Community and Population Characteristics and Future Potential Habitats Under Climate Change of Juniperus Species in Yunnan, Southwestern China
Abstract
1. Introduction
2. Materials and Methods
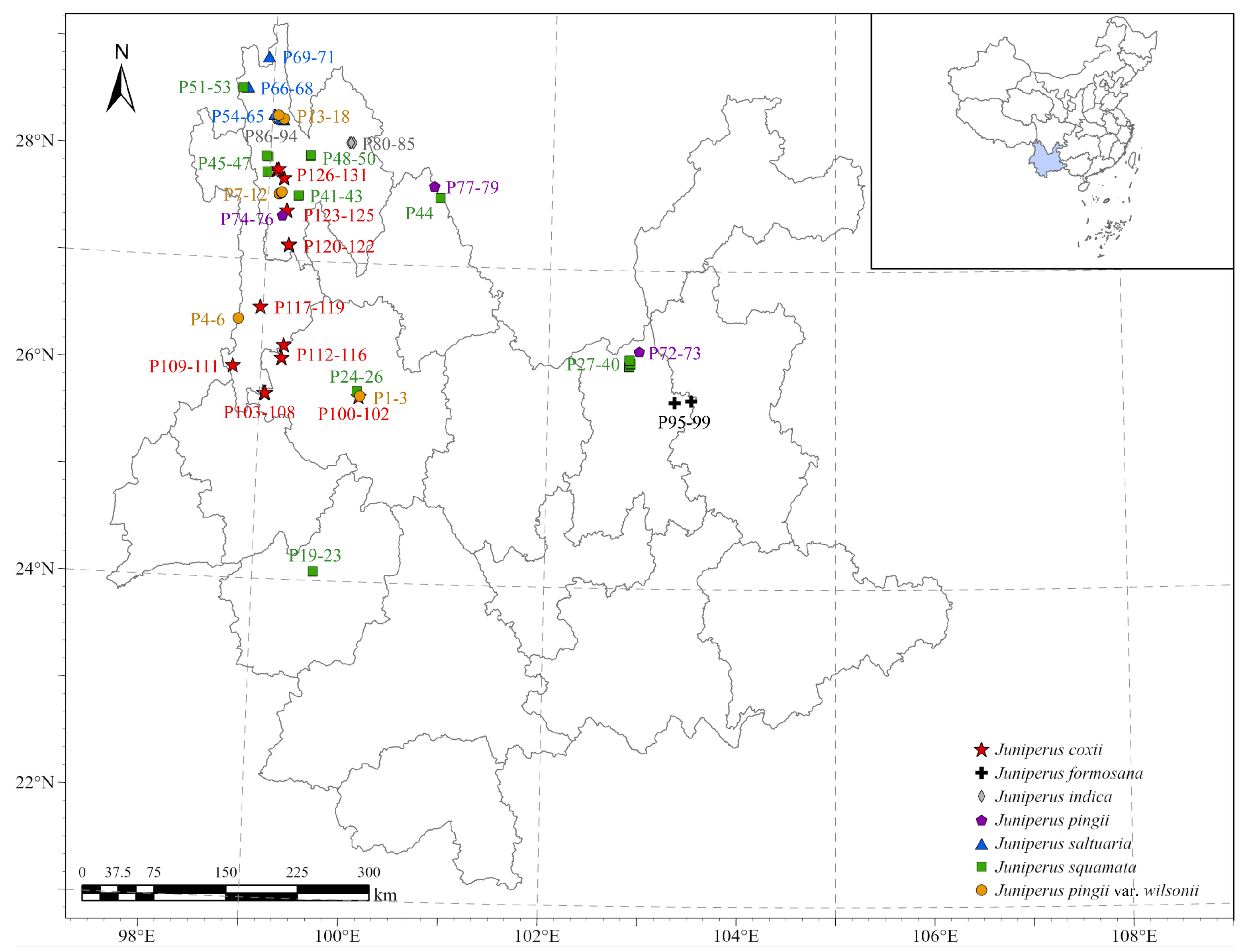
2.1. Study Area
2.2. Community and Population Characteristics
2.2.1. Field Investigation
2.2.2. Forest Structure
2.2.3. Community Classification
2.2.4. Species Diversity and Floristic Features
2.2.5. Phylogenetic Analysis
2.2.6. Regeneration Analysis
2.3. Future Potential Habitats Under Climate Change
2.3.1. Occurrence Data
2.3.2. Climate Data
2.3.3. Climate Variable Screening
2.3.4. Parameter Optimization
2.3.5. Future Suitable Habitat Prediction
3. Results
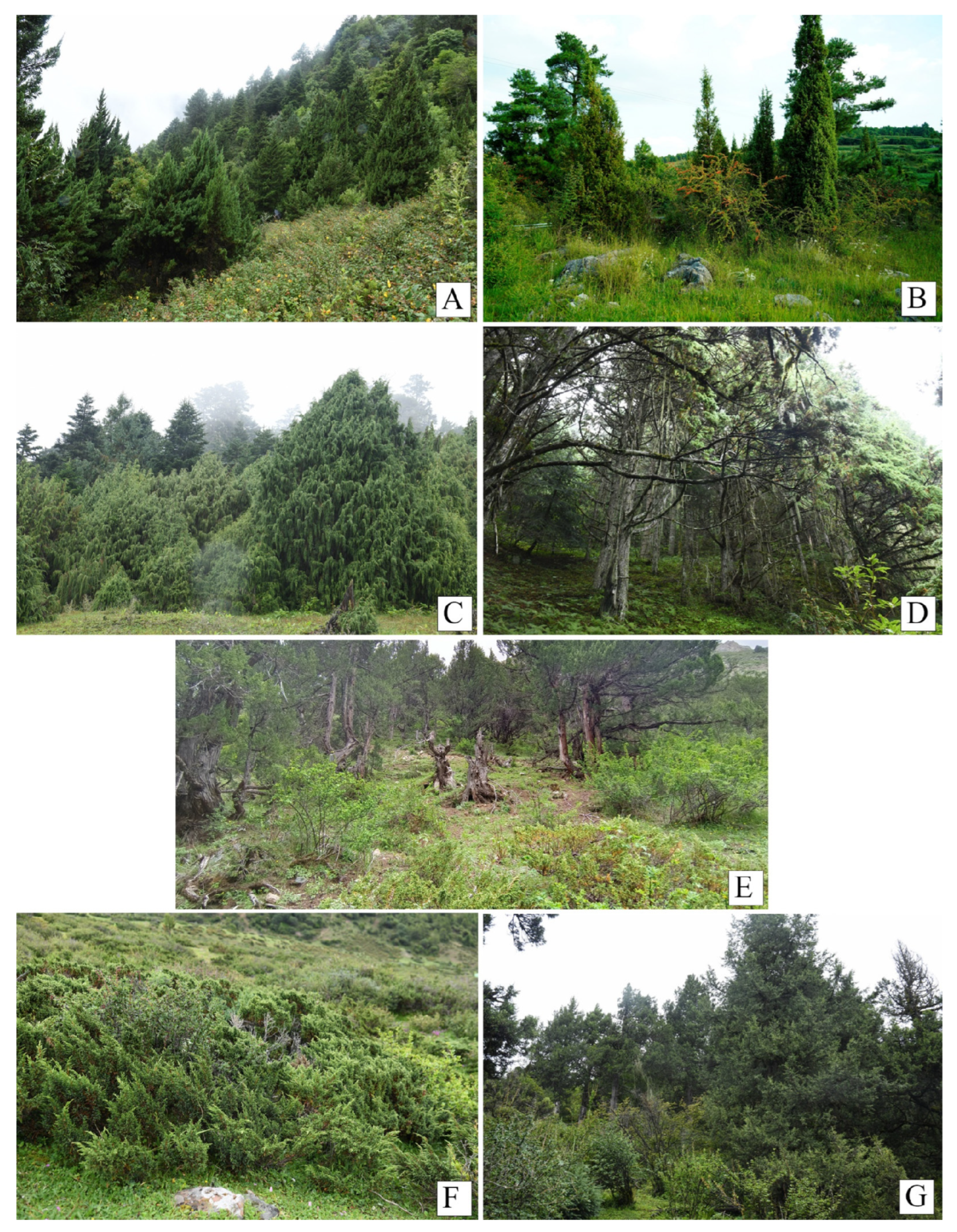
3.1. Community Characteristics
3.1.1. Community Types and Stratification
3.1.2. Species Diversity and Phylogenetic Diversity
3.2. Population Structure
DBH-Class Structure and Regeneration
3.3. Predicted Habitats Under Climate Change
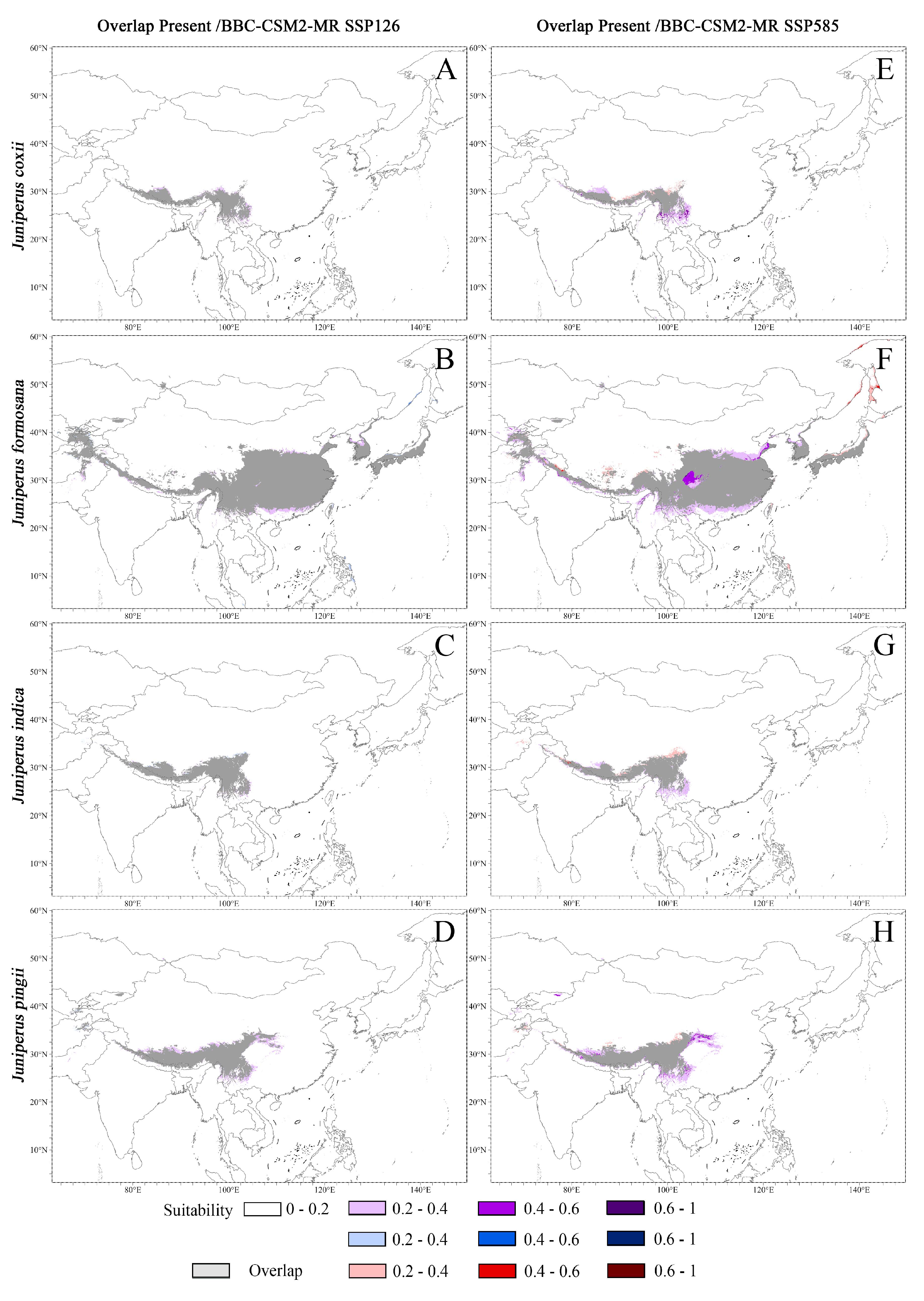
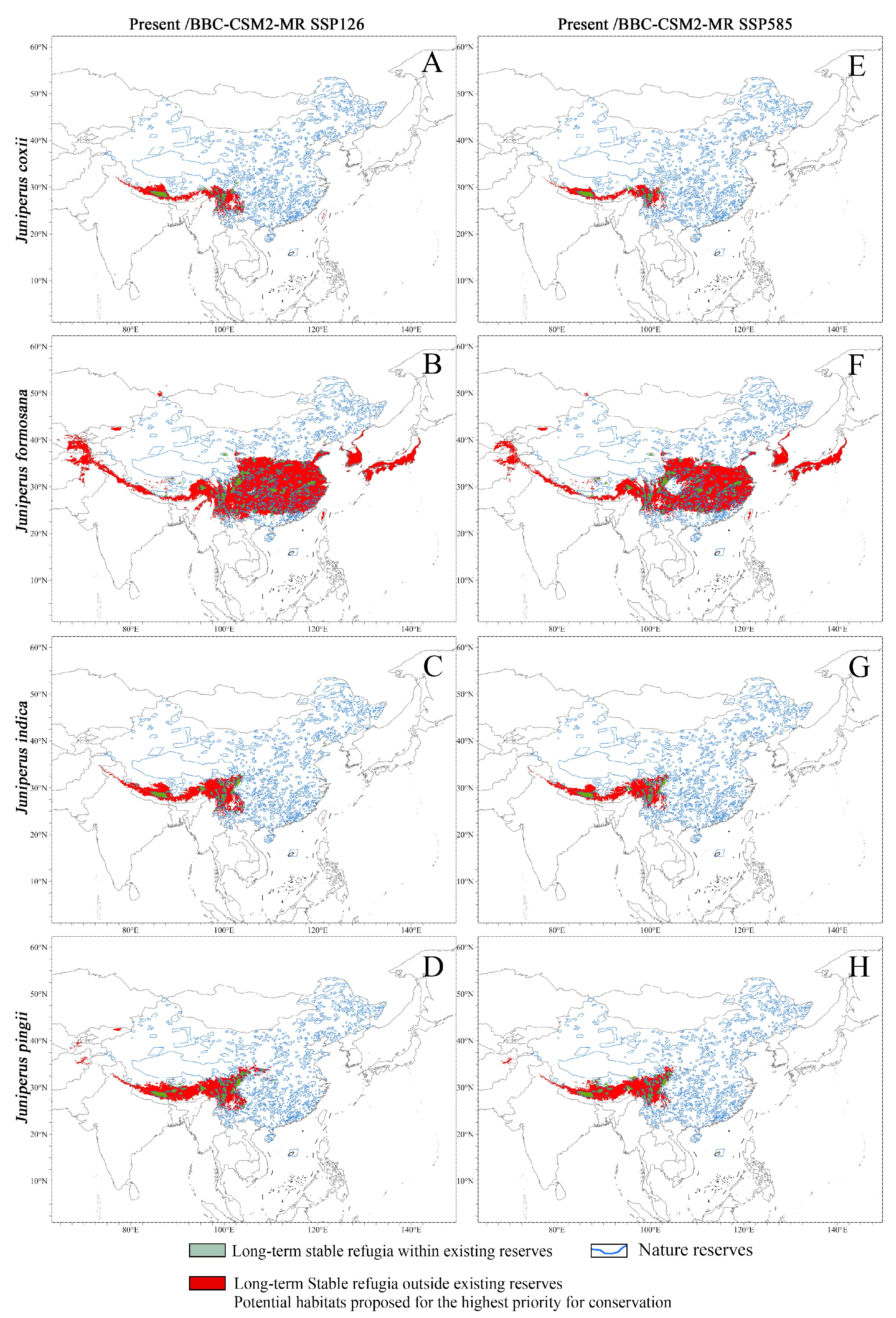
3.3.1. Potential Distribution Under Current Climate
3.3.2. Potential Distribution Under Future Climate
4. Discussion
4.1. Habitats and Regeneration
4.2. Species Diversity
4.3. Regeneration
4.4. Phylogenetic Diversity
4.5. Management and Conservation Recommendations
4.6. Potential Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Thorne, R.F. Major Disjunctions in the Geographic Ranges of Seed Plants. Q. Rev. Biol. 1972, 47, 365–411. [Google Scholar] [CrossRef]
- Farjon, A. A Monograph of Cupressaceae and Sciadopitys; Royal Botanic Gardens Kew: London, UK, 2005. [Google Scholar]
- Adams, R.P. Junipers of the World: The Genus Juniperus; Trafford Publishing: Vancouver, BC, Canada, 2014. [Google Scholar]
- Kvaček, Z. A new juniper from the Palaeogene of Central Europe. Feddes Repert. 2002, 113, 492–502. [Google Scholar] [CrossRef]
- Farjon, A. Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press and Missouri Botanical Garden Press: Beijing, China, 1999; Volume 4. [Google Scholar]
- Spicer, R.A.; Harris, N.B.W.; Widdowson, M.; Herman, A.B.; Guo, S.; Valdes, P.J.; Wolfe, J.A.; Kelley, S.P. Constant Elevation of Southern Tibet over the Past 15 Million Years. Nature 2003, 421, 622–624. [Google Scholar] [CrossRef]
- Liu, J.Q.; Wang, Y.J.; Wang, A.L.; Hideaki, O.; Abbott, R.J. Radiation and Diversification within the Ligularia–Cremanthodium–Parasenecio Complex (Asteraceae) Triggered by Uplift of the Qinghai-Tibetan Plateau. Mol. Phylogenet. Evol. 2006, 38, 31–49. [Google Scholar] [CrossRef]
- Miller, R.F.; Bates, J.D.; Svejcar, T.J.; Pierson, F.B.; Eddleman, L.E. Biology, Ecology, and Management of Western Juniper (Juniperus occidentalis) (Oregon State University); Agricultural Experiment Station, Oregon State University: Corvallis, OR, USA, 2010. [Google Scholar]
- Singh, A.; Samant, S.S. Population and Community Structure Pattern of Juniperous polycarpos K. Koch with Climate Change Effect in the Cold Desert Trans Himalayan Region, India. Arid. Ecosyst. 2020, 10, 17–26. [Google Scholar] [CrossRef]
- Garcίa, D.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Age Structure of Juniperus communis L. in the Iberian Peninsula: Conservation of Remnant Populations in Mediterranean Mountains. Biol. Conserv. 1999, 87, 215–220. [Google Scholar] [CrossRef]
- Mao, K.; Hao, G.; Liu, J.; Adams, R.; Milne, R. Diversification and Biogeography of Juniperus (Cupressaceae): Variable Diversification Rates and Multiple Intercontinental Dispersals. New Phytol. 2010, 188, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P.; Schwarzbach, A.E. Phylogeny of Juniperus Using nrDNA and Four cpDNA Regions. Phytologia 2013, 95, 179–187. [Google Scholar]
- Rahimian Boogar, A.; Salehi, H.; Pourghasemi, H.; Blaschke, T. Predicting Habitat Suitability and Conserving Juniperus spp. Habitat Using SVM and Maximum Entropy Machine Learning Techniques. Water 2019, 11, 2049. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Peng, S.; Yirdaw, E.; Wu, N. Spatial Structure of Alpine Trees in Mountain Baima Xueshan on the Southeast Tibetan Plateau. Silva Fenn. 2009, 43, 197–208. [Google Scholar] [CrossRef]
- Zhu, A.; Fan, W.; Adams, R.P.; Mower, J.P. Phylogenomic Evidence for Ancient Recombination between Plastid Genomes of the Cupressus-Juniperus-Xanthocyparis Complex (Cupressaceae). BMC Evol. Biol. 2018, 18, 137. [Google Scholar] [CrossRef]
- Dakhil, M.A.; Halmy, M.W.A.; Hassan, W.A.; El-Keblawy, A.; Pan, K.; Abdelaal, M. Endemic Juniperus Montane Species Facing Extinction Risk under Climate Change in Southwest China: Integrative Approach for Conservation Assessment and Prioritization. Biology 2021, 10, 63. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/en (accessed on 15 June 2025).
- Qin, H.; Yang, Y.; Dong, S.-Y.; He, Q.; Jia, Y.; Zhao, L.; Yu, S.-X.; Huiyuan, L.; Liu, B.; Yan, Y.-H.; et al. Threatened Species List of China’s Higher Plants. Biodivers. Sci. 2017, 25, 696–744. [Google Scholar] [CrossRef]
- Zhao, J.; Maitituersun, A.; Li, C.; Li, Q.; Xu, F.; Liu, T. Evaluation on Analgesic and Anti-Inflammatory Activities of Total Flavonoids from Juniperus sabina. Evid. Based Complement. Alternat. Med. 2018, 2018, 7965306. [Google Scholar] [CrossRef] [PubMed]
- Jim-Min, F.; Ching-Kuo, L.; Yu-Shia, C. Lignans from Leaves of Juniperus chinensis. Phytochemistry 1992, 31, 3659–3661. [Google Scholar] [CrossRef]
- Basas-Jaumandreu, J.; López, J.F.; de las Heras, F.X.C. Labdane-Type Diterpenoids from Juniperus communis Needles. Ind. Crop. Prod. 2015, 76, 333–345. [Google Scholar] [CrossRef]
- Meng, X.; Li, D.; Zhou, D.; Wang, D.; Liu, Q.; Fan, S. Chemical Composition, Antibacterial Activity and Related Mechanism of the Essential Oil from the Leaves of Juniperus rigida Sieb. et Zucc against Klebsiella pneumoniae. J. Ethnopharmacol. 2016, 194, 698–705. [Google Scholar] [CrossRef]
- Monk, C.D.; Child, G.I.; Nicholson, S.A. Species Diversity of a Stratified Oak-Hickory Community. Ecology 1969, 50, 468–470. [Google Scholar] [CrossRef]
- Loya, Y. Community Structure and Species Diversity of Hermatypic Corals at Eilat, Red Sea. Mar. Biol. 1972, 13, 100–123. [Google Scholar] [CrossRef]
- Tunnicliffe, V. High Species Diversity and Abundance of the Epibenthic Community in an Oxygen-Deficient Basin. Nature 1981, 294, 354–356. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Miller, T.E. Effects of Different Resource Additions of Species Diversity in an Annual Plant Community. Ecology 1990, 71, 213–225. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and Community Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Prinzing, A.; Durka, W.; Klotz, S.; Brandl, R. The Niche of Higher Plants: Evidence for Phylogenetic Conservatism. Proc. R. Soc. Lond. B 2001, 268, 2383–2389. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Cornwell, W.K.; Webb, C.O.; Ackerly, D.D. Trait Evolution, Community Assembly, and the Phylogenetic Structure of Ecological Communities. Am. Nat. 2007, 170, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J.; Kozak, K.H.; Fine, P.V.A.; Kembel, S.W. The Merging of Community Ecology and Phylogenetic Biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef]
- Leadley, P.; Alkemade, R.; Fernandez, J.; Proença, V.; Scharlemann, J. Biodiversity Scenarios: Projections of 21st Century Change in Biodiversity and Associated Ecosystem Services. Secr. Conv. Biol. Divers. 2010, 1–132. [Google Scholar]
- Gottfried, M.; Pauli, H.; Reiter, K.; Grabherr, G. A Fine-Scaled Predictive Model for Changes in Species Distribution Patterns of High Mountain Plants Induced by Climate Warming. Divers. Distrib. 1999, 5, 241–251. [Google Scholar] [CrossRef]
- Matsui, T.; Yagihashi, T.; Nakaya, T.; Tanaka, N.; Taoda, H. Climatic Controls on Distribution of Fagus Crenata Forests in Japan. J. Veg. Sci. 2004, 15, 57–66. [Google Scholar] [CrossRef]
- Wiens, J.J.; Graham, C.H. Niche Conservatism: Integrating Evolution, Ecology, and Conservation Biology. Annu. Rev. Ecol. Evol. S. 2005, 36, 519–539. [Google Scholar] [CrossRef]
- Tsuyama, I.; Nakao, K.; Higa, M.; Matsui, T.; Shichi, K.; Tanaka, N. What Controls the Distribution of the Japanese Endemic Hemlock, Tsuga Diversifolia? Footprint of Climate in the Glacial Period on Current Habitat Occupancy. J. For. Res. 2014, 19, 154–165. [Google Scholar] [CrossRef]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.-F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T.; et al. Identifying Long-Term Stable Refugia for Relict Plant Species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef]
- Tang, C.Q.; Ohashi, H.; Matsui, T.; Herrando-Moraira, S.; Dong, Y.-F.; Li, S.; Han, P.-B.; Huang, D.-S.; Shen, L.-Q.; Li, Y.-F.; et al. Effects of Climate Change on the Potential Distribution of the Threatened Relict Dipentodon sinicus of Subtropical Forests in East Asia: Recommendations for Management and Conservation. Glob. Ecol. Conserv. 2020, 23, e01192. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive Habitat Distribution Models in Ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Haase, C.G.; Yang, A.; McNyset, K.M.; Blackburn, J.K. GARPTools: R Software for Data Preparation and Model Evaluation of GARP Models. Ecography 2021, 44, 1790–1796. [Google Scholar] [CrossRef]
- Ma, R.; Ban, J.; Wang, Q.; Zhang, Y.; Yang, Y.; Li, S.; Shi, W.; Zhou, Z.; Zang, J.; Li, T. Full-Coverage 1 Km Daily Ambient PM2.5 and O3 Concentrations of China in 2005–2017 Based on a Multi-Variable Random Forest Model. Earth Syst. Sci. Data 2022, 14, 943–954. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with MAXENT: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive Performance of Presence-Only Species Distribution Models: A Benchmark Study with Reproducible Code. Ecol. Monogr. 2022, 92, e01486. [Google Scholar] [CrossRef]
- Pérez-Suárez, M.; Ramírez-Albores, J.E.; Martínez-Campos, Á.R. Predicting the Impacts of Climate Change on Potential Suitability Habitats of Three Juniperus Trees in Mexico. Plant Ecol. 2024, 225, 37–51. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Hu, Y.; Wang, H.; Wen, M.; Liu, C.; Ding, P. Prediction of Potentially Suitable Distribution Areas for Juniperus tibetica Based on MaxEnt Model. J. Cent. South Univ. For. Technol. 2023, 43, 41–51. [Google Scholar]
- Turkmenoglu, G.; Sarikaya, A.G.; Uzun, A.; Fakir, H. Prediction of Present and Future Distribution Areas of Juniperus drupacea Labill and Determination of Ethnobotany Properties in Antalya Province, Türkiye. Open Life Sci. 2024, 19, 20220883. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Q.; Lu, X.; Du, M.-R.; Xiao, S.L.; Li, S.; Han, P.B.; Zeng, J.L.; Wen, J.R.; Yao, S.Q.; Shi, Y.C.; et al. Forest Characteristics and Population Structure of a Threatened Palm Tree Caryota obtusa in the Karst Forest Ecosystem of Yunnan, China. J. Plant Ecol. 2022, 15, 829–843. [Google Scholar] [CrossRef]
- Tang, C.Q.; Chen, Q.; Shi, Y.C.; Li, Q.; Pei, K.D.; Li, S.; Han, P.B.; Xiao, S.L.; Du, M.R.; Peng, M.C.; et al. Endangered Taxus wallichiana var. Wallichiana—Its Forest Characteristics, Population Structure, and Regeneration Status in Yunnan, Southwestern China. Diversity 2024, 16, 642. [Google Scholar]
- Tang, C.Q.; Yao, S.Q.; Han, P.B.; Wen, J.R.; Li, S.; Peng, M.C.; Wang, C.Y.; Matsui, T.; Li, Y.P.; Lu, S.; et al. Forest Characteristics, Population Structure and Growth Trends of Threatened Relict Pseudotsuga fzZorrestii in China. Plant Divers. 2023, 45, 422–433. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M. PC-ORD: Multivariate Analysis of Ecological Data, Version 7.0 for Windows; Wild Blueberry Media: Corvallis, OR, USA, 2016. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R.; Springer: New York, NY, USA, 2011; Volume 2. [Google Scholar] [CrossRef]
- Pielou, E.G.; Hill, M.O. An Introduction to Mathematical Ecology. J. Ecol. 1970, 58, 896. [Google Scholar] [CrossRef]
- Lande, R. Statistics and Partitioning of Species Diversity, and Similarity among Multiple Communities. Oikos 1996, 76, 5. [Google Scholar] [CrossRef]
- Tang, C.Q.; Han, P.-B.; Li, S.; Shen, L.-Q.; Huang, D.-S.; Li, Y.-F.; Peng, M.-C.; Wang, C.-Y.; Li, X.-S.; Li, W.; et al. Species Richness, Forest Types and Regeneration of Schima in the Subtropical Forest Ecosystem of Yunnan, Southwestern China. For. Ecosyst. 2020, 7, 35. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Wu, Z. The areal-types of Chinese genera of seed plants. Acta Bot. Yunnanica 1991, 13, 1. [Google Scholar]
- Wu, Z. The Areal types of the World Families of Seed Plant. Acta Bot. Yunnanica 2003, 25, 245–257. [Google Scholar]
- Zhang, J.; Liu, B.; Liu, S.; Feng, Z.; Jiang, K. Plantlist: Looking Up the Status of Plant Scientific Names Based on the Plant List Database, Searching the Chinese Names and Making Checklists of Plants. R Package Version 4.3.2. 2019. Available online: https://github.com/helixcn/plantlist/ (accessed on 26 August 2025).
- Smith, S.A.; Brown, J.W. Constructing a Broadly Inclusive Seed Plant Phylogeny. Am. J. Bot. 2018, 105, 302–314. [Google Scholar] [CrossRef]
- Jin, Y.; Qian, H.V. PhyloMaker: An R Package That Can Generate Very Large Phylogenies for Vascular Plants. Ecography 2019, 42, 1353–1359. [Google Scholar] [CrossRef]
- Jin, Y.; Qian, H.V. PhyloMaker2: An Updated and Enlarged R Package That Can Generate Very Large Phylogenies for Vascular Plants. Plant Divers. 2022, 44, 335–339. [Google Scholar] [CrossRef]
- Kembel, S.W.; Hubbell, S.P. The Phylogenetic Structure of a Neotropical Forest Tree Community. Ecology 2006, 87, S86–S99. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xing, X. CMIP6 Scenario Model Intercomparison Project (ScenarioMIP). Clim. Change Res. 2019, 15, 519–525. [Google Scholar]
- Feng, X.; Park, D.S.; Walker, C.; Peterson, A.T.; Merow, C.; Papeş, M. A Checklist for Maximizing Reproducibility of Ecological Niche Models. Nat. Ecol. Evol. 2019, 3, 1382–1395. [Google Scholar] [CrossRef] [PubMed]
- Vignali, S.; Barras, A.G.; Arlettaz, R.; Braunisch, V. SDMtune: An R Package to Tune and Evaluate Species Distribution Models. Ecol. Evol. 2020, 10, 11488–11506. [Google Scholar] [CrossRef]
- Alkishe, A.A.; Peterson, A.T.; Samy, A.M. Climate Change Influences on the Potential Geographic Distribution of the Disease Vector Tick Ixodes Ricinus. PLoS ONE 2017, 12, e0189092. [Google Scholar] [CrossRef] [PubMed]
- Shcheglovitova, M.; Anderson, R.P. Estimating Optimal Complexity for Ecological Niche Models: A Jackknife Approach for Species with Small Sample Sizes. Ecol. Model. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- James, A.M.; Burdett, C.; McCool, M.J.; Fox, A.; Riggs, P. The Geographic Distribution and Ecological Preferences of the American Dog Tick, Dermacentor Variabilis (Say), in the U.S.A. Med. Vet. Entomol. 2015, 29, 178–188. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting Thresholds for the Prediction of Species Occurrence with Presence-only Data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Harrison, T.M.; Copeland, P.; Kidd, W.S.F.; Yin, A. Raising Tibet. Science 1992, 255, 1663–1670. [Google Scholar] [CrossRef]
- Chung, S.L.; Lo, C.H.; Lee, T.Y.; Zhang, Y.; Xie, Y.; Li, X.; Wang, K.L.; Wang, P.L. Diachronous Uplift of the Tibetan Plateau Starting 40 Myr Ago. Nature 1998, 394, 769–773. [Google Scholar] [CrossRef]
- Guo, Z.; Ruddiman, W.; Hao, Q.; Wu, H.B.; Qiao, Y.; Zhu, R.; Peng, S.; Wei, J.J.; Yuan, B.Y.; Liu, T.S. Onset of Asian Desertification by 22 Myr Ago Inferred from Loess Deposits in China. Nature 2002, 416, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Yachi, S.; Loreau, M. Biodiversity and Ecosystem Productivity in a Fluctuating Environment: The Insurance Hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Muhammad, Z.; Majeed, M.; Aziz, R.; Khan, A.; Mangrio, W.M.; Abdo, H.G.; Almohamad, H.; Al Dughairi, A.A. Vegetation Diversity Pattern during Spring Season in Relation to Topographic and Edaphic Variables in Sub-Tropical Zone. Bot. Stud. 2023, 64, 25. [Google Scholar] [CrossRef]
- Lepcha, P. Climate Change and Its Impact on Mountainous Plant Species: A Review; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Helmus, M.R.; Keller, W.; Paterson, M.J.; Yan, N.D.; Cannon, C.H.; Rusak, J.A. Communities Contain Closely Related Species during Ecosystem Disturbance. Ecol. Lett. 2010, 13, 162–174. [Google Scholar] [CrossRef]
- Verdú, M.; Pausas, J.G. Fire Drives Phylogenetic Clustering in Mediterranean Basin Woody Plant Communities. J. Ecol. 2007, 95, 1316–1323. [Google Scholar] [CrossRef]
- Ojeda, F.; Pausas, J.G.; Verdú, M. Soil Shapes Community Structure through Fire. Oecologia 2010, 163, 729–735. [Google Scholar] [CrossRef]
- Dinnage, R. Disturbance Alters the Phylogenetic Composition and Structure of Plant Communities in an Old Field System. PLoS ONE 2009, 4, e7071. [Google Scholar] [CrossRef]
- Fang, J.; Zhu, J.; Shi, Y. The Responses of Ecosystems to Global Warming. Chin. Sci. Bull. 2017, 63, 136–140. [Google Scholar] [CrossRef]
- Chung, M.Y.; López-Pujol, J.; Chung, M.G. The Role of the Baekdudaegan (Korean Peninsula) as a Major Glacial Refugium for Plant Species: A Priority for Conservation. Biol. Conserv. 2017, 206, 236–248. [Google Scholar] [CrossRef]





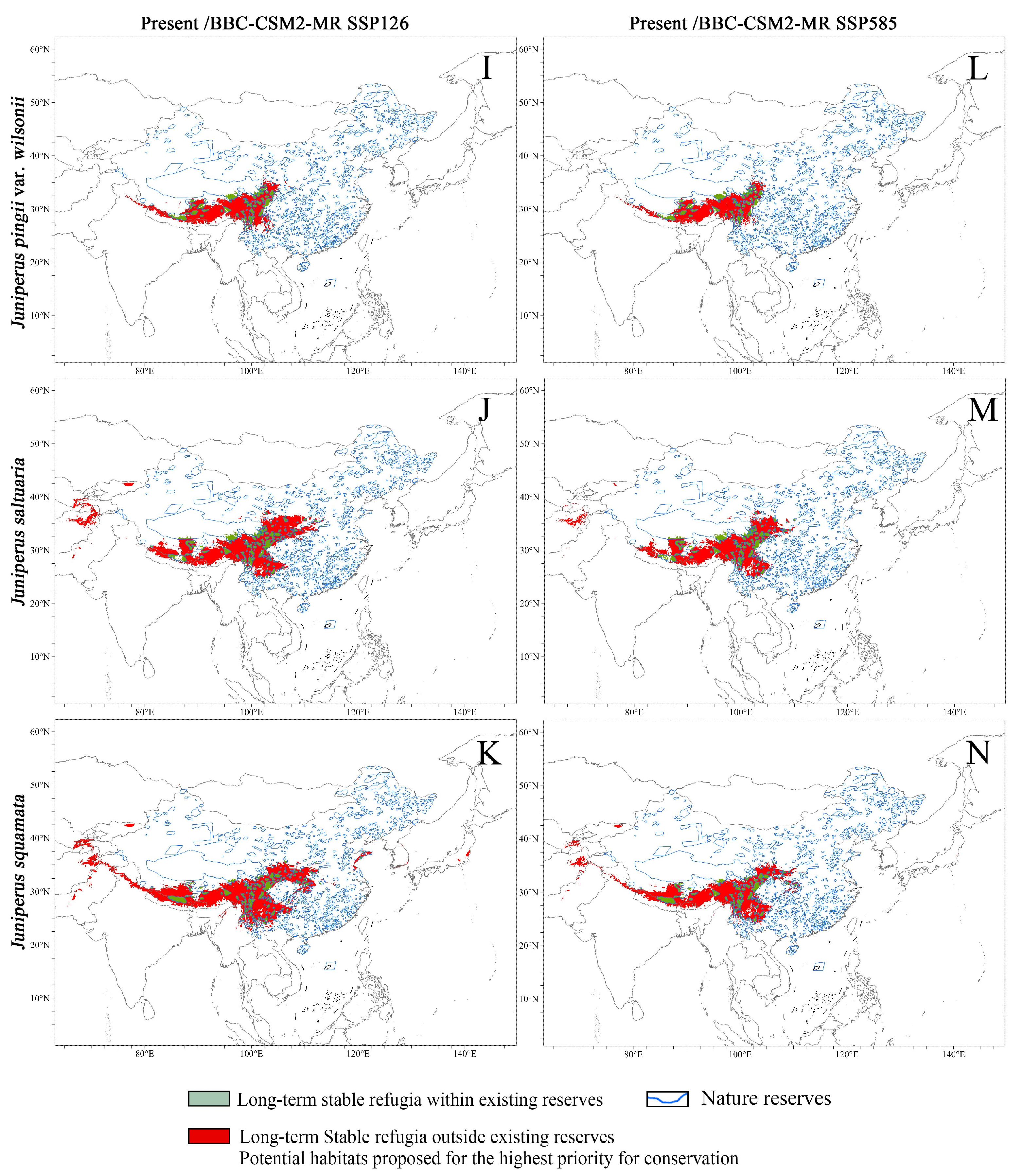
| Area of Stable Refugia (×104 km2) | Area of Stable Refugia Within Nature Reserves (×104 km2 and %) | Area of Stable Refugia Outside Nature Reserve (×104 km2 and %) | ||
|---|---|---|---|---|
| JC | Present | 65.16 | 12.76 (19.58) | 52.4 (80.42) |
| BCC-CSM2-MR 126 | 50.46 | 10.69 (21.19) | 39.77 (78.81) | |
| BCC-CSM2-MR 585 | 41.82 | 9.48 (22.66) | 32.34 (77.34) | |
| JF | Present | 431.79 | 58.7 (13.59) | 373.09 (86.41) |
| BCC-CSM2-MR 126 | 393.32 | 54.82 (13.94) | 338.5 (86.06) | |
| BCC-CSM2-MR 585 | 316.92 | 50.73 (16.01) | 266.19 (83.99) | |
| JI | Present | 86.54 | 17.42 (20.13) | 69.12 (79.87) |
| BCC-CSM2-MR 126 | 82.20 | 16.66 (20.27) | 65.54 (79.73) | |
| BCC-CSM2-MR 585 | 70.91 | 15.63 (22.04) | 55.28 (77.96) | |
| JP | Present | 127.73 | 26.95 (21.1) | 100.78 (78.9) |
| BCC-CSM2-MR 126 | 96.63 | 20.72 (21.44) | 75.91 (78.56) | |
| BCC-CSM2-MR 585 | 83.84 | 20.44 (24.37) | 63.4 (75.63) | |
| JPW | Present | 105.16 | 26.25 (24.96) | 78.91 (75.04) |
| BCC-CSM2-MR 126 | 90.48 | 23.81 (26.31) | 66.68 (73.69) | |
| BCC-CSM2-MR 585 | 100.48 | 35.25 (35.08) | 65.23 (64.92) | |
| JSA | Present | 184.81 | 35.73 (19.33) | 149.08 (80.67) |
| BCC-CSM2-MR 126 | 154.81 | 31.47 (20.33) | 123.34 (79.67) | |
| BCC-CSM2-MR 585 | 159.52 | 48.33 (30.3) | 111.19 (69.7) | |
| JSQ | Present | 239.64 | 40.35 (16.84) | 199.29 (83.16) |
| BCC-CSM2-MR 126 | 172.32 | 31.66 (18.37) | 140.66 (81.63) | |
| BCC-CSM2-MR 585 | 139.26 | 32.61 (23.41) | 106.65 (76.59) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.-C.; Chen, Q.; Du, M.-R.; Xiao, S.-L.; Li, S.-F.; Wang, X.-F.; Li, Q.; Tang, C.Q. Community and Population Characteristics and Future Potential Habitats Under Climate Change of Juniperus Species in Yunnan, Southwestern China. Plants 2025, 14, 2754. https://doi.org/10.3390/plants14172754
Shi Y-C, Chen Q, Du M-R, Xiao S-L, Li S-F, Wang X-F, Li Q, Tang CQ. Community and Population Characteristics and Future Potential Habitats Under Climate Change of Juniperus Species in Yunnan, Southwestern China. Plants. 2025; 14(17):2754. https://doi.org/10.3390/plants14172754
Chicago/Turabian StyleShi, You-Cai, Qing Chen, Min-Rui Du, Shu-Li Xiao, Shuai-Feng Li, Xiao-Fan Wang, Qiao Li, and Cindy Q. Tang. 2025. "Community and Population Characteristics and Future Potential Habitats Under Climate Change of Juniperus Species in Yunnan, Southwestern China" Plants 14, no. 17: 2754. https://doi.org/10.3390/plants14172754
APA StyleShi, Y.-C., Chen, Q., Du, M.-R., Xiao, S.-L., Li, S.-F., Wang, X.-F., Li, Q., & Tang, C. Q. (2025). Community and Population Characteristics and Future Potential Habitats Under Climate Change of Juniperus Species in Yunnan, Southwestern China. Plants, 14(17), 2754. https://doi.org/10.3390/plants14172754






