid | Emcapa03 × Robusta2258 | Cultivar |
| 120 | BAG24 | Hybrid | Emcapa03 × Robusta1675 | AGB Embrapa |
| 121 | BRS3210 | Hybrid | Emcapa03 × Robusta2258 | Cultivar |
| 122 | BAG33 | Hybrid | Open pollination | AGB Embrapa |
| 123 | BAG34 | Hybrid | Open pollination | AGB Embrapa |
| 124 | BAG23 | Hybrid | Open pollination | AGB Embrapa |
| 125 | BAG22 | Hybrid | Emcapa03 × Robusta2258 | AGB Embrapa |
| 126 | VP148 | Hybrid | Open pollination | Public domain 5 |
| 127 | BRSOuroPreto120 | Conilon | Open pollination | Cultivar |
| 128 | BRSOuroPreto125 | Conilon | Open pollination | Cultivar |
| 129 | Con854 | Conilon | Open pollination | AGB Embrapa |
| 130 | Con1048 | Conilon | Open pollination | AGB Embrapa |
| 131 | BRS3137 | Hybrid | Open pollination | Cultivar |
| 132 | BRS2336 | Hybrid | Open pollination | Cultivar |
| 133 | BRSOuroPreto203 | Conilon | Open pollination | Cultivar |
| 134 | BRS2299 | Hybrid | Open pollination | Cultivar |
| 135 | BRSOuroPreto130 | Conilon | Open pollination | Cultivar |
| 136 | BRSOuroPreto199 | Hybrid | Open pollination | Cultivar |
| 137 | BRSOuroPreto184 | Conilon | Open pollination | Cultivar |
| 138 | BRSOuroPreto155 | Conilon | Open pollination | Cultivar |
| 139 | BRS3193 | Hybrid | Open pollination | Cultivar |
| 140 | BRS2314 | Hybrid | Emcapa03 × Robusta640 | Cultivar |
| Resistance | Loci | Marker | Primers | Temp. | References |
|---|---|---|---|---|---|
| Hemileia vastatrix | A | SSR016 | F: ACCCGAAAGAAAGAACCAAG R: CCACACAACTCTCCTCATTC | 65 | [22,23] |
| B | CaRHv9 | F: TGATGAAGAAGAGCGCATAGC R: GTCTAAGACCAGAATCAGATGG | 65 | [23] | |
| C | CARF005 | F: GGACATCAACACCAACCTC R: ATCCCTACCATCCACTTCAAC | 60 | [8,15] | |
| D | RLK 2 | F: GCTCACAGGTCCGATTCCTCTG R: TTTGGGAATAGGCCCGGAAAGA | 66 | [23] | |
| Colletotrichum kahawae | E | Sat235 | F: TCGTTCTGTCATTAAATCGTCAA R: GCAAATCATGAAAATAGTTGGTG | 50 | [24,25] |
| F | Sat207 | R: GAAGCCGTTTCAAGCC F: CAATCTCTTTCCGATGCTCT | 50 | [24,25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.C.A.; Silva, L.d.F.; Rocha, R.B.; Teixeira, A.L.; Leichtweis, B.G.; Nascimento, M.; Caixeta, E.T. Genetic Diversity and Disease Resistance Genes Profiling in Cultivated Coffea canephora Genotypes via Molecular Markers. Plants 2025, 14, 2781. https://doi.org/10.3390/plants14172781
Silva ACA, Silva LdF, Rocha RB, Teixeira AL, Leichtweis BG, Nascimento M, Caixeta ET. Genetic Diversity and Disease Resistance Genes Profiling in Cultivated Coffea canephora Genotypes via Molecular Markers. Plants. 2025; 14(17):2781. https://doi.org/10.3390/plants14172781
Chicago/Turabian StyleSilva, Ana Carolina Andrade, Letícia de Faria Silva, Rodrigo Barros Rocha, Alexsandro Lara Teixeira, Bruno Grespan Leichtweis, Moysés Nascimento, and Eveline Teixeira Caixeta. 2025. "Genetic Diversity and Disease Resistance Genes Profiling in Cultivated Coffea canephora Genotypes via Molecular Markers" Plants 14, no. 17: 2781. https://doi.org/10.3390/plants14172781
APA StyleSilva, A. C. A., Silva, L. d. F., Rocha, R. B., Teixeira, A. L., Leichtweis, B. G., Nascimento, M., & Caixeta, E. T. (2025). Genetic Diversity and Disease Resistance Genes Profiling in Cultivated Coffea canephora Genotypes via Molecular Markers. Plants, 14(17), 2781. https://doi.org/10.3390/plants14172781







