Assessment of Habitat Suitability for the Invasive Vine Sicyos angulatus Under Current and Future Climate Change Scenarios
Abstract
1. Introduction
2. Results
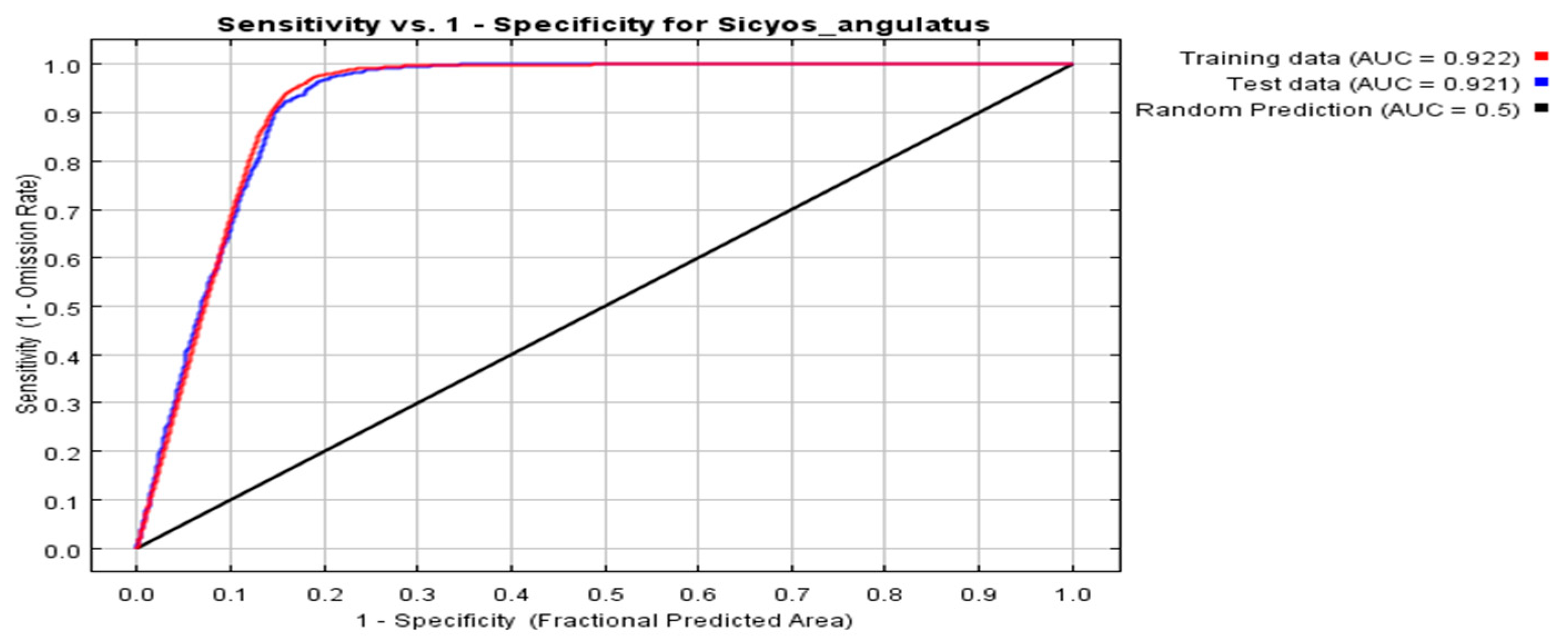
2.1. Assessment of Prediction Accuracy via the MaxEnt Model
2.2. Key Climatic Variables Influencing the Distribution of S. angulatus
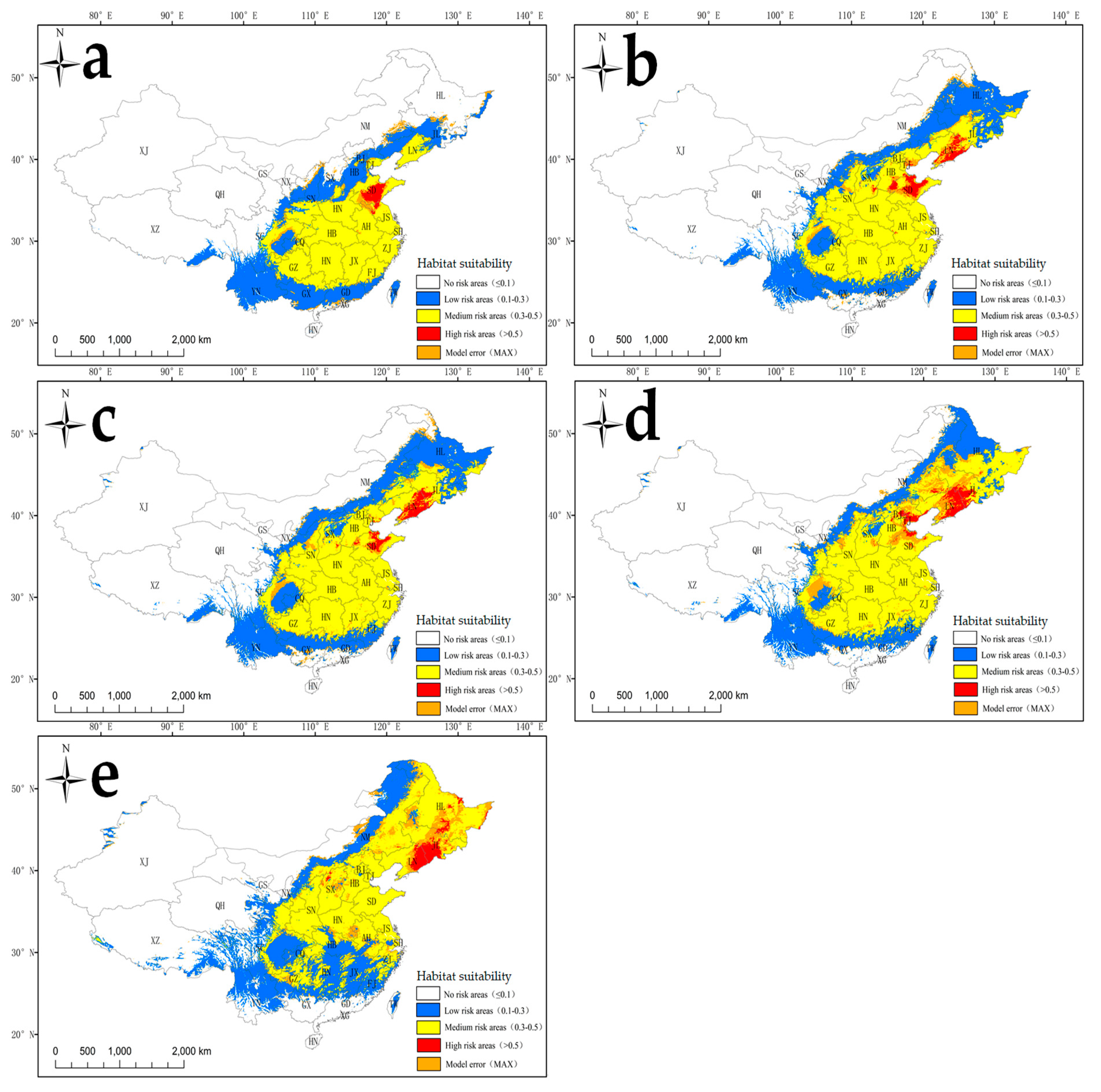
2.3. Current Habitat Suitability for S. angulatus According to the MaxEnt Model
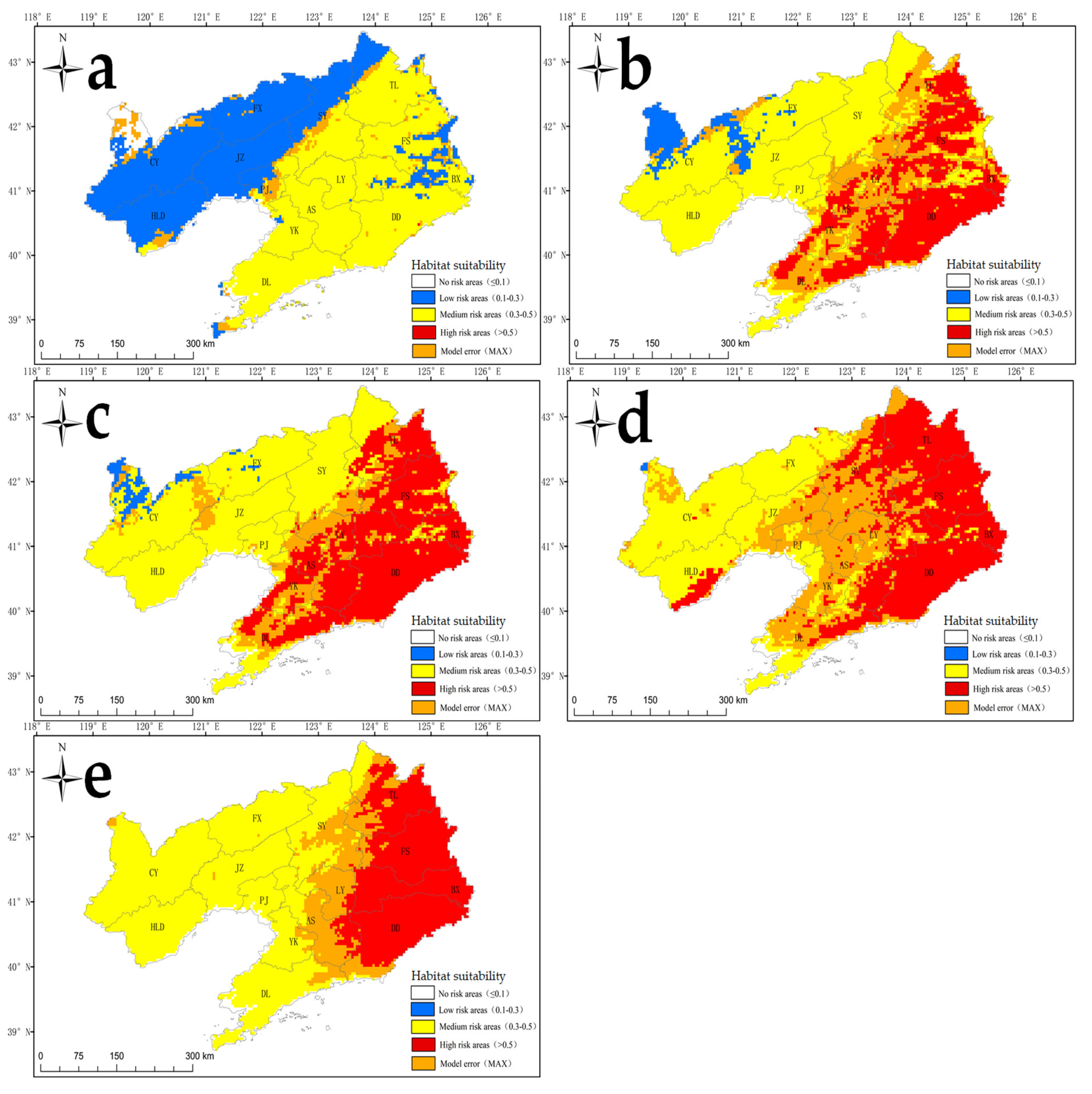
2.4. Future Habitat Suitability for S. angulatus According to the MaxEnt Model
3. Discussion
3.1. Performance of the MaxEnt Model
3.2. Importance of Precipitation for S. angulatus’ Habitat Suitability
3.3. Importance of Temperature for S. angulatus’ Habitat Suitability
3.4. Invasion Risk and Management Under Current and Future Climate Scenarios
4. Materials and Methods
4.1. Data Acquisition
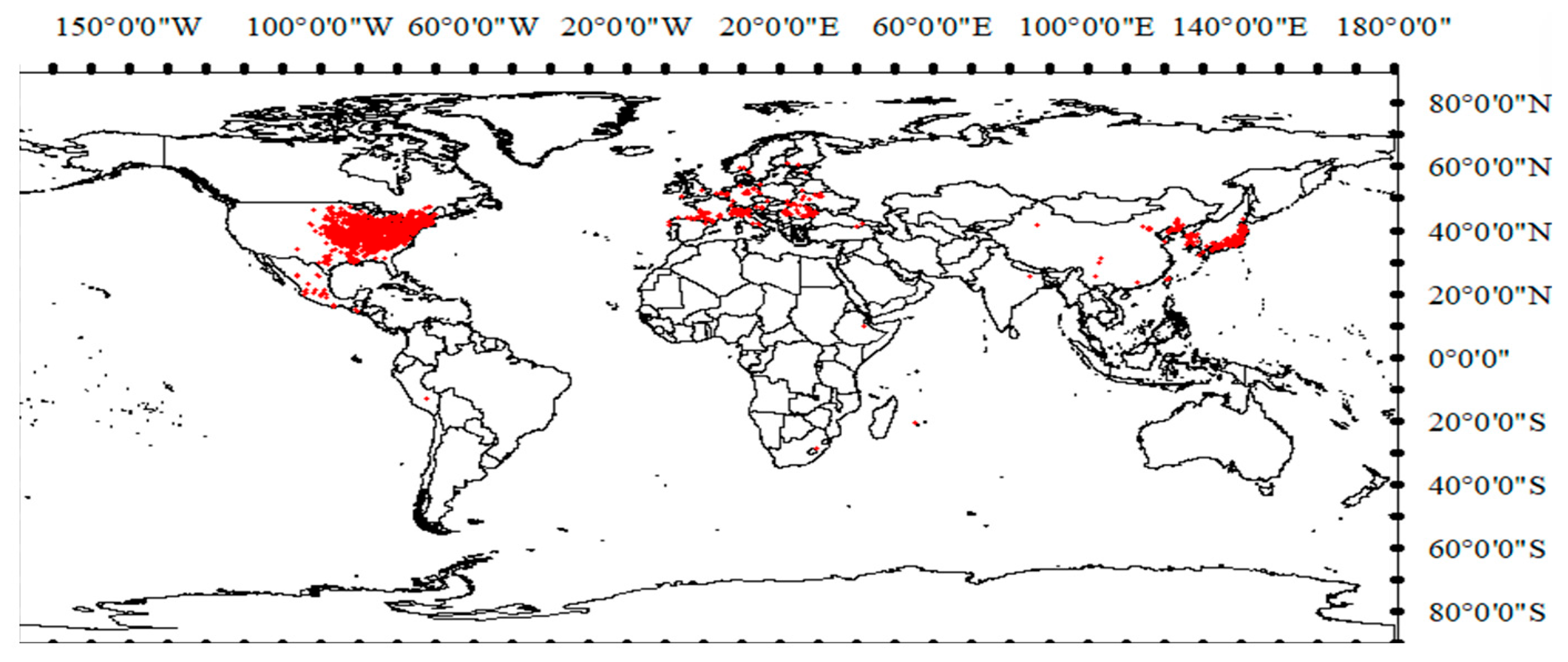
4.1.1. Occurrence Records of S. angulatus Worldwide
4.1.2. Climatic Variables
4.2. Methods
4.2.1. Model Establishment and Accuracy Evaluation
4.2.2. Classification of Habitat Suitability
4.2.3. Habitat Suitability Expectations Under Future Climate Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, F.; Yan, S.; Li, M.; Liu, X.; Zhang, X.; Cao, Y.; Zhao, H. Adaptive Strategies of Structures that Enhance Invasion in Sicyos angulatus. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1323–1330. [Google Scholar] [CrossRef]
- Arifin, M.; Okamoto, T. Pollination biology of Sicyos angulatus: An invasive plant primarily pollinated by the native insects and introduced honeybee Apis mellifera. Plant Species Biol. 2023, 38, 95–108. [Google Scholar] [CrossRef]
- Osawa, T.; Okawa, S.; Kurokawa, S.; Ando, S. Generating an agricultural risk map based on limited ecological information: A case study using Sicyos angulatus. Ambio 2016, 45, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Son, D.C. Genetic diversity pattern reveals the primary determinant of burcucumber (Sicyos angulatus L.) invasion in Korea. Front. Plant Sci. 2022, 13, 997521. [Google Scholar] [CrossRef]
- Adhikari, P.; Jeon, J.-Y.; Kim, H.W.; Shin, M.-S.; Adhikari, P.; Seo, C. Potential impact of climate change on plant invasion in the Republic of Korea. J. Ecol. Environ. 2019, 43, 36. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, G.; Fu, W.; Zhang, Y.; Zhao, Z.; Li, Z.; Qin, Y. Impacts of climate change on climatically suitable regions of two invasive Erigeron weeds in China. Front. Plant Sci. 2023, 14, 1238656. [Google Scholar] [CrossRef]
- Yazlık, A.; Ambarlı, D. Do non-native and dominant native species carry a similar risk of invasiveness? A case study for plants in Turkey. NeoBiota 2022, 76, 53–72. [Google Scholar] [CrossRef]
- Önen, H.; Farooq, S.; Tad, S.; Özaslan, C.; Gunal, H.; Chauhan, B.S. The Influence of Environmental Factors on Germination of Burcucumber (Sicyos angulatus) Seeds: Implications for Range Expansion and Management. Weed Sci. 2018, 66, 494–501. [Google Scholar] [CrossRef]
- Jocienė, L.; Krokaitė, E.; Rekašius, T.; Juškaitytė, E.; Ielciu, I.; Galanina, O.; Kupčinskienė, E. The Molecular Evidence for Invasive Climber Echinocystis lobata (Michx.) Torr. & A. Gray in Eastern and Central Europe. Diversity 2023, 15, 1084. [Google Scholar] [CrossRef]
- He, L.L.; Liu, J.C.; Chen, B. Potential Distribution and Agricultural Economic Loss Prediction of Alien Invasive Plant Sicyos angulatus in Liaoning Province. J. Shenyang Agric. Univ. 2022, 53, 119–127. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, J.; Wang, Y.; Zhang, L.; Dong, J.; Song, S. An invasive alien weed Sicyos angulatus L. causes great harm to corn. Plant Prot. 2014, 40, 187–188. [Google Scholar] [CrossRef]
- Byun, C.; Jeong, Y.; Hong, S.H. Synergistic effects of soil nutrient level and native species identity and diversity on biotic resistance to Sicyos angulatus, an invasive species. Oecologia 2022, 200, 221–230. [Google Scholar] [CrossRef]
- Farooq, S.; Tad, S.; Onen, H.; Gunal, H.; Caldiran, U.; Ozaslan, C. Range expansion potential of two co-occurring invasive vines to marginal habitats in Turkey. Acta Oecol. 2017, 84, 23–33. [Google Scholar] [CrossRef]
- Zhang, M.; Cong, P.; Liu, Y.; Wang, P.; Liu, L. Research progress on the invasion of strategies and control measures of Sicyos angulatus. J. Biosaf. 2025, 34, 27–33. [Google Scholar] [CrossRef]
- Niculescu, M.; Iancu, P.; Păniță, O.F. Invasion of Sicyos angulatus in Riparian Habitats in the Jiu and Danube Area (Romania). Diversity 2024, 16, 400. [Google Scholar] [CrossRef]
- Asaeda, T.; Rashid, M.H.; Kotagiri, S.; Uchida, T. The role of soil characteristics in the succession of two herbaceous lianas in a modified river floodplain. River Res. Appl. 2011, 27, 591–601. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.G. Temporal and spatial variations of vegetation in a riparian zone of South Korea. J. Ecol. Environ. 2020, 44, 9. [Google Scholar] [CrossRef]
- Rashid, M.H.; Uddin, M.N.; Sarkar, A.; Parveen, M.; Asaeda, T. The growth and nutrient uptake of invasive vines on contrasting riverbank soils. River Res. Appl. 2019, 35, 749–758. [Google Scholar] [CrossRef]
- Ampt, E.A.; van Ruijven, J.; Zwart, M.P.; Raaijmakers, J.M.; Termorshuizen, A.J.; Mommer, L. Plant neighbours can make or break the disease transmission chain of a fungal root pathogen. New Phytol. 2021, 233, 1303–1316. [Google Scholar] [CrossRef]
- Changjun, G.; Yanli, T.; Linshan, L.; Bo, W.; Yili, Z.; Haibin, Y.; Xilong, W.; Zhuoga, Y.; Binghua, Z.; Bohao, C. Predicting the potential global distribution of Ageratina adenophora under current and future climate change scenarios. Ecol. Evol. 2021, 11, 12092–12113. [Google Scholar] [CrossRef]
- Yan, H.; Feng, L.; Zhao, Y.; Feng, L.; Wu, D.; Zhu, C. Prediction of the spatial distribution of Alternanthera philoxeroides in China based on ArcGIS and MaxEnt. Glob. Ecol. Conserv. 2020, 21, e00856. [Google Scholar] [CrossRef]
- Looney, B.; Miyauchi, S.; Morin, E.; Drula, E.; Courty, P.E.; Kohler, A.; Kuo, A.; LaButti, K.; Pangilinan, J.; Lipzen, A.; et al. Evolutionary transition to the ectomycorrhizal habit in the genomes of a hyper-diverse lineage of mushroom-forming fungi. New Phytol. 2021, 233, 2294–2309. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Rivas Sáenz, S.; Penas, A. Worldwide bioclimatic classification system. Glob. Geobot. 2011, 1, 1–638. [Google Scholar] [CrossRef]
- Zeng, J.; Li, C.; Liu, J.; Li, Y.; Hu, Z.; He, M.; Zhang, H.; Yan, H. Ecological assessment of current and future Pogostemon cablin Benth. potential planting regions in China based on MaxEnt and ArcGIS models. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100308. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Jiang, X.; Chen, H.; Liu, M.; Wang, R. Potential geographical distribution of the edangred plant Isoetes under human activities using MaxEnt and GARP. Glob. Ecol. Conserv. 2022, 38, e02186. [Google Scholar] [CrossRef]
- Britton, T.G.; Brodribb, T.J.; Richards, S.A.; Ridley, C.; Hovenden, M.J. Canopy damage during a natural drought depends on species identity, physiology and stand composition. New Phytol. 2021, 233, 2058–2070. [Google Scholar] [CrossRef]
- Zhou, J.; Cieraad, E.; van Bodegom, P.M. Global analysis of trait-trait relationships within and between species. New Phytol. 2021, 233, 1643–1656. [Google Scholar] [CrossRef]
- Ray, D.; Behera, M.D.; Jacob, J. Evaluating Ecological Niche Models: A Comparison Between Maxent and GARP for Predicting Distribution of Hevea brasiliensis in India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 88, 1337–1343. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Atwater, D.; Callaway, R. Extended consequences of selection by exotic invaders on natives. New Phytol. 2022, 233, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Tang, X.; Zhang, H.; Zu, M.; Zhang, X.; Yuan, Y. Analysis of niche shift and potential suitable distributions of Dendrobium under the impact of global climate change. Environ. Sci. Pollut. Res. Int. 2023, 30, 11978–11993. [Google Scholar] [CrossRef]
- Tu, W.; Xiong, Q.; Qiu, X.; Zhang, Y. Dynamics of invasive alien plant species in China under climate change scenarios. Ecol. Indic. 2021, 129, 107919. [Google Scholar] [CrossRef]
- Fan, L.; Mi, C.; Li, J.; Zhang, Y.; Zhang, H.; Zhang, G.; Wang, H. Projecting global shifts in the invasive potential of Bidens pilosa L. under climate change using species distribution models. Front. Plant Sci. 2025, 16, 1580278. [Google Scholar] [CrossRef]
- Mandrekar, J. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Waheed, M.; Walas, Ł.; Alipour, S.; Arshad, F.; Jameel, M.A.; Siddiqui, M.H.; Alamri, S.; Haq, S.M.; Bussmann, R.W. Global climate change increases the risk of invasion and the expansion of paper mulberry in the subtropical region. Glob. Ecol. Conserv. 2024, 54, e03088. [Google Scholar] [CrossRef]
- Liu, W.; Tao, Y.; He, P.; Liu, J.; Zhang, W. Assessing the impacts of climate change on suitable distribution areas and ecological risks of the invasive grass (Spartina alterniflora) in China. J. Nat. Conserv. 2025, 87, 126985. [Google Scholar] [CrossRef]
- Luo, X.; Shen, S.; Liao, K.; Li, S.; Pan, Q.; Ma, J.; Li, W.; Yang, X. Invasion Status, Mechanisms, and Future Distribution Prediction of Solidago canadensis in the Trade Port Region: A Case Study of Ningbo Port, China. Plants 2025, 14, 1546. [Google Scholar] [CrossRef] [PubMed]
- Guinard, K.; Mailhot, A.; Caya, D. Projected changes in characteristics of precipitation spatial structures over North America. Int. J. Climatol. 2014, 35, 596–612. [Google Scholar] [CrossRef]
- Mauget, S.A. Multidecadal Regime Shifts in U.S. Streamflow, Precipitation, and Temperature at the End of the Twentieth Century. J. Clim. 2003, 16, 3905–3916. [Google Scholar] [CrossRef]
- Pan, M.; Roundy, J.K.; Wood, E.F.; Yuan, X. CFSv2-Based Seasonal Hydroclimatic Forecasts over the Conterminous United States. J. Clim. 2013, 26, 4828–4847. [Google Scholar] [CrossRef]
- Blunden, J.; Arndt, D.S. State of the Climate in 2016. Bull. Am. Meteorol. Soc. 2017, 98, S1–S280. [Google Scholar] [CrossRef]
- Saigusa, N.; Yamamoto, S.; Hirata, R.; Ohtani, Y.; Ide, R.; Asanuma, J.; Gamo, M.; Hirano, T.; Kondo, H.; Kosugi, Y.; et al. Temporal and spatial variations in the seasonal patterns of CO2 flux in boreal, temperate, and tropical forests in East Asia. Agric. For. Meteorol. 2008, 148, 700–713. [Google Scholar] [CrossRef]
- Sakata, M.; Marumoto, K.; Narukawa, M.; Asakura, K. Regional variations in wet and dry deposition fluxes of trace elements in Japan. Atmos. Environ. 2006, 40, 521–531. [Google Scholar] [CrossRef]
- Tanaka, N.; Lai, Y.-J.; Im, S.; Mahali, M.B.; Tuankrua, V.; Kuraji, K.; Cleophas, F.; Tantasirin, C.; Gomyo, M.; Tseng, C.-W.; et al. Climate Elasticity of Annual Runoff: Observation in Fifteen Forested Catchments on a Latitudinal Gradient in East Asia. Atmosphere 2023, 14, 629. [Google Scholar] [CrossRef]
- Lee, S.-M.; Byun, H.-R.; Tanaka, H.L. Spatiotemporal Characteristics of Drought Occurrences over Japan. J. Appl. Meteorol. Climatol. 2012, 51, 1087–1098. [Google Scholar] [CrossRef]
- Sun, S.; Deng, Z. Analysis of a Potentially Suitable Habitat for Solanum aculeatissimum in Southwest China Under Climate Change Scenarios. Plants 2025, 14, 1979. [Google Scholar] [CrossRef]
- Bonan, G.B. Frost Followed the Plow: Impacts of Deforestation on the Climate of the United States. Ecol. Appl. 1999, 9, 1305–1315. [Google Scholar] [CrossRef]
- Oh, S.-G.; Son, S.-W.; Min, S.-K. Possible impact of urbanization on extreme precipitation–temperature relationship in East Asian megacities. Weather Clim. Extrem. 2021, 34, 100401. [Google Scholar] [CrossRef]
- Wan, J.-Z.; Zhang, Z.-X.; Wang, C.-J. Identifying potential distributions of 10 invasive alien trees: Implications for conservation management of protected areas. Environ. Monit. Assess. 2018, 190, 739. [Google Scholar] [CrossRef]
- Jia, T.; Qi, Y.; Zhao, H.; Xian, X.; Li, J.; Huang, H.; Yu, W.; Liu, W.-x. Estimation of climate-induced increased risk of Centaurea solstitialis L. invasion in China: An integrated study based on biomod2. Front. Ecol. Evol. 2023, 11, 1113474. [Google Scholar] [CrossRef]
- Fallah, B.; Rostami, M. Exploring the impact of the recent global warming on extreme weather events in Central Asia using the counterfactual climate data ATTRICI v1.1. Clim. Change 2024, 177, 80. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, J.; Zhang, F. Spatiotemporal trends of temperature and precipitation extremes across contrasting climatic zones of China during 1956–2015. Theor. Appl. Climatol. 2019, 138, 1877–1897. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, V.P. Changes in extreme precipitation in Texas. J. Geophys. Res. Atmos. 2010, 115, D14106. [Google Scholar] [CrossRef]
- Terefe, S.; Bantider, A.; Teferi, E.; Abi, M. Spatiotemporal trends in mean and extreme climate variables over 1981–2020 in Meki watershed of central rift valley basin, Ethiopia. Heliyon 2022, 8, e11684. [Google Scholar] [CrossRef]
- Hua, W.; Chen, H.; Sun, S.; Zhou, L. Assessing climatic impacts of future land use and land cover change projected with the CanESM2 model. Int. J. Climatol. 2015, 35, 3661–3675. [Google Scholar] [CrossRef]
- Tian, D.; Dong, W.; Zhang, H.; Guo, Y.; Yang, S.; Dai, T. Future changes in coverage of 1.5 °C and 2 °C warming thresholds. Sci. Bull. 2017, 62, 1455–1463. [Google Scholar] [CrossRef]
- Byun, C.; Oh, M.; Lee, E.J.; Kang, H. Seed density is as important as limiting similarity, diversity effect, and propagule pressure in plant restoration to control invasion. Ecol. Eng. 2020, 144, 105712. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, K.; Wurzburger, N.; Zhang, J. Relationships between plant diversity and soil microbial diversity vary across taxonomic groups and spatial scales. Ecosphere 2020, 11, e02999. [Google Scholar] [CrossRef]
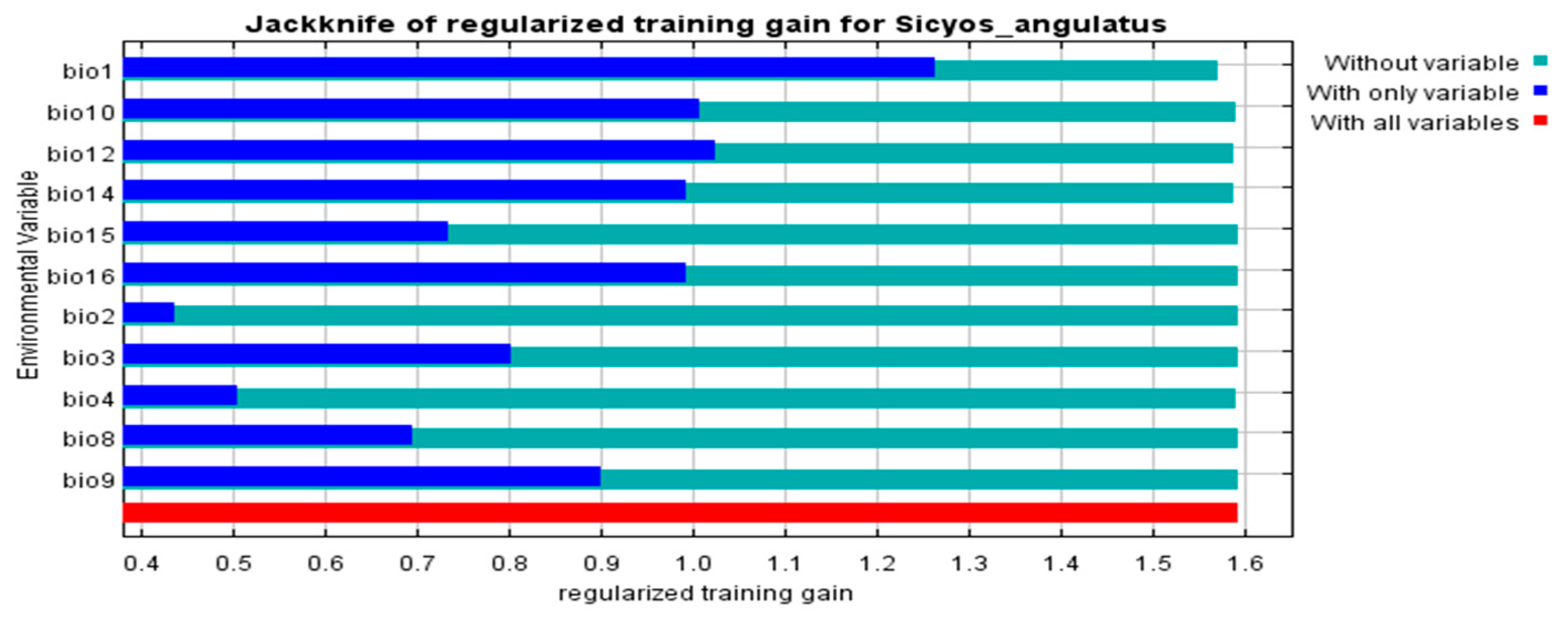
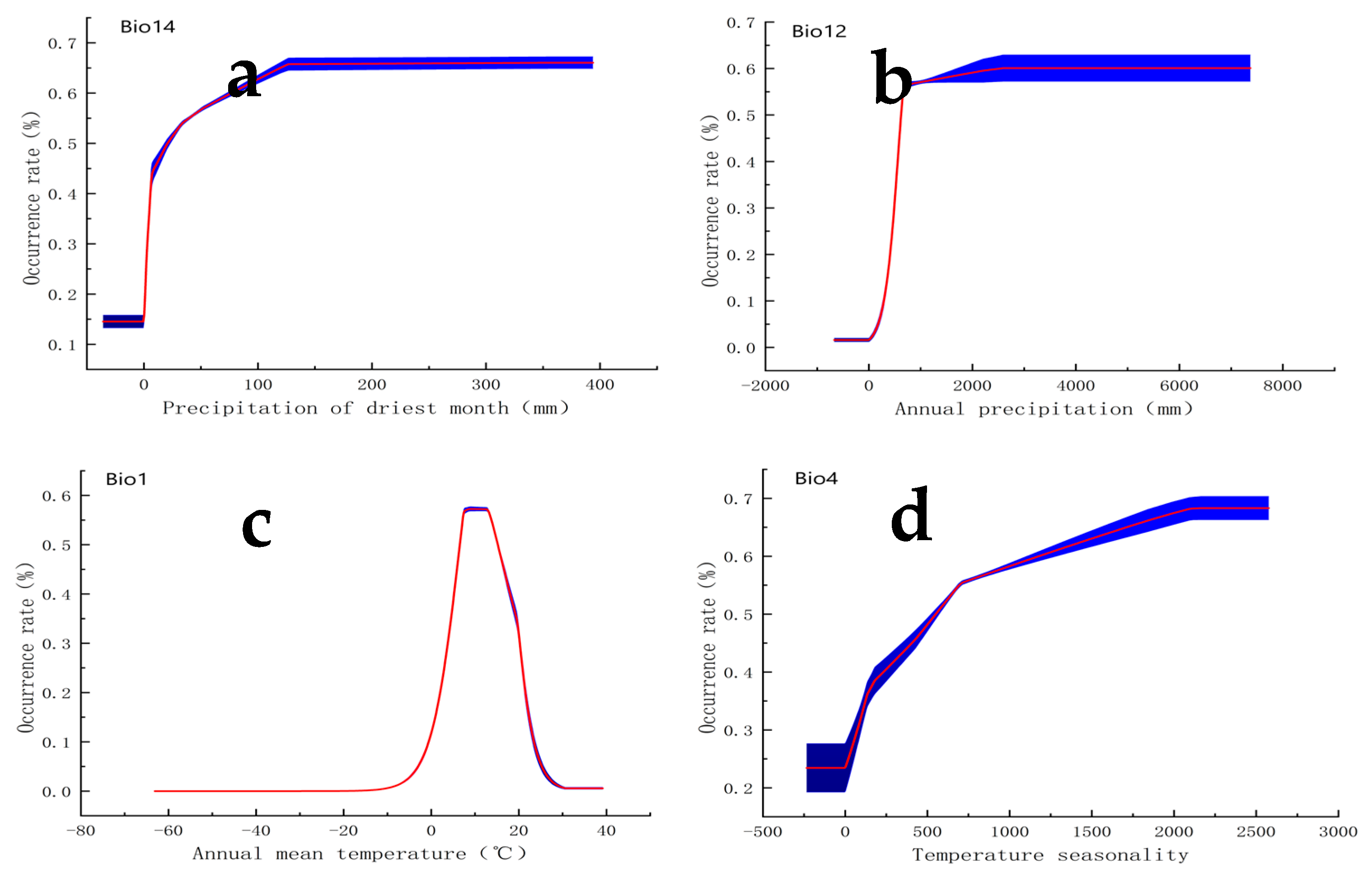

| Labels | Climatic Variables | Percent Contribution (%) | Permutation Importance (%) |
|---|---|---|---|
| Bio1 | Average annual temperature (°C) | 18.1 ± 0.7 | 57.5 ± 2.3 |
| Bio2 | Mean diurnal range (mean monthly value of (maximum temperature−minimum temperature)) (°C) | 0.2 ± 0.2 | 0.4 ± 0.1 |
| Bio3 | Isothermality (BIO2/BIO7) (×100) | 1.4 ± 0.7 | 18.7 ± 3.2 |
| Bio4 | Temperature seasonality (standard deviation ×100) | 14.9 ± 0.6 | 0.9 ± 0.2 |
| Bio5 | Maximum temperature in the warmest month (°C) | 0 | 0 |
| Bio6 | Minimum temperature in the coldest month (°C) | 0 | 0 |
| Bio7 | Temperature annual range (BIO5–BIO6) (°C) | 0 | 0 |
| Bio8 | Mean temperature in the wettest quarter (i.e., a period of three months) (°C) | 0.1 ± 0.1 | 0.4 ± 0.1 |
| Bio9 | Mean temperature in the driest quarter (°C) | 0.1 ± 0.1 | 0.6 ± 0.3 |
| Bio10 | Mean temperature in the warmest quarter (°C) | 0.1 ± 0.2 | 0.4 ± 0.1 |
| Bio11 | Mean temperature in the coldest quarter (°C) | 0 | 0 |
| Bio12 | Annual precipitation (mm) | 26.8 ± 2.6 | 16.0 ± 1.5 |
| Bio13 | Precipitation in the wettest month (mm) | 0 | 0 |
| Bio14 | Precipitation in the driest month (mm) | 37.4 ± 2.5 | 4.7 ± 0.5 |
| Bio15 | Precipitation seasonality (coefficient of variation) | 0.4 ± 0.4 | 0.3 ± 0.1 |
| Bio16 | Precipitation in the wettest quarter (mm) | 0.4 ± 0.2 | 0.2 ± 0.1 |
| Bio17 | Precipitation in the driest quarter (mm) | 0 | 0 |
| Bio18 | Precipitation in the warmest quarter (mm) | 0 | 0 |
| Bio19 | Precipitation in the coldest quarter (mm) | 0 | 0 |
| Climate Scenarios | Habitat Suitability | ||||
|---|---|---|---|---|---|
| <0.1 | 0.1–0.3 | 0.3–0.5 | >0.5 | ||
| Global (%) | Contemporary era (1970–2000) | 89.5 ± 0.1 | 6.4 ± 0.1 | 2.9 ± 0.1 | 1.2 ± 0.1 |
| 2050s under RCP2.6 | 84.7 ± 0.1 | 7.8 ± 0.1 | 5.1 ± 0.1 | 2.4 ± 0.1 | |
| 2090s under RCP2.6 | 84.1 ± 0.2 | 8.1 ± 0.1 | 5.5 ± 0.1 | 2.3 ± 0.1 | |
| 2050s under RCP8.5 | 82.3 ± 0.1 | 8.8 ± 0.1 | 6.2 ± 0.1 | 2.8 ± 0.1 | |
| 2090s under RCP8.5 | 78.2 ± 0.1 | 9.1 ± 0.1 | 8.8 ± 0.1 | 3.9 ± 0.1 | |
| China (%) | Contemporary era (1970–2000) | 66.4 ± 0.5 | 14.9 ± 0.5 | 18.1 ± 0.3 | 0.6 ± 0.2 |
| 2050s under RCP2.6 | 57.9 ± 0.6 | 18.5 ± 0.5 | 22.4 ± 0.6 | 1.2 ± 0.5 | |
| 2090s under RCP2.6 | 57.0 ± 0.5 | 19.1 ± 0.5 | 22.9 ± 0.8 | 1.1 ± 0.4 | |
| 2050s under RCP8.5 | 54.9 ± 0.4 | 17.3 ± 0.9 | 26.3 ± 0.8 | 1.6 ± 0.8 | |
| 2090s under RCP8.5 | 51.2 ± 0.3 | 21.5 ± 1.2 | 25.9 ± 0.9 | 1.5 ± 0.8 | |
| Liaoning (%) | Contemporary era (1970–2000) | 3.1 ± 0.9 | 44.6 ± 1.3 | 52.2 ± 1.7 | 0.1 ± 0.1 |
| 2050s under RCP2.6 | 0 | 7.0 ± 1.7 | 65.7 ± 10.5 | 27.3 ± 10.4 | |
| 2090s under RCP2.6 | 0 | 4.1 ± 1.1 | 61.7 ± 9.0 | 34.2 ± 9.1 | |
| 2050s under RCP8.5 | 0 | 1.5 ± 1.5 | 59.8 ± 12.7 | 38.8 ± 13.3 | |
| 2090s under RCP8.5 | 0 | 1.6 ± 2.4 | 71.3 ± 9.4 | 27.0 ± 10.2 | |
| Items | Climate Change Scenarios | Area Change | ||||||
|---|---|---|---|---|---|---|---|---|
| World | Asia | Africa | North America | South America | Europe | Oceania | ||
| Expansion | 2050s under RCP2.6 | 5680 (32.11%) | 1021 (26.01%) | 84 (19.34%) | 2225 (37.89%) | 117 (12.16%) | 1941 (32.47%) | 293 (56.1%) |
| 2090s under RCP2.6 | 6088 (33.65%) | 1357 (31.87%) | 83 (19.14%) | 2045 (35.93%) | 118 (12.27%) | 2289 (36.19%) | 196 (46.11%) | |
| 2050s under RCP8.5 | 8514 (41.49%) | 2117 (42.18%) | 54 (13.34%) | 2998 (45.11%) | 128 (13.19%) | 3012 (42.73%) | 206 (47.35%) | |
| 2090s under RCP8.5 | 15,577 (56.47%) | 4739 (62.02%) | 59 (14.46%) | 6160 (62.81%) | 297 (26.08%) | 4092 (50.34%) | 229 (50.07%) | |
| Stable | 2050s under RCP2.6 | 11,101 (62.76%) | 2691 (68.59%) | 217 (49.94%) | 3576 (60.91%) | 469 (48.95%) | 3951 (66.1%) | 197 (37.74%) |
| 2090s under RCP2.6 | 10,980 (60.67%) | 2668 (62.62%) | 178 (40.99%) | 3542 (62.22%) | 443 (46.1%) | 3946 (62.38%) | 204 (48.06%) | |
| 2050s under RCP8.5 | 10,742 (52.35%) | 2644 (52.68%) | 147 (36.27%) | 3485 (52.45%) | 380 (39.2%) | 3914 (55.53%) | 172 (39.67%) | |
| 2090s under RCP8.5 | 8993 (32.6%) | 1983 (25.95%) | 66 (15.99%) | 2874 (29.31%) | 297 (26.09%) | 3616 (44.48%) | 157 (34.35%) | |
| Reduction | 2050s under RCP2.6 | 906 (5.12%) | 212 (5.4%) | 134 (30.72%) | 70 (1.2%) | 373 (38.89%) | 85 (1.43%) | 32 (6.16%) |
| 2090s under RCP2.6 | 1028 (5.68%) | 235 (5.51%) | 173 (39.87%) | 105 (1.85%) | 400 (41.63%) | 91 (1.44%) | 25 (5.83%) | |
| 2050s under RCP8.5 | 1265 (6.16%) | 258 (5.15%) | 204 (50.39%) | 162 (2.43%) | 462 (47.6%) | 123 (1.74%) | 56 (12.99%) | |
| 2090s under RCP8.5 | 3014 (10.93%) | 919 (12.03%) | 285 (69.55%) | 773 (7.88%) | 545 (47.82%) | 421 (5.18%) | 71 (15.58%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, C.; Ye, J.; Zhang, H.; Qin, Y.; Yan, R.; Xu, G.; Zhou, H. Assessment of Habitat Suitability for the Invasive Vine Sicyos angulatus Under Current and Future Climate Change Scenarios. Plants 2025, 14, 2745. https://doi.org/10.3390/plants14172745
Xiao C, Ye J, Zhang H, Qin Y, Yan R, Xu G, Zhou H. Assessment of Habitat Suitability for the Invasive Vine Sicyos angulatus Under Current and Future Climate Change Scenarios. Plants. 2025; 14(17):2745. https://doi.org/10.3390/plants14172745
Chicago/Turabian StyleXiao, Cui, Ji Ye, Haibo Zhang, Yonghui Qin, Ruihuan Yan, Guanghao Xu, and Haili Zhou. 2025. "Assessment of Habitat Suitability for the Invasive Vine Sicyos angulatus Under Current and Future Climate Change Scenarios" Plants 14, no. 17: 2745. https://doi.org/10.3390/plants14172745
APA StyleXiao, C., Ye, J., Zhang, H., Qin, Y., Yan, R., Xu, G., & Zhou, H. (2025). Assessment of Habitat Suitability for the Invasive Vine Sicyos angulatus Under Current and Future Climate Change Scenarios. Plants, 14(17), 2745. https://doi.org/10.3390/plants14172745






