Synergistic Effects of Lavandula angustifolia and a Bacterial Consortium on Bioremediation of a Heavy Metal-Contaminated Soil
Abstract
1. Introduction
2. Results
2.1. Plant Growth, Soil pH, and EC
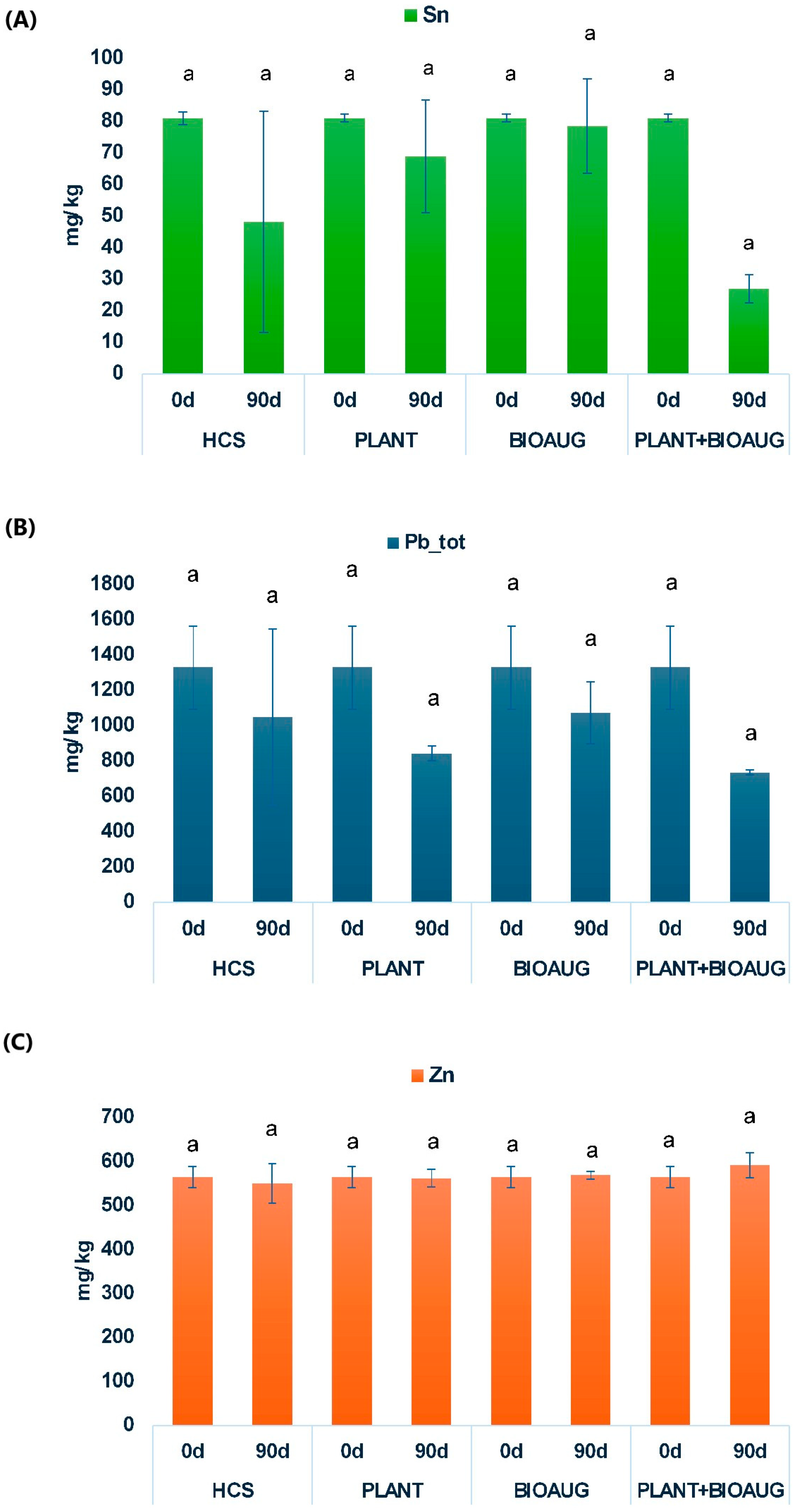
2.2. Contaminant Removal and Plant Uptake
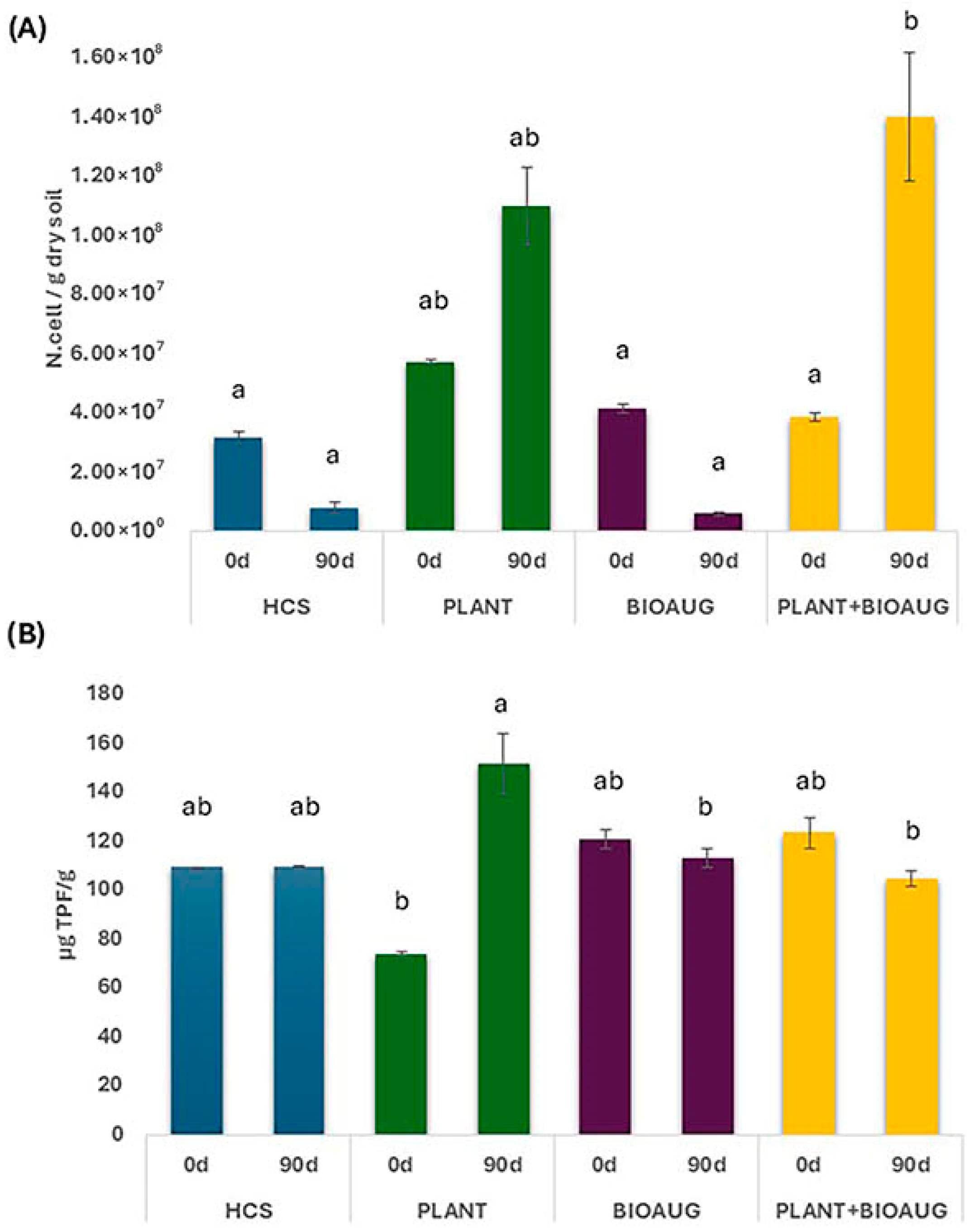
2.3. Microbial Abundance and Activity
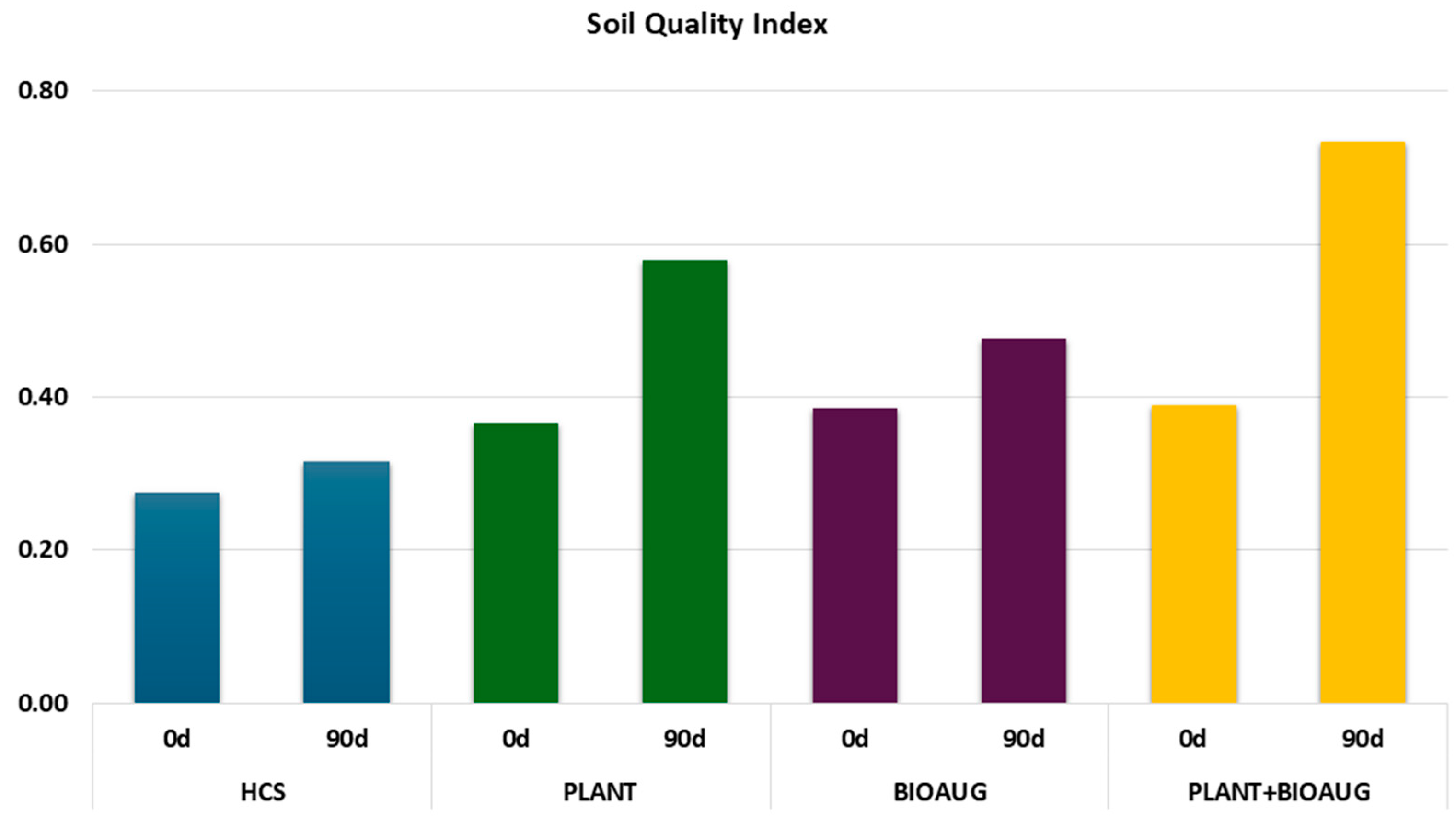
2.4. Soil Quality Index
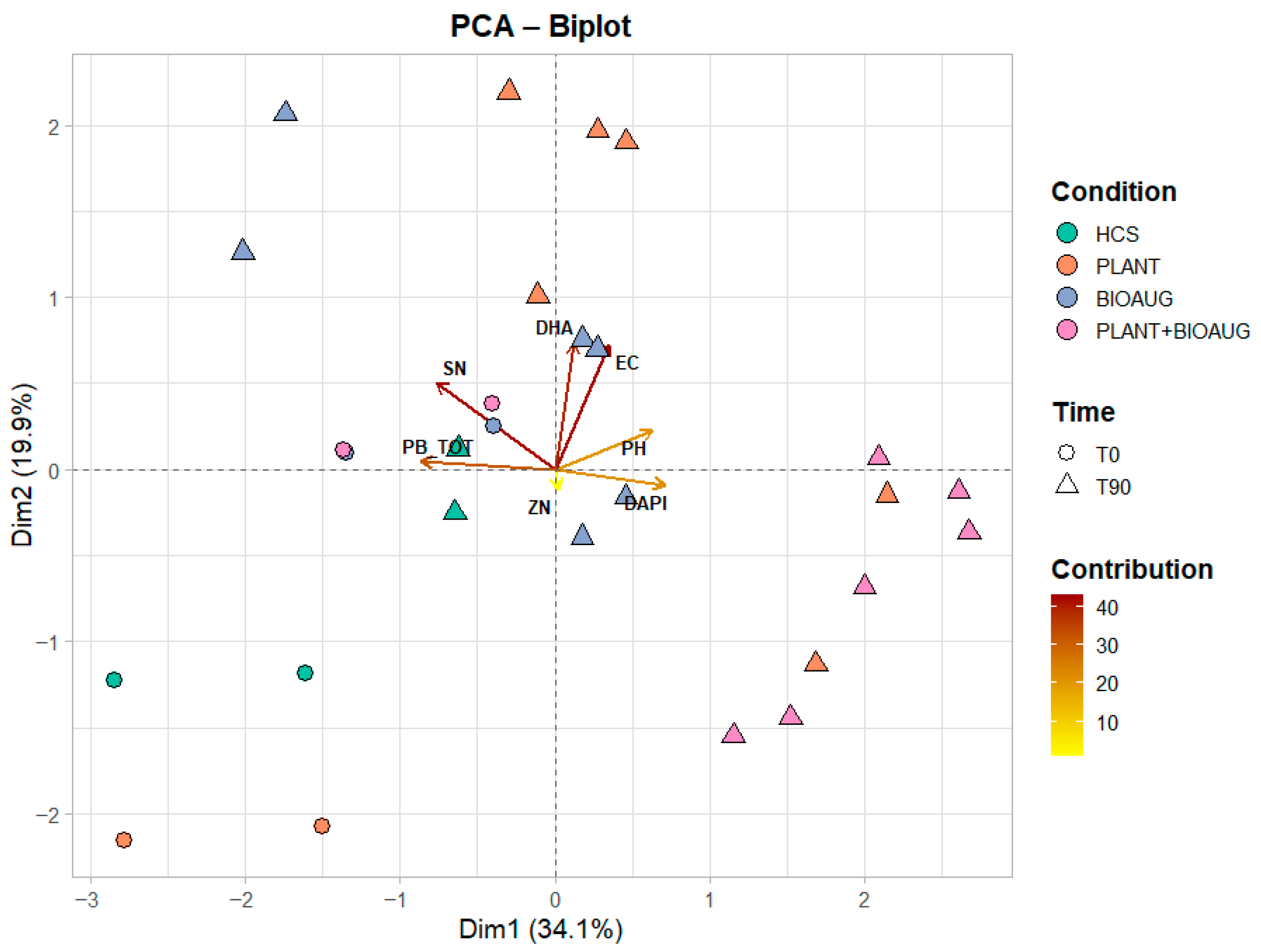
2.5. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Study Area and Sample Collection
4.2. Plant, Bacterial Consortium, and Experimental Design
4.3. Plant Growth Analysis
4.4. Physico-Chemical Analysis of the Soil and Pollutant Plant Uptake Assessment
4.5. Analysis of the Microbiological Soil Community
4.6. Soil Quality Index (SQI)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shetty, B.R.; Jagadeesha, P.B.; Salmataj, S.A. Heavy metal contamination and its impact on the food chain: Exposure, bioaccumulation, and risk assessment. CYTA-J. Food 2025, 23, 2438726. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; KC, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Waheed, A.; Zhang, Q.; Xu, H.; Dou, H.; Muhammad, M.; Aili, A.; Alshaharni, M.O. Mitigation of cadmium stress by salicylic acid: Physiological and biochemical responses in NM-2006, NM-92, and Mash-88 mung bean varieties. J. Hazard. Mater. 2025, 485, 136878. [Google Scholar] [CrossRef]
- Omotayo, A.O.; Omotayo, O.P. Potentials of microbe-plant assisted bioremediation in reclaiming heavy metal polluted soil environments for sustainable agriculture. Environ. Sustain. Indic. 2024, 22, 100396. [Google Scholar] [CrossRef]
- Shamoogardiani, E.; Navidjouy, N. Phytoremediation of Heavy Metals Polluted Environments. 2022. Available online: http://hsm.umsu.ac.ir (accessed on 10 May 2025).
- Min, X.; Zhang, K.; Chen, J.; Chai, L.; Lin, Z.; Zou, L.; Liu, W.; Ding, C.; Shi, Y. Bacteria-driven copper redox reaction coupled electron transfer from Cr(VI) to Cr(III): A new and alternate mechanism of Cr(VI) bioreduction. J. Hazard. Mater. 2024, 461, 132485. [Google Scholar] [CrossRef]
- Jan, M.; Mir, T.A.; Khare, R.K. Phytoremediation: The Way Forward. In Aquatic Contamination: Tolerance and Bioremediation; Wiley: Hoboken, NJ, USA, 2023; pp. 145–164. [Google Scholar] [CrossRef]
- Oro, C.E.D.; Puton, B.M.S.; Venquiaruto, L.D.; Dallago, R.M.; Tres, M.V. Effective Microbial Strategies to Remediate Contaminated Agricultural Soils and Conserve Functions. Agronomy 2024, 14, 2637. [Google Scholar] [CrossRef]
- Zhang, Q.; Waheed, A.; Aili, A.; Xu, H.; Kuerban, A.; Muhammad, M.; Ali, S. Copper sulfate-induced stress in Spinach: Metabolic pathway disruption and plant response. Sci. Hortic. 2024, 337, 113575. [Google Scholar] [CrossRef]
- Vaishnavi, J.; Osborne W., J. Phyto-rhizoremediation potential of C. zizanioides augmented with Bacillus infantis (VITVJ8) for the uptake of heavy metals (Cr, Pb and Zn). Front. Soil Sci. 2025, 5, 1484039. [Google Scholar] [CrossRef]
- Rostami, S.; Azhdarpoor, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef]
- Narciso, A.; Grenni, P.; Spataro, F.; De Carolis, C.; Rauseo, J.; Patrolecco, L.; Garbini, G.L.; Rolando, L.; Iannelli, M.A.; Bustamante, M.A.; et al. Effects of sulfamethoxazole and copper on the natural microbial community from a fertilized soil. Appl. Microbiol. Biotechnol. 2024, 108, 516. [Google Scholar] [CrossRef] [PubMed]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, F.; Hassanpouraghdam, M.B.; Pirsarandib, Y.; Aazami, M.A.; Asadi, M.; Ercisli, S.; Mehrabani, L.V.; Puglisi, I.; Baglieri, A. Improvements in the biochemical responses and Pb and Ni phytoremediation of lavender (Lavandula angustifolia L.) plants through Funneliformis mosseae inoculation. BMC Plant Biol. 2023, 23, 252. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kostova, I. Health effects of heavy metal contaminants Vis-à-Vis microbial response in their bioremediation. Inorganica Chim. Acta 2024, 568, 122068. [Google Scholar] [CrossRef]
- Ancona, V.; Rascio, I.; Aimola, G.; Barra Caracciolo, A.; Grenni, P.; Uricchio, V.F.; Borello, D. Chapter 2—Plant-assisted bioremediation: Soil recovery and energy from biomass. In Assisted Phytoremediation; Pandey, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 25–48. ISBN 9780128228937. [Google Scholar] [CrossRef]
- Çolak, S.; Yilmaz, Ş.B.A.; Öztekin, E. Bioaccumulation Factors of Heavy Metal(loid)s in Some Medicinal and Aromatic Plants Species: Example of Zonguldak/Türkiye. Water Air Soil Pollut. 2023, 234, 522. [Google Scholar] [CrossRef]
- Kaveh, H. Heavy Metal and Micro Elements Distribution Patterns and Inter-element Relationships in Market-sourced Lavender (Lavandula spp.) from Khorasan Razavi Region, Iran. J. Med. Plants By-Prod. 2025, in press. [Google Scholar] [CrossRef]
- Caprari, C.; Fantasma, F.; Divino, F.; Bucci, A.; Iorizzi, M.; Naclerio, G.; Ranalli, G.; Saviano, G. Chemical profile, in vitro biological activity and comparison of essential oils from fresh and dried flowers of Lavandula angustifolia L. Molecules 2021, 26, 5317. [Google Scholar] [CrossRef]
- Angelova, V.R.; Grekov, D.F.; Kisyov, V.K.; Ivanov, K.I. Potential of Lavender (Lavandula vera L.) for Phytoremediation of Soils Contaminated with Heavy Metals. Int. J. Agric. Biol. Eng. 2015, 9, 522–529. [Google Scholar]
- Ancona, V.; Gatto, A.; Aimola, G.; Rascio, I. Microcosm Experiment for Assessing Sunflower Capability to Grow on a PCB and HM Contaminated Soil. Available online: https://www.researchgate.net/publication/359732911 (accessed on 6 April 2022).
- Hlihor, R.M.; Roșca, M.; Hagiu-Zaleschi, L.; Simion, I.M.; Daraban, G.M.; Stoleru, V. Medicinal Plant Growth in Heavy Metals Contaminated Soils: Responses to Metal Stress and Induced Risks to Human Health. Toxics 2022, 10, 499. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Usman, M.; Zaman, Q.U.; Deng, G.; Chen, S.; Alwahibi, M.S.; Rizwana, H.; Iqbal, J.; Ahmad, S.; et al. Protective Effects of Multiple-Chemical Engineered Biochar On Hormonal Signalling, Antioxidant Pathways and Secondary Metabolites in Lavender Exposed to Chromium and Fluoride Toxicity. J. Crop Health 2025, 77, 64. [Google Scholar] [CrossRef]
- Pirsarandib, Y.; Hassanpouraghdam, M.B.; Rasouli, F.; Aazami, M.A.; Puglisi, I.; Baglieri, A. Phytoremediation of Soil Contaminated with Heavy Metals via Arbuscular Mycorrhiza (Funneliformis mosseae) Inoculation Ameliorates the Growth Responses and Essential Oil Content in Lavender (Lavandula angustifolia L.). Agronomy 2022, 12, 1221. [Google Scholar] [CrossRef]
- Sabina, R.O.; Santos, E.S.; Abreu, M.M. Accumulation of Mn and Fe in aromatic plant species from the abandoned Rosalgar Mine and their potential risk to human health. Appl. Geochem. 2019, 104, 42–50. [Google Scholar] [CrossRef]
- Gayke, A.; Nalavday, A.; Tiple, A. Extraction and Characterization of Natural Dyes from Transvaal Daisy and Lavender Petals: A Sustainable Approach. Academic 2024, 2, 12. Available online: www.theacademic.in (accessed on 1 December 2024).
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef]
- Bender, L.A.; Demarco, C.F.; Pieniz, S.; Carlos, F.S.; Quadro, M.S.; Andreazza, R. An Integrated Approach to Pb Bioremediation: Role of Bacteria in Enhancing Phytoremediation. Sustainability 2025, 17, 1386. [Google Scholar] [CrossRef]
- Nnaji, N.D.; Anyanwu, C.U.; Miri, T.; Onyeaka, H. Mechanisms of Heavy Metal Tolerance in Bacteria: A Review. Sustainability 2024, 16, 11124. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Terenzi, V. Rhizosphere microbial communities and heavy metals. Microorganisms 2021, 9, 1462. [Google Scholar] [CrossRef]
- Cavone, C.; Monaco, P.; Fantasma, F.; Rizzo, P.; Tarracchini, C.; Petraro, S.; Ventura, M.; Milani, C.; Celico, F.; Naclerio, G.; et al. Natural Hydrocarbon-Contaminated Springs as a Reservoir of Microorganisms Useful for Bioremediation: Isolation and Multilevel Analysis of Hydrocarbonoclastic Bacteria from the Agri Valley (Southern Italy). Sustainability 2025, 17, 3083. [Google Scholar] [CrossRef]
- Hegyi, A.; Nguyen, T.B.K.; Posta, K. Metagenomic analysis of bacterial communities in agricultural soils from vietnam with special attention to phosphate solubilizing bacteria. Microorganisms 2021, 9, 1796. [Google Scholar] [CrossRef]
- Presentato, A.; Piacenza, E.; Cappelletti, M.; Turner, R.J. Interaction of Rhodococcus with Metals and Biotechnological Applications. In Biology of Rhodococcus; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–357. [Google Scholar] [CrossRef]
- Khidr, R.; Qurbani, K.; Muhammed, V.; Salim, S.; Abdulla, S.; Wsw, H. Synergistic effects of indigenous bacterial consortia on heavy metal tolerance and reduction. Environ. Geochem. Health 2025, 47, 79. [Google Scholar] [CrossRef]
- Conlon, R.; Dowling, D.N.; Germaine, K.J. Assessing Microbial Activity and Rhizoremediation in Hydrocarbon and Heavy Metal-Impacted Soil. Microorganisms 2025, 13, 848. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.-L.; Chen, Y.-S.; Chen, Y.-D.; Qian, L.-W.; Xiong, W.-L.; Xu, J.-H.; Jiang, J.-P. Phytomanagement of a chromium-contaminated soil by a high-value plant: Phytostabilization of heavy metal contaminated sites. Bioresources 2020, 15, 3545–3565. [Google Scholar] [CrossRef]
- Cao, Z.; Yan, W.; Ding, M.; Yuan, Y. Construction of microbial consortia for microbial degradation of complex compounds. Front. Bioeng. Biotechnol. 2022, 10, 1051233. [Google Scholar] [CrossRef]
- Duncker, K.E.; Holmes, Z.A.; You, L. Engineered microbial consortia: Strategies and applications. Microb. Cell Fact. 2021, 20, 211. [Google Scholar] [CrossRef]
- Biswas, S.; Jayaram, S.; Philip, I.; Balasubramanian, B.; Pappuswamy, M.; Barceló, D.; Chelliapan, S.; Kamyab, H.; Sarojini, S.; Vasseghian, Y. Appraisal of the potential of endophytic bacterium Bacillus amyloliquefaciens from Alternanthera philoxeroides: A triple approach to heavy metal bioremediation, diesel biodegradation, and biosurfactant production. J. Environ. Chem. Eng. 2024, 12, 113454. [Google Scholar] [CrossRef]
- Chaudhry, H.; Vasava, H.B.; Chen, S.; Saurette, D.; Beri, A.; Gillespie, A.; Biswas, A. Evaluating the Soil Quality Index Using Three Methods to Assess Soil Fertility. Sensors 2024, 24, 864. [Google Scholar] [CrossRef]
- Sharma, I.; Kashyap, S.; Agarwala, N. Biotic stress-induced changes in root exudation confer plant stress tolerance by altering rhizospheric microbial community. Front. Plant Sci. 2023, 14, 1132824. [Google Scholar] [CrossRef]
- Shen, H.; Choi, C.; Masa, J.; Li, X.; Qiu, J.; Jung, Y.; Sun, Z. Electrochemical ammonia synthesis: Mechanistic understanding and catalyst design. Chem 2021, 7, 1708–1754. [Google Scholar] [CrossRef]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial Interventions in Bioremediation of Heavy Metal Contaminants in Agroecosystem. Front. Microbiol. 2022, 13, 824084. [Google Scholar] [CrossRef] [PubMed]
- Barra Caracciolo, A.; Grenni, P.; Garbini, G.L.; Rolando, L.; Campanale, C.; Aimola, G.; Fernandez-Lopez, M.; Fernandez-Gonzalez, A.J.; Villadas, P.J.; Ancona, V. Characterization of the Belowground Microbial Community in a Poplar-Phytoremediation Strategy of a Multi-Contaminated Soil. Front. Microbiol. 2020, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Barra Caracciolo, A.; Bustamante, M.A.; Nogues, I.; Di Lenola, M.; Luprano, M.L.; Grenni, P. Changes in microbial community structure and functioning of a semiarid soil due to the use of anaerobic digestate derived composts and rosemary plants. Geoderma 2015, 245–246, 89–97. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Biniecka, P.; Bondarczuk, K.; Piotrowska-Seget, Z. Metagenomic Functional Profiling Reveals Differences in Bacterial Composition and Function During Bioaugmentation of Aged Petroleum-Contaminated Soil. Front. Microbiol. 2020, 11, 02106. [Google Scholar] [CrossRef]
- Mei, T.; Zeng, Q.; Chen, R.; Tan, W. Soil microbial necromass carbon contributions to soil organic carbon after three decades of citrus cultivation. Front. Microbiol. 2025, 16, 1589966. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, Y.; Yang, K.; Wang, Y.; Zhang, C.; Ji, M. Application oriented bioaugmentation processes: Mechanism, performance improvement and scale-up. Bioresour. Technol. 2022, 344, 126192. [Google Scholar] [CrossRef]
- Franzetti, A.; Caredda, P.; La Colla, P.; Pintus, M.; Tamburini, E.; Papacchini, M.; Bestetti, G. Cultural factors affecting biosurfactant production by Gordonia sp. BS29. Int. Biodeterior Biodegrad. 2009, 63, 943–947. [Google Scholar] [CrossRef]
- Sánchez-Corona, C.G.; Gonzalez-Avila, L.U.; Hernández-Cortez, C.; Rojas-Vargas, J.; Castro-Escarpulli, G.; Castelán-Sánchez, H.G. Impact of Heavy Metal and Resistance Genes on Antimicrobial Resistance: Ecological and Public Health Implications. Genes 2025, 16, 625. [Google Scholar] [CrossRef] [PubMed]
- Henao, S.G.; Ghneim-Herrera, T. Heavy Metals in Soils and the Remediation Potential of Bacteria Associated with the Plant Microbiome. Front. Environ. Sci. 2021, 9, 604216. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Khalili, E.; Zuleta Mediavilla, D.P.; Khorami, M.; Aminabhavi, T.M.; Vasseghian, Y. Nanobioremediation of heavy metals using microorganisms. J. Environ. Manag. 2025, 392, 126736. [Google Scholar] [CrossRef]
- Emiliani, G.; Mengoni, A.; Maida, I.; Perrin, E.; Chiellini, C.; Fondi, M.; Gallo, E.; Gori, L.; Maggini, V.; Vannacci, A.; et al. Linking bacterial endophytic communities to essential oils: Clues from Lavandula angustifolia mill. Evid.-Based Complement. Altern. Med. 2014, 2014, 650905. [Google Scholar] [CrossRef]
- Sui, M.; Qin, X.; Sun, N.; Liu, Y.; Yang, C.; Guan, L.; Zhang, Y.; Wang, H.; Zhang, M.; Mao, Y.; et al. Effect of Elaeagnus angustifolia Linn. on the Physicochemical Properties and Microbial Community Structure of Inter-Rhizosphere Soils. Plants 2025, 14, 1242. [Google Scholar] [CrossRef]
- Vidican, R.; Mihaiescu, T.; Plesa, A.D.; Malinas, A.; Pop, B. The phytoremediation potential of Lavandula angustifolia Mill. grown in soils historically polluted with heavy metals: A case study from Baia Mare, Romania. J. Appl. Life Sci. Environ. 2023, 55/2022, 495–504. [Google Scholar] [CrossRef]
- Mazzon, M.; Cionci, N.B.; Buscaroli, E.; Alberoni, D.; Baffoni, L.; Di Gioia, D.; Marzadori, C.; Barbanti, L.; Toscano, A.; Braschi, I. Pot experimental trial for assessing the role of different composts on decontamination and reclamation of a polluted soil from an illegal dump site in Southern Italy using Brassica juncea and Sorghum bicolor. Environ. Sci. Pollut. Res. 2024, 31, 2640–2656. [Google Scholar] [CrossRef]
- Ancona, V.; Barra Caracciolo, A.; Grenni, P.; Di Lenola, M.; Campanale, C.; Calabrese, A.; Uricchio, V.F.; Mascolo, G.; Massacci, A. Plant-assisted bioremediation of a historically PCB and heavy metal-contaminated area in Southern Italy. New Biotechnol. 2017, 38, 65–73. [Google Scholar] [CrossRef]
- Ancona, V.; Rascio, I.; Aimola, G.; Campanale, C.; Grenni, P.; di Lenola, M.; Garbini, G.L.; Uricchio, V.F.; Caracciolo, A.B. Poplar-assisted bioremediation for recovering a PCB and heavy-metal-contaminated area. Agriculture 2021, 11, 689. [Google Scholar] [CrossRef]
- Ministro per le Politiche. Agricole Metodi Ufficiali di Analisi Chimica del Suolo; Decreto Ministeriale del 13 September 1999; Ministro per le Politiche: Rome, Italy, 1999. [Google Scholar]
- Angelini, A.; Scelsi, E.; Ancona, V.; Aimola, G.; Pastore, C. Performic acid pre-treatment of poplar biomasses grown on a contaminated area for enhanced enzymatic digestibility: A viable route to obtain fine-products and recovery of contaminants. J. Clean. Prod. 2022, 369, 133346. [Google Scholar] [CrossRef]
- Suliman, K.H.; Gaafar, A.R.Z.; Abdelmalik, A.M.; AlMunqedhi, B.M.; Elzein, A.; Hodhod, M.S. Microbial dynamics and dehydrogenase activity in tomato (Lycopersicon esculentum Mill.) rhizospheres: Impacts on growth and soil health across different soil types. Open Chem. 2024, 22, 20230209. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Sarikhani, M.R.; Shiri, J.; Shahbazi, F. Modeling soil enzyme activity using easily measured variables: Heuristic alternatives. Appl. Soil Ecol. 2021, 157, 103753. [Google Scholar] [CrossRef]
- Grenni, P.; Rodríguez-Cruz, M.S.; Herrero-Hernández, E.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Caracciolo, A.B. Effects of wood amendments on the degradation of terbuthylazine and on soil microbial community activity in a clay loam soil. Water Air Soil Pollut. 2012, 223, 5401–5412. [Google Scholar] [CrossRef]
- Santorufo, L.; Memoli, V.; Panico, S.C.; Esposito, F.; Vitale, L.; Di Natale, G.; Trifuoggi, M.; Barile, R.; De Marco, A.; Maisto, G. Impact of anthropic activities on soil quality under different land uses. Int. J. Environ. Res. Public Health 2021, 18, 8423. [Google Scholar] [CrossRef]
- Marzaioli, R.; D’Ascoli, R.; De Pascale, R.A.; Rutigliano, F.A. Soil quality in a Mediterranean area of Southern Italy as related to different land use types. Appl. Soil Ecol. 2010, 44, 205–212. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The Soil Management Assessment Framework. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]

| Treatments | Days | pH | EC |
|---|---|---|---|
| Mean ± st.dv | Mean ± st.dv | ||
| HCS | 0 | 6.64 ± 0.23 a | 0.99 ± 0.01 |
| 90 | 7.35 ± 0.08 c | 1.20 ± 0.04 | |
| PLANT | 0 | 6.64 ± 0.23 a | 0.99 ± 0.01 |
| 90 | 7.13 ± 0.00 b | 1.38 ± 0.18 | |
| BIOAUG | 0 | 7.31 ± 0.06 c | 1.22 ± 0.01 |
| 90 | 7.28 ± 0.04 b,c | 1.50 ± 0.08 | |
| PLANT+BIOAUG | 0 | 7.31 ± 0.06 c | 1.22 ± 0.01 |
| 90 | 7.38 ± 0.07 b,c | 1.39 ± 0.20 |
| Zn | Sn | Pb | ||
|---|---|---|---|---|
| PLANT | Leaves Roots TF | 37.15 ± 5.38 39.88 ± 16.67 0.93 | 0.10 ± 0.03 0.50 ± 0.02 0.20 | 5.74 ± 2.51 26.49 ± 6.59 0.22 |
| PLANT+BIOAUG | Leaves Roots TF | 11.26 ± 2.96 20.33 ± 11.53 0.55 | 0.10 ± 0.04 0.51 ± 0.03 0.20 | 15.46 ± 5.20 20.39 ± 6.21 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavone, C.; Rutigliano, A.; Cotugno, P.; Rolando, L.; De Carolis, C.; Barra Caracciolo, A.; Grenni, P.; Savino, I.; Bucci, A.; Naclerio, G.; et al. Synergistic Effects of Lavandula angustifolia and a Bacterial Consortium on Bioremediation of a Heavy Metal-Contaminated Soil. Plants 2025, 14, 2734. https://doi.org/10.3390/plants14172734
Cavone C, Rutigliano A, Cotugno P, Rolando L, De Carolis C, Barra Caracciolo A, Grenni P, Savino I, Bucci A, Naclerio G, et al. Synergistic Effects of Lavandula angustifolia and a Bacterial Consortium on Bioremediation of a Heavy Metal-Contaminated Soil. Plants. 2025; 14(17):2734. https://doi.org/10.3390/plants14172734
Chicago/Turabian StyleCavone, Cristina, Aurora Rutigliano, Pietro Cotugno, Ludovica Rolando, Chiara De Carolis, Anna Barra Caracciolo, Paola Grenni, Ilaria Savino, Antonio Bucci, Gino Naclerio, and et al. 2025. "Synergistic Effects of Lavandula angustifolia and a Bacterial Consortium on Bioremediation of a Heavy Metal-Contaminated Soil" Plants 14, no. 17: 2734. https://doi.org/10.3390/plants14172734
APA StyleCavone, C., Rutigliano, A., Cotugno, P., Rolando, L., De Carolis, C., Barra Caracciolo, A., Grenni, P., Savino, I., Bucci, A., Naclerio, G., Celico, F., Uricchio, V. F., & Ancona, V. (2025). Synergistic Effects of Lavandula angustifolia and a Bacterial Consortium on Bioremediation of a Heavy Metal-Contaminated Soil. Plants, 14(17), 2734. https://doi.org/10.3390/plants14172734












