1. Introduction
Access to safe and nutritious food is essential for human survival, making food safety and resilience of food supply chains critical global challenges. However, population growth, climate change, and increased urbanization are some of the key factors influencing the effectiveness and implementation of interventions aimed at addressing these challenges [
1]. According to United Nations projections, the global population is expected to reach 11 billion by 2100 [
2], intensifying pressure on food systems. Additionally, studies indicate that rising food demand has already led to the expansion of cropland over the past two decades [
3], with estimates suggesting an additional 18% increase by 2050 [
4]. This increase is attributed to the significant shrinkage of forestland and grassland areas, contributing to biodiversity loss and terrestrial ecosystem degradation [
3,
5,
6]. In contrast, Gao et al. [
7] projected a 12.8% decline of global cropland by the end of the 21st century, whilst considering the objective of maintaining the temperature increase to 1.5 °C above pre-industrial levels, as outlined in the Paris Agreement. These contrasting projections highlight the urgent need for integrated land use strategies that reconcile food production, biodiversity conservation, and climate mitigation.
Recent data from the Food and Agriculture Organization (FAO) reveal that over 713 million people currently suffer from undernutrition, with an alarming additional 152 million individuals potentially facing hunger between 2019 and 2023 [
8]. Africa has been identified as the most affected region; projections suggest 582 million people could experience chronic undernourished by 2030, with the majority of these individuals residing in African nations [
8]. While most prevalent in low-income countries, deficiencies in key vitamins (A and D) and minerals (zinc and iron) have also been observed in high-income nations [
9,
10]. Indeed, a key dimension of the global food crisis is micronutrient malnutrition, commonly referred to as “hidden hunger”, which extends beyond caloric deficiency, affecting over two billion people worldwide [
11,
12,
13]. Critical micronutrients such as iron (Fe), zinc (Zn), iodine (I), and selenium (Se) are often absent from the daily diet, a phenomenon that can be attributed to soil nutrient depletion due to degradation. This depletion not only undermines crop nutritional quality but also poses a significant threat to global food security and nutrition [
14,
15]. In response, global initiatives such as the Sustainable Development Goals (SDGs), particularly Zero Hunger, and the Voluntary Guidelines on Food Systems and Nutrition (VGFSyN) [
16,
17] have been implemented to address these challenges.
Dietary diversification, nutrient supplementation, and food fortification are fundamental strategies for combating nutrient deficiencies. However, biofortification—enhancing crop nutrient content through breeding, genetic engineering, or agronomic practices—has been identified as the most sustainable and cost-effective solution [
18,
19,
20,
21]. Biofortification strategies are being pursued to enhance the nutrient content of high demand crops with preferred characteristics, such as high yield, focusing on malnourished rural populations who have limited access to fortified foods and supplements [
22]. Unlike, conventional fortification, which involves the addition of fortificants during processing, biofortification enriches crops during cultivation [
21,
23]. Among biofortification strategies, agronomic biofortification—using nutrient-enhanced fertilizers—offers an effective and immediate strategy for enhancing crop nutrient content. Further optimization through advanced fertilizer formulations could maximize its potential in addressing global malnutrition [
24,
25].
Se constitutes an essential trace element for human health, playing a pivotal role in numerous biological functions within the human body. It contributes to cardiovascular, immune, and endocrine system functions and exhibits antioxidant properties linked to cancer prevention [
26,
27,
28]. Se is the structural component of selenoproteins, which contain the amino acid selenocysteine and significantly influence biological activity in humans [
28,
29]. Both prolonged deficiency and excess Se intake can have detrimental effects, with extreme toxicity (selenosis) reported in cases of overconsumption [
28]. To ensure adequate Se intake, the European Food Safety Authority (EFSA) has established age-specific recommendations: 70 μg day
−1 for adults, 15 μg day
−1 for infants aged 7–11 months, and 85 μg day
−1 for lactating women, to compensate for Se secreted in breast milk [
30]. The upper safe intake limit is set at 400 μg day
−1 by multiple regulatory bodies [
31,
32,
33].
Beyond human nutrition, Se has been studied for its effects on plant performance. According to [
34] sodium selenate (Na
2SeO
4) application in the NS of a floating system enhanced Se accumulation in chicory and lettuce, improving yield and postharvest life. Additionally, Se application at an optimal concentration can mitigate abiotic stresses (e.g., drought and salinity) in vegetable crops [
35,
36]. Nevertheless, excessive Se can be phytotoxic, negatively impacting plant growth [
37,
38]. Plants cannot absorb SeO
2 directly via their rootzone, and consequently it must be first converted into bioavailable forms such as SeO
42− or SeO
32− [
39]. One possible mechanism for the indirect accumulation of SeO
2 is the reaction of SeO
2 with water to form H
2SeO
3, which is a form of Se that is available to plants [
40]. Selenate is more soluble, less phytotoxic, and is absorbed more easily by plants compared to selenite [
41], while the toxicity of SeO
2 is influenced by its conversion to selenious acid. Consequently, selecting the optimal dose and species are critical for successful biofortification.
Neglected and underutilised species (NUS), are defined as wild, native crops that are not widely cultivated for commercial purposes and remain unknown to most consumers [
42,
43]. They are recognised for their nutritional value, resilience under harsh environmental conditions, and low crop input requirements [
44,
45,
46,
47,
48,
49,
50]. NUS can be integrated into local, small-scale cultivation systems, enhancing dietary diversity and sustainability. Their role in addressing food insecurity and biodiversity loss has garnered significant research attention [
42,
51,
52,
53,
54]. In alignment with Sustainable Development Goal 2 (SDG2), Zero Hunger, FAO launched the Future Smart Food Initiative, which seeks to improve food system sustainability and equity in hunger-prone regions [
55,
56]. The growing demand for nutritious and healthy foods has led to a significant increase in the consumption of NUS in both rural and urban areas [
57].
Despite the heightened level of interest from researchers in the biofortification approach, an essential research gap remains with regard to the evaluation of biofortification strategies for neglected and underutilised leafy vegetables. Although these species have considerable potential and could be included in biofortification protocols due to their physiological attributes, they have not yet been thoroughly investigated. Therefore, the present study aims to examine the potential role of Se biofortification in the performance, nutritional value, and antioxidant properties of three underutilised leafy vegetables ordinarily encountered in the MED region:
Portulaca oleracea L.,
Taraxacum officinale L., and
Mesembryanthemum crystallinum L., commonly known as purslane, dandelion, and iceplant, respectively. Purslane is a globally distributed nutrient-rich wild leafy vegetable that is consumed in countries across Europe, Africa, the Middle East, Asia, Australia, and the Americas [
58]. It contains essential bioactive compounds and it is also a valuable source of omega-3 fatty acids [
58] making it a nutrient-rich food that should be included in the human diet [
58]. Iceplant is a succulent known for its antioxidant and anti-inflammatory effects, attributed to its rich content of vitamins, minerals, and antioxidant compounds [
59]. Iceplant is usually grown in dry regions [
60] and it is extensively used as a food for its nutritional properties in several areas, such as India, Australia, New Zealand, and some EU countries [
61]. Dandelion is a species of significant nutritional value that can thrive in a range of climatic conditions and is found in several regions [
62]. Dandelion has been used traditionally in diets due to its high nutrient content and antioxidant properties, while several studies have focused on its additional pharmaceutical effects [
63]. Finally, this study will ascertain the optimal Se dosage in relation to plant performance while considering recommended dietary intake levels. Our findings will advance understanding of Se biofortification in soilless cultivation systems, supporting sustainable agriculture and improved nutrition, particularly in Se-deficient regions (China, New Zealand, and northern Europe) [
64].
3. Discussion
This study demonstrates that moderate SeO
2 supplementation (1–2 μM) enhances growth in all three neglected and underutilised crops, whereas a high dose (4 μM) induces mild stress in iceplant. Numerous studies suggest a positive correlation between optimal Se concentrations and improved crop physiological characteristics, whether Se is applied exogenously via foliar spray, through the nutrient solution, or directly into the soil [
34,
65,
66,
67]. However, at elevated concentrations, the outcomes are often contradictory, including reduced growth, yield decline, and phytotoxicity [
38,
68,
69]. No significant yield differences across Se treatments (0 μM, 2.6 μM, 3.9 μM and 5.2 μM) in lettuce, wild rocket, spinach, and purslane were observed by [
70]. The upper tolerable Se limits for plants depend on their accumulation capacity and tissue tolerance, with species-specific variation. Consequently, plants have been classified according to this ability as hyperaccumulators (>1000 mg Se kg
−1 DW), secondary accumulators (100–1000 mg Se kg
−1 DW), and non-accumulators (<100 mg Se kg
−1 DW) [
71]. Although the three NUS we examined are known for their potential to accumulate diverse metals, none of them have been qualified as Se hyperaccumulators or secondary accumulators. The only detrimental effect observed was on iceplant at 4 μM SeO
2, which exhibited altered growth traits. The findings of our study suggest that SeO
2 biofortification at 1, 2, and 4 μM in the NS positively impacted growth performance and nutritional value in the three species. In contrast, 1 μM and 4 μM SeO
2 were most effective in enhancing antioxidant activity, although the higher dose (4 μM) may stress certain species.
The effectiveness of biofortification depends on two primary factors: the form of Se employed, (organic or inorganic), and the biofortification method used (foliar, through NS, or soil application). Since plants do not absorb Se directly in the form of SeO
2, it must first be converted into bioavailable forms such as SeO
42− or SeO
32− [
39] for root uptake. Consequently, SeO
2 has not been widely adopted in biofortification strategies. However, ref. [
72] demonstrated that SeO
2 at 2.0 μM enhanced total yield in both ungrafted and grafted cherry tomatoes grown in a soilless system. Similarly, in the current study, SeO
2 application via the rootzone improved growth characteristics of the examined species. More specifically, purslane’s fresh biomass, number of leaves, and leaf area increased uniformly at 1, 2, and 4 μM SeO
2. Dandelion’s fresh weight increased only at the 4 μM SeO
2 dose, while the number of leaves and the leaf area showed an increase at 2 and 4 μM SeO
2. Iceplant’s growth traits improved at 1 and 2 μM SeO
2 treatments, while the highest SeO
2 dose suppressed growth. These outcomes align with [
73] who reported that foliar Na
2SeO
4 (260 μM) significantly increased biomass, stem length, number of stems, and diameter in wild
P. oleracea compared to the cultivated Makovey variety, underlining the ability of Se to act as a growth stimulator at specific concentrations. Reference [
68] observed similar dose-dependent effects in hydroponically cultivated
Valerianella locusta L. treated with four distinct doses of Se using Na
2SeO
4 via the NS solution (0, 5, 10, 10, and 20 μM). The study concluded that plants exposed to a 5 μM Se dose increased their fresh and dry weight when harvested at 66 days. In contrast, 20 μM Se resulted in reduced growth, highlighting the narrow window between beneficial and toxic Se levels. The positive impact of Se on plants’ growth performance can be attributed to the improvement of a plant’s photosynthetic capacity and root growth, as well as improved nutritional status [
35,
74]. Se’s toxic effects are often attributed to malformed selenoproteins, which arise from the incorrect incorporation of Se-containing amino acids (selenocysteine or selenomethionine) in place of cysteine or methionine [
75]. This misincorporation can lead to Se toxicity, disrupting cellular processes. Additionally, Se may act as a pro-oxidant, contributing to oxidative stress, despite its well-known antioxidant properties [
76,
77]. Studies have also linked Se toxicity with lipid peroxidation and cell membranes damage [
78]. Increased lipid peroxidation in lettuce plants has been observed under high Se concentrations, particularly in plants treated with Na
2SeO
3 [
78]. This outcome is supported by the strong negative correlation between Se leaf content and MDA content. Similar results were obtained by [
79], who reported that the high Se toxicity in broccoli plants with low S nutrition was attributed to an increased Se/total Se ratio in proteins, which enhanced the generation of ROS and elevated lipid peroxidation. This caused increased cell membrane damage and reduced antioxidant enzyme activities. As shown in
Table 1, purslane’s dry matter content (DMC), a common stress indicator related to growth rate [
80,
81], was not significantly altered under SeO
2 treatments. On the contrary, dandelion’s DMC decreased significantly with increasing SeO
2 doses, suggesting a potential role of Se in modulating antioxidant responses. The results of iceplant’s growth traits are in line with the observed increase in DMC in both the 0 and 4 μM SeO
2 treatments, which may reflect mild stress under high Se exposure.
The effect of Se on macronutrient accumulation remains a contentious research topic due to its dependence on multiple factors, including plant species, Se chemical form, environmental conditions, and biofortification methods. The findings of the present study indicate that the nutritional status of dandelion and iceplant with regard to macronutrient concentration was affected by varying SeO
2 levels. Conversely, the concentration of macronutrients in the above-ground biomass of purslane remained unaffected. More specifically, in iceplant, the total nitrogen and NO
3− content of the above-ground parts decreased under SeO
2 exposure. In contrast, dandelion exhibited a significant increase in NO
3− concentration at 2 and 4 μM SeO
2 and in total N at 4 μM SeO
2. The P content in both species (iceplant and dandelion) reduced at 2 and 4 μM SeO
2, an outcome that could be attributed to the fact that Se, mostly in the form of selenite, shares the same transport channel with P in plants [
82,
83]. The impacts of Se on Ca and Mg concentrations were observed exclusively in iceplant, where a decline was mainly exhibited at 4 μM SeO
2, while Na increased at this Se level. In the research conducted by [
84] mature lettuce and basil plants were treated with different concentrations of Na
2SeO
4 (0, 1, and 3 mg Se L
−1 for lettuce and 0, 2, and 3 mg Se L
−1 for basil) prior to transplantation. While the macronutrient concentrations (P, K, Na, Ca) remained unaffected in both species, the NO
3− concentration in lettuce decreased under the 3 mg Se L
−1 treatment, whereas basil showed no significant change. Conversely, Mg content in basil plants increased at the highest dose (3 mg Se L
−1). Similarly, ref. [
85] evaluated the effects of two Na
2SeO
4 doses on the nutritional status of two
Valerianella locusta cultivars (Baron and Gala), revealing differential interactions between Se and macronutrient accumulation, even within the same plant species. At the highest Se level, the Baron cultivar increased its K, P, Ca, Mg, and S leaf contents, whereas the Gala cultivar showed elevated S and P levels under both Se doses (10 μM and 40 μM Se). Contrasting findings have been reported regarding Se’s influence on nitrate (NO
3−) absorption. The study by [
86] observed no significant effects of Na
2SeO
4 on NO
3− uptake in spinach, while [
87] reported reduced NO
3− content in green and purple basil, tatsoi, and coriander microgreens under Se application. This discrepancy may stem from the competitive relationship of Se with N assimilation. Since nitrate content in the edible part of leafy vegetables is a significant quality indicator, the European Union has established maximum permissible levels under the Commission Regulation (EU) No 1258/2011 [
88]. In our study, the nitrate content of the three cultivated NUS was well below the EU regulatory thresholds, accounting for seasonal growth conditions.
The Se biofortification intervention had a significant impact on the micronutrient status of the three domestic NUS. However, the effects varied considerably among plant species. In dandelion, B content decreased with Se application, whereas in iceplant, 1 μM and 2 μΜ SeO
2 enhanced B absorption. In contrast, purslane biomass exhibited no change in B content. Fe accumulation in the three NUS was unaffected by Se doses, in contrast with other studies that reported an interaction between Se and Fe [
84,
87,
89], suggesting that Se-nutrient interactions may be context- and species-dependent. SeO
2 application significantly reduced Mn concentration only in iceplant, which is consistent with the findings of [
70] that highlighted negative Se and Mn correlations. However, ref. [
74] observed increased Mn content in lettuce following soil application of Se nanomaterials, further underscoring the variability in Se effects, while Zn absorption responded differentially across species. Specifically, in purslane, 1 μM and 2 μM SeO
2 increased Zn uptake, while the highest dose had an inhibitory effect. Dandelion also exhibited increased an Zn concentration at 2 μM SeO
2. On the contrary, Zn concentration in iceplant declined progressively with increasing SeO
2 doses. Scientific literature presents conflicting results regarding Se’s impact on Zn uptake. For instance, ref. [
90] reported increased Zn absorption in both roots and leaves of Chinese cabbage when the soil was supplemented with L-Selenomethionine. Conversely, another study [
91] indicated that excessive foliar SeO
32− (100 and 200 mg L
−1) reduced Zn content in
Brassica rapa var. rapa L., whereas the lowest dose (50 mg L
−1) had no effect [
92]. Statistical analysis in the present study demonstrated that the application of 2 μM SeO
2 enhanced Cu concentration in both purslane and dandelion above-ground biomass. However, the Cu content in iceplant remained unaffected. Existing literature shows considerable heterogeneity in Se’s impact on Cu uptake— in particular, the results of studies reporting Se-induced Cu accumulation in leafy vegetables [
74,
84]. Conversely, other studies observed reduced Cu uptake when different forms of Se (Na
2SeO
4 and SeO
32−) were applied via NS or foliar application [
89,
91]. Reference [
93] further highlights this variability. In lettuce, fertigation with Na
2SeO
3 in nutrient solutions at eight different Se concentrations (0, 5, 10, 20, 40, 60, 80, and 120 µM) increased Cu absorption, reaching its highest levels at 60 μM, beyond which Cu declined. Contrary to this, Na
2SeO
4 application reduced Cu content compared to the Se-free control. For all the above findings, it is evident that the interaction between Se and both macro- and microelements are highly context-dependent, and influenced by factors such as Se form, dose, application method, and plant species. While some studies show increased mineral levels, others report reductions. This indicates a complex, genotype-specific interaction rather than a universal trend.
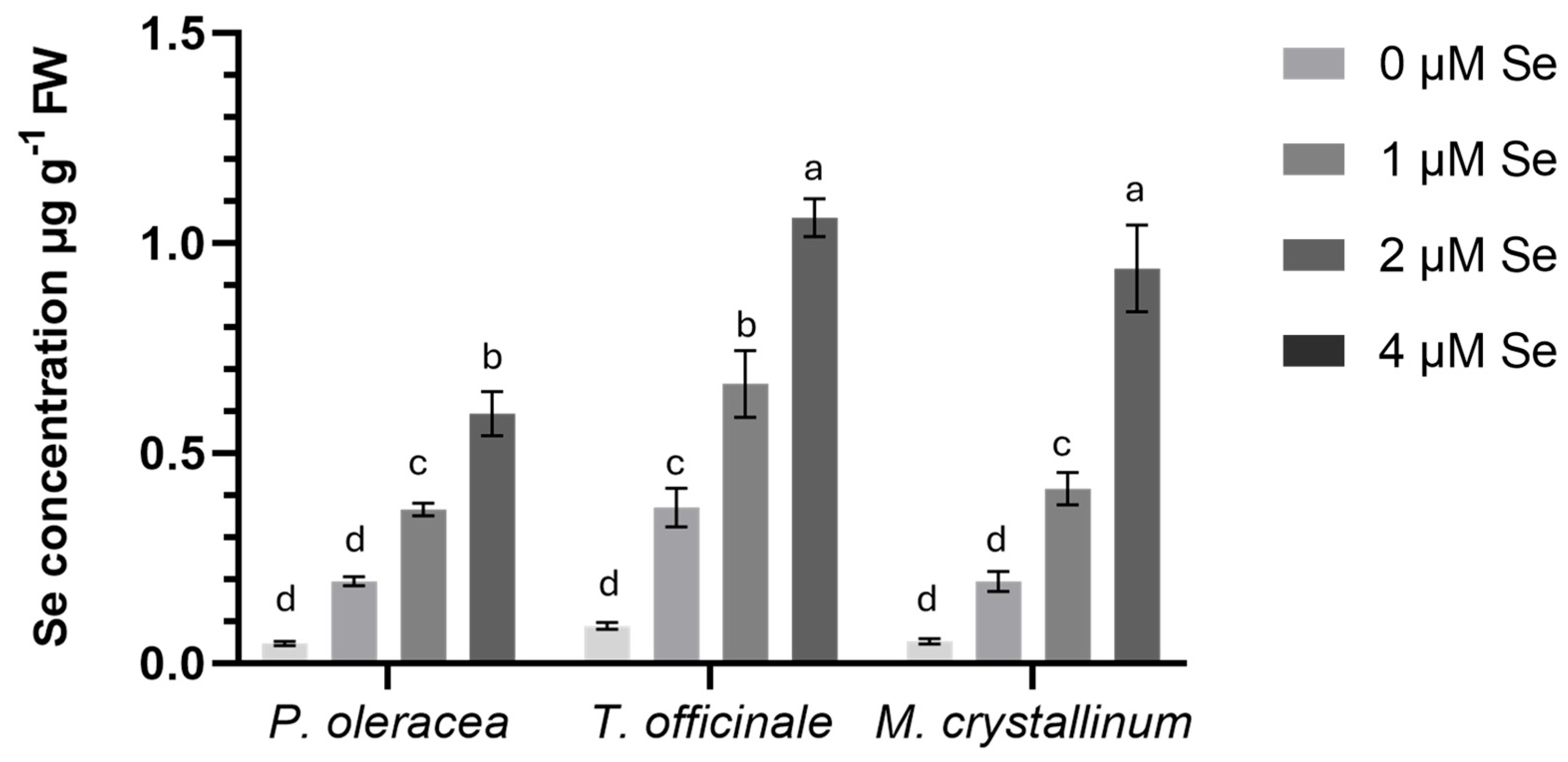
As illustrated in
Figure 1, the accumulation of Se enhanced progressively in all three examined NUS with increasing SeO
2 doses. These findings are consistent with those of numerous studies on Se biofortification across different plant species, Se forms and doses, and cropping systems [
68,
70,
72,
73,
84,
85,
86,
92,
94,
95,
96,
97,
98,
99,
100,
101]. Among the three cultivated species, dandelion and iceplant exhibited the highest Se accumulation at 4 μM SeO
2, with concentrations of 1.1 μg g
−1 and 0.9 μg g
−1 Se (fresh biomass), respectively.
Portulaca exhibited lower Se uptake, with a mean value of 0.6 μg g
−1 Se (fresh above-ground tissue) at the same dose. These results contrast with a study [
95] reporting that aeroponically grown dandelion treated with 7 μg ml
−1 Se as Na
2SeO
4 accumulated less than parsley, lamb’s lettuce, and chicory. This phenomenon, according to the authors, has been ascribed to the dandelion’s underdeveloped root system. For iceplant, ref. [
102] found that selenate (SeO
42−) treatment at 100 μΜ, 300 μΜ, and 400 μM led to high Se accumulation in leaves, suggesting iceplant’s potential as a Se hyperaccumulator. On the other hand, selenite (SeO
32−) caused significant growth inhibition at 300 μM and total damage at 500 μM. Purslane, a nutrient-rich species, has been widely studied in Se biofortification. Studies have shown that purslane can accumulate Se in various forms (Na
2SeO
4 and Na
2SeO
3) and through different application methods (foliar application, soil drenching, fertigation) [
73,
84,
100]. The effectiveness of Se biofortification and its dietary impact on consumers is summarised in
Table 5. Such indices are analysed in [
103]. The corresponding indicators are considered important to include in studies related to biofortification strategies of trace elements, to improve and ensure both efficacy and consumer safety.
It is well documented that, at certain concentrations, selenium is capable of mitigating oxidative stress in plants by stimulating their antioxidant activity [
76,
104,
105]. The findings of the present study reveal species-specific variations in the Se concentrations that positively affect plant antioxidant capacity and secondary metabolite levels. Notably, purslane exhibited the highest antioxidant capacity, total phenolic, and flavonoid content at 1 μM SeO
2, while iceplant also showed improved antioxidant capacity at the same concentration. In contrast, dandelion achieved its highest antioxidant capacity at elevated SeO
2 levels. These outcomes are consistent with the respective yield performance of each species. In [
105], it is reported that foliar or NS application of Na
2SeO
4 at 10 μM enhanced antioxidant activity and total phenolic content in sweet basil, although total flavonoid content remained unaffected by Se doses or application method. Conversely, another study on
Valerianella locusta L., found that moderate Na
2SeO
4 concentrations in the NS increased antioxidant capacity, total phenolic and flavonoid content, whereas higher doses diminished these parameters [
68]. These results are consistent with those observed in purslane and iceplant. In addition, elevated Na
2SeO
3 concentrations (8 and 10 mg L
−1) reduced total phenolic and flavonoid content of dandelion seedlings, whereas lower doses have been found to promote these characteristics in comparison to Se-untreated plants [
82]. The relationship between Se and the antioxidant properties of plants has been widely documented [
72,
99,
101,
103]. Antioxidant activity in plants is essential critical indicator influenced by a complex biochemical interaction within plant extracts [
105], although the precise mechanisms of Se’s effects remain incompletely understood. The positive impact of Se, at low concentrations, on the antioxidant properties of plants may be attributed to two key factors: (a) increased non-enzymatic antioxidants such as phenolic compounds, flavonoids, and ascorbic acid [
99,
104,
105], and (b) a correlation between Se and the biosynthesis of glutathione peroxidase (GSx), guaiacol peroxidase (GPOX), thioredoxin reductase (TrxR), and antioxidant enzymes, that play a pivotal role in protecting cells from oxidative damage by scavenging reactive oxygen species (ROS) [
82,
99,
103,
104]. However, enzyme responses vary by species and Se dosage. For instance, ref. [
106] observed decreased GSH-Px and POD activity at high Se levels, while SOD activity increased progressively. On the other hand, ref. [
107] reported elevated SOD, CAT, POD, APX, and GR activity in wild quinoa under increasing Se concentrations. Another study [
105] suggested that Se’s influence on antioxidant activity is more likely mediated by its effect on secondary metabolite biosynthesis rather than direct antioxidant action. In conclusion, the decline in antioxidant capacity in purslane and iceplant can be ascribed to the pro-oxidant effect that is evident at elevated concentrations of Se [
108,
109]. An alternative hypothesis, that requires further examination, is that excessive Se may upregulate antioxidant enzyme activities (POD, SOD, and GSx), while suppressing secondary metabolite pathways, explaining the reduced antioxidant properties observed in purslane and iceplant under excess Se.