Buddleja globosa Leaf Methanolic Extract Acts Against Trypanosoma cruzi Parasites by Inducing Mitochondrial Inner Membrane Hyperpolarization
Abstract
1. Introduction
2. Results
2.1. NMR Characterization of the Iridoid Present in the BG500 Fraction
2.2. Antioxidant Activity and Polyphenol Identification and Quantification
2.2.1. Antioxidant Activity of MET and Fractions
2.2.2. Polyphenol Identification and Quantification
2.3. In Vitro Cytotoxic Activity of MET and the BG500 Fraction
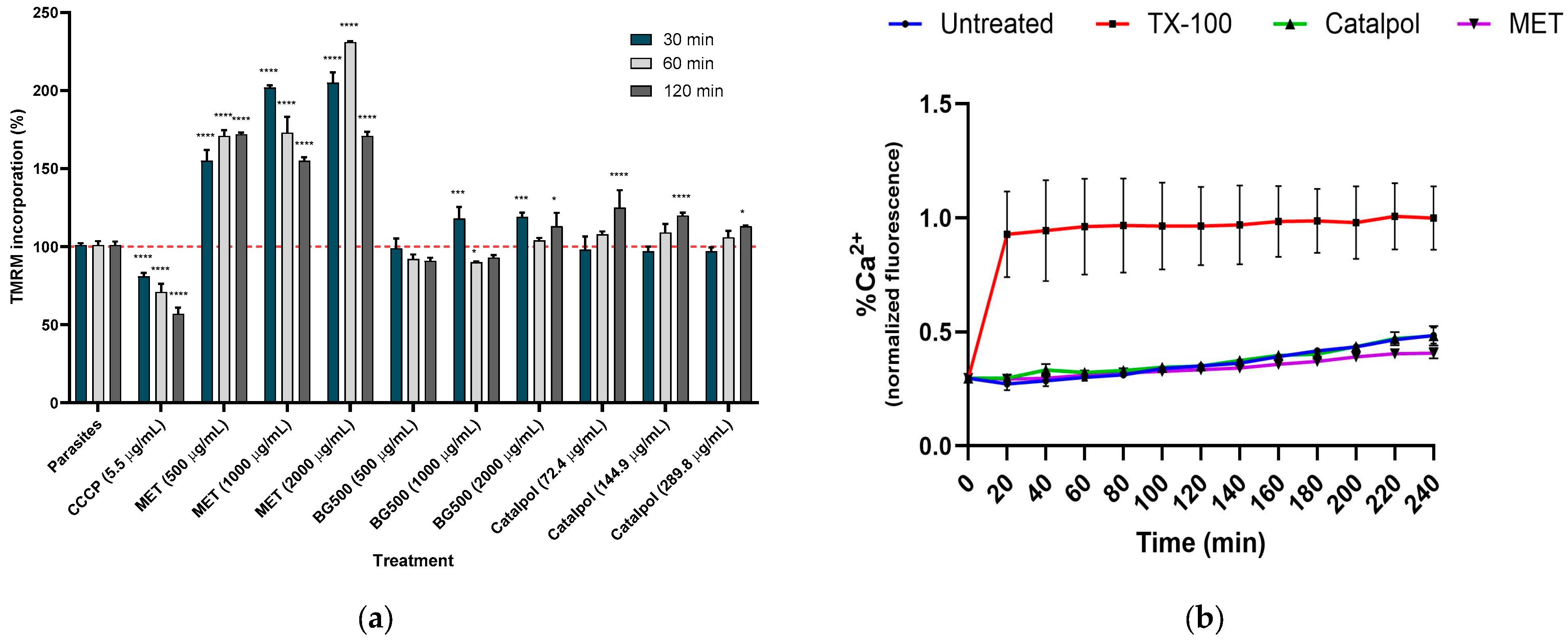
2.4. Effect of MET and Catalpol on ΔΨm
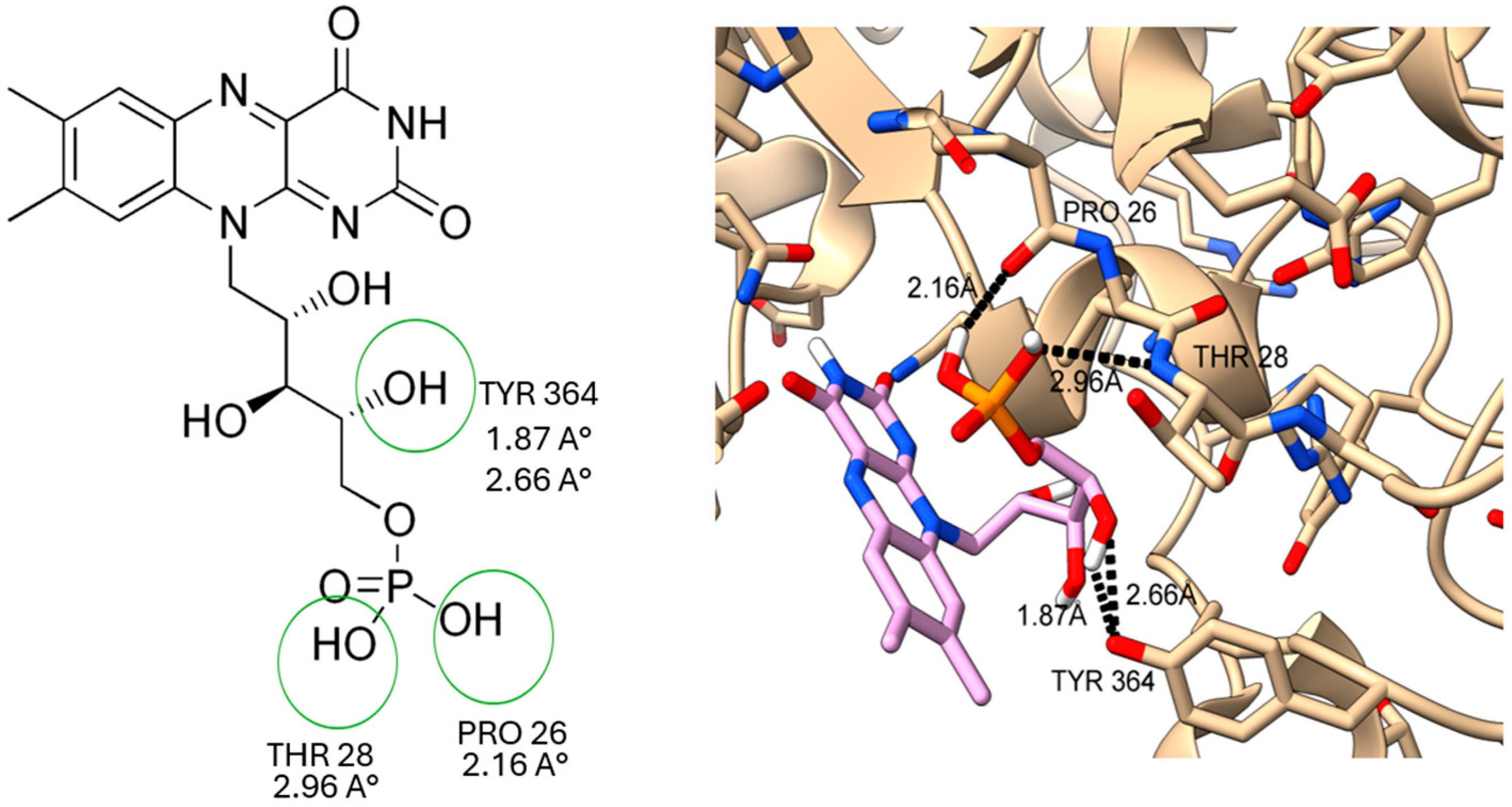
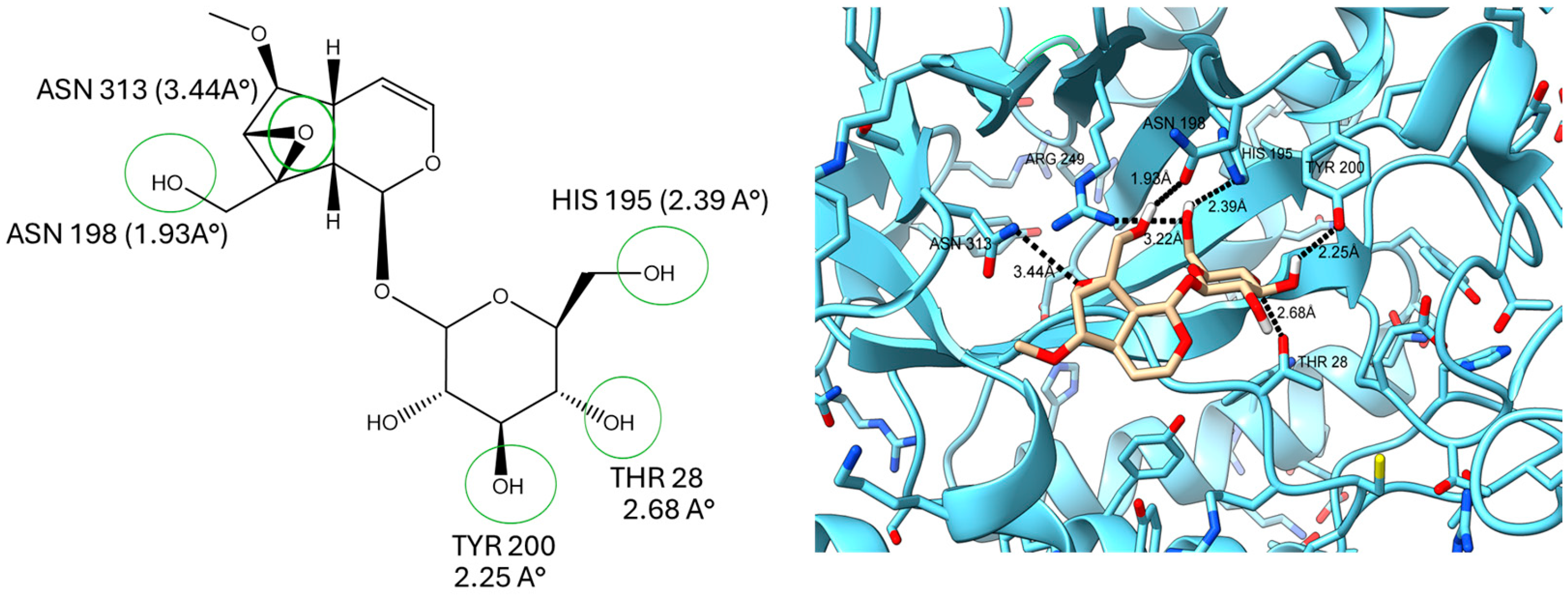
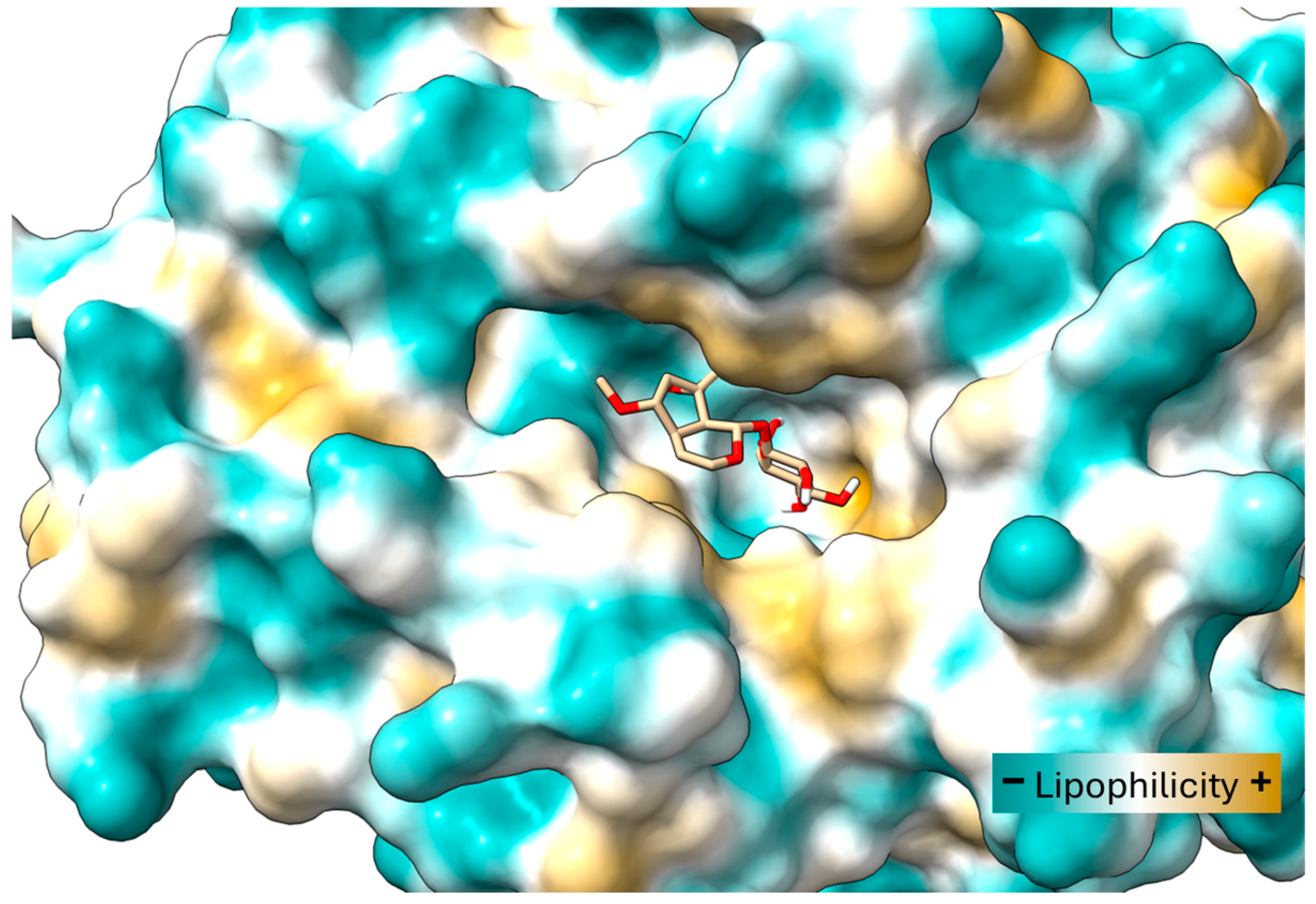
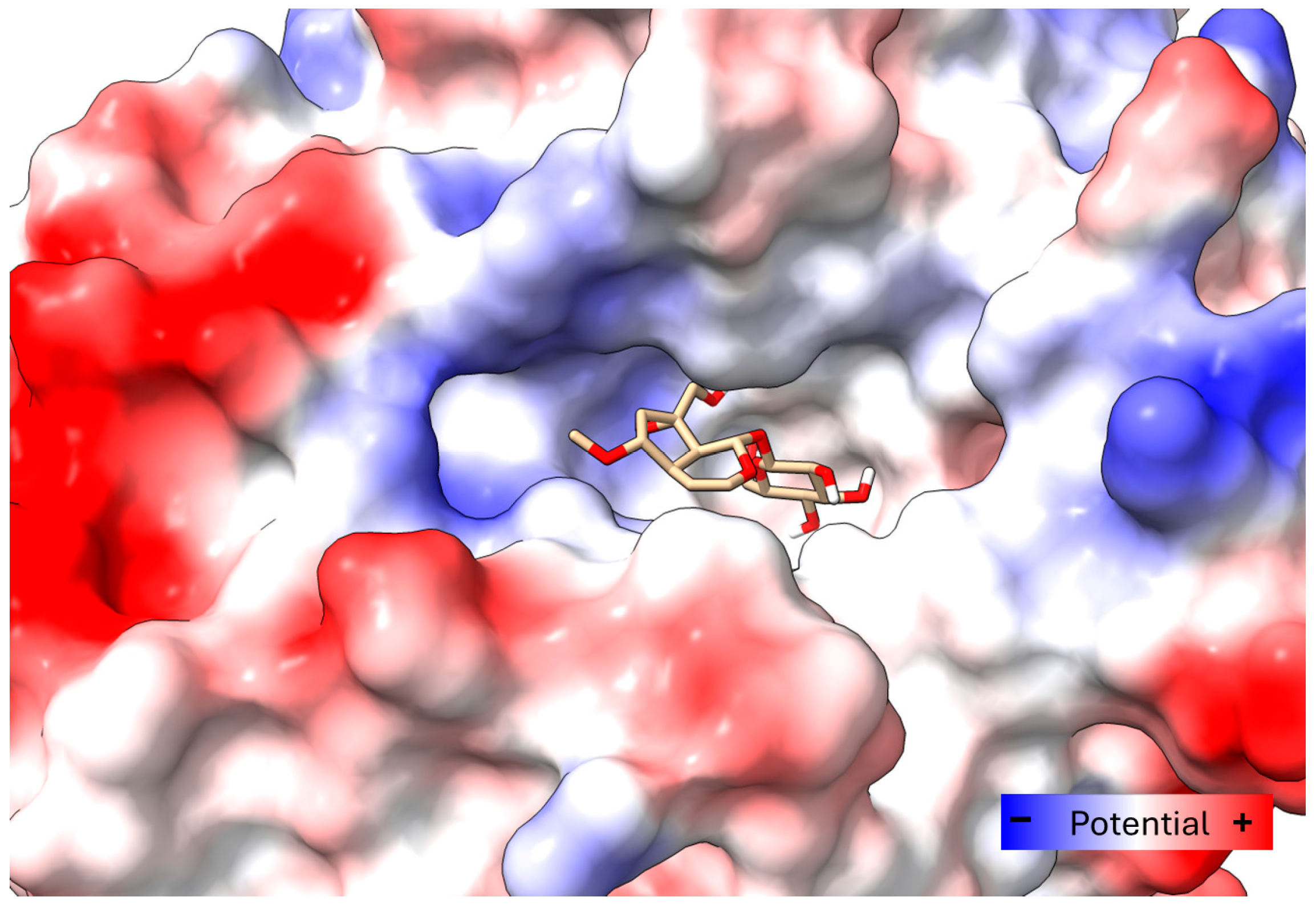
2.5. Docking Study on T. cruzi Oxidoreductases
3. Discussion
4. Materials and Methods
4.1. Plant Material
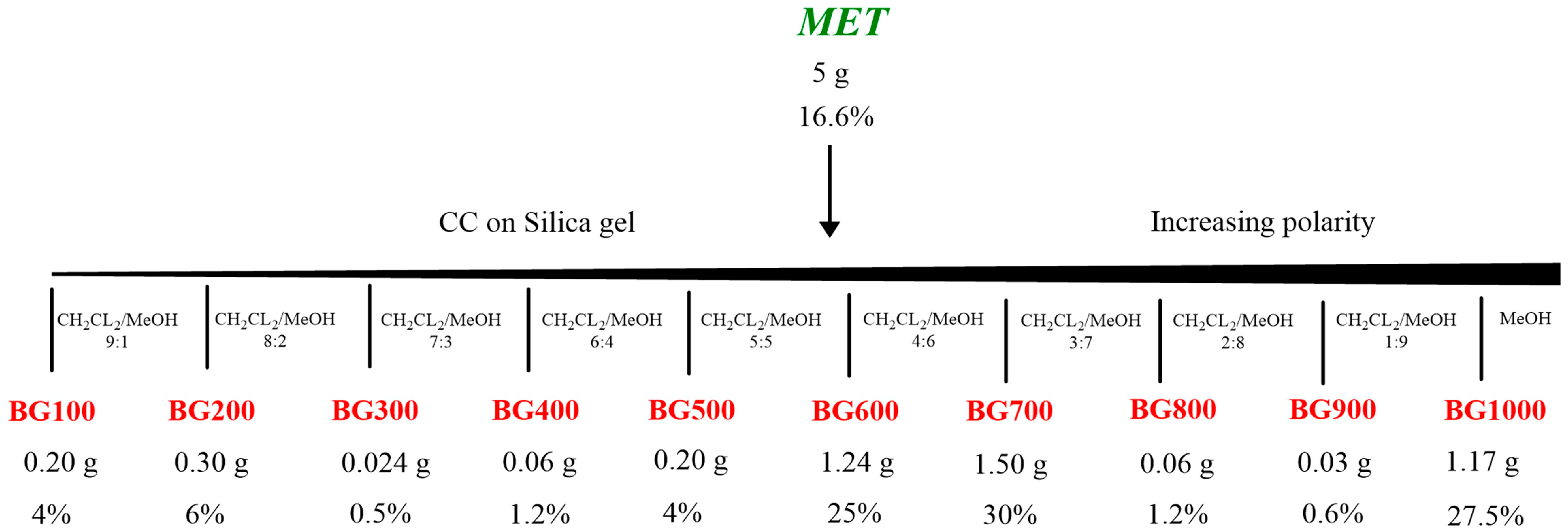
4.2. Extration Procedures and MET Fractionation
4.3. Characterization via Nuclear Magnetic Resonance (NMR)
4.4. UPLC-ESI-MS/MS Analysis from MET
4.5. Determination of Total Phenolic Content (TPC) Assay
4.6. Determination of Total Flavonoid Content (TFC)
4.7. Antioxidant Capacity Assay
4.7.1. Ferric Reducing Antioxidant Power (FRAP)
4.7.2. 2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid Radical Cation (ABTS•+)) Scavenging Activity
4.7.3. DPPH Free Radical Scavenging Activity
4.8. Parasites
4.9. Cell Viability Assay
4.10. Assessment of Mitochondrial Membrane Potential (ΔΨm) and Intracellular Calcium (Ca2+) Levels
4.11. Molecular Docking Study of 6-O-Methylcatalpol and Catalpol Binding with Old Yellow Enzyme (OYE)
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MET | Methanolic extract of B. globosa leaves |
| CD | Chagas disease |
| BNZ | Benznidazole |
| NADPH | Reduced forms of nicotinamide adenine dinucleotide phosphate |
| 13C NMR | Carbon-13 nuclear magnetic resonance |
| 1H NMR | Proton nuclear magnetic resonance |
| UPLC-ESI- MS/MS | Ultra-performance liquid chromatography–electrospray ionization–tandem mass spectrometry |
| LOD | Limits of detection |
| LOQ | Limit of quantification |
| Ppb | Parts per billion |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| FRAP | Ferric reducing antioxidant power |
| ABTS+ | 2,2′-azinobis-3-ethylbenzthiazolin-6-Sulfonic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| GAE | Gallic acid equivalents |
| QE | Quercetin equivalent |
| TE | Trolox equivalent |
| Abs | Absorbance |
| RPMI | Roswell Park Memorial Institute Medium |
| FBS | Fetal bovine serum |
| MTT | (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) |
| IC50 | Concentration required to decrease 50% cell viability. |
| SI | Selectivity Index |
| SDS | Sodium dodecyl sulfate |
| DMSO | Dimethysulfoxide |
| ΔΨm | Mitochondrial membrane potential |
| TMRM | Tetramethylrhodamine methyl ester |
| CCCP | Carbonyl cyanide m-chlorophenylhydrazone |
| PBS | Phosphate-buffered saline |
| ANOVA | Analyses of variance |
| 3D | Three-dimensional |
| 2D | Two-dimensional |
| SD | Standard deviation |
| COSY | Correlation spectroscopy |
| HMBC | Heteronuclear multiple bond correlation |
| DEPT | Distortionless enhancement by polarization transfer |
| NA | No activity |
| NE | No evaluated |
| NADH | Reduced forms of the coenzymes nicotinamide adenine dinucleotide |
| FMN | Flavin mononucleotide |
| CD3OD | Deuterated methanol |
| TMS | Tetramethylsilane |
| OYE | Old Yellow Enzyme |
| TYR | Tyrosine |
| THR | Threonine |
| TR | Trypanothione reductase |
| PRO | Proline |
| ALA | Alanine |
| ESP | Coulombic electrostatic potential |
| CC | Column chromatography |
| Cp | Cruzipain |
| RMSD | Root-Mean-Square Deviation |
References
- Wilke, A.B.B.; Farina, P.; Ajelli, M.; Canale, A.; Dantas-Torres, F.; Otranto, D.; Benelli, G. Human migrations, anthropogenic changes, and insect-borne diseases in Latin America. Parasit. Vectors 2025, 18, 4. [Google Scholar] [CrossRef]
- PAHO. Actualización de la Estimación de la Enfermedad de Chagas en Los Países Endémicos de Las Américas 2018; Pan American Health Organization: Washington, DC, USA, 2018. [Google Scholar]
- Lynn, M.K.; Rodriguez Aquino, M.S.; Buendia Rivas, C.A.; Landrove, A.M.; Portillo de Juarez, A.M.; Aguila Ceron, R.; Garcia Lopez, D.; Nolan, M.S.; El Salvador Congenital Chagas, t. Prenatal Chagas disease screening in Latin America: The current policy landscape and potential utility of an expanded maternal-familial Trypanosoma cruzi testing framework. Lancet Reg. Health Am. 2025, 47, 101139. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Sun, Z.S.; Zheng, J.X.; Zhang, S.X.; Yin, J.X.; Zhao, H.Q.; Shen, H.M.; Baneth, G.; Chen, J.H.; Kassegne, K. Prevalence and attributable health burdens of vector-borne parasitic infectious diseases of poverty, 1990-2021: Findings from the Global Burden of Disease Study 2021. Infect. Dis. Poverty 2024, 13, 96. [Google Scholar] [CrossRef]
- Cruz, C.V.; Rabinovich, A.; Moscatelli, G.; Moroni, S.; Gonzalez, N.; Garcia-Bournissen, F.; Ballering, G.; Freilij, H.; Altcheh, J. Adverse events associated with benznidazole treatment for Chagas disease in children and adults. Br. J. Clin. Pharmacol. 2024, 90, 3334–3347. [Google Scholar] [CrossRef]
- Berenstein, A.J.; Falk, N.; Moscatelli, G.; Moroni, S.; Gonzalez, N.; Garcia-Bournissen, F.; Ballering, G.; Freilij, H.; Altcheh, J. Adverse Events Associated with Nifurtimox Treatment for Chagas Disease in Children and Adults. Antimicrob. Agents Chemother. 2021, 65, e01135–20. [Google Scholar] [CrossRef]
- OPS. Guía Para El Diagnóstico Y El Tratamiento de la Enfermedad de Chagas; Organización Panamericana de la Salud: Washington, DC, USA, 2018. [Google Scholar]
- Filardi, L.S.; Brener, Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 755–759. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar] [CrossRef]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; Del Carmen Bangher, M.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascon, J.; et al. Benznidazole and Posaconazole in Eliminating Parasites in Asymptomatic T. cruzi Carriers: The STOP-CHAGAS Trial. J. Am. Coll. Cardiol. 2017, 69, 939–947. [Google Scholar] [CrossRef]
- Torrico, F.; Gascon, J.; Ortiz, L.; Alonso-Vega, C.; Pinazo, M.J.; Schijman, A.; Almeida, I.C.; Alves, F.; Strub-Wourgaft, N.; Ribeiro, I.; et al. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: A proof-of-concept, randomised, placebo-controlled trial. Lancet Infect. Dis. 2018, 18, 419–430. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Forsyth, C.; Losada, I.; Esteban, E.T.; Garcia-Rodriguez, M.; Villegas, M.L.; Molina, I.; Crespillo-Andujar, C.; Gallego, M.; Ballart, C.; et al. Efficacy and safety of fexinidazole for treatment of chronic indeterminate Chagas disease (FEXI-12): A multicentre, randomised, double-blind, phase 2 trial. Lancet Infect. Dis. 2024, 24, 395–403. [Google Scholar] [CrossRef]
- Padilla, A.M.; Wang, W.; Akama, T.; Carter, D.S.; Easom, E.; Freund, Y.; Halladay, J.S.; Liu, Y.; Hamer, S.A.; Hodo, C.L.; et al. Discovery of an orally active benzoxaborole prodrug effective in the treatment of Chagas disease in non-human primates. Nat. Microbiol. 2022, 7, 1536–1546. [Google Scholar] [CrossRef]
- Betu Kumeso, V.K.; Kalonji, W.M.; Rembry, S.; Valverde Mordt, O.; Ngolo Tete, D.; Pretre, A.; Delhomme, S.; Ilunga Wa Kyhi, M.; Camara, M.; Catusse, J.; et al. Efficacy and safety of acoziborole in patients with human African trypanosomiasis caused by Trypanosoma brucei gambiense: A multicentre, open-label, single-arm, phase 2/3 trial. Lancet Infect. Dis. 2023, 23, 463–470. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Malchiodi, E.; Ioset, J.R.; Bivona, A.; Gollob, K.J.; Dutra, W.O. Challenges and advancements in the development of vaccines and therapies against Chagas disease. Lancet Microbe 2024, 5, 100972. [Google Scholar] [CrossRef]
- de Menezes, R.P.B.; de Assis, E.B.; de Sousa, N.F.; de Souza, J.M.S.; Rodrigues, K.A.D.; Scotti, L.; Tavares, J.F.; da Silva, M.S.; Scotti, M.T. Exploring Lamiaceae diterpenoids as potential multitarget therapeutics for leishmaniasis and chagas disease. Mol. Divers. 2025, ahead of print. [Google Scholar] [CrossRef]
- Bridi, R.; Lino von Poser, G.; Gomez, M.; Andia, M.E.; Oyarzun, J.E.; Nunez, P.; Vasquez Arias, A.J.; Espinosa-Bustos, C. Hepatoprotective species from the Chilean medicinal flora: Junellia spathulata (Verbenaceae). J. Ethnopharmacol. 2021, 267, 113543. [Google Scholar] [CrossRef]
- Díaz-Forestier, J.; León-Lobos, P.; Marticorena, A.; Celis-Diez, J.L.; Giovannini, P. Native Useful Plants of Chile: A Review and Use Patterns. Econ. Bot. 2019, 73, 112–126. [Google Scholar] [CrossRef]
- Ministerio De Salud. Decreto Supremo 3: Aprueba Reglamento Del Sistema Nacional de Control de Los Productos Farmacéuticos de Uso Humano; Decreto Supremo 3; Biblioteca del Congreso Nacional de Chile: Santiago, Chile, 2011. [Google Scholar]
- Irigoin, F.; Cibils, L.; Comini, M.A.; Wilkinson, S.R.; Flohe, L.; Radi, R. Insights into the redox biology of Trypanosoma cruzi: Trypanothione metabolism and oxidant detoxification. Free Radic. Biol. Med. 2008, 45, 733–742. [Google Scholar] [CrossRef]
- Díaz-Viraqué, F.; Chiribao, M.L.; Trochine, A.; González-Herrera, F.; Castillo, C.; Liempi, A.; Kemmerling, U.; Maya, J.D.; Robello, C. Old Yellow Enzyme from Trypanosoma cruzi Exhibits In Vivo Prostaglandin F2α Synthase Activity and Has a Key Role in Parasite Infection and Drug Susceptibility. Front. Immunol. 2018, 9, 456. [Google Scholar] [CrossRef]
- Machado-Silva, A.; Cerqueira, P.G.; Grazielle-Silva, V.; Gadelha, F.R.; Peloso Ede, F.; Teixeira, S.M.; Machado, C.R. How Trypanosoma cruzi deals with oxidative stress: Antioxidant defence and DNA repair pathways. Mutat. Res. Rev. Mutat. Res. 2016, 767, 8–22. [Google Scholar] [CrossRef]
- Larit, F.; Elokely, K.M.; Nael, M.A.; Benyahia, S.; Leon, F.; Cutler, S.J.; Ghoneim, M.M. Proposed Mechanism for the Antitrypanosomal Activity of Quercetin and Myricetin Isolated from Hypericum afrum Lam.: Phytochemistry, In Vitro Testing and Modeling Studies. Molecules 2021, 26, 1009. [Google Scholar] [CrossRef]
- Marin, C.; Ramirez-Macias, I.; Lopez-Cespedes, A.; Olmo, F.; Villegas, N.; Diaz, J.G.; Rosales, M.J.; Gutierrez-Sanchez, R.; Sanchez-Moreno, M. In vitro and in vivo trypanocidal activity of flavonoids from Delphinium staphisagria against Chagas disease. J. Nat. Prod. 2011, 74, 744–750. [Google Scholar] [CrossRef]
- Conserva, G.A.; Costa-Silva, T.A.; Quiros-Guerrero, L.M.; Marcourt, L.; Wolfender, J.L.; Queiroz, E.F.; Tempone, A.G.; Lago, J.H.G. Kaempferol-3-O-alpha-(3,4-di-E-p-coumaroyl)-rhamnopyranoside from Nectandra oppositifolia releases Ca2+ from intracellular pools of Trypanosoma cruzi affecting the bioenergetics system. Chem. Biol. Interact. 2021, 349, 109661. [Google Scholar] [CrossRef]
- Quintero-Pertuz, H.; Veas-Albornoz, R.; Carrillo, I.; Gonzalez-Herrera, F.; Lapier, M.; Carbono-Delahoz, E.; Del Olmo, E.; Feliciano, A.S.; Kemmerling, U.; Olea-Azar, C.; et al. Trypanocidal effect of alcoholic extract of Castanedia santamartensis (Asteraceae) leaves is based on altered mitochondrial function. Biomed. Pharmacother. 2022, 148, 112761. [Google Scholar] [CrossRef]
- Bombaca, A.C.S.; Dossow, D.V.; Barbosa, J.M.C.; Paz, C.; Burgos, V.; Menna-Barreto, R.F.S. TrypanocidalActivity of Natural Sesquiterpenoids Involves Mitochondrial Dysfunction, ROS Production and Autophagic Phenotype in Trypanosoma cruzi. Molecules 2018, 23, 2800. [Google Scholar] [CrossRef]
- Otero, C.; Klagges, C.; Morales, B.; Sotomayor, P.; Escobar, J.; Fuentes, J.A.; Moreno, A.A.; Llancalahuen, F.M.; Arratia-Perez, R.; Gordillo-Fuenzalida, F.; et al. Anti-Inflammatory Chilean Endemic Plants. Pharmaceutics 2023, 15, 897. [Google Scholar] [CrossRef]
- Torres-Vega, J.; Gomez-Alonso, S.; Perez-Navarro, J.; Alarcon-Enos, J.; Pastene-Navarrete, E. Polyphenolic Compounds Extracted and Purified from Buddleja globosa Hope (Buddlejaceae) Leaves Using Natural Deep Eutectic Solvents and Centrifugal Partition Chromatography. Molecules 2021, 26, 2192. [Google Scholar] [CrossRef]
- Pastene-Navarrete, E.; Torres-Vega, J. Buddleja globosa Hope. In Medicinal and Aromatic Plants of South America Vol. 2: Argentina, Chile and Uruguay; Máthé, Á., Bandoni, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 135–144. [Google Scholar]
- Dinda, B. Occurrence and Distribution of Iridoids. In Pharmacology and Applications of Naturally Occurring Iridoids; Springer International Publishing: Cham, Switzerland, 2019; pp. 17–82. [Google Scholar]
- Houghton, P.J.; Hikino, H. Anti-hepatotoxic activity of extracts and constituents of Buddleja species. Planta Med. 1989, 55, 123–126. [Google Scholar] [CrossRef]
- Houghton, P.J. Buddleja globosa: A Medicinal plant of Chile, their chemistry, biological activity and traditional uses. BLACPMA 2003, 2, 6. [Google Scholar]
- Gonçalves, G.A.; Eifler-Lima, V.L.; von Poser, G.L. Revisiting nature: A review of iridoids as a potential antileishmanial class. Phytochem. Rev. 2022, 21, 101–126. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Yu, M.; Li, J.; Xie, Z.; Gao, B.; Liu, Y. Anti-inflammatory effect and mechanism of catalpol in various inflammatory diseases. Drug Dev. Res. 2023, 84, 1376–1394. [Google Scholar] [CrossRef]
- Wu, C.; Shi, Z.; Ge, Q.; Xu, H.; Wu, Z.; Tong, P.; Jin, H. Catalpol promotes articular cartilage repair by enhancing the recruitment of endogenous mesenchymal stem cells. J. Cell Mol. Med. 2024, 28, e18242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qiang, Y. Catalpol ameliorates inflammation and oxidative stress via regulating Sirt1 and activating Nrf2/HO-1 signaling against acute kidney injury. Environ. Toxicol. 2023, 38, 2182–2191. [Google Scholar] [CrossRef]
- Gao, X.; Xu, J.; Liu, H. Protective effects of catalpol on mitochondria of hepatocytes in cholestatic liver injury. Mol. Med. Rep. 2020, 22, 2424–2432. [Google Scholar] [CrossRef]
- Duff, R.B.; Bacon, J.S.; Mundie, C.M.; Farmer, V.C.; Russell, J.D.; Forrester, A.R. Catalpol and Methylcatalpol: Naturally Occurring Glycosides in Plantago and Buddleia Species. Biochem. J. 1965, 96, 1–5. [Google Scholar] [CrossRef]
- Hamedi, A.; Zengin, G.; Aktumsek, A.; Selamoglu, Z.; Pasdaran, A. In vitro and in silico approach to determine neuroprotective properties of iridoid glycosides from aerial parts of Scrophularia amplexicaulis by investigating their cholinesterase inhibition and anti-oxidant activities (Scrophulariaceae). Biointerface Res. Appl. Chem. 2020, 10, 5429–5454. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, H.; Zhang, L. Review: Bioactive constituents form Buddleja species. Pak. J. Pharm. Sci. 2019, 32, 721–741. [Google Scholar]
- Stevens, J.F.; Revel, J.S.; Maier, C.S. Mitochondria-Centric Review of Polyphenol Bioactivity in Cancer Models. Antioxid. Redox Signal. 2018, 29, 1589–1611. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Okamoto, N.; Tokuoka, K.; Sugiyama, S.; Uchiyama, N.; Matsumura, H.; Inaka, K.; Urade, Y.; Inoue, T. Structure of the inhibitor complex of old yellow enzyme from Trypanosoma cruzi. J. Synchrotron Radiat. 2011, 18, 66–69. [Google Scholar] [CrossRef]
- Barbosa, H.; Thevenard, F.; Reimao, J.Q.; Tempone, A.G.; Honorio, K.M.; Lago, J.H.G. The Potential of Secondary Metabolites from Plants as Drugs or Leads against Trypanosoma-An Update from 2012 to 2021. Curr. Top. Med. Chem. 2023, 23, 159–213. [Google Scholar] [CrossRef]
- Thabet, A.A.; Ayoub, I.M.; Youssef, F.S.; Al-Sayed, E.; Efferth, T.; Singab, A.N.B. Phytochemistry, structural diversity, biological activities and pharmacokinetics of iridoids isolated from various genera of the family Scrophulariaceae Juss. Phytomedicine Plus 2022, 2, 100287. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Li, X.; Jiang, Z.; Sun, L.; Zhao, G.; Zhou, G.; Zhang, H.; Shang, J.; Wang, T. Mitochondrial fusion/fission process involved in the improvement of catalpol on high glucose-induced hepatic mitochondrial dysfunction. Acta Biochim. Biophys. Sin. 2015, 47, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Sokol-Letowska, A.; Oszmianski, J.; Piorecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Bouzidi, C.; Marie, A.; Acquaviva, R.; Cappello, A.R.; Deguin, B. Iridoid- and flavonoid-enriched fractions of Cornus sanguinea and Cornus mas exert antioxidant and anti-inflammatory effects and inhibit key enzymes in the treatment of metabolic disorders. Food Funct. 2023, 14, 8838–8853. [Google Scholar] [CrossRef]
- Mitra, S.; Tareq, A.; Das, R.; Bin Emran, T.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A first evidence in the synergism and bioactivities. Food Rev. Int. 2023, 39, 4419–4441. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Theoduloz, C.; Sus, N.; Burgos-Edwards, A.; Schmeda-Hirschmann, G.; Frank, J.; Jimenez-Aspee, F. Iridoids and polyphenols from chilean Gaultheria spp. berries decrease the glucose uptake in Caco-2 cells after simulated gastrointestinal digestion. Food Chem. 2022, 369, 130940. [Google Scholar] [CrossRef]
- Przybylska, D.; Kucharska, A.Z.; Piórecki, N.; Sozanski, T. The Health-Promoting Quality Attributes, Polyphenols, Iridoids and Antioxidant Activity during the Development and Ripening of Cornelian Cherry (Cornus mas L.). Antioxidants 2024, 13, 229. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kim, H.J.; Lee, K.H.; Kang, S.C.; Zee, O.P. Antioxidative iridoid glycosides and phenolic compounds from Veronica peregrina. Arch. Pharm. Res. 2009, 32, 207–213. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.A.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.G.; et al. The Potential of Secondary Metabolites from Plants as Drugs or Leads Against Protozoan Neglected Diseases—Part II. Curr. Med. Chem. 2012, 19, 2176–2228. [Google Scholar] [CrossRef]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Seydi, E.; Rahimpour, Z.; Salimi, A.; Pourahmad, J. Selective toxicity of chrysin on mitochondria isolated from liver of a HCC rat model. Bioorg Med. Chem. 2019, 27, 115163. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, J.Y.; Sha, B.B.; Ma, Y.E.; Hu, T.; Ma, Y.C.; Sun, H.; Shi, J.X.; Dong, Z.M.; Li, P. Luteolin inhibits cell proliferation and induces cell apoptosis via down-regulation of mitochondrial membrane potential in esophageal carcinoma cells EC1 and KYSE450. Oncotarget 2017, 8, 27471–27480. [Google Scholar] [CrossRef]
- Dos Santos, A.L.; Amaral, M.; Hasegawa, F.R.; Lago, J.H.G.; Tempone, A.G.; Sartorelli, P. (-)-T-Cadinol-a Sesquiterpene Isolated from Casearia sylvestris (Salicaceae)-Displayed In Vitro Activity and Causes Hyperpolarization of the Membrane Potential of Trypanosoma cruzi. Front. Pharmacol. 2021, 12, 734127. [Google Scholar] [CrossRef]
- Murakami, M.T.; Rodrigues, N.C.; Gava, L.M.; Honorato, R.V.; Canduri, F.; Barbosa, L.R.; Oliva, G.; Borges, J.C. Structural studies of the Trypanosoma cruzi Old Yellow Enzyme: Insights into enzyme dynamics and specificity. Biophys. Chem. 2013, 184, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lira, C.; Tapia, R.A.; Herrera, A.; Lapier, M.; Maya, J.D.; Soto-Delgado, J.; Oliver, A.G.; Graham Lappin, A.; Uriarte, E. New benzimidazolequinones as trypanosomicidal agents. Bioorg. Chem. 2021, 111, 104823. [Google Scholar] [CrossRef] [PubMed]
- Varas Condori, M.A.; Arias-Sante, M.F.; Bridi, R.; Rincon-Cervera, M.A.; Porras, O.; Reyes-Jara, A.; de Camargo, A.C. Phenolics of Maqui Leaf Residues Exhibit Antioxidant Properties Against Ozone-Induced Oxidation in Fish Model Systems. Antioxidants 2025, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Merma, P.R.; Arias-Sante, M.F.; Rincon-Cervera, M.A.; Porras, O.; Bridi, R.; Rhein, S.; Sanchez-Contreras, M.; Hernandez-Pino, P.; Tobar, N.; Puente-Diaz, L.; et al. Phenolic Fractions from Walnut Milk Residue: Antioxidant Activity and Cytotoxic Potential. Plants 2024, 13, 3473. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Speisky, H.; Bridi, R.; Nunez Pizarro, P.; Larena, A.; Pinaffi-Langley, A.; Shahidi, F.; Schwember, A.R. Chickpeas from a Chilean Region Affected by a Climate-Related Catastrophe: Effects of Water Stress on Grain Yield and Flavonoid Composition. Molecules 2022, 27, 691. [Google Scholar] [CrossRef]
- Perez, M.; Dominguez-Lopez, I.; Lamuela-Raventos, R.M. The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Velásquez, P.; Rodríguez, K.; Retamal, M.; Giordano, A.; Valenzuela, L.M.; Montenegro, G. Relation between composition, antioxidant and antibacterial activities and botanical origin of multi-floral bee pollen. J. Appl. Bot. Food Qual. 2017, 90, 306–314. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Vieira, T.; Regitano-D’Arce, M.A.B.; Calori-Domingues, M.A.; Canniatti-Brazaca, S.G. Gamma radiation effects on peanut skin antioxidants. Int. J. Mol. Sci. 2012, 13, 3073–3084. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.F.; DaRocha, W.D.; Freitas, J.M.; Oliveira, L.A.; Kitten, G.T.; Machado, C.R.; Pena, S.D.; Chiari, E.; Macedo, A.M.; Teixeira, S.M. Cell culture and animal infection with distinct Trypanosoma cruzi strains expressing red and green fluorescent proteins. Int. J. Parasitol. 2008, 38, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bugnon, M.; Rohrig, U.F.; Goullieux, M.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Röhrig, U.F.; Goullieux, M.; Bugnon, M.; Zoete, V. Attracting Cavities 2.0: Improving the Flexibility and Robustness for Small-Molecule Docking. J. Chem. Inf. Model. 2023, 63, 3925–3940. [Google Scholar] [CrossRef]
- Zoete, V.; Schuepbach, T.; Bovigny, C.; Chaskar, P.; Daina, A.; Röhrig, U.F.; Michielin, O. Attracting Cavities for Docking. Replacing the Rough Energy Landscape of the Protein by a Smooth Attracting Landscape. J. Comput. Chem. 2016, 37, 437–447. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.R.C.; Meng, E.E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018, 27, 14–25. [Google Scholar] [CrossRef]
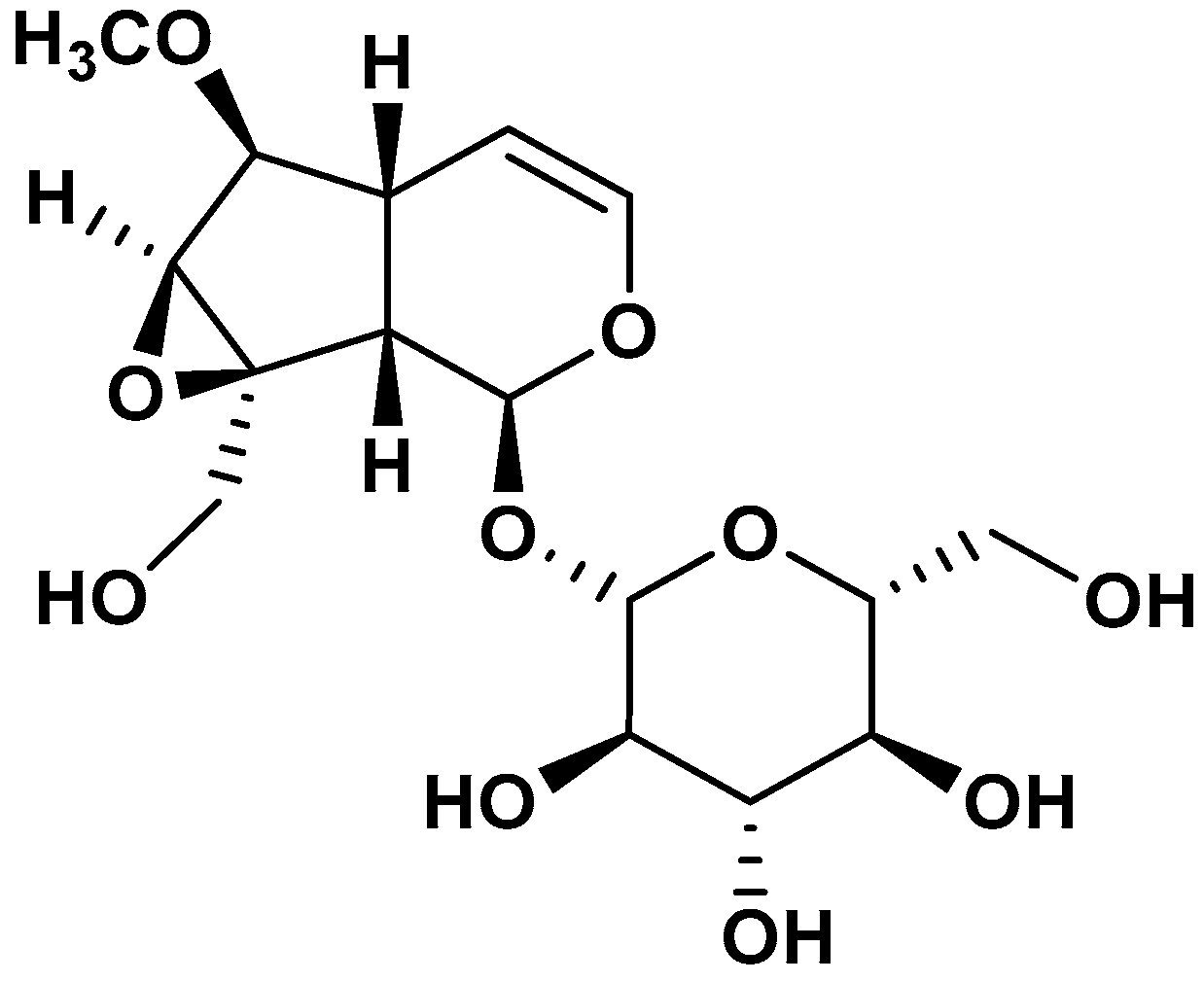
| N° | δ C | δ H (J in Hz) |
|---|---|---|
| 1 | 93.81 | 5.07 (d, J = 9.7 Hz) |
| 3 | 140.58 | 6.36 (d, J = 5.9 Hz) |
| 4 | 102.59 | 5.03 (m) |
| 5 | 35.95 | 2.35 (m) |
| 6 | 87.15 | 3.65 (m) |
| 7 | 57.77 | 3.68 (brs) |
| 8 | 65.01 | - |
| 9 | 41.83 | 2.54 (t, J = 8.1 Hz) |
| 10 | 60.16 | 4.16 (d, J = 13.1 Hz); 3.82 (d, J = 13.1 Hz) |
| OCH3 | 56.62 | 3.50 (s) |
| 1′ | 98.29 | 4.79 (d, J = 7.8 Hz) |
| 2′ | 73.42 | 3.30 (m) |
| 3′ | 77.23 | 3.30 (m) |
| 4′ | 70.36 | 3.30 (m) |
| 5′ | 76.29 | 3.45–3.40 (m) |
| 6′ | 61.54 | 3.93 (d, J = 12.2 Hz); 3.66 (m) |
| Extract or Fractions | TPC ‡ μmol GAE/g dw | TFC ‡ μmol QE/g dw | FRAP ‡ μmol TE/g dw | ABTS+ ‡ μmol TE/g dw | DPPH ‡ μmol TE/g dw |
|---|---|---|---|---|---|
| MET | 1614.7 ± 105.9 a | 271.1 ± 3.0 a | 1021.5 ± 33.6 a | 849.0 ± 33.9 a | 849.7 ± 27.4 a |
| BG100 | NA | NA | 46.2 ± 1.1 b | NA | NA |
| BG200 | 44.8 ± 5.3 b | 655.6 ± 4.6 b | 226.0 ± 1.4 c | NA | NA |
| BG300 | 662.5 ± 9.5 c | NE | 558.0 ± 1.7 d | 505.2 ± 10.9 b | 259.5 ± 5.6 b |
| BG400 | 1036.1 ± 14.5 d | NE | 903.0 ± 1.4 e | 968.2 ± 18.6 c | 431.0 ± 11.4 c |
| BG500 | 648.9 ± 5.5 c | 131.8 ± 4.9 c,d | 537.7 ± 2.9 d | 381.3 ± 17.9 d | 312.7 ± 9.5 d |
| BG600 | 1224.9 ± 43.6 e | 118.2 ± 5.1 c | 749.9 ± 12.4 f | 517.3 ± 14.3 b | 605.3 ± 16.2 d |
| BG700 | 308.2 ± 13.1 f,g | 186.8 ± 5.1 e | 559.2 ± 18.9 d | 212.4 ± 11.6 e | 326.9 ± 7.3 e |
| BG800 | 213.3 ± 8.9 f | 221.1 ± 5.0 f | 273.9 ± 11.3 g | 198.6 ± 10.4 e | 110.3 ± 1.5 f |
| BG900 | 331.1 ± 38.4 g | 145.2 ± 3.7 d | 333.2 ± 11.8 h | 194.2 ± 11.6 e | 118.6 ± 2.9 f |
| BG1000 | NA | 136.0 ± 4.2 d | 150.5 ± 5.3 i | 83.5 ± 5.6 f | NA |
| Compound | Rt | [M–H]− | MS/MS | MET | BG500 Fraction |
|---|---|---|---|---|---|
| (min) | mg/100g | mg/100g | |||
| Syringic acid | 4.30 | 197.0 | 181.9 | 0.81 ± 0.04 | 1.48 ± 0.13 |
| Chlorogenic acid | 3.85 | 353.1 | 191.0 | 0.22 ± 0.03 | 0.36 ± 0.03 |
| Caffeic acid | 3.85 | 178.9 | 135.0 | 3.27 ± 0.27 | 0.04 ± 0.02 |
| Rutin | 5.61 | 609.0 | 299.8 | 154.94 ± 7.24 | nd |
| Chrysin | 11.38 | 253.0 | 208.9 | 2.44 ± 1.01 | 6.00 ± 1.25 |
| Quercetin | 8.10 | 301.0 | 150.9 | 45.88 ± 3.07 | 43.66 ± 3.60 |
| Luteolin | 7.98 | 285.0 | 133.0 | 6.34 ± 0.50 | 54.65 ± 1.07 |
| Cryptochlorogenic acid | 3.60 | 353.0 | 173.0 | 1.80 ± 0.12 | nd |
| IC50 for Trypomastigotes (μg/mL) | IC50 for Vero Cells (μg/mL) | SI | |||
|---|---|---|---|---|---|
| R2 | R2 | ||||
| MET | 280 ± 3.5 | 0.99 | >5000 | 0.97 | >20 |
| BG500 fraction | 358 ± 4.2 | 0.98 | >5000 | 0.98 | >15 |
| Nifurtimox | 5.0 ± 0.5 | 0.96 | 184 ± 1.3 ‡ | 0.99 | >20 |
| Enzyme | Binding Energy of the Inhibitor |
|---|---|
| Old Yellow enzyme (PDBID: 4E2B) | −6.411 Kcal/mol |
| Trypanothione reductase (PDBID: 1NDA) | −6.018 Kcal/mol |
| Cruzipain (PDBID:1eWL) | −5.841 Kcal/mol |
| SOD-Tc (PDBID:4dvh) | −5.051 Kcal/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Pertuz, H.; Valenzuela-Bass, V.; Lapier, M.; Ortega-Campos, J.; Alfaro, S.; von Poser, G.L.; Espinosa-Bustos, C.; de Camargo, A.C.; González-Herrera, F.; Maya, J.D.; et al. Buddleja globosa Leaf Methanolic Extract Acts Against Trypanosoma cruzi Parasites by Inducing Mitochondrial Inner Membrane Hyperpolarization. Plants 2025, 14, 2749. https://doi.org/10.3390/plants14172749
Quintero-Pertuz H, Valenzuela-Bass V, Lapier M, Ortega-Campos J, Alfaro S, von Poser GL, Espinosa-Bustos C, de Camargo AC, González-Herrera F, Maya JD, et al. Buddleja globosa Leaf Methanolic Extract Acts Against Trypanosoma cruzi Parasites by Inducing Mitochondrial Inner Membrane Hyperpolarization. Plants. 2025; 14(17):2749. https://doi.org/10.3390/plants14172749
Chicago/Turabian StyleQuintero-Pertuz, Helena, Vicente Valenzuela-Bass, Michel Lapier, José Ortega-Campos, Sebastián Alfaro, Gilsane Lino von Poser, Christian Espinosa-Bustos, Adriano Costa de Camargo, Fabiola González-Herrera, Juan D. Maya, and et al. 2025. "Buddleja globosa Leaf Methanolic Extract Acts Against Trypanosoma cruzi Parasites by Inducing Mitochondrial Inner Membrane Hyperpolarization" Plants 14, no. 17: 2749. https://doi.org/10.3390/plants14172749
APA StyleQuintero-Pertuz, H., Valenzuela-Bass, V., Lapier, M., Ortega-Campos, J., Alfaro, S., von Poser, G. L., Espinosa-Bustos, C., de Camargo, A. C., González-Herrera, F., Maya, J. D., & Bridi, R. (2025). Buddleja globosa Leaf Methanolic Extract Acts Against Trypanosoma cruzi Parasites by Inducing Mitochondrial Inner Membrane Hyperpolarization. Plants, 14(17), 2749. https://doi.org/10.3390/plants14172749









