Multifunctional Roles of Medicinal Plants in the Meat Industry: Antioxidant, Antimicrobial, and Color Preservation Perspectives
Abstract
1. Introduction
2. Research Methodology
3. Phytochemical Composition of Medicinal Plants and Their General Biological Properties
4. Antioxidant Capacity of Medicinal Plants in Meat
5. Antimicrobial Activity of Medicinal Plants in Meat
6. Plants That Remove Specific Meat Aromas and Improve/Protect Meat Color
7. New Potential Medicinal Plants for Application in Meat Products
8. Clean-Label Meat Products Enriched with Medicinal Plants
9. Emerging Technologies for the Application of Medicinal Plants in Meat Systems
10. Safety Considerations: Toxicological and Allergenic Aspects of Medicinal Plants
11. Challenges in Choosing Plants for the Meat Industry
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aminzare, M.; Hashemi, M.; Ansarian, E.; Bimkar, M.; Azar, H.H.; Mehrasbi, M.R.; Daneshamooz, S.; Raeisi, M.; Jannat, B.; Afshari, A. Using Natural Antioxidants in Meat and Meat Products as Preservatives: A Review. Adv. Anim. Vet. Sci. 2019, 7, 417–426. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant Extracts as Natural Antioxidants in Meat and Meat Products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. A Comparative Study between Natural and Synthetic Antioxidants: Evaluation of Their Performance after Incorporation into Biscuits. Food Chem. 2017, 216, 342–346. [Google Scholar] [CrossRef]
- Regulation-1333/2008-EN-Additives-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/oj (accessed on 4 April 2024).
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural Antioxidants against Lipid–Protein Oxidative Deterioration in Meat and Meat Products: A Review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, F.A. Advances in Use of Natural Antioxidants as Food Additives for Improving the Oxidative Stability of Meat Products. Madridge J. Food Technol. 2016, 1, 10–17. [Google Scholar] [CrossRef]
- Boskovic, M.; Glisic, M.; Djordjevic, J.; Vranesevic, J.; Djordjevic, V.; Baltic, M.Z. Preservation of Meat and Meat Products Using Nanoencapsulated Thyme and Oregano Essential Oils. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 333, p. 012038. [Google Scholar] [CrossRef]
- Bojorges, H.; Ríos-Corripio, M.A.; Hernández-Cázares, A.S.; Hidalgo-Contreras, J.V.; Contreras-Oliva, A. Effect of the Application of an Edible Film with Turmeric (Curcuma longa L.) on the Oxidative Stability of Meat. Food Sci. Nutr. 2020, 8, 4308–4319. [Google Scholar] [CrossRef]
- Alañón, M.E.; Alarcón, M.; Marchante, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Extraction of Natural Flavorings with Antioxidant Capacity from Cooperage By-Products by Green Extraction Procedure with Subcritical Fluids. Ind. Crops Prod. 2017, 103, 222–232. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural Antioxidants as Food and Feed Additives to Promote Health Benefits and Quality of Meat Products: A Review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Ahn, J.; Grun, I.; Mustapha, A. Effects of Plant Extracts on Microbial Growth, Color Change, and Lipid Oxidation in Cooked Beef. Food Microbiol. 2007, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Aguirre, G.; Piñeiro-Vázquez, Á.T.; Sanginés-García, J.R.; Sánchez Zárate, A.; Ochoa-Flores, A.A.; Segura-Campos, M.R.; Vargas-Bello-Pérez, E.; Chay-Canul, A.J. Using Plant-Based Compounds as Preservatives for Meat Products: A Review. Heliyon 2023, 9, e17071. [Google Scholar] [CrossRef] [PubMed]
- Petcu, C.D.; Mihai, O.D.; Tăpăloagă, D.; Gheorghe-Irimia, R.-A.; Pogurschi, E.N.; Militaru, M.; Borda, C.; Ghimpețeanu, O.-M. Effects of Plant-Based Antioxidants in Animal Diets and Meat Products: A Review. Foods 2023, 12, 1334. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, K.; Rifky, M.; Zokirov, K.; Jesfar, M.; Farmonov, J.; Sanaev, E.; Makhmayorov, J.; Samadiy, M. Impact of Curing Salt (Nitrites) on the Processed Meat Products and Its Alternatives: A Review. New Mater. Compd. Appl. 2024, 8, 254–264. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Jia, J.; Peng, H.; Qian, Q.; Pan, Z.; Liu, D. Nitrite and Nitrate in Meat Processing: Functions and Alternatives. Curr. Res. Food Sci. 2023, 6, 100470. [Google Scholar] [CrossRef]
- Sorour, M.; Mehanni, A.-H.; Mahmoud, E.-S.; El-Hawashy, R. Nitrate, Nitrite and N-Nitrosamine in Meat Products. J. Sohag Agrisci. (JSAS) 2023, 8, 121–135. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef]
- Cygan-Szczegielniak, D.; Karolina, S. Effect of Freezing and Cooking on Sodium Nitrite Content in Processed Meat Products. 2023. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4578613 (accessed on 3 August 2024).
- Cui, H.; Gabriel, A.A.; Nakano, H. Antimicrobial Efficacies of Plant Extracts and Sodium Nitrite against Clostridium botulinum. Food Control 2010, 21, 1030–1036. [Google Scholar] [CrossRef]
- Orădan, A.C.; Tocai (Moțoc), A.C.; Rosan, C.A.; Vicas, S.I. Fruit Extracts Incorporated into Meat Products as Natural Antioxidants, Preservatives, and Colorants. Processes 2024, 12, 2756. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and Antioxidant Efficiency of Nanoemulsion-Based Edible Coating Containing Ginger (Zingiber officinale) Essential Oil and Its Effect on Safety and Quality Attributes of Chicken Breast Fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Omoregbe, O.; Makaka, G.; Nwokolo, N.; Mukumba, P. Bioenergy from Bio-Waste: A Bibliometric Analysis of the Trend in Scientific Research from 1998–2018. Biomass Convers. Biorefin. 2022, 12, 1077–1092. [Google Scholar] [CrossRef]
- Omoregbe, O.; Mustapha, A.N.; Steinberger-Wilckens, R.; El-Kharouf, A.; Onyeaka, H. Carbon Capture Technologies for Climate Change Mitigation: A Bibliometric Analysis of the Scientific Discourse during 1998–2018. Energy Rep. 2020, 6, 1200–1212. [Google Scholar] [CrossRef]
- Fadda, A.; Sanna, D.; Sakar, E.H.; Gharby, S.; Mulas, M.; Medda, S.; Yesilcubuk, N.S.; Karaca, A.C.; Gozukirmizi, C.K.; Lucarini, M.; et al. Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils. Sustainability 2022, 14, 849. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Oukerrou, M.A.; Tilaoui, M.; Mouse, H.A.; Leouifoudi, I.; Jaafari, A.; Zyad, A. Chemical Composition and Cytotoxic and Antibacterial Activities of the Essential Oil of Aloysia citriodora Palau Grown in Morocco. Adv. Pharmacol. Pharm. Sci. 2017, 2017, 7801924. [Google Scholar] [CrossRef]
- Hosseini, M.; Jamshidi, A.; Raeisi, M.; Azizzadeh, M. The Antibacterial and Antioxidant Effects of Clove (Syzygium aromaticum) and Lemon Verbena (Aloysia citriodora) Essential Oils. J. Hum. Environ. Health Promot. 2019, 5, 86–93. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Salama, Y.; Al-Hajj, N.; Jaradat, N.; Jobran, N.T.; Warad, I.; Hamdan, L.; Alrob, M.A.; Sawafta, A.; Hidmi, A. Chemical Composition, Anticancer, Antimicrobial Activity of Aloysia citriodora Palau Essential Oils from Four Different Locations in Palestine. BMC Complement. Med. Ther. 2024, 24, 94. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Fraisse, D.; Besson, C.; Vasson, M.-P.; Texier, O. Bioavailability of Lemon Verbena (Aloysia triphylla) Polyphenols in Rats: Impact of Colonic Inflammation. Br. J. Nutr. 2014, 111, 1773–1781. [Google Scholar] [CrossRef]
- Polumackanycz, M.; Petropoulos, S.A.; Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Plenis, A.; Viapiana, A. Chemical Composition and Antioxidant Properties of Common and Lemon Verbena. Antioxidants 2022, 11, 2247. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.-W.; Ko, C.-H.; Yue, G.G.-L.; Lee, J.K.-M.; Li, K.-K.; Lee, M.; Li, G.; Fung, K.-P.; Leung, P.-C.; Lau, C.B.-S. Green Tea (Camellia sinensis) Extract Inhibits Both the Metastasis and Osteolytic Components of Mammary Cancer 4T1 Lesions in Mice. J. Nutr. Biochem. 2014, 25, 395–403. [Google Scholar] [CrossRef]
- Yan, Y.; Ren, Y.; Li, X.; Zhang, X.; Guo, H.; Han, Y.; Hu, J. A Polysaccharide from Green Tea (Camellia sinensis L.) Protects Human Retinal Endothelial Cells against Hydrogen Peroxide-Induced Oxidative Injury and Apoptosis. Int. J. Biol. Macromol. 2018, 115, 600–607. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Balaji, G.; Chalamaiah, M.; Hanumanna, P.; Vamsikrishna, B.; Jagadeesh Kumar, D.; Venu Babu, V. Mast Cell Stabilizing and Anti-Anaphylactic Activity of Aqueous Extract of Green Tea (Camellia sinensis). Int. J. Vet. Sci. Med. 2014, 2, 89–94. [Google Scholar] [CrossRef]
- Santiago, J.A.; Cardoso, M.D.G.; Batista, L.R.; Castro, E.M.D.; Teixeira, M.L.; Pires, M.F. Essential Oil from Chenopodium ambrosioides L.: Secretory Structures, Antibacterial and Antioxidant Activities. Acta Sci. Biol. Sci. 2016, 38, 139. [Google Scholar] [CrossRef]
- Kasali, F.M.; Tusiimire, J.; Kadima, J.N.; Agaba, A.G. Ethnomedical Uses, Chemical Constituents, and Evidence-Based Pharmacological Properties of Chenopodium ambrosioides L.: Extensive Overview. Future J. Pharm. Sci. 2021, 7, 153. [Google Scholar] [CrossRef]
- Kamdem, B.P.; Le Doux Kamto, E.; Paumo, H.K.; Katata-Seru, L.M.; Pegnyemb, D.E.; Igne, F.E. Chemical Constituents, Ethnomedicinal Uses, Pharmacology, and Toxicity of Dysphania ambrosioides (L.) Mosyakin & Clemants, Formerly Chenopodium ambrosioides L. Nat. Prod. J. 2022, 12, e200921196661. [Google Scholar] [CrossRef]
- Muhayimana, A.; Chalchat, J.-C.; Garry, R.-P. Chemical Composition of Essential Oils of Chenopodium ambrosioides L. from Rwanda. J. Essent. Oil Res. 1998, 10, 690–692. [Google Scholar] [CrossRef]
- Ouadja, B.; Katawa, G.; Toudji, G.A.; Layland, L.; Gbekley, E.H.; Ritter, M.; Anani, K.; Ameyapoh, Y.; Karou, S.D. Anti-Inflammatory, Antibacterial and Antioxidant Activities of Chenopodium ambrosioides L. (Chenopodiaceae) Extracts. J. Appl. Biosci. 2021, 162, 16764–16794. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Kunnath, K.; Alfarhan, A.; Rajagopal, R.; Ramesh, V. Chemical Composition of Cinnamomum verum Leaf and Flower Essential Oils and Analysis of Their Antibacterial, Insecticidal, and Larvicidal Properties. Molecules 2021, 26, 6303. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Mrabti, H.N.; El Hachlafi, N.; El Kamili, T.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G.; et al. Integrated Analysis of Antimicrobial, Antioxidant, and Phytochemical Properties of Cinnamomum verum: A Comprehensive In Vitro and In Silico Study. Biochem. Syst. Ecol. 2023, 110, 104700. [Google Scholar] [CrossRef]
- Farias, A.P.P.; Monteiro, O.D.S.; Da Silva, J.K.R.; Figueiredo, P.L.B.; Rodrigues, A.A.C.; Monteiro, I.N.; Maia, J.G.S. Chemical Composition and Biological Activities of Two Chemotype-Oils from Cinnamomum verum J. Presl Growing in North Brazil. J. Food Sci. Technol. 2020, 57, 3176–3183. [Google Scholar] [CrossRef]
- Phu, H.H.; Pham Van, K.; Tran, T.H.; Pham, D.T.N. Extraction, Chemical Compositions and Biological Activities of Essential Oils of Cinnamomum verum Cultivated in Vietnam. Processes 2022, 10, 1713. [Google Scholar] [CrossRef]
- Kuttithodi, A.M.; Narayanankutty, A.; Visakh, N.U.; Job, J.T.; Pathrose, B.; Olatunji, O.J.; Alfarhan, A.; Ramesh, V. Chemical Composition of the Cinnamomum malabatrum Leaf Essential Oil and Analysis of Its Antioxidant, Enzyme Inhibitory and Antibacterial Activities. Antibiotics 2023, 12, 940. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Ahmed, H.; Dixit, R.K.; Saraf, S.A. Crocus sativus L.: A Comprehensive Review. Pharmacogn. Rev. 2010, 4, 200. [Google Scholar] [CrossRef]
- Maggi, M.A.; Bisti, S.; Picco, C. Saffron: Chemical Composition and Neuroprotective Activity. Molecules 2020, 25, 5618. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qiu, X.; Wu, Q.; Chang, F.; Zhou, T.; Zhou, M.; Pei, J. Active Constituents of Saffron (Crocus sativus L.) and Their Prospects in Treating Neurodegenerative Diseases (Review). Exp. Ther. Med. 2023, 25, 235. [Google Scholar] [CrossRef]
- Ayatollahi, H.; Javan, A.O.; Khajedaluee, M.; Shahroodian, M.; Hosseinzadeh, H. Effect of Crocus sativus L. (Saffron) on Coagulation and Anticoagulation Systems in Healthy Volunteers. Phytother. Res. 2014, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Moras, B.; Loffredo, L.; Rey, S. Quality Assessment of Saffron (Crocus sativus L.) Extracts via UHPLC-DAD-MS Analysis and Detection of Adulteration Using Gardenia Fruit Extract (Gardenia jasminoides Ellis). Food Chem. 2018, 257, 325–332. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Nevárez-Moorillón, G.V.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Cid-Pérez, T.S. Detection of Saffron’s Main Bioactive Compounds and Their Relationship with Commercial Quality. Foods 2022, 11, 3245. [Google Scholar] [CrossRef]
- Li, R.; Jiang, Z. Chemical Composition of the Essential Oil of Cuminum cyminum L. from China. Flavour Fragr. J. 2004, 19, 311–313. [Google Scholar] [CrossRef]
- Bettaieb, I.; Bourgou, S.; Sriti, J.; Msaada, K.; Limam, F.; Marzouk, B. Essential Oils and Fatty Acids Composition of Tunisian and Indian Cumin (Cuminum cyminum L.) Seeds: A Comparative Study. J. Sci. Food Agric. 2011, 91, 2100–2107. [Google Scholar] [CrossRef]
- Allahghadri, T.; Rasooli, I.; Owlia, P.; Nadooshan, M.J.; Ghazanfari, T.; Taghizadeh, M.; Astaneh, S.D.A. Antimicrobial Property, Antioxidant Capacity, and Cytotoxicity of Essential Oil from Cumin Produced in Iran. J. Food Sci. 2010, 75, H54–H61. [Google Scholar] [CrossRef]
- Wanner, J.; Bail, S.; Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Gochev, V.; Girova, T.; Atanasova, T.; Stoyanova, A. Chemical Composition and Antimicrobial Activity of Cumin Oil (Cuminum cyminum, Apiaceae). Nat. Prod. Commun. 2010, 5, 1934578X1000500904. [Google Scholar] [CrossRef]
- Srinivasan, K. Cumin (Cuminum cyminum) and Black Cumin (Nigella sativa) Seeds: Traditional Uses, Chemical Constituents, and Nutraceutical Effects. Food Qual. Saf. 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Prasad, S.; Yuan, W.; Li, S.; Aggarwal, B.B. Identification of a Novel Compound (β-Sesquiphellandrene) from Turmeric (Curcuma longa) with Anticancer Potential: Comparison with Curcumin. Investig. New Drugs 2015, 33, 1175–1186. [Google Scholar] [CrossRef]
- Guimarães, A.; Vinhas, A.C.; Gomes, A.; Souza, L.; Krepsky, P. Essential Oil of Curcuma longa L. Rhizomes Chemical Composition, Yield Variation and Stability. Química Nova 2020, 43, 909–913. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Vo, T.T.B.C.; Vo, T.T.T.N.; Lai, T.N.H. Turmeric (Curcuma longa L.): Chemical Components and Their Effective Clinical Applications. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 883–898. [Google Scholar] [CrossRef]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A. Scientific Basis for the Therapeutic Use of Cymbopogon citratus, Stapf (Lemon Grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3. [Google Scholar] [CrossRef]
- Wahyuni, D.K.; Kharisma, V.D.; Murtadlo, A.A.A.; Rahmawati, C.T.; Syukriya, A.J.; Prasongsuk, S.; Subramaniam, S.; Wibowo, A.T.; Purnobasuki, H. The Antioxidant and Antimicrobial Activity of Ethanolic Extract in Roots, Stems, and Leaves of Three Commercial Cymbopogon Species. BMC Complement. Med. Ther. 2024, 24, 272. [Google Scholar] [CrossRef]
- Kiani, H.S.; Ali, A.; Zahra, S.; Hassan, Z.U.; Kubra, K.T.; Azam, M.; Zahid, H.F. Phytochemical Composition and Pharmacological Potential of Lemongrass (Cymbopogon) and Impact on Gut Microbiota. Appl. Chem. 2022, 2, 229–246. [Google Scholar] [CrossRef]
- Sengupta, A.; Bhattacharjee, S. Cardamom (Elettaria cardamomum) and Its Active Constituent, I,8-Cineole. In Molecular Targets and Therapeutic Uses of Spices; World Scientific: Singapore, 2009; pp. 65–85. ISBN 978-981-283-790-5. [Google Scholar]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Warkentin, T.D. Botany, Traditional Uses, Phytochemistry and Biological Activities of Cardamom [Elettaria cardamomum (L.) Maton]—A Critical Review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef] [PubMed]
- Moulai-Hacene, F.; Boufadi, M.Y.; Keddari, S.; Homrani, A. Chemical Composition and Antimicrobial Properties of Elettaria cardamomum Extract. Pharmacogn. J. 2020, 12, 1058–1063. [Google Scholar] [CrossRef]
- Souissi, M.; Azelmat, J.; Chaieb, K.; Grenier, D. Antibacterial and Anti-Inflammatory Activities of Cardamom (Elettaria cardamomum) Extracts: Potential Therapeutic Benefits for Periodontal Infections. Anaerobe 2020, 61, 102089. [Google Scholar] [CrossRef]
- Almohammed, H.I.; Alkhaibari, A.M.; Alanazi, A.D. Antiparasitic Effects of Elettaria cardamomum L. Essential Oil and Its Main Compounds, 1-8 Cineole Alone and in Combination with Albendazole against Echinococcus granulosus Protoscoleces. Saudi J. Biol. Sci. 2022, 29, 2811–2818. [Google Scholar] [CrossRef]
- Sreedharan, S.; Nair, V.; Cisneros-Zevallos, L. Protective Role of Phenolic Compounds from Whole Cardamom (Elettaria cardamomum (L.) Maton) against LPS-Induced Inflammation in Colon and Macrophage Cells. Nutrients 2023, 15, 2965. [Google Scholar] [CrossRef]
- Zeng, Q.Y.; Wu, J.; Lin, P.C. Chemical Composition and Antimicrobial Activity of the Essential Oil from Epilobium angustifolium. Chem. Nat. Compd. 2016, 52, 1113–1115. [Google Scholar] [CrossRef]
- Nowak, A.; Zagórska-Dziok, M.; Ossowicz-Rupniewska, P.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Adamiak-Giera, U.; Prowans, P.; Czapla, N.; Bargiel, P.; et al. Epilobium angustifolium L. Extracts as Valuable Ingredients in Cosmetic and Dermatological Products. Molecules 2021, 26, 3456. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Cybulska, K.; Makuch, E.; Kucharski, Ł.; Różewicka-Czabańska, M.; Prowans, P.; Czapla, N.; Bargiel, P.; Petriczko, J.; Klimowicz, A. In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 329. [Google Scholar] [CrossRef]
- de Oliveira, F.M.; Romão, W.; Kuster, R.M. Identification of Phenolic Compounds in Eugenia uniflora Leaves by FTICR MS in Association with Different Ionization Sources. Anal. Methods 2018, 10, 1647–1655. [Google Scholar] [CrossRef]
- Bagatini, L.; Zandoná, G.P.; Hoffmann, J.F.; De Souza Cardoso, J.; Teixeira, F.C.; Moroni, L.S.; Junges, A.; Kempka, A.P.; Stefanello, F.M.; Rombaldi, C.V. Evaluation of Eugenia uniflora L. Leaf Extracts Obtained by Pressurized Liquid Extraction: Identification of Chemical Composition, Antioxidant, Antibacterial, and Allelopathic Activity. Sustain. Chem. Pharm. 2023, 35, 101214. [Google Scholar] [CrossRef]
- Santos, R.M.; Fortes, G.A.C.; Ferri, P.H.; Santos, S.C. Influence of Foliar Nutrients on Phenol Levels in Leaves of Eugenia uniflora. Rev. Bras. De Farmacogn. 2011, 21, 581–586. [Google Scholar] [CrossRef]
- Oriola, A.O.; Miya, G.M.; Singh, M.; Oyedeji, A.O. Flavonol Glycosides from Eugenia uniflora Leaves and Their In Vitro Cytotoxicity, Antioxidant and Anti-Inflammatory Activities. Sci. Pharm. 2023, 91, 42. [Google Scholar] [CrossRef]
- Fidelis, E.M.; Savall, A.S.P.; De Oliveira Pereira, F.; Quines, C.B.; Ávila, D.S.; Pinton, S. Pitanga (Eugenia uniflora L.) as a Source of Bioactive Compounds for Health Benefits: A Review. Arab. J. Chem. 2022, 15, 103691. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical Composition and Antimicrobial Activity of Laurus nobilis L. Essential Oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef]
- Mssillou, I.; Agour, A.; El Ghouizi, A.; Hamamouch, N.; Lyoussi, B.; Derwich, E. Chemical Composition, Antioxidant Activity, and Antifungal Effects of Essential Oil from Laurus nobilis L. Flowers Growing in Morocco. J. Food Qual. 2020, 2020, 8819311. [Google Scholar] [CrossRef]
- Awada, F.; Hamade, K.; Kassir, M.; Hammoud, Z.; Mesnard, F.; Rammal, H.; Fliniaux, O. Laurus nobilis Leaves and Fruits: A Review of Metabolite Composition and Interest in Human Health. Appl. Sci. 2023, 13, 4606. [Google Scholar] [CrossRef]
- Mkaddem Guedri, M.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Bouajila, J. Chemical Composition and Antimicrobial and Antioxidant Activities of Tunisian, France and Austrian Laurus nobilis (Lauraceae) Essential Oils. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1929–1940. [Google Scholar] [CrossRef]
- Stefanova, G.; Girova, T.; Gochev, V.; Stoyanova, M.; Petkova, Z.; Stoyanova, A.; Zheljazkov, V.D. Comparative Study on the Chemical Composition of Laurel (Laurus nobilis L.) Leaves from Greece and Georgia and the Antibacterial Activity of Their Essential Oil. Heliyon 2020, 6, e05491. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Chen, Y.; Han, X.; Zhan, Z.; Tian, S.; Cui, Q.; Wang, Y. Chemical Composition of Essential Oils of Litsea cubeba Harvested from Its Distribution Areas in China. Molecules 2012, 17, 7057–7066. [Google Scholar] [CrossRef]
- Yang, K.; Wang, C.F.; You, C.X.; Geng, Z.F.; Sun, R.Q.; Guo, S.S.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Bioactivity of Essential Oil of Litsea cubeba from China and Its Main Compounds against Two Stored Product Insects. J. Asia-Pac. Entomol. 2014, 17, 459–466. [Google Scholar] [CrossRef]
- Yang, N.-Q.; Wang, X.-Y.; Shen, G.-D.; Lu, S.-C.; Fan, Y.-N.; Li, B.-T.; Wang, Y.-S.; Yang, J.-H. Chemical Constituents of Litsea cubeba. Chem. Nat. Compd. 2023, 59, 135–137. [Google Scholar] [CrossRef]
- Li, W.-R.; Shi, Q.-S.; Liang, Q.; Xie, X.-B.; Huang, X.-M.; Chen, Y.-B. Antibacterial Activity and Kinetics of Litsea cubeba Oil on Escherichia coli. PLoS ONE 2014, 9, e110983. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, J.; Muranyi, P. Review on the Chemical Composition of Litsea cubeba Essential Oils and the Bioactivity of Its Major Constituents Citral and Limonene. J. Essent. Oil Res. 2019, 31, 361–378. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The Composition, Pharmacological and Economic Importance of Essential Oil of Litsea cubeba (Lour.) Pers. Food Sci. Technol. 2022, 42, e35720. [Google Scholar] [CrossRef]
- Berté, K.A.S.; Beux, M.R.; Spada, P.K.W.D.S.; Salvador, M.; Hoffmann-Ribani, R. Chemical Composition and Antioxidant Activity of Yerba-Mate (Ilex paraguariensis A.St.-Hil., Aquifoliaceae) Extract as Obtained by Spray Drying. J. Agric. Food Chem. 2011, 59, 5523–5527. [Google Scholar] [CrossRef]
- Rząsa-Duran, E.; Kryczyk-Poprawa, A.; Drabicki, D.; Podkowa, A.; Sułkowska-Ziaja, K.; Szewczyk, A.; Kała, K.; Opoka, W.; Zięba, P.; Fidurski, M.; et al. Yerba Mate as a Source of Elements and Bioactive Compounds with Antioxidant Activity. Antioxidants 2022, 11, 371. [Google Scholar] [CrossRef]
- Teselkin, Y.O.; Babenkova, I.V.; Kochetova, A.A.; Osipov, A.N. Inhibitory Effect of Aqueous Extract from Yerba Mate (Ilex paraguariensis) on the Process of Lipid Peroxidation of Liposomal Membranes. Biophysics 2022, 67, 541–548. [Google Scholar] [CrossRef]
- Pachura, N.; Kupczyński, R.; Sycz, J.; Kuklińska, A.; Zwyrzykowska-Wodzińska, A.; Wińska, K.; Owczarek, A.; Kuropka, P.; Nowaczyk, R.; Bąbelewski, P.; et al. Biological Potential and Chemical Profile of European Varieties of Ilex. Foods 2021, 11, 47. [Google Scholar] [CrossRef]
- Filip, R.; Davicino, R.; Anesini, C. Antifungal Activity of the Aqueous Extract of Ilex paraguariensis against Malassezia furfur. Phytother. Res. 2010, 24, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, S.; Asano, R.; Iwahori, Y.; Narui, T.; Okada, Y.; Singab, A.N.B.; Okuyama, T. Hematological Studies on Black Cumin Oil from the Seeds of Nigella sativa L. Biol. Pharm. Bull. 2001, 24, 307–310. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A Review on Therapeutic Potential of Nigella sativa: A Miracle Herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Bazzaz, B.S.F.; Hosseinzadeh, H. Black Cumin (Nigella sativa) and Its Constituent (Thymoquinone): A Review on Antimicrobial Effects. Iran. J. Basic Med. Sci. 2014, 17, 929–938. [Google Scholar]
- Tembhurne, S.V.; Feroz, S.; More, B.H.; Sakarkar, D.M. A Review on Therapeutic Potential of Nigella sativa (Kalonji) Seeds. J. Med. Plants Res. 2014, 8, 167–177. [Google Scholar] [CrossRef]
- Cheikh-Rouhou, S.; Besbes, S.; Hentati, B.; Blecker, C.; Deroanne, C.; Attia, H. Nigella sativa L.: Chemical Composition and Physicochemical Characteristics of Lipid Fraction. Food Chem. 2007, 101, 673–681. [Google Scholar] [CrossRef]
- Hannan, A.; Saleem, S.; Chaudhary, S.; Barkaat, M.; Arshad, M.U. Anti Bacterial Activity of Nigella sativa against Clinical Isolates of Methicillin Resistant Staphylococcus aureus. J. Ayub Med. Coll. Abbottabad 2008, 20, 72–74. [Google Scholar]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical Composition and Bioactivity of Different Oregano (Origanum vulgare) Extracts and Essential Oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Milenković, A.; Stanojević, J.; Cvetković, D. The Yield, Chemical Composition, and Antioxidant Activities of Essential Oils from Different Plant Parts of the Wild and Cultivated Oregano (Origanum vulgare L.). Horticulturae 2022, 8, 1042. [Google Scholar] [CrossRef]
- Rao, G.; Mukhopadhyay, T.; Annamalai, T.; Radhakrishnan, N.; Sahoo, M. Chemical Constituents and Biological Studies of Origanum vulgare Linn. Pharmacogn. Res. 2011, 3, 143. [Google Scholar] [CrossRef] [PubMed]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Pandian, A.; Warkentin, T.D. Phytochemistry and Therapeutic Potential of Black Pepper [Piper nigrum (L.)] Essential Oil and Piperine: A Review. Clin. Phytosci. 2021, 7, 52. [Google Scholar] [CrossRef]
- Feitosa, B.D.S.; Ferreira, O.O.; Franco, C.D.J.P.; Karakoti, H.; Kumar, R.; Cascaes, M.M.; Jawarkar, R.D.; Mali, S.N.; Cruz, J.N.; De Menezes, I.C.; et al. Chemical Composition of Piper nigrum L. Cultivar Guajarina Essential Oils and Their Biological Activity. Molecules 2024, 29, 947. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Umadevi, N.P.; Kuttan, R. Antioxidant, Anti-Inflammatory and Antinociceptive Properties of Black Pepper Essential Oil (Piper nigrum Linn). J. Essent. Oil Bear. Plants 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Andriana, Y.; Xuan, T.D.; Quy, T.N.; Tran, H.-D.; Le, Q.-T. Biological Activities and Chemical Constituents of Essential Oils from Piper Cubeba Bojer and Piper nigrum L. Molecules 2019, 24, 1876. [Google Scholar] [CrossRef] [PubMed]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Marjanovic Vicentic, J.; Popovic, J.; Golic Grdadolnik, S.; Markovic, D.; Sanković-Babić, S.; Glamoclija, J.; et al. Apigenin-7-O-Glucoside versus Apigenin: Insight into the Modes of Anticandidal and Cytotoxic Actions. EXCLI J. 2017, 16, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Valencia, D.; Rueda Puente, E.O.; Leyva Peralta, M.A.; Mazón-López, S.R.; Ortega-García, J. Bioactive Compounds, Antioxidant Activity and Profile of Fatty Acids in Mesquite Seed Oil (Prosopis spp). Arch. Latinoam. De Nutr. 2020, 70, 50–59. [Google Scholar] [CrossRef]
- Zhong, J.; Lu, P.; Wu, H.; Liu, Z.; Sharifi-Rad, J.; Setzer, W.N.; Suleria, H.A.R. Current Insights into Phytochemistry, Nutritional, and Pharmacological Properties of Prosopis Plants. Evid.-Based Complement. Altern. Med. 2022, 2022, 2218029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cai, J.; Duan, C.-Q.; Reeves, M.; He, F. A Review of Polyphenolics in Oak Woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef]
- Burlacu, E.; Nisca, A.; Tanase, C. A Comprehensive Review of Phytochemistry and Biological Activities of Quercus Species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Othón-Díaz, E.D.; Fimbres-García, J.O.; Flores-Sauceda, M.; Silva-Espinoza, B.A.; López-Martínez, L.X.; Bernal-Mercado, A.T.; Ayala-Zavala, J.F. Antioxidants in Oak (Quercus spp.): Potential Application to Reduce Oxidative Rancidity in Foods. Antioxidants 2023, 12, 861. [Google Scholar] [CrossRef]
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal Uses, Phytochemistry, and Pharmacological Activities of Quercus Species. Evid.-Based Complement. Altern. Med. 2020, 2020, 1920683. [Google Scholar] [CrossRef]
- Morales, D. Oak Trees (Quercus spp.) as a Source of Extracts with Biological Activities: A Narrative Review. Trends Food Sci. Technol. 2021, 109, 116–125. [Google Scholar] [CrossRef]
- Duskaev, G.K.; Rakhmatullin, S.G.; Kazachkova, N.M.; Sheida, Y.V.; Mikolaychik, I.N.; Morozova, L.A.; Galiev, B.H. Effect of the Combined Action of Quercus Cortex Extract and Probiotic Substances on the Immunity and Productivity of Broiler Chickens. Vet. World 2018, 11, 1416–1422. [Google Scholar] [CrossRef]
- Elbanna, K.; Assiri, A.M.A.; Tadros, M.; Khider, M.; Assaeedi, A.; Mohdaly, A.A.A.; Ramadan, M.F. Rosemary (Rosmarinus officinalis) Oil: Composition and Functionality of the Cold-Pressed Extract. Food Meas. 2018, 12, 1601–1609. [Google Scholar] [CrossRef]
- Ulbricht, C.; Abrams, T.R.; Brigham, A.; Ceurvels, J.; Clubb, J.; Curtiss, W.; Kirkwood, C.D.; Giese, N.; Hoehn, K.; Iovin, R.; et al. An Evidence-Based Systematic Review of Rosemary (Rosmarinus officinalis) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2010, 7, 351–413. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.; Batool, R.; Akhtar, W.; Shahzad, T.; Malik, A.; Shah, M.A.; Iqbal, S.; Rauf, A.; Zengin, G.; Bouyahya, A.; et al. Rosemary Species: A Review of Phytochemicals, Bioactivities and Industrial Applications. S. Afr. J. Bot. 2022, 151, 3–18. [Google Scholar] [CrossRef]
- Elyemni, M.; El Ouadrhiri, F.; Lahkimi, A.; Elkamli, T.; Bouia, A.; Eloutassi, N. Chemical Composition and Antimicrobial Activity of Essential Oil of Wild and Cultivated Rosmarinus officinalis from Two Moroccan Localities. J. Ecol. Eng. 2022, 23, 214–222. [Google Scholar] [CrossRef]
- Begum, A.; Sandhya, S.; Shaffath Ali, S.; Vinod, K.R.; Reddy, S.; Banji, D. An In-Depth Review on the Medicinal Flora Rosmarinus officinalis (Lamiaceae). Acta Sci. Pol. Technol. Aliment. 2013, 12, 61–73. [Google Scholar] [PubMed]
- Jafari-Sales, A.; Pashazadeh, M. Study of Chemical Composition and Antimicrobial Properties of Rosemary (Rosmarinus officinalis) Essential Oil on Staphylococcus aureus and Escherichia coli in Vitro. Int. J. Life Sci. Biotechnol. 2020, 3, 62–69. [Google Scholar] [CrossRef]
- Payet, B.; Shum Cheong Sing, A.; Smadja, J. Comparison of the Concentrations of Phenolic Constituents in Cane Sugar Manufacturing Products with Their Antioxidant Activities. J. Agric. Food Chem. 2006, 54, 7270–7276. [Google Scholar] [CrossRef]
- Singh, A.; Lal, U.; Mukhtar, H.; Singh, P.; Shah, G.; Dhawan, R. Phytochemical Profile of Sugarcane and Its Potential Health Aspects. Pharmacogn. Rev. 2015, 9, 45. [Google Scholar] [CrossRef]
- Duarte-Almeida, J.M.; Negri, G.; Salatino, A.; De Carvalho, J.E.; Lajolo, F.M. Antiproliferative and Antioxidant Activities of a Tricin Acylated Glycoside from Sugarcane (Saccharum officinarum) Juice. Phytochemistry 2007, 68, 1165–1171. [Google Scholar] [CrossRef]
- Ali, S.E.; El Gedaily, R.A.; Mocan, A.; Farag, M.A.; El-Seedi, H.R. Profiling Metabolites and Biological Activities of Sugarcane (Saccharum officinarum Linn.) Juice and Its Product Molasses via a Multiplex Metabolomics Approach. Molecules 2019, 24, 934. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.E.; Yuan, Q.; Wang, S.; Farag, M.A. More than Sweet: A Phytochemical and Pharmacological Review of Sugarcane (Saccharum officinarum L.). Food Biosci. 2021, 44, 101431. [Google Scholar] [CrossRef]
- Capek, P.; Hříbalová, V. Water-Soluble Polysaccharides from Salvia officinalis L. Possessing Immunomodulatory Activity. Phytochemistry 2004, 65, 1983–1992. [Google Scholar] [CrossRef]
- Porte, A.; Godoy, R.L.O.; Maia-Porte, L.H. Chemical Composition of Sage (Salvia officinalis L.) Essential Oil from the Rio de Janeiro State (Brazil). Rev. Bras. De Plantas Med. 2013, 15, 438–441. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological Properties of Salvia officinalis and Its Components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Lima, C.F.; Valentao, P.C.R.; Andrade, P.B.; Seabra, R.M.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Water and Methanolic Extracts of Salvia officinalis Protect HepG2 Cells from T-BHP Induced Oxidative Damage. Chem.-Biol. Interact. 2007, 167, 107–115. [Google Scholar] [CrossRef] [PubMed]
- El Euch, S.K.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis Essential Oil: Chemical Analysis and Evaluation of Anti-Enzymatic and Antioxidant Bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- Jažo, Z.; Glumac, M.; Paštar, V.; Bektić, S.; Radan, M.; Carev, I. Chemical Composition and Biological Activity of Salvia officinalis L. Essential Oil. Plants 2023, 12, 1794. [Google Scholar] [CrossRef] [PubMed]
- Golparvar, A.R.; Gheisari, M.M.; Hadipanah, A.; Khorrami, M. Antibacterial, Antifungal Properties and Chemical Composition of Essential Oils of Satureja hortensis L. and Satureja khuzestanica Jamzad. J. Herbs Drug. 2018, 8, 243–249. [Google Scholar] [CrossRef]
- Davoodi, M.; Nejad-Ebrahimi, S.; Rustaiyan, A.; Esmaeili, D. The Chemical Composition and Antibacterial Activity of a Methanolic Extract of Satureja khuzistanica. Braz. J. Pharm. Sci. 2022, 58, e19233. [Google Scholar] [CrossRef]
- Karami, F.; Dastan, D.; Fallah, M.; Matini, M. In Vitro Antitrichomonal Activity of Satureja khuzestanica and Main Essential Oil Components Carvacrol, Thymol, and Eugenol. J. Infect. Dev. Ctries. 2023, 17, 80–85. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M.; Šolić, M.E.; Jerković-Mujkić, A.; Bešta, R. Chemical Composition and Antioxidant and Antimicrobial Activity of Two Satureja Essential Oils. Food Chem. 2008, 111, 648–653. [Google Scholar] [CrossRef]
- Fallahi, S.; Beyranvand, M.; Mahmoudvand, H.; Nayebzadeh, H.; Kheirandish, F.; Jahanbakhsh, S. Chemical Composition, Acute and Sub-Acute Toxicity of Satureja khuzestanica Essential Oil in Mice. Marmara Pharm. J. 2017, 21, 515. [Google Scholar] [CrossRef]
- Skočibušić, M.; Bezić, N. Chemical Composition and Antidiarrhoeal Activities of Winter Savory (Satureja montana L.) Essential Oil. Pharm. Biol. 2003, 41, 622–626. [Google Scholar] [CrossRef]
- Abdelshafeek, K.A.; Osman, A.F.; Mouneir, S.M.; Elhenawy, A.A.; Abdallah, W.E. Phytochemical Profile, Comparative Evaluation of Satureja montana Alcoholic Extract for Antioxidants, Anti-Inflammatory and Molecular Docking Studies. BMC Complement. Med. Ther. 2023, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, S.; Abdollahi, M. An Update on Pharmacology of Satureja Species; From Antioxidant, Antimicrobial, Antidiabetes and Anti-Hyperlipidemic to Reproductive Stimulation. Int. J. Pharmacol. 2010, 6, 346–353. [Google Scholar] [CrossRef]
- Santos, J.D.C.; Coelho, E.; Silva, R.; Passos, C.P.; Teixeira, P.; Henriques, I.; Coimbra, M.A. Chemical Composition and Antimicrobial Activity of Satureja montana Byproducts Essential Oils. Ind. Crops Prod. 2019, 137, 541–548. [Google Scholar] [CrossRef]
- Miladi, H.; Ben Slama, R.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Chemical Composition and Cytotoxic and Antioxidant Activities of Satureja montana L. Essential Oil and Its Antibacterial Potential against Salmonella Spp. Strains. J. Chem. 2013, 2013, 275698. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Xiang, Z.; Wang, S.; Cong, Z.; Gao, P.; Liu, X. Recent Advances in Nutritional Composition, Phytochemistry, Bioactive, and Potential Applications of Syzygium aromaticum L. (Myrtaceae). Front. Nutr. 2022, 9, 1002147. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Srivastava, S.; Ashish; Dash, K.K.; Singh, R.; Dar, A.H.; Singh, T.; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive Properties of Clove (Syzygium aromaticum) Essential Oil Nanoemulsion: A Comprehensive Review. Heliyon 2024, 10, e22437. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Parker, T.L. Anti-Inflammatory Activity of Clove (Eugenia caryophyllata) Essential Oil in Human Dermal Fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhu, X.; Cao, P.; Wei, S.; Lu, Y. Antibacterial and Antibiofilm Activities of Eugenol from Essential Oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (Clove) Leaf against Periodontal Pathogen Porphyromonas Gingivalis. Microb. Pathog. 2017, 113, 396–402. [Google Scholar] [CrossRef]
- Jaouadi, R.; Cardoso, S.M.; Silva, A.M.S.; Ben Hadj Yahia, I.; Boussaid, M.; Zaouali, Y. Variation of Phenolic Constituents of Tunisian Thymus capitatus (L.) Hoff. et Link. Populations. Biochem. Syst. Ecol. 2018, 77, 10–15. [Google Scholar] [CrossRef]
- Ben Miri, Y.; Djenane, D. Antifungal, Anti-Aflatoxigenic, Antioxidant Activity and in Vivo Efficacy of Essential Oil of the Aerial Parts of Thymus capitatus (L.) Hoffmanns & Link. Phytothérapie 2019, 17, 299–309. [Google Scholar] [CrossRef]
- Belén Sabater-Jara, A.; Pina Funes, M.; Angeles Pedreño, M.; Belchí-Navarro, S. Essential Oils of Thymbra capitata and Thymus hyemalis and Their Uses Based on Their Bioactivity. In Thymus; Rezaei, N., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-133-5. [Google Scholar]
- Benoutman, A.; Erbiai, E.H.; Edderdaki, F.Z.; Cherif, E.K.; Saidi, R.; Lamrani, Z.; Pintado, M.; Pinto, E.; Esteves Da Silva, J.C.G.; Maouni, A. Phytochemical Composition, Antioxidant and Antifungal Activity of Thymus capitatus, a Medicinal Plant Collected from Northern Morocco. Antibiotics 2022, 11, 681. [Google Scholar] [CrossRef]
- Tagnaout, I.; Zerkani, H.; Hadi, N.; El Moumen, B.; El Makhoukhi, F.; Bouhrim, M.; Al-Salahi, R.; Nasr, F.A.; Mechchate, H.; Zair, T. Chemical Composition, Antioxidant and Antibacterial Activities of Thymus broussonetii Boiss and Thymus capitatus (L.) Hoffmann and Link Essential Oils. Plants 2022, 11, 954. [Google Scholar] [CrossRef]
- Maniki, E.; Kostoglou, D.; Paterakis, N.; Nikolaou, A.; Kourkoutas, Y.; Papachristoforou, A.; Giaouris, E. Chemical Composition, Antioxidant, and Antibiofilm Properties of Essential Oil from Thymus capitatus Plants Organically Cultured on the Greek Island of Lemnos. Molecules 2023, 28, 1154. [Google Scholar] [CrossRef]
- Rasooli, I.; Mirmostafa, S.A. Bacterial Susceptibility to and Chemical Composition of Essential Oils from Thymus kotschyanus and Thymus persicus. J. Agric. Food Chem. 2003, 51, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Variations in Chemical Composition and Bioactive Compounds of Thymus kotschyanus Boiss. & Hohen Populations Originated from Different Collection Sites. J. Essent. Oil Bear. Plants 2018, 21, 1272–1283. [Google Scholar] [CrossRef]
- Ghasemi, G.; Alirezalu, A.; Ghosta, Y.; Jarrahi, A.; Safavi, S.A.; Abbas-Mohammadi, M.; Barba, F.J.; Munekata, P.E.S.; Domínguez, R.; Lorenzo, J.M. Composition, Antifungal, Phytotoxic, and Insecticidal Activities of Thymus kotschyanus Essential Oil. Molecules 2020, 25, 1152. [Google Scholar] [CrossRef]
- Khanavi, M.; Hajimehdipoor, H.; Emadi, F.; Khandani, N.K. Essential Oil Compositions of Thymus kotschyanus Boiss. Obtained by Hydrodistillation and Microwave Oven Distillation. J. Essent. Oil Bear. Plants 2013, 16, 117–122. [Google Scholar] [CrossRef]
- Zhiani, R.; Dolatabadi, S.; Imani, H.; Moradi, M.; Emrani, S. A Comparison of the Chemical Composition of Flowering Shoot Thymus kotschyanus and Thymus vulgaris by Using GC-Mass and Antimicrobial Effects of the Bacteria Staphylococcus aureus and Pseudomonas aeruginosa. J. Essent. Oil Bear. Plants 2016, 19, 1639–1647. [Google Scholar] [CrossRef]
- Mahboubi, M.; Heidarytabar, R.; Mahdizadeh, E.; Hosseini, H. Antimicrobial Activity and Chemical Composition of Thymus Species and Zataria multiflora Essential Oils. Agric. Nat. Resour. 2017, 51, 395–401. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical Composition, Antimicrobial, Antioxidant and Antitumor Activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. Essential Oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Tazabayeva, K.; Sylibaeva, B. Chemical Composition of The Essential Oil and Flavonoids of Thymus serpyllum L., Growing on Territory of the East Kazakhstan. Acta Pol. Pharm.-Drug Res. 2018, 75, 1329–1337. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Gonçalves Rodrigues, A.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.; Martinez-de-Oliveira, J. Antifungal Activity of Thymus Oils and Their Major Compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef]
- Pruteanu, A.; Popescu, C.; Vladut, V.; Gageanu, G. Biochemical Analysis of Some Vegetal Extracts Obtained from Indigenous Spontaneous Species of Thymus serpyllum L. Rom. Biotechnol. Lett. 2018, 23, 14013–14024. [Google Scholar]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Kačániová, M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. [Google Scholar] [CrossRef]
- Bairwa, R.; Rajawat, B.; Sodha, R. Trachyspermum ammi. Phcog. Rev. 2012, 6, 56. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; JebelliJavan, A.; Sharifzadeh, A. Chemical Constituents, Antimicrobial and Antioxidative Effects of Trachyspermum ammi Essential Oil: Ajwan EO’s Antimicrobial and Antioxidative Effects. J. Food Process. Preserv. 2014, 38, 1690–1695. [Google Scholar] [CrossRef]
- Moein, M.R.; Zomorodian, K.; Pakshir, K.; Yavari, F.; Motamedi, M.; Zarshenas, M.M. Trachyspermum ammi (L.) Sprague: Chemical Composition of Essential Oil and Antimicrobial Activities of Respective Fractions. J. Evid.-Based Complement. Altern. Med. 2015, 20, 50–56. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; Catalan, C.; De Lampasona, M.P. Chemical Constituents, Antifungal and Antioxidative Effects of Ajwain Essential Oil and Its Acetone Extract. J. Agric. Food Chem. 2004, 52, 3292–3296. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.-S.S.; Bazaid, S.A.; Al Zahrani, O.; El-Halmouch, Y.; El-Sayed, M.M.; El-Wakil, E. Chemical Composition of Volatile Components, Antimicrobial and Anticancer Activity of n-Hexane Extract and Essential Oil from Trachyspermum ammi L. Seeds. Orient. J. Chem. 2014, 30, 1653–1662. [Google Scholar] [CrossRef]
- Mazzara, E.; Scortichini, S.; Fiorini, D.; Maggi, F.; Petrelli, R.; Cappellacci, L.; Morgese, G.; Morshedloo, M.R.; Palmieri, G.F.; Cespi, M. A Design of Experiment (DoE) Approach to Model the Yield and Chemical Composition of Ajowan (Trachyspermum ammi L.) Essential Oil Obtained by Microwave-Assisted Extraction. Pharmaceuticals 2021, 14, 816. [Google Scholar] [CrossRef] [PubMed]
- Naicker, N.; Nagiah, S.; Phulukdaree, A.; Chuturgoon, A. Trigonella foenum-graecum Seed Extract, 4-Hydroxyisoleucine, and Metformin Stimulate Proximal Insulin Signaling and Increase Expression of Glycogenic Enzymes and GLUT2 in HepG2 Cells. Metab. Syndr. Relat. Disord. 2016, 14, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Singh, P.; Rani, A. Antimicrobial Activity of Trigonella foenum-graecum L. (Fenugreek). Eur. J. Exp. Biol. 2017, 7, 1–4. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chaudhary, P.S.; Chikara, S.K.; Sharma, M.C.; Iriti, M. Review on Fenugreek (Trigonella foenum-graecum L.) and Its Important Secondary Metabolite Diosgenin. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 22–31. [Google Scholar] [CrossRef]
- Premanath, R.; Sudisha, J.; Devi, N.L.; Aradhya, S.M. Antibacterial and Anti-Oxidant Activities of Fenugreek (Trigonella foenum graecum L.) Leaves. Res. J. Med. Plant 2011, 5, 695–705. [Google Scholar] [CrossRef][Green Version]
- Visuvanathan, T.; Than, L.T.L.; Stanslas, J.; Chew, S.Y.; Vellasamy, S. Revisiting Trigonella foenum-graecum L.: Pharmacology and Therapeutic Potentialities. Plants 2022, 11, 1450. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging Nettle (Urtica dioica L.): A Reservoir of Nutrition and Bioactive Components with Great Functional Potential. Food Meas. 2017, 11, 423–433. [Google Scholar] [CrossRef]
- Devkota, H.P.; Paudel, K.R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N.K.; Das, N.; Singh, S.K.; Chellappan, D.K.; et al. Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties. Molecules 2022, 27, 5219. [Google Scholar] [CrossRef] [PubMed]
- Semwal, P.; Rauf, A.; Olatunde, A.; Singh, P.; Zaky, M.Y.; Islam, M.M.; Khalil, A.A.; Aljohani, A.S.M.; Al Abdulmonem, W.; Ribaudo, G. The Medicinal Chemistry of Urtica dioica L.: From Preliminary Evidence to Clinical Studies Supporting Its Neuroprotective Activity. Nat. Prod. Bioprospect. 2023, 13, 16. [Google Scholar] [CrossRef]
- Đurović, S.; Pavlić, B.; Šorgić, S.; Popov, S.; Savić, S.; Petronijević, M.; Radojković, M.; Cvetanović, A.; Zeković, Z. Chemical Composition of Stinging Nettle Leaves Obtained by Different Analytical Approaches. J. Funct. Foods 2017, 32, 18–26. [Google Scholar] [CrossRef]
- Tarasevičienė, Ž.; Vitkauskaitė, M.; Paulauskienė, A.; Černiauskienė, J. Wild Stinging Nettle (Urtica dioica L.) Leaves and Roots Chemical Composition and Phenols Extraction. Plants 2023, 12, 309. [Google Scholar] [CrossRef]
- Đurović, S.; Kojić, I.; Radić, D.; Smyatskaya, Y.A.; Bazarnova, J.G.; Filip, S.; Tosti, T. Chemical Constituents of Stinging Nettle (Urtica dioica L.): A Comprehensive Review on Phenolic and Polyphenolic Compounds and Their Bioactivity. Int. J. Mol. Sci. 2024, 25, 3430. [Google Scholar] [CrossRef]
- Gandomi, H.; Misaghi, A.; Basti, A.A.; Bokaei, S.; Khosravi, A.; Abbasifar, A.; Javan, A.J. Effect of Zataria multiflora Boiss. Essential Oil on Growth and Aflatoxin Formation by Aspergillus flavus in Culture Media and Cheese. Food Chem. Toxicol. 2009, 47, 2397–2400. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Mirbadie, S.R.; Sadooghian, S.; Harandi, M.F.; Jahanbakhsh, S.; Saedi Dezaki, E. Chemical Composition and Scolicidal Activity of Zataria multiflora Boiss Essential Oil. J. Essent. Oil Res. 2017, 29, 42–47. [Google Scholar] [CrossRef]
- Karimi, A.; Krähmer, A.; Herwig, N.; Schulz, H.; Hadian, J.; Meiners, T. Variation of Secondary Metabolite Profile of Zataria multiflora Boiss. Populations Linked to Geographic, Climatic, and Edaphic Factors. Front. Plant Sci. 2020, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Ramezani, M.; Salmani, G. Antinociceptive, Anti-Inflammatory and Acute Toxicity Effects of Zataria multiflora Boiss Extracts in Mice and Rats. J. Ethnopharmacol. 2000, 73, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sharififar, F.; Moshafi, M.H.; Mansouri, S.H.; Khodashenas, M.; Khoshnoodi, M. In Vitro Evaluation of Antibacterial and Antioxidant Activities of the Essential Oil and Methanol Extract of Endemic Zataria multiflora Boiss. Food Control 2007, 18, 800–805. [Google Scholar] [CrossRef]
- Pilevar, Z.; Haglund, K.; Ranaei, V.; Taghizadeh, M.; Maghboli Balasjin, N.; Hosseini, H. Emerging Studies on Zataria multiflora Boiss L.: Pioneering the Antimicrobial and Antifungal Characteristics–A Systematic Review. Appl. Food Biotechnol. 2024, 11, e20. [Google Scholar] [CrossRef]
- Nishidono, Y.; Saifudin, A.; Nishizawa, M.; Fujita, T.; Nakamoto, M.; Tanaka, K. Identification of the Chemical Constituents in Ginger (Zingiber officinale) Responsible for Thermogenesis. Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Yu, M.; Luo, D.; Chen, S.; Liu, W.; Zhang, Y.; Zhang, L.; Zhao, T. Essential Oils of Zingiber officinale: Chemical Composition, in Vivo Alleviation Effects on TPA Induced Ear Swelling in Mice and in Vitro Bioactivities. Front. Nutr. 2022, 9, 1043175. [Google Scholar] [CrossRef]
- Munda, S.; Dutta, S.; Haldar, S.; Lal, M. Chemical Analysis and Therapeutic Uses of Ginger (Zingiber officinale Rosc.) Essential Oil: A Review. J. Essent. Oil Bear. Plants 2018, 21, 994–1002. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Zhang, Y. Research Progress on Chemical Constituents of Zingiber officinale Roscoe. BioMed Res. Int. 2019, 2019, 5370823. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M.M.; Hafez, R.M.; El-Ansary, A.E. Chemical Constituents and Biological Activities of Different Extracts from Ginger Plant (Zingiber officinale). Chem. Biol. Technol. Agric. 2023, 10, 14. [Google Scholar] [CrossRef]
- Li, M.-Y.; Liu, H.-Y.; Wu, D.-T.; Kenaan, A.; Geng, F.; Li, H.-B.; Gunaratne, A.; Li, H.; Gan, R.-Y. L-Theanine: A Unique Functional Amino Acid in Tea (Camellia sinensis L.) with Multiple Health Benefits and Food Applications. Front. Nutr. 2022, 9, 853846. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Critical Overview of the Use of Plant Antioxidants in the Meat Industry: Opportunities, Innovative Applications and Future Perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef] [PubMed]
- Özalp Özen, B.; Soyer, A. Effect of Plant Extracts on Lipid and Protein Oxidation of Mackerel (Scomber scombrus) Mince during Frozen Storage. J. Food Sci. Technol. 2018, 55, 120–127. [Google Scholar] [CrossRef]
- Jongberg, S.; Tørngren, M.; Skibsted, L. Dose-Dependent Effects of Green Tea or Maté Extracts on Lipid and Protein Oxidation in Brine-Injected Retail-Packed Pork Chops. Medicines 2018, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Delgado, L.H.; González-Mondragón, E.G.; Salazar Govea, A.Y.; Andrade, J.R.; Santiago-Castro, J.T. Potential Application of Epazote (Chenopodium ambrosioides L.) as Natural Antioxidant in Raw Ground Pork. LWT 2017, 84, 306–313. [Google Scholar] [CrossRef]
- Mancini, S.; Preziuso, G.; Dal Bosco, A.; Roscini, V.; Szendrő, Z.; Fratini, F.; Paci, G. Effect of Turmeric Powder (Curcuma longa L.) and Ascorbic Acid on Physical Characteristics and Oxidative Status of Fresh and Stored Rabbit Burgers. Meat Sci. 2015, 110, 93–100. [Google Scholar] [CrossRef]
- De Carvalho, F.A.L.; Munekata, P.E.S.; Lopes De Oliveira, A.; Pateiro, M.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Turmeric (Curcuma longa L.) Extract on Oxidative Stability, Physicochemical and Sensory Properties of Fresh Lamb Sausage with Fat Replacement by Tiger Nut (Cyperus esculentus L.) Oil. Food Res. Int. 2020, 136, 109487. [Google Scholar] [CrossRef]
- Boeira, C.P.; Piovesan, N.; Soquetta, M.B.; Flores, D.C.B.; Lucas, B.N.; Rosa, C.S.D.; Terra, N.N. Extraction of Bioactive Compounds of Lemongrass, Antioxidant Activity and Evaluation of Antimicrobial Activity in Fresh Chicken Sausage. Cienc. Rural 2018, 48, e20180477. [Google Scholar] [CrossRef]
- Dilek, N.M.; Gümrükçüoğlu, A.; Demirel, G.; Durmaz, A.; Torunoğlu, E.I.; Aytar, E.C.; Ünal, K. Antioxidant and Preservative Effects of Epilobium angustifolium Extract in Beef Burgers Products: Physicochemical Properties, Color Stability, Lipid Oxidation, and Molecular Docking Analyses. Food Sci. Nutr. 2025, 13, e70125. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of Pitanga Leaf Extracts on Lipid and Protein Oxidation of Pork Burger during Shelf-Life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef]
- De Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of Guarana (Paullinia cupana) Seed and Pitanga (Eugenia uniflora L.) Leaf Extracts on Lamb Burgers with Fat Replacement by Chia Oil Emulsion during Shelf Life Storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef]
- Chauhan, P.; Das, A.K.; Nanda, P.K.; Kumbhar, V.; Yadav, J.P. Effect of Nigella sativa Seed Extract on Lipid and Protein Oxidation in Raw Ground Pork during Refrigerated Storage. Nutr. Food Sci. 2018, 48, 2–15. [Google Scholar] [CrossRef]
- Al-Hijazeen, M.; Lee, E.; Mendonca, A.; Ahn, D. Effect of Oregano Essential Oil (Origanum vulgare subsp. hirtum) on the Storage Stability and Quality Parameters of Ground Chicken Breast Meat. Antioxidants 2016, 5, 18. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Pan, D.-D.; Cao, J.-X.; Shao, X.-F.; Chen, Y.-J.; Sun, Y.-Y.; Ou, C.-R. Effect of Black Pepper Essential Oil on the Quality of Fresh Pork during Storage. Meat Sci. 2016, 117, 130–136. [Google Scholar] [CrossRef]
- Ramírez-Rojo, M.I.; Vargas-Sánchez, R.D.; Torres-Martínez, B.D.M.; Torrescano-Urrutia, G.R.; Lorenzo, J.M.; Sánchez-Escalante, A. Inclusion of Ethanol Extract of Mesquite Leaves to Enhance the Oxidative Stability of Pork Patties. Foods 2019, 8, 631. [Google Scholar] [CrossRef]
- Soriano, A.; Alañón, M.E.; Alarcón, M.; García-Ruíz, A.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Oak Wood Extracts as Natural Antioxidants to Increase Shelf Life of Raw Pork Patties in Modified Atmosphere Packaging. Food Res. Int. 2018, 111, 524–533. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Fu, S.; Yang, D.; Yu, Y.; Chen, J.; Chen, Y. Effects of Rosemary (Rosmarinus officinalis L.) Extracts and Dry Ice on the Physicochemical Stability of Omega-3 Fatty-acid-fortified Surimi-like Meat Products. J. Sci. Food Agric. 2019, 99, 3843–3851. [Google Scholar] [CrossRef]
- Kahraman, T.; Issa, G.; Bingol, E.B.; Kahraman, B.B.; Dumen, E. Effect of Rosemary Essential Oil and Modified-Atmosphere Packaging (MAP) on Meat Quality and Survival of Pathogens in Poultry Fillets. Braz. J. Microbiol. 2015, 46, 591–599. [Google Scholar] [CrossRef]
- Dumandan, N.G.; Kagaoan, A.C.T.; Acda, R.D.; Tumambing, C.R.; Esteban, M.A.S.; Leonardo, L.P.A.; Lim, L.R.A.; Pham, L.J. Protective Effects of Sugarcane Phenolic Extract against Lipid Oxidative Damages in Raw Ground Pork and Beef during Refrigerated Storage Conditions. Heliyon 2023, 9, e14486. [Google Scholar] [CrossRef]
- Šojić, B.; Ikonić, P.; Pavlić, B.; Zeković, Z.; Tomović, V.; Kocić-Tanackov, S.; Džinić, N.; Škaljac, S.; Ivić, M.; Jokanović, M.; et al. The Effect of Essential Oil from Sage (Salvia officinalis L.) Herbal Dust (Food Industry by-Product) on the Microbiological Stability of Fresh Pork Sausages. LWT—Food Sci. Technol. 2017, 89, 749–755. [Google Scholar] [CrossRef]
- Šojić, B.; Pavlić, B.; Tomović, V.; Ikonić, P.; Zeković, Z.; Kocić-Tanackov, S.; Đurović, S.; Škaljac, S.; Jokanović, M.; Ivić, M. Essential Oil versus Supercritical Fluid Extracts of Winter Savory (Satureja montana L.)—Assessment of the Oxidative, Microbiological and Sensory Quality of Fresh Pork Sausages. Food Chem. 2019, 287, 280–286. [Google Scholar] [CrossRef]
- Zahid, M.A.; Choi, J.Y.; Seo, J.-K.; Parvin, R.; Ko, J.; Yang, H.-S. Effects of Clove Extract on Oxidative Stability and Sensory Attributes in Cooked Beef Patties at Refrigerated Storage. Meat Sci. 2020, 161, 107972. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, X.; Li, X.; Wu, J.; Guo, X. The Application of Clove Extract Protects Chinese-Style Sausages against Oxidation and Quality Deterioration. Korean J. Food Sci. Anim. Resour. 2017, 37, 114–122. [Google Scholar] [CrossRef]
- Šojić, B.; Tomović, V.; Kocić-Tanackov, S.; Kovačević, D.B.; Putnik, P.; Mrkonjić, Ž.; Đurović, S.; Jokanović, M.; Ivić, M.; Škaljac, S.; et al. Supercritical Extracts of Wild Thyme (Thymus serpyllum L.) by-Product as Natural Antioxidants in Ground Pork Patties. LWT 2020, 130, 109661. [Google Scholar] [CrossRef]
- Engy, F.; Zaki, E. Incorporation of Fenugreek Seed Powder in the Manufacturing of Rabbit Sausage and its Effects on the Quality Properties During Frozen Storage. J. Adv. Food Sci. 2018, 5, 8–14. [Google Scholar]
- Amiri, E.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R. Combined Antioxidant and Sensory Effects of Corn Starch Films with Nanoemulsion of Zataria multiflora Essential Oil Fortified with Cinnamaldehyde on Fresh Ground Beef Patties. Meat Sci. 2019, 153, 66–74. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M. Manipulating the Fatty Acid Composition of Meat to Improve Nutritional Value and Meat Quality. In New Aspects of Meat Quality; Elsevier: Amsterdam, The Netherlands, 2017; pp. 501–535. ISBN 978-0-08-100593-4. [Google Scholar]
- Montalvo-Puente, A.P.; Torres-Gallo, R.; Acevedo-Correa, D.; Montero-Castillo, P.M.; Tirado, D.F. Nutritional Comparison of Beef, Pork and Chicken Meat from Maracaibo City (Venezuela). Adv. J. Food Sci. Technol. 2018, 15, 218–224. [Google Scholar] [CrossRef]
- Dinh, T.T.N.; To, K.V.; Schilling, M.W. Fatty Acid Composition of Meat Animals as Flavor Precursors. Meat Muscle Biol. 2021, 5, 1–16. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Namal Senanayake, S.P.J. Green Tea Extract: Chemistry, Antioxidant Properties and Food Applications—A Review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Food and Feed Information Portal Database|FIP. Available online: https://ec.europa.eu/food/food-feed-portal/screen/food-additives/search/details/POL-FAD-IMPORT-2985 (accessed on 21 October 2024).
- Eremeeva, N.B.; Makarova, N.V. Antioxidant Properties of Medicinal Plants and Their Effect on Microbial Spoilage of Semi-Finished Meat, Poultry and Fish. Izv. Vuzov. Prikl. Khim. Biotekhnol. 2022, 11, 590–602. [Google Scholar] [CrossRef]
- Meshaal, A.K.; Hetta, H.F.; Yahia, R.; Abualnaja, K.M.; Mansour, A.T.; Al-Kadmy, I.M.S.; Alghamdi, S.; Dablool, A.S.; Emran, T.B.; Sedky, H.; et al. In Vitro Antimicrobial Activity of Medicinal Plant Extracts against Some Bacterial Pathogens Isolated from Raw and Processed Meat. Life 2021, 11, 1178. [Google Scholar] [CrossRef]
- Abid, M.M.; Hussain, T.K.; Hwaidi, E.H.; Kadhim, H.I. Effect of Aromatic Oil of Three Plants of Labiatae Gene on Microorganism by Using in Preservation of Minced Meat. J. Pharm. Negat. Results 2022, 13, 686–689. [Google Scholar] [CrossRef]
- Al-Moghazy, M.; Ammar, M.S.; Sief, M.M.; Mohamed, S.R. Evaluation the Antimicrobial Activity of Artemisia and Portulaca Plant Extracts in Beef Burger. Evaluation 2017, 1, 31–42. [Google Scholar] [CrossRef]
- Manilal, A.; Sabu, K.R.; Woldemariam, M.; Aklilu, A.; Biresaw, G.; Yohanes, T.; Seid, M.; Merdekios, B. Antibacterial Activity of Rosmarinus officinalis against Multidrug-Resistant Clinical Isolates and Meat-Borne Pathogens. Evid.-Based Complement. Altern. Med. 2021, 2021, 6677420. [Google Scholar] [CrossRef]
- Elmaadawy, A.A. Antimicrobial activity of some plant extracts using food model media. Maj. Dirasat Buḥuth Tarbia Naw‘ia. 2022, 8, 3–21. [Google Scholar] [CrossRef]
- Rastgou, M.; Rezaee Danesh, Y.; Ercisli, S.; Sayyed, R.Z.; El Enshasy, H.A.; Dailin, D.J.; Alfarraj, S.; Ansari, M.J. The Effect of Some Wild Grown Plant Extracts and Essential Oils on Pectobacterium betavasculorum: The Causative Agent of Bacterial Soft Rot and Vascular Wilt of Sugar Beet. Plants 2022, 11, 1155. [Google Scholar] [CrossRef]
- Gyawali, R.; Hayek, S.A.; Ibrahim, S.A. Plant Extracts as Antimicrobials in Food Products. In Handbook of Natural Antimicrobials for Food Safety and Quality; Elsevier: Amsterdam, The Netherlands, 2015; pp. 49–68. ISBN 978-1-78242-034-7. [Google Scholar]
- Hosseini, M.; Jamshidi, A.; Raeisi, M.; Azizzadeh, M. Effect of Sodium Alginate Coating Containing Clove (Syzygium aromaticum) and Lemon Verbena (Aloysia citriodora) Essential Oils and Different Packaging Treatments on Shelf Life Extension of Refrigerated Chicken Breast. J. Food Process. Preserv. 2021, 45, e14946. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Bher, A.; Mulla, M.; Jacob, H.; Auras, R. Active Chicken Meat Packaging Based on Polylactide Films and Bimetallic Ag–Cu Nanoparticles and Essential Oil. J. Food Sci. 2018, 83, 1299–1310. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; McClements, D.J.; Hamishehkar, H. Multifunctional Halochromic Packaging Materials: Saffron Petal Anthocyanin Loaded-Chitosan Nanofiber/Methyl Cellulose Matrices. Food Hydrocoll. 2021, 111, 106237. [Google Scholar] [CrossRef]
- Fatimeh, S.; Gholamreza, J.K.; Nabi, S. Chitosan/Cumin (Cuminum cyminum L.) Essential Oil Edible biodegradable Coating: Its Effect on Microbial, Physicaland Sensory Properties of Chicken Meat During referigeration. Carpathian J. Food Sci. Technol. 2021, 13, 75–89. [Google Scholar] [CrossRef]
- Yang, H.-J.; Song, K.B. Application of Lemongrass Oil-Containing Polylactic Acid Films to the Packaging of Pork Sausages. Korean J. Food Sci. Anim. Resour. 2016, 36, 421–426. [Google Scholar] [CrossRef][Green Version]
- Cui, H.; Wu, J.; Li, C.; Lin, L. Promoting Anti-listeria Activity of Lemongrass Oil on Pork Loin by Cold Nitrogen Plasma Assist. J. Food Saf. 2017, 37, e12316. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional Chitosan-Based Coating with Liposomes Containing Laurel Essential Oils and Nanosilver for Pork Preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef]
- Konuk Takma, D.; Korel, F. Active Packaging Films as a Carrier of Black Cumin Essential Oil: Development and Effect on Quality and Shelf-Life of Chicken Breast Meat. Food Packag. Shelf Life 2019, 19, 210–217. [Google Scholar] [CrossRef]
- Moraes-Lovison, M.; Marostegan, L.F.P.; Peres, M.S.; Menezes, I.F.; Ghiraldi, M.; Rodrigues, R.A.F.; Fernandes, A.M.; Pinho, S.C. Nanoemulsions Encapsulating Oregano Essential Oil: Production, Stability, Antibacterial Activity and Incorporation in Chicken Pâté. LWT 2017, 77, 233–240. [Google Scholar] [CrossRef]
- Šojić, B.; Tomović, V.; Pavlić, B.; Ikonić, P.; Škaljac, S.; Jokanović, M.; Ivić, M. The Effect of Winter Savory (Satureja montana L.) Extract on the Quality of Cooked Pork Sausages. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 333, p. 012103. [Google Scholar] [CrossRef]
- Ruiz, A.; Williams, S.K.; Djeri, N.; Hinton, A.; Rodrick, G.E. Nisin, Rosemary, and Ethylenediaminetetraacetic Acid Affect the Growth of Listeria Monocytogenes on Ready-to-Eat Turkey Ham Stored at Four Degrees Celsius for Sixty-Three Days. Poult. Sci. 2009, 88, 1765–1772. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-Friendly Active Packaging Consisting of Nanostructured Biopolymer Matrix Reinforced with TiO2 and Essential Oil: Application for Preservation of Refrigerated Meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Sirocchi, V.; Devlieghere, F.; Peelman, N.; Sagratini, G.; Maggi, F.; Vittori, S.; Ragaert, P. Effect of Rosmarinus officinalis L. Essential Oil Combined with Different Packaging Conditions to Extend the Shelf Life of Refrigerated Beef Meat. Food Chem. 2017, 221, 1069–1076. [Google Scholar] [CrossRef]
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus officinalis Essential Oils in Chitosan-Benzoic Acid Nanogel with Enhanced Antibacterial Activity in Beef Cutlet against Salmonella typhimurium during Refrigerated Storage. LWT 2017, 84, 394–401. [Google Scholar] [CrossRef]
- Danilović, B.; Đorđević, N.; Milićević, B.; Šojić, B.; Pavlić, B.; Tomović, V.; Savić, D. Application of Sage Herbal Dust Essential Oils and Supercritical Fluid Extract for the Growth Control of Escherichia coli in Minced Pork during Storage. LWT 2021, 141, 110935. [Google Scholar] [CrossRef]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of Chitosan Coatings Incorporating with Free or Nano-Encapsulated Satureja Plant Essential Oil on Quality Characteristics of Lamb Meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- El Abed, N.; Kaabi, B.; Smaali, M.I.; Chabbouh, M.; Habibi, K.; Mejri, M.; Marzouki, M.N.; Ben Hadj Ahmed, S. Chemical Composition, Antioxidant and Antimicrobial Activities of Thymus capitata Essential Oil with Its Preservative Effect against Listeria monocytogenes Inoculated in Minced Beef Meat. Evid.-Based Complement. Altern. Med. 2014, 2014, 152487. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-Starch Film Containing Pomegranate Peel Extract and Thymus kotschyanus Essential Oil Can Prolong the Shelf Life of Beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef]
- Boskovic, M.; Djordjevic, J.; Ivanovic, J.; Janjic, J.; Zdravkovic, N.; Glisic, M.; Glamoclija, N.; Baltic, B.; Djordjevic, V.; Baltic, M. Inhibition of Salmonella by Thyme Essential Oil and Its Effect on Microbiological and Sensory Properties of Minced Pork Meat Packaged under Vacuum and Modified Atmosphere. Int. J. Food Microbiol. 2017, 258, 58–67. [Google Scholar] [CrossRef]
- Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barberá, E. Antimicrobial Active Packaging Including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat. Foods 2016, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Azarifar, M.; Ghanbarzadeh, B.; Sowti Khiabani, M.; Akhondzadeh Basti, A.; Abdulkhani, A. The Effects of Gelatin-CMC Films Incorporated with Chitin Nanofiber and Trachyspermum ammi Essential Oil on the Shelf Life Characteristics of Refrigerated Raw Beef. Int. J. Food Microbiol. 2020, 318, 108493. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Movlan, H.S.; Yaghoubi, M.; Pateiro, M.; Lorenzo, J.M. Ɛ-Polylysine Coating with Stinging Nettle Extract for Fresh Beef Preservation. Meat Sci. 2021, 176, 108474. [Google Scholar] [CrossRef]
- Raeisi, M.; Hashemi, M.; Sadeghi, A.R.; Aminzare, M.; Khodadadi, M.; Ahmadzadeh, A.M.; Afshari, A. Salmonella typhimurium and Listeria monocytogenes Growth Inhibition by Zataria multiflora Essential Oil in Ground Meat. J. Hum. Environ. Health Promot. 2017, 2, 261–269. [Google Scholar] [CrossRef][Green Version]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Suriyaprom, S.; Mosoni, P.; Leroy, S.; Kaewkod, T.; Desvaux, M.; Tragoolpua, Y. Antioxidants of Fruit Extracts as Antimicrobial Agents against Pathogenic Bacteria. Antioxidants 2022, 11, 602. [Google Scholar] [CrossRef]
- Rehman Nengroo, Z.; Rauf, A.; Danish, M.; Sharief Dar, M. Evaluation of Fatty Acid Composition and Antimicrobial Activity of Eight Medicinal Plants from Kashmir. Orient. J. Chem. 2020, 36, 44–53. [Google Scholar] [CrossRef]
- Albakry, Z.; Karrar, E.; Ahmed, I.A.M.; Oz, E.; Proestos, C.; El Sheikha, A.F.; Oz, F.; Wu, G.; Wang, X. Nutritional Composition and Volatile Compounds of Black Cumin (Nigella sativa L.) Seed, Fatty Acid Composition and Tocopherols, Polyphenols, and Antioxidant Activity of Its Essential Oil. Horticulturae 2022, 8, 575. [Google Scholar] [CrossRef]
- Türkmen, M.; Koçer, O. Variation of Components in Laurel (Laurus nobilis L.) Fixed Oil Extracted by Different Methods. Int. J. Chem. Technol. 2021, 5, 167–171. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Khider, M.; Abulreesh, H.H.; Assiri, A.M.A.; Elsanhoty, R.M.; Assaeedi, A.; Elbanna, K. Cold Pressed Rosemary (Rosmarinus officinalis) Oil. In Cold Pressed Oils; Elsevier: Amsterdam, The Netherlands, 2020; pp. 683–694. ISBN 978-0-12-818188-1. [Google Scholar]
- Rashid, H.M.; Mahmod, A.I.; Afifi, F.U.; Talib, W.H. Antioxidant and Antiproliferation Activities of Lemon Verbena (Aloysia citrodora): An In Vitro and In Vivo Study. Plants 2022, 11, 785. [Google Scholar] [CrossRef]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and Pharmacological Importance of Stinging Nettle (Urtica dioica L.): A Review. Heliyon 2022, 8, e09717. [Google Scholar] [CrossRef]
- Pathak, R.; Sharma, H. A Review on Medicinal Uses of Cinnamomum verum (Cinnamon). J. Drug Deliv. Ther. 2021, 11, 161–166. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Ivanauskas, L.; Bezruk, I.; Sidorenko, L.; Lesyk, R.; Georgiyants, V. Characterization of Phytochemical Components of Crocus sativus Leaves: A New Attractive By-Product. Sci. Pharm. 2021, 89, 28. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.-M.; Elahi, F.; Chelliah, R.; Lee, B.-H.; Oh, D.-H. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Front. Sustain. Food Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
- Wu, X.; Dong, S.; Chen, H.; Guo, M.; Sun, Z.; Luo, H. Perilla frutescens: A Traditional Medicine and Food Homologous Plant. Chin. Herb. Med. 2023, 15, 369–375. [Google Scholar] [CrossRef]
- Huang, P.; Wang, Z.; Shi, Y.; Zhang, R.; Feng, X.; Kan, J. Deodorizing Effects of Rosemary Extract on Silver Carp (Hypophthalmichthys molitrix) and Determination of Its Deodorizing Components. J. Food Sci. 2022, 87, 636–650. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Bañón, S. Use of Dietary Rosemary Diterpenes to Inhibit Rancid Volatiles in Lamb Meat Packed under Protective Atmosphere. Animal 2016, 10, 1391–1401. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, B.; Zhong, F.; Chen, J.; Zhang, T. Effect of Microbial Fermentation on the Fishy-Odor Compounds in Kelp (Laminaria japonica). Foods 2021, 10, 2532. [Google Scholar] [CrossRef]
- Soto-Simenta, S.; Caro, I.; Mateo, J.; Quinto, E.J. Effect of Cooking Lamb Using Maguey Leaves (Agave salmiana) on Meat Volatile Composition. Available online: https://www.researchgate.net/publication/302584181_Effect_of_cooking_lamb_using_maguey_leaves_Agave_salmiana_on_meat_volatile_composition (accessed on 4 April 2024).
- Dias, P.C.G.; Ferreira, E.M.M.; Santos, I.J.; Carlis, M.; Vicente, A.C.S.; Silva, A.S.A.; Assis, R.G.; Comelli, J.H.; Biava, J.S.S.; Soares, L.C.B.; et al. PSVI-16 The Essential Oil of Arnica Montana Positively Modulates the Fatty Acid Profile of Lamb Meat. J. Anim. Sci. 2022, 100, 403–404. [Google Scholar] [CrossRef]
- Purnamayanti, L.; Jamhari, J.; Hanim, C.; Irawan, A. Physicochemical Properties, Oxidative Stability, and Sensory Quality of Lamb Sausage Added with Green Tea Leaves (Camelia sinensis) Powder. Trop. Anim. Sci. J. 2020, 43, 57–63. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Wafa, M.M. The Antibacterial Activity of Laurus nobilis Leaf Extract and Its Potential Use as a Preservative for Fresh Lamb Meat. Afr. J. Microbiol. Res. 2020, 14, 617–624. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Ros, G.; Nieto, G. Synthetic vs. Natural Hydroxytyrosol for Clean Label Lamb Burgers. Antioxidants 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, H.; Saad, S.M.; Sabeq, I.I.; Hamad, A.; Edris, S.N. Impact of Cold Pressed Cardamom Oil (Elettaria cardamomum) on The Sensory Attributes and Color Stability of Frozen Chicken Burger. Benha J. Appl. Sci. 2025, 10, 7–13. [Google Scholar]
- Šojić, B.; Tomović, V.; Savanović, J.; Kocić-Tanackov, S.; Pavlić, B.; Jokanović, M.; Milidrag, A.; Martinović, A.; Vujadinović, D.; Vukić, M. Sage (Salvia officinalis L.) Essential Oil as a Potential Replacement for Sodium Nitrite in Dry Fermented Sausages. Processes 2021, 9, 424. [Google Scholar] [CrossRef]
- Sarewicz, M.; Pintscher, S.; Pietras, R.; Borek, A.; Bujnowicz, Ł.; Hanke, G.; Cramer, W.A.; Finazzi, G.; Osyczka, A. Catalytic Reactions and Energy Conservation in the Cytochrome bc1 and b6f Complexes of Energy-Transducing Membranes. Chem. Rev. 2021, 121, 2020–2108. [Google Scholar] [CrossRef]
- Demir, T.; Ağaoğlu, S. Antioxidant, Antimicrobial and Metmyoglobin Reducing Activity of Artichoke (Cynara scolymus) Powder Extract-Added Minced Meat during Frozen Storage. Molecules 2021, 26, 5494. [Google Scholar] [CrossRef]
- Palanisamy, S.; Singh, A.; Zhang, B.; Zhao, Q.; Benjakul, S. Effects of Different Phenolic Compounds on the Redox State of Myoglobin and Prevention of Discoloration, Lipid and Protein Oxidation of Refrigerated Longtail Tuna (Thunnus tonggol) Slices. Foods 2024, 13, 1238. [Google Scholar] [CrossRef]
- Zinchenko, R.; Slyva, Y. Analysis of the Use of Plant Components in the Production of Meat Products. Anim. Sci. Food Technol. 2022, 13, 19–29. [Google Scholar] [CrossRef]
- Saeed, F.; Mehjabeen; Jahan, N.; Ahmad, M. In Vivo Evaluation & Safety Profile Evaluation of Arctostaphylos uva-ursi (L.) Spreng. Extract in Rabbits. Pak. J. Pharm. Sci. 2014, 27, 2197–2205. [Google Scholar] [PubMed]
- Lamien-Meda, A.; Lukas, B.; Schmiderer, C.; Franz, C.; Novak, J. Validation of a Quantitative Assay of Arbutin Using Gas Chromatography in Origanum majorana and Arctostaphylos uva-ursi Extracts. Phytochem. Anal. 2009, 20, 416–420. [Google Scholar] [CrossRef]
- Tocai (Moţoc), A.C.; Kokeric, T.; Tripon, S.; Barbu-Tudoran, L.; Barjaktarevic, A.; Cupara, S.; Vicas, S.I. Sanguisorba minor Scop.: An Overview of Its Phytochemistry and Biological Effects. Plants 2023, 12, 2128. [Google Scholar] [CrossRef]
- Tocai (Moţoc), A.-C.; Ranga, F.; Teodorescu, A.G.; Pallag, A.; Vlad, A.M.; Bandici, L.; Vicas, S.I. Evaluation of Polyphenolic Composition and Antimicrobial Properties of Sanguisorba officinalis L. and Sanguisorba minor Scop. Plants 2022, 11, 3561. [Google Scholar] [CrossRef] [PubMed]
- Erener, G.; Turan, C.; Gungor, E.; Altop, A. Effect of Chamomile (Matricaria chamomilla L.), Linden (Tilia cordata Mill.), and Green Tea (Camellia sinensis L.) Aqueous Extract Administration in the Drinking Water during Pre-Slaughter Feed Withdrawal Period in Broiler Chickens. Trop. Anim. Health Prod. 2023, 55, 252. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Ugurlu, A.; Zengin, G.; Baloglu, M.C.; Altunoglu, Y.C.; Mollica, A.; Custodio, L.; Neng, N.R.; Nogueira, J.M.F.; Mahomoodally, M.F. Novel in Vitro and in Silico Insights of the Multi-Biological Activities and Chemical Composition of Bidens tripartita L. Food Chem. Toxicol. 2018, 111, 525–536. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez-Salazar, J.A.; Jaime-Patlán, M.; Sosa-Morales, M.E.; Lorenzo, J.M. Plant Extracts Obtained with Green Solvents as Natural Antioxidants in Fresh Meat Products. Antioxidants 2021, 10, 181. [Google Scholar] [CrossRef]
- Khadijah, H.A.H.; Nurul, A.M.A.; Shalyda, S. Alchemilla vulgaris and Filipendula ulmaria Extracts as Potential Natural Preservatives in Beef Patties. MAJS 2017, 21, 986–995. [Google Scholar] [CrossRef][Green Version]
- Mantzourani, I.; Daoutidou, M.; Dasenaki, M.; Nikolaou, A.; Alexopoulos, A.; Terpou, A.; Thomaidis, N.; Plessas, S. Plant Extract and Essential Oil Application against Food-Borne Pathogens in Raw Pork Meat. Foods 2022, 11, 861. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Song, Z.; Kerry, J.P.; O’Sullivan, M.G.; Hamill, R.M. Addressing Clean Label Trends in Commercial Meat Processing: Strategies, Challenges and Insights from Consumer Perspectives. Foods 2023, 12, 2062. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making Sense of the “Clean Label” Trends: A Review of Consumer Food Choice Behavior and Discussion of Industry Implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, R.; Tian, Y.; Xu, S.; Meng, X. Curcumin Nanopreparations: Recent Advance in Preparation and Application. Biomed. Mater. 2024, 19, 052009. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, L.; Yang, Y.; Su, Y.; Chang, C.; Gu, L.; Yu, H. A Comprehensive Review in the Formulation of Nanocurcumin and Its Potential Application in Functional Foods. Food Rev. Int. 2024, 40, 3487–3507. [Google Scholar] [CrossRef]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of Curcumin-Loaded Nanocarriers for Food, Drug and Cosmetic Purposes. Trends Food Sci. Technol. 2019, 88, 445–458. [Google Scholar] [CrossRef]
- Gadapa, S.; Battula, S.N.; Pushpadass, H.A.; Naik, L.N.; Emerald, M.E. Optimization of Green Tea Catechin Loaded Niosomes by Thin Film Hydration Technique Using Food Grade Surfactants. J. Sci. Res. Rep. 2024, 30, 177–187. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, D.; Kumari, A.; Yadav, S.K. Encapsulation of Catechin and Epicatechin on BSA NPS Improved Their Stability and Antioxidant Potential. EXCLI J. 2014, 13, 331–346. [Google Scholar]
- Ojeda-Piedra, S.A.; Zambrano-Zaragoza, M.L.; González-Reza, R.M.; García-Betanzos, C.I.; Real-Sandoval, S.A.; Quintanar-Guerrero, D. Nano-Encapsulated Essential Oils as a Preservation Strategy for Meat and Meat Products Storage. Molecules 2022, 27, 8187. [Google Scholar] [CrossRef]
- Tsironi, M.; Kosma, I.S.; Badeka, A.V. The Effect of Whey Protein Films with Ginger and Rosemary Essential Oils on Microbiological Quality and Physicochemical Properties of Minced Lamb Meat. Sustainability 2022, 14, 3434. [Google Scholar] [CrossRef]
- Bolumar, T.; Orlien, V.; Sikes, A.; Aganovic, K.; Bak, K.H.; Guyon, C.; Stübler, A.; De Lamballerie, M.; Hertel, C.; Brüggemann, D.A. High-pressure Processing of Meat: Molecular Impacts and Industrial Applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 332–368. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical Constituents, Advanced Extraction Technologies and Techno-Functional Properties of Selected Mediterranean Plants for Use in Meat Products. A Comprehensive Review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Ahmad, N.H.; Yaacob, J.S.; Lim, S.A.H.; Rahim, M.H.A. Food processing to reduce antinutrients in plant-based foods. Int. Food. Res. J. 2023, 30, 25–45. [Google Scholar] [CrossRef]
- Anjali; Garg, D.; Pragi; Kumar, V.; Dimple; Rohilla, M. A Review of Therapeutic Properties and Uses of Salvia officinalis: Review Article. J. Pharma Insights Res. 2024, 2, 146–154. [Google Scholar] [CrossRef]
- Ilie, C.; Golet, I.; Craciunescu, M.; Hogea, E.; Popescu, R.; Horhat, F. Chemical Composition and Antimicrobial Activity of Essential Oil of Western Romanian Salvia officinalis. Rev. Chim. 2016, 67, 131–133. [Google Scholar]
- Regulation-1334/2008-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2008/1334/oj/eng (accessed on 25 August 2025).
- Oniga, I.; Oprean, R.; Toiu, A.; Benedec, D. Chemical Composition of the Essential Oil of Salvia officinalis L. from Romania. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2010, 114, 593–595. [Google Scholar]
- Bedrood, Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicological Effects of Camellia sinensis (Green Tea): A Review. Phytother. Res. 2018, 32, 1163–1180. [Google Scholar] [CrossRef]
- Girolametti, F.; Annibaldi, A.; Illuminati, S.; Damiani, E.; Carloni, P.; Truzzi, C. Essential and Potentially Toxic Elements (PTEs) Content in European Tea (Camellia sinensis) Leaves: Risk Assessment for Consumers. Molecules 2023, 28, 3802. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific Opinion on the Safety of Green Tea Catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef]
- Elmusa, F.; Elmusa, M. Mini-Review on Coumarins: Sources, Biosynthesis, Bioactivity, Extraction and Toxicology. J. Turk. Chem. Soc. Sect. A Chem. 2024, 11, 933–944. [Google Scholar] [CrossRef]
- Coumarin in Flavourings and Other Food Ingredients with Flavouring Properties—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC)|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/793 (accessed on 25 August 2025).
- Curcumin—61st Joint FAO/WHO Expert Committee on Food Additives (JECFA) Meeting—Chemical and Technical Assessment (CTA). 2003. Available online: https://openknowledge.fao.org/items/25fb60b8-3481-4a7e-ba05-a611af0bf17f (accessed on 25 August 2025).
- Bend, J.; Hattan, D.G.; Kawamura, Y.; Knaap, A.G.A.C.; Kuznesof, P.M.; Larsen, J.C.; Meyland, I.; Rao, M.V.; Schlatter, J.; Toledo, M.C.; et al. WHO Technical Report Series: Evaluation of Certain Food Additives: Eugenol. World Health Organ.-Tech. Rep. Ser. 2005, 928, 1–156. [Google Scholar]
- Aurich, S.; Spiric, J.; Engin, A.; Simon, J.; Mahler, V.; Treudler, R. Report of a Case of IgE-Mediated Anaphylaxis to Fenugreek. J. Investig. Allergol. Clin. Immunol. 2019, 29, 56–58. [Google Scholar] [CrossRef]
- Joseph, N.I.; Slavin, E.; Peppers, B.P.; Hostoffer, R.W. Fenugreek Anaphylaxis in a Pediatric Patient. Allergy Rhinol. 2018, 9, 2152656718764134. [Google Scholar] [CrossRef] [PubMed]
- Alessandrello, C.; Sanfilippo, S.; Gangemi, S.; Pioggia, G.; Minciullo, P.L. Fenugreek: New Therapeutic Resource or Emerging Allergen? Appl. Sci. 2024, 14, 9195. [Google Scholar] [CrossRef]
- Seiller, H.; Kurihara, F.; Chasset, F.; Soria, A.; Barbaud, A. Tert-butylhydroquinone Is a Marker for Sensitivity to Nigella sativa Oil Allergy: Five New Cases. Contact Dermat. 2021, 84, 447–449. [Google Scholar] [CrossRef]
- Perić, A.; Sotirović, J.; Jovančević, L.; Medić, S.P. Occupational Rhinitis Caused by Hypersensitivity to Black Pepper. Occup. Med. 2023, 73, 167–169. [Google Scholar] [CrossRef]
- Jung, S.K.; Choi, D.W.; Jung, C.H.; Kim, Y.-J.; Jung, S.Y.; Shon, D.-H. Piper nigrum Fruit Extract Prevents TMA-Induced Allergic Contact Dermatitis by Regulating Th2 Cytokine Production. J. Agric. Sci. 2015, 7, 135–146. [Google Scholar] [CrossRef]
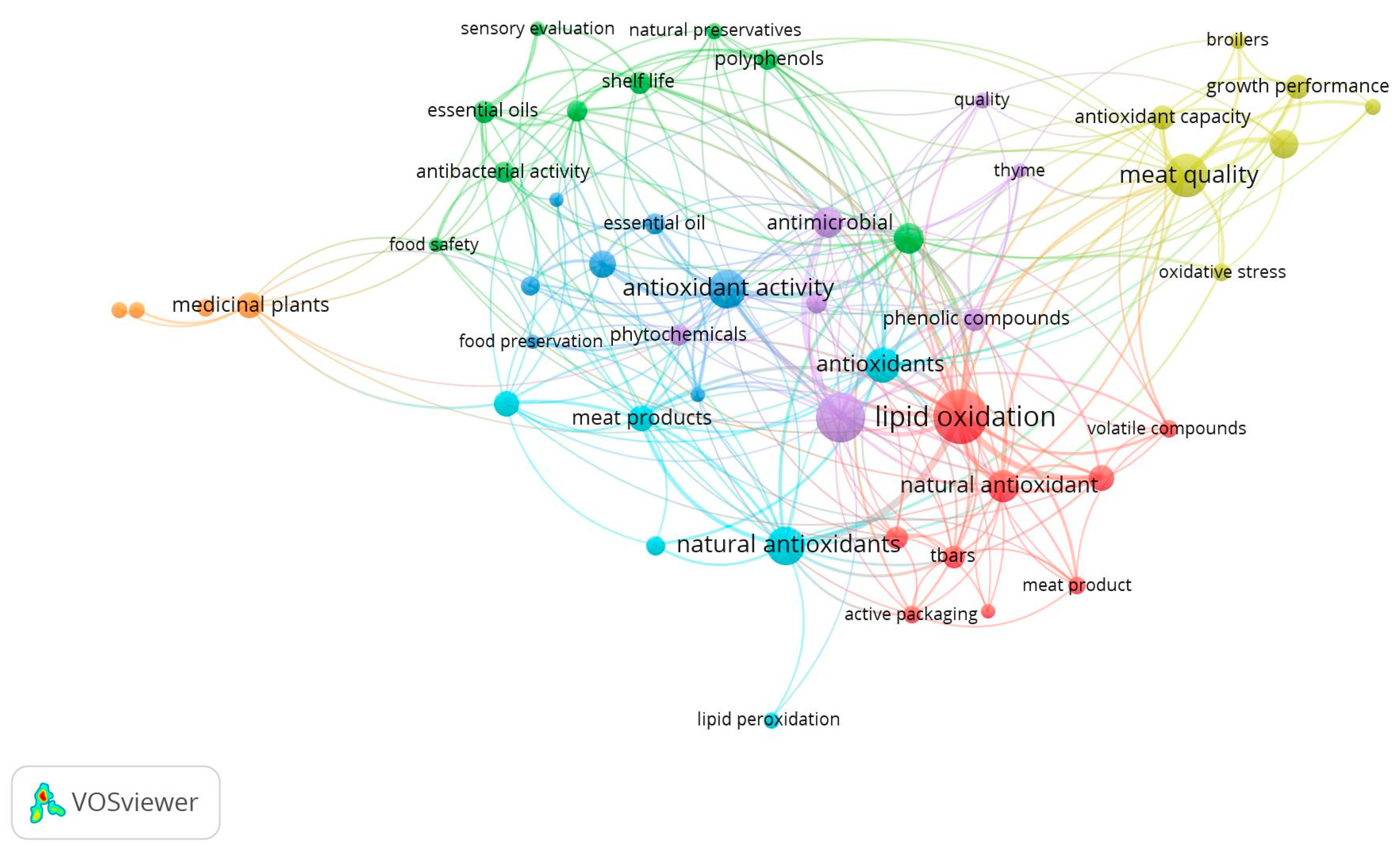
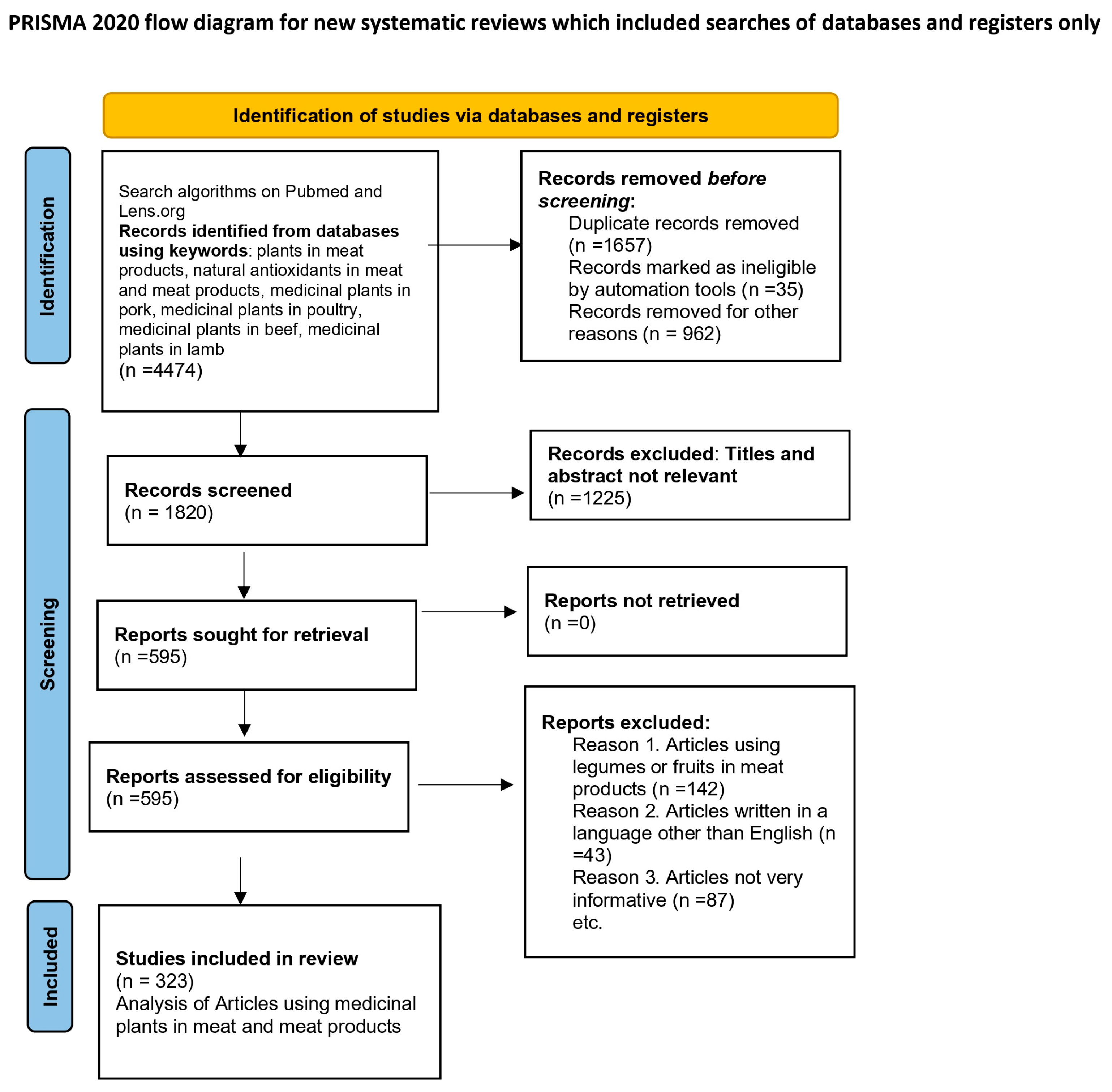
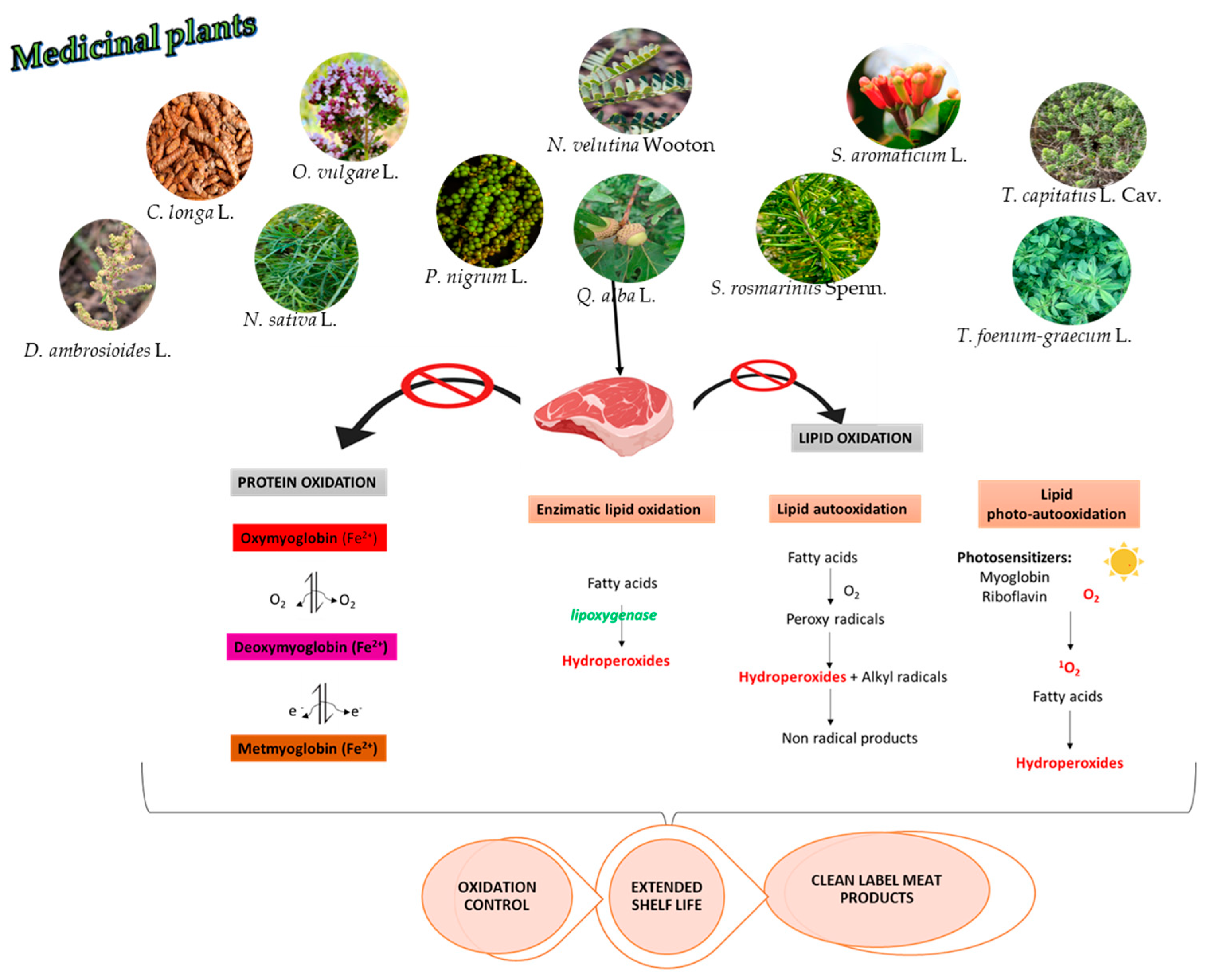
| Plants Family | Years of Publications | No. of Publications |
|---|---|---|
| Amaranthaceae | 2016, 2017, 2021–2022 | 7 |
| Apiaceae | 2004, 2011–2012, 2014–2015, 2018, 2020–2021 | 20 |
| Aquifoliaceae | 2010, 2011, 2019, 2021–2022 | 9 |
| Fabaceae | 2011, 2015, 2017, 2018, 2021 | 11 |
| Fagaceae | 2015, 2018–2023 | 10 |
| Iridaceae | 2010, 2014, 2018, 2020–2023 | 8 |
| Lamiaceae | 2000, 2003–2004, 2007, 2009, 2011, 2013–2014, 2016–2025 | 99 |
| Lauraceae | 2012, 2014, 2017–2023 | 21 |
| Myrtaceae | 2011, 2017, 2018–2024 | 17 |
| Onagraceae | 2025 | 1 |
| Piperaceae | 2014, 2016, 2019, 2021, 2024 | 6 |
| Poaceae | 2006, 2007, 2011, 2015, 2019, 2021–2024 | 14 |
| Ranunculaceae | 2001, 2007–2008, 2013, 2014, 2018–2019 | 9 |
| Theaceae | 2014, 2018, 2022 | 8 |
| Urticaceae | 2017, 2021–2024 | 8 |
| Verbenaceae | 2014, 2017, 2019, 2021–2022, 2024 | 7 |
| Zingerberaceae | 2009, 2015, 2018–2023 | 25 |
| TOTAL | 280 |
| Keyword | Appearances | Cluster/Category |
|---|---|---|
| Lipid oxidation | 70 | /Red bubbles (lipid oxidation and chemical compounds) |
| Antioxidant | 57 | /Blue bubbles (antioxidant activity or natural antioxidants) |
| Meat quality | 44 | /Green and yellow bubbles (meat quality) |
| Antioxidant activity | 36 | /Blue bubbles (antioxidant activity or natural antioxidants) |
| Natural antioxidant | 35 | /Blue bubbles (antioxidant activity or natural antioxidants) |
| Meat | 23 | /Green and yellow bubbles (meat quality) |
| Medicinal plants | 15 | /Orange bubbles (medicinal plants) |
| Family | Scientific/Common Name | Photos | Phytochemical Composition | Biological Properties |
|---|---|---|---|---|
| Verbenaceae | Aloysia citrodora Paláu/lemon verbena |  | Flavones: luteolin 7-diglucuronide, apigenin, scutellarein, and pedalitin; Flavonols: kaempferol; Phenylethanoid glycosides: verbascoside; Essential oils: geranial, neral, α-curcumene, spathulenol, and caryophyllene oxide [27,28,29]. | Antibacterial, antiviral, antioxidant, anticancer, anti-inflammatory, insecticidal, and immunomodulatory properties [27,30,31]. |
| Theaceae | Camellia sinensis (L.) Kuntze/tea plant |  | Flavones: myricetin glycosides; Flavonols: quercetin glycosides, camelliquercetiside, quercetin-3-O-β-D- glucopyranoside, and rutin; Flavanols: epigallocatechin 3-O-gallate, epicatechin, and catechin; Triterpenoid saponins: teasperol and teasperin; Alkaloids: caffeine, theophylline, and theobromine; Nutrients: calcium, magnesium, iron, and manganese; Other: L-theanine [32,33,34]. | Antioxidant, antibacterial, antiviral, anticancer, antidiabetic, and neuroprotective effects [33,35]. |
| Amaranthaceae | Dysphania ambrosioides (L.) Mosyakin & Clemants/Jerusalem tea |  | Flavonols: quercetin 3-O-rutinoside, quercetin 3-O-glucoside, quercetin O-rhamnosyl-glucoside, and kaempferol 3-O-rutinoside; Phenolic acids: p-coumaric acid and ferulic acid; Nutrients: potassium, calcium, magnesium, iron, fructose, glucose, sucrose, malic, ascorbic, citric, and fumaric acids [36,37,38]. | Antioxidant, anti-inflammatory, antipyretic, analgesic, antidiabetic, antimalarial, antipyretic, and insecticidal effects [39,40]. |
| Lauraceae | Cinnamomum verum J.Presl/Ceylon cinnamon tree |  | Flavanols: proanthocyanidins A and B and catechin; Flavonols: kaempferol and quercetin; Phenolic acids: vanillic acid, caffeic acid, gallic acid, p-coumaric acid, ferulic acid, and chlorogenic acid; Essential oils: linalool and (E)-cinnamyl acetate β-caryophyllene; Nutrients: cinnamic acid, vitamin A, vitamin C, thiamin, riboflavin, vitamin B6, calcium, magnesium, and iron [41,42]. | Antibacterial, antifungal, antioxidant, anti-inflammatory, antidiabetic, and anticancer activities [43,44,45]. |
| Iridaceae | Crocus sativus L./saffron crocus |  | Flavonols: astragalin, kaempferol-3- glycopyranosyl (1-2)-6 acetylglucopyranoside, kaempferol-3-glucopyranosyl (1-2)-glucopyranoside, myricetin, and quercetin; Anthocyanins: cyanidin-3-glucoside, delphinidin, and petunidin; Carotenoids: crocin and crocetin; Essential oils: safranal and picrocrocin; Nutrients: riboflavin, thiamine, potassium, manganese, magnesium, zinc, and sodium [46,47,48]. | Antioxidant, anti-inflammatory, anticancer, and antidepressant functions [49,50,51]. |
| Apiaceae | Cuminum cyminum L./cumin |  | Flavones: apigenin, luteolin, amentoflavone, and 5,7-dihydroxy-3,4-dimethoxyflavone; Flavonols: kaempferol and quercetin; Phenolic acids: caffeic acid, ferulic acid and p-coumaric acid, and protocatechuic acid; Essential oils: cuminaldehyde, cymene, terpenoids, p-menthal,3-dien-7-al, p-mentha-l,4-dien-7-al, α-terpinene, p-cymene, and β-pinene; Nutrients: calcium, iron, magnesium, and phosphorus, and niacin [52,53]. | Antimicrobial, diuretic, antihypertensive, antidiabetic, anticancer, immune-modulatory, anthelmintic, analgesic, anti-inflammatory, spasmolytic, bronchodilator, gastroprotective, hepatoprotective, and renal-protective properties [54,55,56]. |
| Zingiberaceae | Curcuma longa L./turmeric |  | Flavones: luteolin; Flavonols: myricetin, quercetin, and kaempferol; Curcuminoids: curcumin, demethoxycurcumin, and bisdemethoxycurcumin; Essential oils: α-turmerone, curlone, γ-turmerone, β-sesquiphellandrene, and the monoterpenes β-pinene, and para-cymene; Nutrients: sodium, iron, magnesium, calcium, and vitamins C and E [57,58]. | Antibacterial, antioxidant, anti-inflammatory, anticarcinogenic, antidiabetic, and wound-healing activities [59,60]. |
| Poaceae | Cymbopogon citratus (DC.) Stapf/lemongrass |  | Flavones: isoorientin 2’-O-rhamnoside, luteolin, and apigenin; Flavonols: quercetin and kaempferol; Phenolic acids: caffeoylquinic acid and chlorogenic acids; Essential oils: β-myrcene, β-ocimene, linalool, citronellal, citronellol, caryophyllene, and β-pinene; Nutrients: citric acid, vitamin D, potassium, sodium, magnesium, manganese, iron, and zinc [61,62]. | Antibacterial, antiamoebic, anti-inflammatory, antimalarial, and ascaricidal activity [61,63]. |
| Zingiberaceae | Elettaria cardamomum (L.) Maton/true cardamom |  | Flavonols: quercetin and kaempferol; Flavones: luteolin; Flavanols: catechin; Phenolic acids: protocatechuic acid, caffeic acid, syringic acid, and 5-O-caffeoylquinic acid, gallic acid, vanillic acid, ferulic acid, and synapic acid; Anthocyanidin: pelargonidin; Essential oils: 1,8-cineole, terpineol, limonene, terpinyl acetates, linalyl acetate, linalool, sabinene, eucalyptol, terpineol, and limonene; Nutrients: vitamin C, calcium, potassium, magnesium, phosphorus, sulfur, and manganese [64,65,66]. | Antioxidant, antitumor, antihypertensive, immunomodulatory, anti-inflammatory, antidiabetic, antiulcerogenic, and insecticidal activities [67,68,69]. |
| Onagraceae | Epilobium angustifolium L./fireweed |  | Flavonols: quercetin-3-O-rhamnoside, quercetin-3-O-glucuronide, kaempferol, and myricetin; Phenolic acids: gallic acid, caffeic acid, ellagic acid, ferulic acid, and protocatechuic acid; Fatty acids: tricosanoic, nervonic, linoleic, palmitic, caprylic, caproic, and butyric acids [70,71,72]. | Antioxidant, anticancer, antiandrogen, immunostimulatory, metal-binding, and antimicrobial activities [71,72]. |
| Myrtaceae | Eugenia uniflora L./Pitanga or Surinam cherry |  | Flavonols: kaempferol pentoside, myricetin galloyl hexoside, myricetin hexoside, myricetin pentoside, myricetin rhamnoside, quercetin galloyl hexoside, quercetin rhamnoside, quercetin hexoside and quercetin pentoside, and rutin; Phenolic acids: gallic acid and ellagic acid, Anthocyanins: cyanidin-3-glucoside and delphinidin-3-glucoside; Carotenoids: lycopene, γ-carotene, and β-cryptoxanthin Essential oils: trans-β-ocimene, cis-ocimene, β-pinene, eugenilones A-N, seline-1,3,7-triene-8-one oxide, and β-caryophyllene; Nutrients: vitamins C and A [73,74,75]. | Antimicrobial, antiviral, antifungal, hepatoprotective, and antioxidant effects [76,77]. |
| Lauraceae | Laurus nobilis L./bay laurel |  | Flavonols: kaempferol-3-O-rhamnoside, kaempferol-3-O-(2′′,4”-di-E-p-coumaroyl)-rhamnoside, kaempferol-3-O-arabinoside, isoquercitrin, quercetin-3-O-rhamnoside, 3′-methoxyquercetin-3-O-glucopyranoside, and rutin; Flavones: luteolin and izovitexin-2′′-rhamnoside; Phenolic acids: gallic, vanillic, and rosmarinic acids; Essential oils: 1,8-cineole, α-terpinyl acetate, and α-terpineol; Fatty acids: lauric, palmitic, oleic (ω-9), and linoleic (ω-6) acids [78,79,80,81]. | Antioxidant, antimicrobial, digestive, antitumor, analgesic, anti-inflammatory, antiproliferative, antimutagenic, anticholinergic, and insect-repellent effects [82,83]. |
| Lauraceae | Litsea cubeba (Lour.) Pers./mountain pepper |  | Flavonols: kaempferol-3 and 7-glucosides, naringin, and quercetin; Phenolic acids: caffeic acid; Essential oils: E-citral (geranial), Z-citral (neral) and D-limonene, β-thujene, β-pinene, α-pinene, 6-methyl-5-hepten-2-one, and linalool; Fatty acids: palmitic acid, stearic acid, and myristoleic acid; Nutrients: vitamins E and A [84,85,86]. | Antibacterial, antioxidant, antiparasitic, anticancer, and cytotoxic effects [87,88,89]. |
| Aquifoliaceae | Mate (Ilex paraguariensis) A.St.-Hil./Yerba mate |  | Flavonols: quercetin, kaempferol, and rutin; Flavones: luteolin; Phenolic acids: 3-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, and caffeic acid; Nutrients: vitamins (A, B1, B2, B3, C, and E) and minerals (potassium, magnesium, calcium, manganese, iron, selenium, phosphorus, and zinc) [90,91,92]. | Anti-inflammatory, antioxidant, hypocholesterolemic, hypotensive, and antidiabetic activities [93,94]. |
| Ranunculaceae | Nigella sativa L./black cumin |  | Flavanols: catechin; Flavones: apigenin; Phenolic acids: chlorogenic acid, gallic acid, and vanillic acid; Alkaloids: nigellicin, nigellidin, and quanazoline; Essential oils: nigellone, thymoquinone, thymohydroquinone, dithymoquinone, thymol, carvacrol, α and β-pinene, d-limonene, and d-citronellol; volatile oils of the seeds: p-cymene, t-anethole, 4-terpineol, and longifoline; Fatty acids: arachidonic (ω-6), eicosadienoic (ω-6), linoleic (ω-6), linolenic (ω-3), oleic (ω-9), palmitoleic (ω-7), palmitic, and stearic acids; Phytosterols: beta-sitosterol, cycloeucalenol, cycloartenol, sterol esters, and sterol glucosides [95,96,97,98]. | Antioxidant, antitussive, gastroprotective, antianxiety, antiulcer, antiasthmatic, anticancer, anti-inflammatory, immunomodulatory antitumor, and hepatoprotective effects, as well as protection against cardiovascular disorders [99,100]. |
| Lamiaceae | Origanum vulgare L./oregano |  | Flavones: luteolin, scutellarein, apigenin, apigenin-7-O-glucoside, and naringenin; Flavonols: quercetin O-hexoside, quercetin dimethyl ether, and quercitrin; Phenolic acids: rosmarinic acid, caffeic acid, gallic acid, and chlorogenic acid; Essential oils: carvacrol and/or thymol, linalool, and p-cymene; Fatty acids: linoleic (ω-6), oleic (ω-9), and stearic palmitic acids; Nutrients: iron, copper, sulfur, chlorine, iodine, and selenium [101,102,103]. | Antimicrobial, antiviral, antioxidant, anti-inflammatory, antispasmodic, antiurolithic, antiproliferative, and neuroprotective effects [104,105]. |
| Piperaceae | Piper nigrum L./black pepper |  | Flavonols: quercetin, kaempferol, and rhamnetin; Phenolic acids: gallic acid, naringenin, moracin C, vanillin, and 6-gingerol; Alkaloids: piperine, pellitorine, and piperolactam D Essential oils: sabinene, 3-carene, D-limonene, α-pinene, caryophyllene, β-phellandrene, α-phellandrene, α-thujene, and β-bisabolene; Nutrients: carbohydrates, proteins, calcium, magnesium, potassium, iron, and vitamin C [106,107]. | Antimicrobial, cytotoxicity, insecticidal, anti-inflammatory, and toxicity effects [108,109]. |
| Fabaceae | Neltuma velutina Wooton/velvet mesquite |  | Flavones: luteolin, apigenin, apigenin-7-O-glucoside, vitexin, and isovitexin; Flavonols: quercetin and kaempferol; Phenolic acids: gallic acid, hydroxybenzoic acid, chlorogenic acid, ferulic acid, caffeic acid, and p-coumaric acid; Fatty acids: stearic acid, linoleic acid (ω-6), oleic acid (ω-9), palmitic acid, and arachidic acid; Nutrients: calcium and potassium [110,111]. | Antibacterial, antihelmintic, insecticidal, antioxidant, and cytotoxic effects [111,112]. |
| Fagaceae | Quercus alba L./white oak |  | Flavonols: quercetin and kaempferol; Flavanols: catechin and epicatechin; Flavanones: naringin; Tannins: castalagin and vescalagin; Phenolic acids: gallic, ellagic, vanillic, p-hydroxybenzoic, syringic, salicylic, p-coumaric, caffeic, ferulic acid, sinapic acid, and protocatechuic acid; Anthocyanins: cyanidin-3-O-glucoside and cyanidin-3-O-sophoroside; Fatty acids: linoleic (ω-6) and palmitic acids; Nutrients: vitamin B12, iron, and potassium [113,114,115]. | Antibacterial, antiviral, antioxidant, anti-inflammatory, and anticancer activities [116,117,118]. |
| Lamiaceae | Salvia rosmarinus Spenn./rosemary |  | Flavones: luteolin and apigenin; Flavanols: gallocatechin and epigallocatechin; Phenolic acids: rosmarinic acid, caffeic acid, ferulic acid, and quinic acid; Phenolic diterpenes: carnosic acid, carnosol Essential oils: 1,8-cineole, camphor, α-pinene, camphene, α-terpineol, Terpenoids: ursolic acid, betulinic acid, carnosic acid, and carnosol; Fatty acids: linoleic acid (ω-6) and oleic acid (ω-9); Nutrients: phosphorus, potassium, copper, vitamin A, vitamin C, thiamin, and riboflavin [119,120,121,122]. | Antifungal, antibacterial, antioxidant, analgesic, anti-inflammatory, antirheumatic, antispasmodic (in renal colic and dysmenorrhea), carminative, and choleretic activities [123,124]. |
| Poaceae | Saccharum officinarum L./sugar cane |  | Flavones: apigenin, luteolin, tricin, orientin, vitexin, schaftoside, and swertisin; Flavonols: kaempferol-3-O-rutinoside, quercetin-3-O-rutinoside, and kaempferol-3-O-glucopyranoside; Phenolic acids: sinapic acid and caffeic acid; Fatty acids: palmitic and linoleic (ω-6) acids Nutrients: sucrose, fibers, and vitamin C [125,126]. | Anti-inflammatory, analgesic, antihyperglycemic, diuretic, hepatoprotective, diuretic, and antithrombotic effects [127,128,129]. |
| Lamiaceae | Salvia officinalis L./sage |  | Flavonols: quercetin, kaempferol, and rutin; Flavones: luteolin 7-O-glucoside; Flavanols: epicatecin and epigallocatechin gallate; Phenolic acids: rosmarinic acid, methyl rosmarinate, caffeic acid, salvianolic acid K, syringic acid, and vanillic acid; Essential oils: α-thujone, (E)-β-caryophyllene, 1,8-cineole, α-humulene, β-pinene, β-thujone, camphor, allo-aromadendrene, borneol, and α-pinene; Nutrients: magnesium, zinc, copper, and vitamins A, C, and E [130,131,132]. | Antioxidant, anticancer, antimutagenic, anti-inflammatory, and antiseptic effects [133,134,135]. |
| Lamiaceae | Satureja khuzestanica Jamzad/savory |  | Flavones: apigenin, galangin, luteolin, cirsilineol, and diosmin; Flavonols: quercetin, epigallocatechin-3-O-gallate, kaempferol, and myrcetin; Phenolic acids: rosmarinic acid, ferulic acid, gallic acid, and vanillic acid; Essential oils: carvacrol, ƴ-terpinene, p-cymene, α-terpinene, and thymol; Fatty acids: linoleic acid (ω-6), palmitic acid, 9-octadecenoic acid, methyl ester, and hexadecanoic acid; Nutrients: potassium and α-tocopherol [136,137,138]. | Antibacterial, antifungal, antioxidant, antidiabetic, antihyperlipidemic, and anti-inflammatory effects [139,140]. |
| Lamiaceae | Satureja montana L./winter savory |  | Flavones: luteolin and apigenin; Flavonols: quercetin, kaempferol, and rutin; Phenolic acids: ellagic, caffeic, p-coumaric, protocatehuic, rosmarinic, and syringic acids; Essential oils: linalool, α-terpineol, cis-sabinene hydrate, and p-cymene; Nutrients: vitamins A, C, B1, B3, and B6; potassium; iron; calcium; magnesium; manganese; zinc; and selenium; Others: ursolic acid and oleanolic acid [141,142,143]. | Antibacterial, antiviral, antioxidant, antiseptic, antifungal, carminative, and digestive properties [143,144,145]. |
| Myrtaceae | Syzygium aromaticum L./clove |  | Flavones: apigenin; Flavonols: quercetin, myricetin, rhamnocitrin, kumatakenin, kaempferol, pachypodol, and isorhamnetin; Phenolic acids: gallic acid, ellagic acid, and salvianolic acid C; Essential oils: eugenol, eugenyl acetate, caryophyllene, and α-humulene; Fatty acids: palmitic, stearic, linoleic (ω-6), and linolenic (ω-3) acids; Nutrients: aspartic acid; glutamic acid; arginine; alanine; vitamins, including B1, B6, C, K, riboflavin, and A; zinc; iron; calcium; and manganese [146,147,148]. | Antibacterial, antiviral, and antifungal activities, as well as hypoglycemic, antitumor, and anti-inflammatory effects [149,150]. |
| Lamiaceae | Thymbra capitatus (L.) Cav./Mediterranean wild thyme |  | Flavones: apigenin and flavone; Flavonols: myristin, quercetin dihydrite, and campherol; Flavanols: catechin and epicatechin; Phenolic acids: gallic acid, chlorogenic acid, and rosmarinic acid; Others: resorcinol, carnosic acid; Essential oils: thymol, carvacrol, γ-terpinene, and p-cymene; Nutrients: vitamins C and E, potassium, magnesium, lignoceric acid, and hexadecanoic acid [151,152,153]. | Antibacterial, antioxidant, analgesic, and antiseptic properties [154,155,156]. |
| Lamiaceae | Thymus kotschyanus Boiss. & Hohen./Kotschyanus thyme |  | Phenolic acids: gallic acid; Essential oils: carvacrol, thymol, p-cymene, and geraniol; Nutrients: β-carotene and vitamins C and E [157,158,159]. | Antifungal, anti-inflammatory, antimicrobial, and expectorant properties [160,161,162]. |
| Lamiaceae | Thymus serpyllum L./wild thyme |  | Flavones: catechol, naringin, and luteolin. Phenolic acids: rosmarinic acid, gallic acid, caffeic acid, p-coumaric acid, ferullic acid, and veratric acid; Essential oils: thymol, carvacrol, p-cymol, linalol, and α -pinene; Nutrients: potassium, iron, vitamin A, vitamin C, and vitamin E [163,164]. | Antimicrobial, antioxidant, antiseptic, antispasmodic, and antihypertensive effects [165,166,167]. |
| Apiaceae | Trachyspermum ammi Sprague/caraway |  | Flavones: apigenin; Flavonols: quercetin; Phenolic acids: gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, and rosmarinic acid; Essential oils: γ-terpinene, ρ-cymene, pulegone, carvacrol, and thymol; Nutrients: calcium, phosphorous, iron, nicotinic acid, and carotene [168,169,170]. | Antifungal, antioxidant, antimicrobial, antinociceptive, cytotoxic activity, hypolipidemic, antihypertensive, antispasmodic, and diuretic properties [171,172,173]. |
| Fabaceae | Trigonella foenum-graecum L./fenugreek |  | Flavones: apigenin, orientin, luteolin, vitexin, and isovitexin; Flavonols: quercetin and kaempferol 7- O-rhamnosyl-(1→2)-glucoside; Phenolic acids: gallic acid, galloyl-coumaric acid pentoside, caffeoyl-coumaroyl-quinic acid, tricaffeoyl-glucosyl-glucoside, and dihydrogallic acid derivative; Saponins: diosgenin, yamogenin, tigogenin, and neotigogenin; Nutrients: arginine; lysine; histidine; calcium; iron; vitamins B, A, and C; and nicotinic acid Others: 4-hydroxyisoleucine [174,175,176]. | Antimicrobial, antioxidant, anticancer, hypoglycaemic, hypocholesterolemic, immunomodulatory, and neuroprotective effects [174,177,178]. |
| Urticaceae | Urtica dioica L./nettle |  | Flavonols: Quercetin 3-O-rutinoside, quercetin 3-O-galactoside; kaempferol 3-O-glucoside, and isorhamnetol 3-O-rutinoside; Flavones: apigenin, luteolin 7-O-neohesperidoside, and luteolin 7-O-b-d-Glucopyranoside; Phenolic acids: chlorogenic, neochlorogenic, cichoric, and caffeoylmalic acids; Essential oils: hexanal, linalool, carvone, cumin aldehyde, and carvacrol; Fatty acids: palmitic acid and linolenic acid (ω-3); Nutrients: glucose, sucrose, vitamin A, potassium, phosphorus, magnesium, sodium, and zinc; Others: inositol and rhamnose [179,180,181]. | Antioxidant, antimicrobial, anti-inflammatory, antiulcer, and analgesic effects [182,183,184]. |
| Lamiaceae | Zataria multiflora Boiss./Shirazi thyme |  | Flavones: apigenin, luteolin, and 6-hydroxyluteolin; Phenolic acids: gallic acid, syringic acid, protocatechuic acid, and 4-hydroxybenzoic acid; Essential oils: carvacrol; gamma-terpinene, alpha-pinene, eucalyptol, globulol, thymol, and linalool; Nutrients: vitamin E; Others: oleanolic acid, β-sitosterol, and betolin [185,186,187]. | Antibacterial, antiseptic, analgesic, and carminative effects [188,189,190]. |
| Zingiberaceae | Zingiber officinalis Roscoe/common ginger |  | Flavonols: quercetin, kaempferol, and rutin; Flavones: naringenin; Flavanols: catechin and epicatechin; Phenolic acids: gallic acid, ferulic acid, caffeic acid, and p-coumaric acid; Essential oils: gingerols (6-gingerol, 8-gingerol, and 10-gingerol), β-bisabolene, α-curcumene, zingiberene, α-farnesene, and β-sesquiphellandrene; Nutrients: vitamin E, iron, potassium, and sodium [191,192,193]. | Antioxidant, anti-inflammatory, antimicrobial, anticancer, antipyretic, antidiabetic, analgesic, antihelmintic, and antiviral activities [194,195,196]. |
| Scientific Plant Name | Type of Anatomical Part Used/Form Used | Type of Meat Used | Quantity Used | Storage Conditions | Phenolic Content and Antioxidant Capacity | Main Outcomes | References |
|---|---|---|---|---|---|---|---|
| C. sinensis (L.) Kuntze | Leaf extract/direct in formula incorporation | Fish mince | 0.01% | 6 months frozen storage at −18 ± 1 °C | Not determined | ↓ level of TBARS ↓ level of peroxide | [199] |
| C. sinensis (L.) Kuntze and I. paraguariensis | Whole-plant extracts/direct in formula incorporation | Brine-injected pork chops | 100, 350, or 700 ppm extract | 7 days at 5 °C | At 100 ppm: TPC: 29 ± 0 ppm GAE (C. sinensis); TPC: 28 ± 4 ppm GAE (I. paraguariensis) At 350 ppm: TPC: 68 ± 13 ppm GAE (C. sinensis); TPC: 83 ± 11 ppm GAE (I. paraguariensis) At 700 ppm: TPC: 105 ± 36 ppm GAE (C. sinensis); TPC:150 ± 33 ppm GAE (I. paraguariensis) | ↑ antioxidant capacity ↓ level of TBARS | [200] |
| D. ambrosioides (L.) Mosyakin & Clemants | Whole plant/water and ethanol extracts | Raw ground pork | 50 mL/kg | 9 days at 4 °C | TPC: 126.3 mg GAE/100 g; TFC: 147.26 mg QE/100 g; DPPH (IC50): 0.97 mg/mL; DPPH: 16.65% of radical inhibition | ↑ antioxidant capacity | [201] |
| C. longa L. | Rhizome powder/direct in formula incorporation | Rabbit patties | 3.5% | 7 days at 4 ± 1 °C | FRAP: 0.94 mmol Trolox; DPPH: 2.51 mmol Trolox | ↑ antioxidant capacity ↓ lipid oxidation | [202] |
| C. longa L. | Roots/extract | Fresh lamb sausage | 0.025, 0.05, and 0.075% | 18 days at 2 ± 1 °C | TPC: 5018.42 mg GAE/100 g; ABTS: 1490.53 mg AAE/100 g; DPPH: 42.92 mg TE/g; FRAP: 980.27 µmol Fe+2/100 g | ↑ antioxidant capacity ↓ lipid oxidation | [203] |
| C. citratus (DC.) Stapf | Whole fruit/cereal alcohol (70%) | Fresh chicken sausage | 0.5 and 1.0% | 42 days at 4 °C | TPC: 133.84 mg GAE/g; TFC: 13.42 mg QE/g; IC50: 0.45 mg/mL | ↑ antioxidant capacity | [204] |
| E. angustifolium L. | Whole plant/methanol plant | Beef burgers | 1 g, 3 g, and 9 g | 4 °C ± 1 °C for a period of 8 days | DPPH: 48.80 ± 3.74% FRAP: 2198.05 ± 78.56 mg/L; TPC: 1263.48 ± 12.13 mg GAE/100 mL; TFC: 278.43 ± 3.27 mg CE/100 mL | ↑ antioxidant capacity | [205] |
| E. uniflora L. | Leaf powder/hydroethanolic extract | Pork burgers | 0.02, 0.05, and 0.1% | Refrigerated storage (2 ± 1 °C) under light to simulate supermarket conditions for 18 days | TPC: 229.38 mg GAE/g; DPPH: 242 µg/mL | ↑ antioxidant capacity ↓ level of TBARS | [206] |
| E. uniflora L. | Leaves/hydroethanolic extract | Lamb burgers | 250 mg/kg | 18 days at 2 °C | TPC: 229.38 mg GAE/g; DPPH: 242 μg/mL; ABTS: 570.97 mg TE/g | ↑ antioxidant capacity ↓ level of TBARS | [207] |
| N. sativa L. | Seed extract/hydroethanolic extract | Fresh minced beef | 1.5% | 9 days at 4 °C | Not determined | ↓ level of TBARS ↓ lipid oxidation | [208] |
| O. vulgare L. | Essential oil/direct in formula incorporation | Ground chicken breast | 100 ppm; 300 ppm; 400 ppm | 7 days at 4 °C | Not determined | ↓ lipid oxidation ↓ protein oxidation | [209] |
| P. nigrum L. | Whole plant/anhydrous ethanol | Fresh pork | 0.1 and 0.5% (v/v) in 20% | 9 days at 4 °C | Not determined | ↓ level of TBARS | [210] |
| N. velutina Wooton | Leaves/ultrasound-assisted extraction—ethanol | Pork patties | 2% | 10 days at 4 °C | TPC: 278.50 mg GAE/g; TFC: 226.8 mg RE/g; DPPH (100 µg/mL): 85.3% radical inhibition | ↑ antioxidant capacity | [211] |
| Q. alba L. | Chips/subcritical water | Pork patties | 0.05, 0.5, and 1.0% | 12 days at 4 °C | TPC: 2180.8 mg GAE/L; ABTS: 32.00 mM TE/L; DPPH: 31.20 mM TE/L | ↑ antioxidant capacity ↓ lipid oxidation | [212] |
| S. rosmarinus Spenn. | Leaves/ethanolic extract 80% (v/v) | Chicken surimi | 200 mg/kg | 14 days at 4 °C | TPC: 24.46 mg/g; TFC: 38.36 mg/g; TDTC: 88.76 mg/g | ↑ antioxidant capacity ↓ level of TBARS | [213] |
| S. rosmarinus Spenn. | Essential oil of leaves/direct in formula incorporation | Poultry fillets | 0.2% | Two different conditions: air-packaging and modified atmosphere | Not determined | ↓ lipid oxidation ↓ rancidity | [214] |
| S. officinarum L. | Whole plant/direct in formula incorporation | Raw ground pork and beef | 50 μg/mL | 14 days at 4 °C | DPPH: 191.00 mg TE/g; ABTS: 359.80 mg TE/g; FRAP: 97.80 mg TE/g | ↑ antioxidant capacity ↓ level of TBARS ↓ lipid oxidation | [215] |
| S. officinalis L. | Whole plant/subcritical fluid extraction | Fresh pork sausages | 0.05, 0.075, and 0.1 μL/g | 8 days at 3 °C | DPPH (IC50): 0.0242 mg/mL | ↑ antioxidant capacity | [216] |
| S. montana L. | Aerial parts/direct in formula incorporation | Fresh pork sausages | 0.075 and 0.150 µL/g | 10 days at 3 °C | DPPH: 26.17–27.87 µg/mL | ↑ antioxidant capacity ↓ lipid oxidation | [217] |
| S. aromaticum L. | Powder/condensed aqueous extract | Beef patties | 0.1% | 10 days at 4 °C | Not determined | ↓ lipid oxidation ↓ level of TBARS | [218] |
| S. aromaticum L. | Dried flower buds/clove extract was dissolved in edible ethanol before being mixed with the ingredients | Chinese-style sausage | 0.25%, 0.5%, 1%, and 2% | 21 days at 4 °C | Not determined | ↓ level of TBARS ↓ lipid oxidation | [219] |
| T. serpyllum L. | Whole plant/subcritical fluid extraction | Ground pork patties | 0.075 and 0.150 µL/g | 3 days at 4 °C | ABTS: 576.7–665.6 µM TE/g; DPPH: 37.5–58.3 µM TE/g | ↑ antioxidant capacity ↓ lipid oxidation | [220] |
| T. foenum-graecum L. | Seed powder/direct in formula incorporation | Rabbit sausage | 5, 10, or 15% | Frozen storage at ̶ 18 °C ± 1 for 3 months | Not determined | ↓ lipid oxidation | [221] |
| Z. multiflora Boiss. | Aerial parts/corn starch films and fortified nanoemulsion | Ground beef patties | 6% | 20 days at 4 ± 1 °C | Not determined | ↓ level of TBARS ↓ level of peroxide | [222] |
| Scientific Plant Name | Type of Anatomical Part Used/Form Used | Type of Meat Used | Quantity Used | Storage Conditions | Antimicrobial Effect | References |
|---|---|---|---|---|---|---|
| A. citrodora Paláu and S. aromaticum L. | Leaves of A. citriodora and flowers of buds of S. aromaticum/sodium alginate-based coatings | Chicken breast | 0.2 and 0.5% | 15 days, refrigerated | Total bacterial count, Pseudomonas, lactic acid bacteria, psychrotrophic bacteria, Enterobacteriaceae, molds, and yeasts | [237] |
| C. verum J. Presl | Cinnamon essential oil/polymer matrix | Chicken meat | 25 and 50% | 21 at 4 °C | S. typhimurium, C. jejuni, and L. monocytogenes | [238] |
| C. sativus L. | Petals/films based on chitosan and methylcellulose nanofiber | Lamb meat | 3% | 25 days at 3 °C | E. coli and S. aureus | [239] |
| C. cyminum L. | Powdered cumin seed/chitosan-based coating | Chicken meat | 0.2, 0.4, and 0.6% | 9 days at 4 °C | Total count of bacteria, Enterobacteriaceae, S. aureus, E. coli, mold, and yeast | [240] |
| C. citratus (DC.) Stapf | Aerial-part essential oil/poly lactic acid film | Pork sausages | 2% | 12 days storage at 4 °C | L. monocytogenes | [241] |
| C. citratus (DC.) Stapf | Leaf oils/direct in formula incorporation | Pork loin | 5 mg/mL | 8 days at 4 °C | L. monocytogenes | [242] |
| L. nobilis L. | Leaf essential oil/liposomes encapsulated with silver nanoparticles | Pork | 1% | 15 days at 4 °C | E. coli and S. aureus | [243] |
| N. sativa L. | Black cumin essential oil/multilayer film based on chitosan and alginate | Chicken meat | 1% | 5 days at 4 °C | S. aureus and E. coli | [244] |
| O. vulgare L. | Leaves/direct nanoemulsion encapsulation | Chicken pâté | 5% | 8 days at (4.0 ± 2 °C) | S. aureus and E. coli | [245] |
| S. montana L. | Supercritical fluid extract of aerial parts | Cooked pork sausages | 0.025, 0.050, 0.075, and 0.100 µL/g | 0, 15, and 30 days at 4 °C | Salmonella spp., E. coli, and L. monocytogenes | [246] |
| S. rosmarinus Spenn. | Leaves/direct in formula incorporation | Turkey ham | 1% | 63 days at 4 °C | L. monocytogenes counts | [247] |
| S. rosmarinus Spenn. | Leaves/whey protein isolate-based film | Lamb meat | 2% | 15 days at (4.0 ± 1 °C) | Total count of psychrotrophic bacteria | [248] |
| S. rosmarinus Spenn. | Leaf essential oil/spraying on packaging | Beef meat | 4% (30% essential oil/70% ethanol) | 4 °C for up to 20 days | Pseudomonas spp., Brochothrix thermosphacta, and Enterobacteriaceae | [249] |
| S. rosmarinus Spenn. | Leaf essential oil/nanogel encapsulation | Beef cutlet | 0.5, 1.0, and 2.0 mg of nanoencapsulated oil per g of meat | 12 days at 4 °C | S. typhimurium | [250] |
| S. officinalis L. | Leaf essential oil/direct in formula incorporation | Minced pork | 0.4 and 0.6 µL/g | 14 days at 4 °C | E. coli | [251] |
| S. khuzestanica Jamzad | Aerial parts/chitosan-based coating | Lamb meat | 1% | 20 days at 4 °C | Pseudomonas, total count of bacteria, and lactic acid bacteria | [252] |
| T. capitatus (L.) Cav. | Leaf essential oil/direct in formula incorporation | Minced beef meat | 0.01, 0.05, 0.25, and 1.25% | 15 days at 7 °C | L. monocytogenes | [253] |
| T. kotschyanus Boiss. & Hohen. | Leaf essential oil/films based on corn starch and chitosan | Beef | 1 and 2% | 21 days at 4 °C | Pseudomonas, lactic acid bacteria, and L. monocytogenes | [254] |
| Thymus spp. | Leaf essential oils/direct in formula incorporation | Fresh pork meat | 0.3, 0.6, and 0.9% | 15 days at 3 ± 1 °C | Salmonella S. enterica ser. Enteritidis S. enterica ser. Typhimurium, S. enterica ser. Montevideo, and S. enterica ser. Infantis) | [255] |
| T. vulgaris L. | Chitosan film with thyme essential oil | Cooked ham | 0%, 0.5%, 1%, and 2% | 21 days at 3 ± 1 °C | Aerobic mesophilic bacteria, lactic acid bacteria, and enterobacteria | [256] |
| T. ammi Sprague | Seed essential oil/films based on gelatin and carboxymethylcellulose with chitin nanofiber | Beef | 0.24, 0.64, and 1% | 15 days at 4 °C | Total viable count, psychotrophic count, Pseudomonas spp., S. aureus, lactic acid bacteria, molds, and yeasts | [257] |
| U. dioica L. | Leaves/ε-polylysine coating | Beef | 3, 6, and 9% | 12 days at 4 °C | Molds and yeasts and total bacterial and coliform counts | [258] |
| Z. multiflora Boiss. | Whole-plant essential oil/direct in formula incorporation | Minced beef meat | 0.03, 0.5, 1, and 2% | 9 days of storage at 7 °C | L. monocytogenes | [259] |
| Z. officinalis Roscoe | Rhizomes of ginger essential oil /nanoemulsion-based edible sodium caseinate | Chicken breast fillets | 3% and 6% | 12 days at 4 °C | L. monocytogenes | [22] |
| Medicinal Plants | Meat Product | Color Parameters | References | |||||
|---|---|---|---|---|---|---|---|---|
| ∆L* | ∆a* | ∆b* | Chroma | ∆E | Hue | |||
| C. sinensis (L.) Kuntze | Brine-injected pork chops | +2.18 | ns | +2.51 | +2.51 | 3.3 | ns | [200] |
| I. paraguariensis A.St.-Hil. | Brine-injected pork chops | +2.57 | ns | +0.88 | +0.89 | 2.7 | ns | [200] |
| D. ambrosioides (L.) Mosyakin & Clemants | Raw ground pork | −0.89 | −0.28 | −1.26 | ns | ns | ns | [201] |
| C. longa L. | Fresh lamb sausage | −0.18 | +2.5 | +0.34 | ns | ns | ns | [203] |
| E. cardamomum | Frozen chicken burger | −1.95 | +0.3 | −1.21 | −1 | 3.44 | −2.29 | [280] |
| E. uniflora L. | Pork burger | −0.05 | +4 | +0.07 | ns | ns | ns | [206] |
| E. uniflora L. | Lamb burgers | +3.02 | −1.38 | +0.88 | ns | ns | ns | [207] |
| N. sativa L. | Fresh minced beef | 0 | −0.9 | ns | ns | ns | ns | [208] |
| O. vulgare L. | Ground chicken breast | +0.87 | +2.14 | +2.56 | ns | ns | ns | [209] |
| P. nigrum L. | Fresh pork | +0.1 | −0.23 | +1.89 | ns | ns | ns | [210] |
| N. velutina Wooton | Pork patties | +2.2 | −2.86 | −2.77 | −6.4 | 1.13 | ns | [211] |
| Q. alba L. | Pork patties | +6.74 | −0.94 | +1.73 | ns | ns | ns | [212] |
| S. rosmarinus Spenn. | Poultry fillets | +2.77 | −1.1 | −0.1 | ns | ns | ns | [214] |
| S. officinarum L. | Raw ground pork and beef | −3.16 | −1.32 | −2.64 | −3.09 | 2.44 | −1.81 | [215] |
| S. officinalis L. | Fresh pork sausages | +1.5 | −0.4 | −0.48 | ns | ns | ns | [281] |
| S. montana L. | Fresh pork sausages | −3.83 | +1.99 | 0.54 | +1.6 | +2.09 | +1.96 | [217] |
| S. aromaticum L. | Beef patties | +1.16 | −1.08 | −0.19 | −0.52 | ns | +4.06 | [218] |
| S. aromaticum L. | Chinese-style sausage | +2.56 | −0.18 | −2.21 | ns | ns | ns | [219] |
| T. serpyllum L. | Ground pork patties | −0.8 | −0.61 | −1 | ns | +1.98 | ns | [220] |
| T. vulgaris L. | Cooked ham | −2.52 | −0.24 | −0.28 | −0.37 | +2.28 | +0.28 | [256] |
| T. foenum-graecum L. | Rabbit sausage | +1.16 | −2.95 | −2.66 | ns | ns | ns | [221] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tocai, A.C.; Rosan, C.A.; Teodorescu, A.G.; Venter, A.C.; Vicas, S.I. Multifunctional Roles of Medicinal Plants in the Meat Industry: Antioxidant, Antimicrobial, and Color Preservation Perspectives. Plants 2025, 14, 2737. https://doi.org/10.3390/plants14172737
Tocai AC, Rosan CA, Teodorescu AG, Venter AC, Vicas SI. Multifunctional Roles of Medicinal Plants in the Meat Industry: Antioxidant, Antimicrobial, and Color Preservation Perspectives. Plants. 2025; 14(17):2737. https://doi.org/10.3390/plants14172737
Chicago/Turabian StyleTocai (Moțoc), Alexandra Cristina, Cristina Adriana Rosan, Andrei George Teodorescu, Alina Cristiana Venter, and Simona Ioana Vicas. 2025. "Multifunctional Roles of Medicinal Plants in the Meat Industry: Antioxidant, Antimicrobial, and Color Preservation Perspectives" Plants 14, no. 17: 2737. https://doi.org/10.3390/plants14172737
APA StyleTocai, A. C., Rosan, C. A., Teodorescu, A. G., Venter, A. C., & Vicas, S. I. (2025). Multifunctional Roles of Medicinal Plants in the Meat Industry: Antioxidant, Antimicrobial, and Color Preservation Perspectives. Plants, 14(17), 2737. https://doi.org/10.3390/plants14172737






