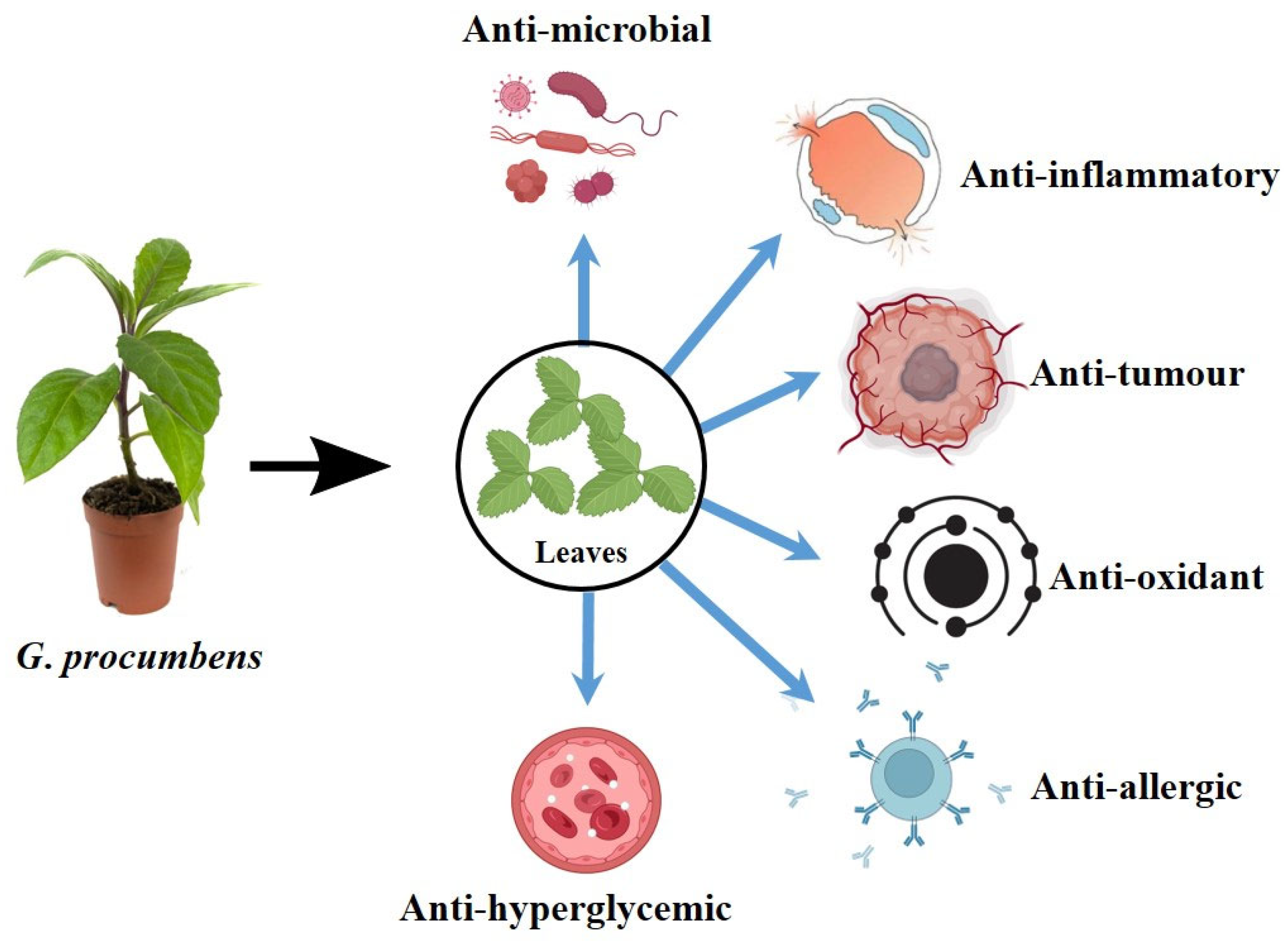
Pharmacological Overview of Bioactive Natural Products from Gynura procumbens (Lour.) Merr
Abstract
1. Introduction
| SL No. | Activity | Mechanisms and Effects | References |
|---|---|---|---|
| 1 | Antioxidant | Scavenges free radicals and upregulates antioxidant enzymes (SOD, CAT, and GPx) | [6,7] |
| 2 | Anti-inflammatory | Inhibits COX-2, NF-κB, TNF-α, and IL-6 pathways | [15] |
| 3 | Antidiabetic | Enhances insulin sensitivity, lowers blood glucose, and inhibits α-glucosidase | [16] |
| 4 | Antihypertensive | Vasodilatory effect and improves endothelial function | [17] |
| 5 | Anticancer | Induces apoptosis, inhibits proliferation, angiogenesis, and metastasis | [18,19] |
| 6 | Hepatoprotective | Protects liver tissue from oxidative and chemical-induced injury | [20] |
| 7 | Antimicrobial | Active against bacteria and fungi; potentiates antibiotics | [14] |
| 8 | Hypolipidemic | Lowers total cholesterol and triglycerides; increases HDL | [21,22] |
| 9 | Wound healing | Promotes tissue regeneration and reduces inflammation | [23] |
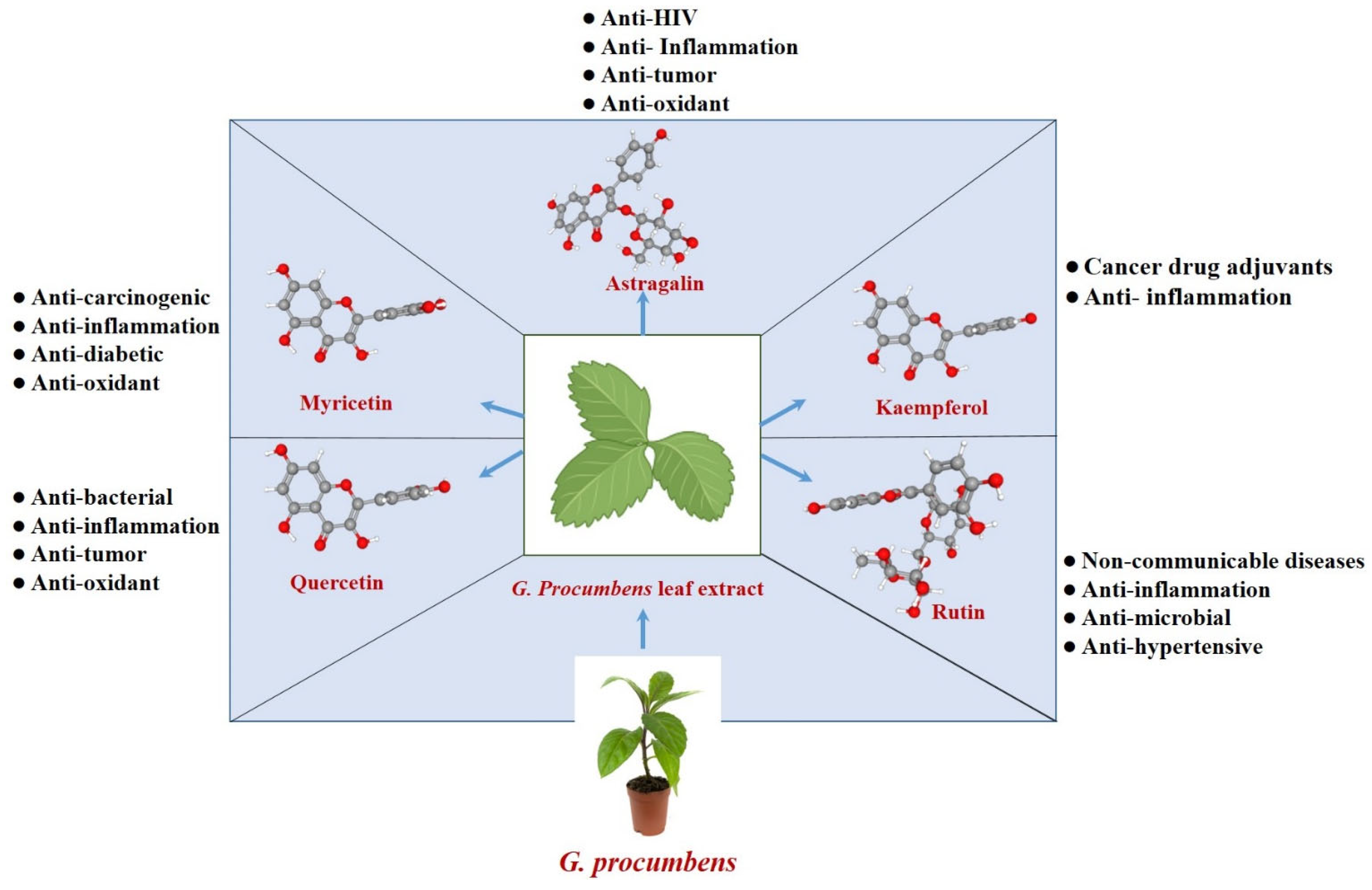
2. Flavonoids Synthesized by G. procumbens
2.1. Astragalin (Kaempferol-3-O-β-D-glucopyranoside)
2.2. Kaempferol
2.3. Myricetin
2.4. Quercetin
2.5. Rutin
3. Broad-Spectrum Pharmacological Activities of G. Procumbens
3.1. In Traditional Medicine
3.2. Antihypertensive Properties
3.3. Antiglycaemic Properties
3.4. Anticancer Properties
3.5. Antimicrobial Properties
3.6. Antioxidant Properties
3.7. Reproductive Function
3.8. Organ Protective Properties
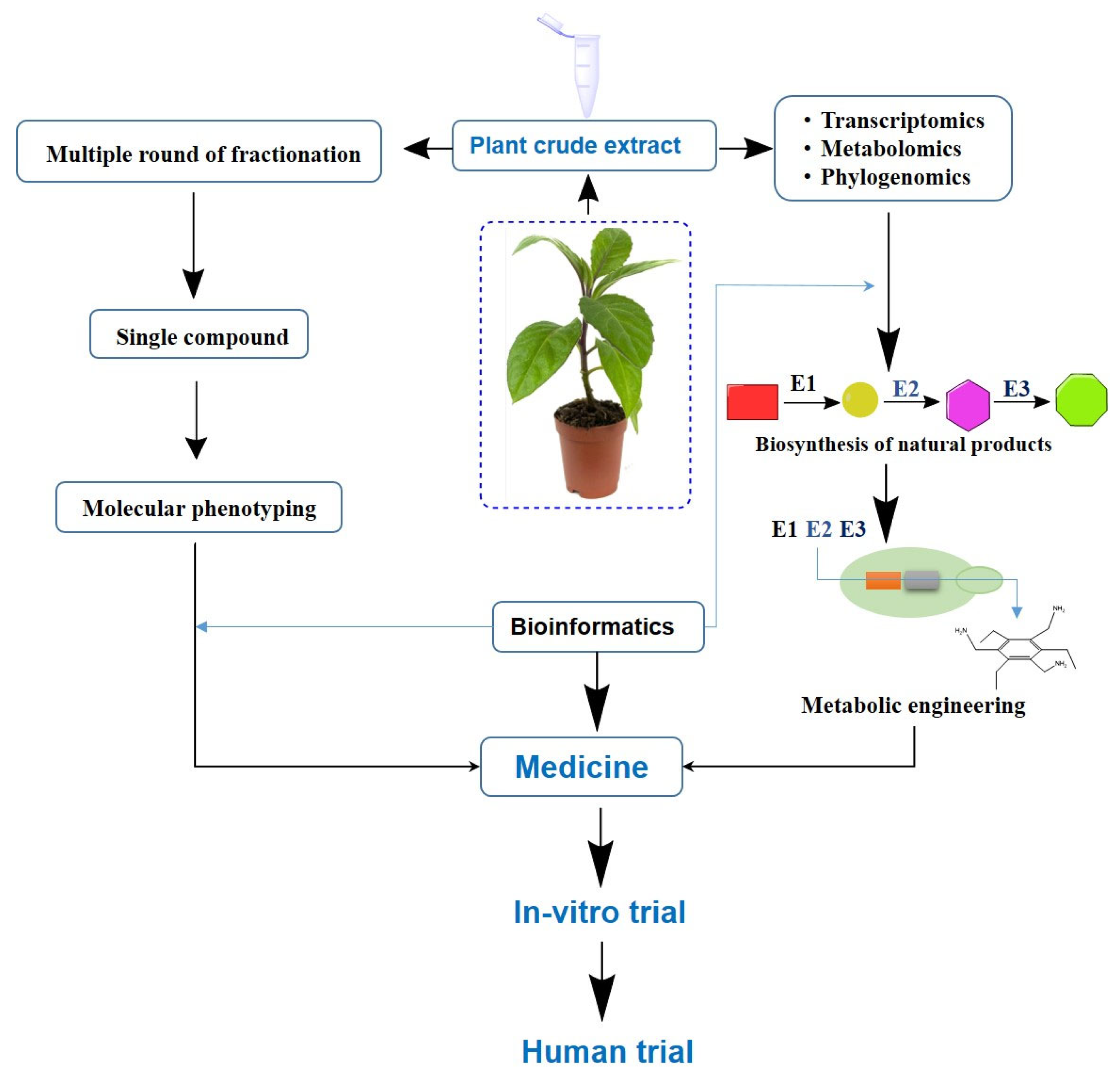
4. Modern Tools in Phytochemical Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SODE | superoxide dismutase |
| CAT | catalase |
| GPx | glutathione peroxidase |
| COX-2 | cyclooxygenase-2 |
| NF-κB | nuclear factor kappa-light chain enhancer of activated B cells |
| TNF-α | tumor necrosis factor-alpha |
| IL-6 | interleukin-6 |
| LDL | low-density lipoprotein |
| HDL | high-density lipoprotein |
| TLR4 | Toll-like receptor 4 |
| HO-1 | heme oxygenase-1 |
| MAPK | mitogen-activated protein kinase |
| BMP | bone morphogenetic protein |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| ATP | adenosine triphosphate |
| ROS | reactive oxygen species |
| VEGF | vascular endothelial growth factor |
| GST | glutathione S-transferase |
| AMPK | AMP-activated protein kinase |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| HPLC | high-performance liquid chromatography |
| GC-MS | gas chromatography–mass spectrometry |
| LC-MS/MS | liquid chromatography–mass spectrometry tandem mass spectrometry |
| HTS | high-throughput screening |
| ADMET | absorption, distribution, metabolism, excretion, and toxicity |
References
- Seth, C.; Schmid, V.; Mueller, S.; Haykowsky, M.; Foulkes, S.J.; Halle, M.; Wernhart, S. Diabetes, obesity, and cardiovascular disease—What is the impact of lifestyle modification? Herz 2025, 1–6. [Google Scholar] [CrossRef]
- Palileo-Villanueva, L.M.; Palafox, B.; Amit, A.M.L.; Pepito, V.C.F.; Ab-Majid, F.; Ariffin, F.; Balabanova, D.; Isa, M.-R.; Mat-Nasir, N.; My, M.; et al. Prevalence, determinants and outcomes of traditional, complementary and alternative medicine use for hypertension among low-income households in Malaysia and the Philippines. BMC Complement. Med. Ther. 2022, 22, 252. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Ghosh, D.; Adhikary, S.; Bhattacherjee, P.; Debnath, S. Herbal Medicine for Health Management and Disease Prevention BT—Herbal Medicine Phytochemistry: Applications and Trends; Izah, S.C., Ogwu, M.C., Akram, M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–35. ISBN 978-3-031-21973-3. [Google Scholar]
- Fayiah, M.; Fayiah, M.S.; Saccoh, S.; Kallon, M.K. Value of Herbal Medicine to Sustainable Development BT—Herbal Medicine Phytochemistry: Applications and Trends; Izah, S.C., Ogwu, M.C., Akram, M., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 1429–1456. ISBN 978-3-031-43199-9. [Google Scholar]
- Rosidah; Mun, Y.; Amirin, S.; Asmawi, M. Antioxidant Potential of Gynura procumbens. Pharm. Biol. 2008, 46, 616–625. [Google Scholar] [CrossRef]
- Kaewseejan, N.; Sutthikhum, V.; Siriamornpun, S. Potential of Gynura procumbens leaves as source of flavonoid-enriched fractions with enhanced antioxidant capacity. J. Funct. Foods 2015, 12, 120–128. [Google Scholar] [CrossRef]
- Hew, C.-S.; Khoo, B.-Y.; Gam, L.-H. The Anti-Cancer Property of Proteins Extracted from Gynura procumbens (Lour.) Merr. PLoS ONE 2013, 8, e68524. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, G.A.; Sadikun, A.; Mariam, A. Flavonoid Identification and Hypoglycaemic Studies of the Butanol Fraction from Gynura procumbens. Pharm. Biol. 2002, 40, 405–410. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Deng, G.-F.; Lin, X.; Xu, X.-R.; Gao, L.-L.; Xie, J.-F.; Li, H.-B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Jobaer, M.A.; Ashrafi, S.; Ahsan, M.; Hasan, C.M.; Rashid, M.A.; Islam, S.N.; Masud, M.M. Phytochemical and Biological Investigation of an Indigenous Plant of Bangladesh, Gynura procumbens (Lour.) Merr.: Drug Discovery from Nature. Molecules 2023, 28, 4186. [Google Scholar] [CrossRef]
- Tristantini, D.; Setiawan, H.; Santoso, L.L. Feasibility Assessment of an Encapsulated Longevity Spinach (Gynura procumbens L.) Extract Plant in Indonesia. Appl. Sci. 2021, 11, 4093. [Google Scholar] [CrossRef]
- Tan, H.-L.; Chan, K.-G.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Gynura procumbens: An Overview of the Biological Activities. Front. Pharmacol. 2016, 7, 52. [Google Scholar] [CrossRef]
- Mascaraque, C.; Aranda, C.; Ocón, B.; Monte, M.J.; Suárez, M.D.; Zarzuelo, A.; Marín, J.J.G.; Martínez-Augustin, O.; de Medina, F.S. Rutin has intestinal antiinflammatory effects in the CD4+ CD62L+ T cell transfer model of colitis. Pharmacol. Res. 2014, 90, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ganjayi, M.S.; Sankaran, K.R.; Meriga, B.; Bhatia, R.; Sharma, S.; Kondepudi, K.K. Astragalin and rutin restore gut microbiota dysbiosis, alleviate obesity and insulin resistance in high-fat diet-fed C57BL/6J mice. Food Sci. Hum. Wellness 2024, 13, 3256–3265. [Google Scholar] [CrossRef]
- Kim, M.-J.; Lee, H.J.; Wiryowidagdo, S.; Kim, H.K. Antihypertensive effects of Gynura procumbens extract in spontaneously hypertensive rats. J. Med. Food 2006, 9, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Paravati, M.R.; Scarlata, G.G.M.; Milanović, M.; Milić, N.; Abenavoli, L. The anticancer activity of quercetin, luteolin, myricetin, and kaempferol in the development of hepatocellular carcinoma: A narrative review. Hepatoma Res. 2024, 10, 43. [Google Scholar] [CrossRef]
- Luo, H.; Gary, O.R.; Lingzhi, L.; Matthew, K.D.; Bing-Hua, J.; Chen, Y.C. Kaempferol Inhibits Angiogenesis and VEGF Expression Through Both HIF Dependent and Independent Pathways in Human Ovarian Cancer Cells. Nutr. Cancer 2009, 61, 554–563. [Google Scholar] [CrossRef]
- Shill, M.C.; Jalal, M.F.B.; Shuma, M.L.; Mollick, P.P.; Muhit, M.A.; Halder, S. Gynura procumbens leaf extract-loaded self-microemulsifying drug delivery system offers enhanced protective effects in the hepatorenal organs of the experimental rats. PLoS ONE 2025, 20, e0304435. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, Z.; Gao, W.; Pu, L.; Wei, J.; Guo, C. Quercetin regulates hepatic cholesterol metabolism by promoting cholesterol-to-bile acid conversion and cholesterol efflux in rats. Nutr. Res. 2016, 36, 271–279. [Google Scholar] [CrossRef]
- Al-Rejaie, S.S.; Aleisa, A.M.; Sayed-Ahmed, M.M.; AL-Shabanah, O.A.; Abuohashish, H.M.; Ahmed, M.M.; Al-Hosaini, K.A.; Hafez, M.M. Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat. BMC Complement. Altern. Med. 2013, 13, 136. [Google Scholar] [CrossRef]
- Li, C.; Hu, M.; Jiang, S.; Liang, Z.; Wang, J.; Liu, Z.; Wang, H.-M.D.; Kang, W. Evaluation Procoagulant Activity and Mechanism of Astragalin. Molecules 2020, 25, 177. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Kamalakkannan, N.; Prince, P.S.M. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kaewseejan, N.; Siriamornpun, S. Bioactive components and properties of ethanolic extract and its fractions from Gynura procumbens leaves. Ind. Crops Prod. 2015, 74, 271–278. [Google Scholar] [CrossRef]
- Krishnan, V.; Ahmad, S.; Mahmood, M. Antioxidant Potential in Different Parts and Callus of Gynura procumbens and Different Parts of Gynura bicolor. Biomed Res. Int. 2015, 2015, 147909. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, Z.; Lu, H.; Liu, F.; Zhou, D.; Zou, Y. Astragalin Exerted Hypoglycemic Effect by Both Inhibiting α-Glucosidase and Modulating AMPK Signaling Pathway. Nutrients 2025, 17, 406. [Google Scholar] [CrossRef]
- Sun, M.; Ye, H.; Zheng, C.; Jin, Z.; Yuan, Y.; Weng, H. Astragalin ameliorates renal injury in diabetic mice by modulating mitochondrial quality control via AMPK-dependent PGC1α pathway. Acta Pharmacol. Sin. 2023, 44, 1676–1686. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, A.; Wang, X.; Sun, H. A kaempferol-3-O-β-d-glucoside, intervention effect of astragalin on estradiol metabolism. Steroids 2019, 149, 108413. [Google Scholar] [CrossRef]
- Li, W.; Hao, J.; Zhang, L.; Cheng, Z.; Deng, X.; Shu, G. Astragalin Reduces Hexokinase 2 through Increasing miR-125b to Inhibit the Proliferation of Hepatocellular Carcinoma Cells in Vitro and in Vivo. J. Agric. Food Chem. 2017, 65, 5961–5972. [Google Scholar] [CrossRef]
- Zeb, A.; Khan, W.; Ul Islam, W.; Khan, F.; Khan, A.; Khan, H.; Khalid, A.; Rehman, N.U.; Ullah, A.; Al-Harrasi, A. Exploring the Anticancer Potential of Astragalin in Triple Negative Breast Cancer Cells by Attenuating Glycolytic Pathway through AMPK/mTOR. Curr. Med. Chem. 2024, 31, 1–14. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, J.; Li, X.; Lin, Q. The flavonoid Astragalin shows anti-tumor activity and inhibits PI3K/AKT signaling in gastric cancer. Chem. Biol. Drug Des. 2021, 98, 779–786. [Google Scholar] [CrossRef]
- Xing, F.; Geng, L.; Guan, H.; Liu, D.; Li, Y.; Zeng, L.; Chen, Y.; Tian, R.; Li, Z.; Cao, R.; et al. Astragalin mitigates inflammatory osteolysis by negatively modulating osteoclastogenesis via ROS and MAPK signaling pathway. Int. Immunopharmacol. 2022, 112, 109278. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.-M.; Guo, Z.; Wang, J.; Ma, H.-Y.; Qin, X.-Y.; Hu, Y.; Lan, R. Astragalin alleviates lipopolysaccharide-induced depressive-like behavior in mice by preserving blood-brain barrier integrity and suppressing neuroinflammation. Free Radic. Biol. Med. 2025, 232, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A.; Allemailem, K.S.; Alwanian, W.M.; Alharbi, B.F.; Alrumaihi, F.; Khan, A.A.; Almatroodi, S.A.; Rahmani, A.H. Effects and Mechanisms of Kaempferol in the Management of Cancers through Modulation of Inflammation and Signal Transduction Pathways. Int. J. Mol. Sci. 2023, 24, 8630. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Wang, Y.; Wang, S.; Li, J.; Li, Z.; Wei, Z.; Li, J.; Bian, Y. Kaempferol regulating macrophage foaming and atherosclerosis through Piezo1-mediated MAPK/NF-κB and Nrf2/HO-1 signaling pathway. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Li, H.; Weng, Q.; Gong, S.; Zhang, W.; Wang, J.; Huang, Y.; Li, Y.; Guo, J.; Lan, T. Kaempferol prevents acetaminophen-induced liver injury by suppressing hepatocyte ferroptosis via Nrf2 pathway activation. Food Funct. 2023, 14, 1884–1896. [Google Scholar] [CrossRef]
- Li, N.; Chen, S.; Deng, W.; Gong, Z.; Guo, Y.; Zeng, S.; Xu, Q. Kaempferol Attenuates Gouty Arthritis by Regulating the Balance of Th17/Treg Cells and Secretion of IL-17. Inflammation 2023, 46, 1901–1916. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Hou, J.; Zheng, Y.; Lin, C.; Ren, J. Protective Effect of Kaempferol on LPS plus ATP-Induced Inflammatory Response in Cardiac Fibroblasts. Inflammation 2015, 38, 94–101. [Google Scholar] [CrossRef]
- Ko, C.-H.; Shen, S.-C.; Lee, T.J.F.; Chen, Y.-C. Myricetin inhibits matrix metalloproteinase 2 protein expression and enzyme activity in colorectal carcinoma cells. Mol. Cancer Ther. 2005, 4, 281–290. [Google Scholar] [CrossRef]
- Hou, D.-D.; Gu, Y.-J.; Wang, D.-C.; Niu, Y.; Xu, Z.-R.; Jin, Z.-Q.; Wang, X.-X.; Li, S.-J. Therapeutic effects of myricetin on atopic dermatitis in vivo and in vitro. Phytomedicine 2022, 102, 154200. [Google Scholar] [CrossRef]
- Huang, D.; Bai, S.; Qiu, G.; Jiang, C.; Huang, M.; Wang, Y.; Zhong, M.; Fang, J.; Cheng, J.; Zhao, X.; et al. Myricetin ameliorates airway inflammation and remodeling in asthma by activating Sirt1 to regulate the JNK/Smad3 pathway. Phytomedicine 2024, 135, 156044. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Lee, J.-H.; Woo, J.-S.; Jung, G.-H.; Jung, S.-H.; Han, E.-J.; Kim, B.; Cho, S.D.; Nam, J.S.; Che, J.H.; et al. Myricetin induces apoptosis and autophagy in human gastric cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Heliyon 2022, 8, e09309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-L.; Zhang, P.-M.; Jiang, M.; Yu, S.-W.; Wang, L. Myricetin induces apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signalling in human colon cancer cells. BMC Complement. Med. Ther. 2020, 20, 209. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Yang, X.; Li, H.; Xiao, X.; You, J.; Li, H.; Zheng, L.; Yi, C.; Li, Z.; et al. Quercetin inhibited LPS-induced cytokine storm by interacting with the AKT1-FoxO1 and Keap1-Nrf2 signaling pathway in macrophages. Sci. Rep. 2024, 14, 20913. [Google Scholar] [CrossRef]
- Lu, X.-L.; Zhao, C.-H.; Yao, X.-L.; Zhang, H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef]
- Sun, G.Y.; Chen, Z.; Jasmer, K.J.; Chuang, D.Y.; Gu, Z.; Hannink, M.; Simonyi, A. Quercetin Attenuates Inflammatory Responses in BV-2 Microglial Cells: Role of MAPKs on the Nrf2 Pathway and Induction of Heme Oxygenase-1. PLoS ONE 2015, 10, e0141509. [Google Scholar] [CrossRef]
- Luo, X.; Bao, X.; Weng, X.; Bai, X.; Feng, Y.; Huang, J.; Liu, S.; Jia, H.; Yu, B. The protective effect of quercetin on macrophage pyroptosis via TLR2/Myd88/NF-κB and ROS/AMPK pathway. Life Sci. 2022, 291, 120064. [Google Scholar] [CrossRef]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac. Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumari, A.; Jagdale, P.; Ayanur, A.; Pant, A.B.; Khanna, V.K. Potential of Quercetin to Protect Cadmium Induced Cognitive Deficits in Rats by Modulating NMDA-R Mediated Downstream Signaling and PI3K/AKT—Nrf2/ARE Signaling Pathways in Hippocampus. NeuroMol. Med. 2023, 25, 426–440. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Li, L.-J.; Dong, Q.-X.; Zhu, J.; Huang, Y.-R.; Hou, S.-J.; Yu, X.-L.; Liu, R.-T. Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J. Neuroinflamm. 2021, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Z.; Dong, R.; Liu, P.; Zhang, X.; Li, Y.; Lai, X.; Cheong, H.-F.; Wu, Y.; Wang, Y.; et al. Rutin ameliorated lipid metabolism dysfunction of diabetic NAFLD via AMPK/SREBP1 pathway. Phytomedicine 2024, 126, 155437. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, X.; Liu, Y.; Zhao, T.; Sun, Z.; Liu, P.; Xiang, Q.; Xiong, J.; Du, X.; Yang, X.; et al. Rutin alleviates EndMT by restoring autophagy through inhibiting HDAC1 via PI3K/AKT/mTOR pathway in diabetic kidney disease. Phytomedicine 2023, 112, 154700. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, X.; Yue, C. Rutin Ameliorates Inflammation and Oxidative Stress in Ulcerative Colitis by Inhibiting NLRP3 Inflammasome Signaling Pathway. Cell Biochem. Biophys. 2024, 82, 3715–3726. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv. Pharmacol. Pharm. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Yang, C.-Z.; Wang, S.-H.; Zhang, R.-H.; Lin, J.-H.; Tian, Y.-H.; Yang, Y.-Q.; Liu, J.; Ma, Y.-X. Neuroprotective effect of astragalin via activating PI3K/Akt-mTOR-mediated autophagy on APP/PS1 mice. Cell Death Discov. 2023, 9, 15. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, K.; Qin, S.; Jing, Y.; Liu, S.; Li, D.; Peng, C. Astragalin: A food-origin flavonoid with therapeutic effect for multiple diseases. Front. Pharmacol. 2023, 14, 1265960. [Google Scholar] [CrossRef]
- Rey, D.; Miranda Sulis, P.; Alves Fernandes, T.; Gonçalves, R.; Silva Frederico, M.J.; Costa, G.M.; Aragon, M.; Ospina, L.F.; Mena Barreto Silva, F.R. Astragalin augments basal calcium influx and insulin secretion in rat pancreaticislets. Cell Calcium 2019, 80, 56–62. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, B.C.; Chung, J.H.; Wiryowidagdo, S.; Chun, W.; Kim, S.S.; Kim, H.; Choe, M. Inhibitory effects of an aqueous extract of Gynura procumbens on human mesangial cell proliferation. Korean J. Physiol. Pharmacol. 2007, 11, 145–148. [Google Scholar]
- Peng, L.; Gao, X.; Nie, L.; Xie, J.; Dai, T.; Shi, C.; Tao, L.; Wang, Y.; Tian, Y.; Sheng, J. Astragalin Attenuates Dextran Sulfate Sodium (DSS)-Induced Acute Experimental Colitis by Alleviating Gut Microbiota Dysbiosis and Inhibiting NF-κB Activation in Mice. Front. Immunol. 2020, 11, 2058. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Tomczyk, M.; Izdebska, W.; Borzym-Kluczyk, M.; Bielawska, A.; Bielawski, K.; Galicka, A. p-Coumaric acid, Kaempferol, Astragalin and Tiliroside Influence the Expression of Glycoforms in AGS Gastric Cancer Cells. Int. J. Mol. Sci. 2022, 23, 8602. [Google Scholar] [CrossRef]
- Yang, M.; Li, W.-Y.; Xie, J.; Wang, Z.-L.; Wen, Y.-L.; Zhao, C.-C.; Tao, L.; Li, L.-F.; Tian, Y.; Sheng, J. Astragalin Inhibits the Proliferation and Migration of Human Colon Cancer HCT116 Cells by Regulating the NF-κB Signaling Pathway. Front. Pharmacol. 2021, 12, 639256. [Google Scholar] [CrossRef]
- Yao, G.; Bai, Z.; Niu, J.; Zhang, R.; Lu, Y.; Gao, T.; Wang, H. Astragalin attenuates depression-like behaviors and memory deficits and promotes M2 microglia polarization by regulating IL-4R/JAK1/STAT6 signaling pathway in a murine model of perimenopausal depression. Psychopharmacology 2022, 239, 2421–2443. [Google Scholar] [CrossRef]
- Kim, E.H.; Shim, Y.Y.; Lee, H.I.; Lee, S.; Reaney, M.J.T.; Chung, M.J. Astragalin and Isoquercitrin Isolated from Aster scaber Suppress LPS-Induced Neuroinflammatory Responses in Microglia and Mice. Foods 2022, 11, 1505. [Google Scholar] [CrossRef] [PubMed]
- Gangarapu, V.; Yıldız, K.; Ince, A.T.; Baysal, B. Role of gut microbiota: Obesity and NAFLD. Turkish J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2014, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Vandana, C.; Nitya, S.; Vasudha, B.; Fatih, O.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Park, S.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.-H. Evaluation of phytochemicals, antioxidants, and antidiabetic efficacy of various solvent fractions of Gynura procumbens (Lour.) Merr. Process Biochem. 2021, 111, 51–62. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Nakamura, Y.; Chang, C.-C.; Mori, T.; Sato, K.; Ohtsuki, K.; Upham, B.L.; Trosko, J.E. Augmentation of differentiation and gap junction function by kaempferol in partially differentiated colon cancer cells. Carcinogenesis 2005, 26, 665–671. [Google Scholar] [CrossRef]
- Hung, H. Inhibition of estrogen receptor alpha expression and function in MCF-7 cells by kaempferol. J. Cell. Physiol. 2004, 198, 197–208. [Google Scholar] [CrossRef]
- Da, J.; Xu, M.; Wang, Y.; Li, W.; Lu, M.; Wang, Z. Kaempferol Promotes Apoptosis While Inhibiting Cell Proliferation via Androgen-Dependent Pathway and Suppressing Vasculogenic Mimicry and Invasion in Prostate Cancer. Anal. Cell. Pathol. 2019, 2019, 1907698. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Daniel, K.G.; Chen, M.S.; Kuhn, D.J.; Landis-Piwowar, K.R.; Dou, Q.P. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem. Pharmacol. 2005, 69, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, X.; Chen, Q.; Chen, T.; Jiang, N.; Guo, Z. Myricetin ameliorates ox-LDL-induced HUVECs apoptosis and inflammation via lncRNA GAS5 upregulating the expression of miR-29a-3p. Sci. Rep. 2021, 11, 19637. [Google Scholar] [CrossRef]
- Xia, S.-F.; Le, G.-W.; Wang, P.; Qiu, Y.-Y.; Jiang, Y.-Y.; Tang, X. Regressive Effect of Myricetin on Hepatic Steatosis in Mice Fed a High-Fat Diet. Nutrients 2016, 8, 799. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, M.; Wang, L.; Yu, S. Anti-tumor effects and associated molecular mechanisms of myricetin. Biomed. Pharmacother. 2019, 120, 109506. [Google Scholar] [CrossRef]
- Park, K.-S.; Chong, Y.; Kim, M.K. Myricetin: Biological activity related to human health. Appl. Biol. Chem. 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Phillips, P.A.; Sangwan, V.; Borja-Cacho, D.; Dudeja, V.; Vickers, S.M.; Saluja, A.K. Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Lett. 2011, 308, 181–188. [Google Scholar] [CrossRef]
- Javed, Z.; Khan, K.; Herrera-Bravo, J.; Naeem, S.; Iqbal, M.J.; Raza, Q.; Sadia, H.; Raza, S.; Bhinder, M.; Calina, D.; et al. Myricetin: Targeting signaling networks in cancer and its implication in chemotherapy. Cancer Cell Int. 2022, 22, 239. [Google Scholar] [CrossRef]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Myricetin: A Multifunctional Flavonol in Biomedicine. Curr. Pharmacol. Rep. 2022, 8, 48–61. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Bazer, F.W.; Song, G. Myricetin suppresses invasion and promotes cell death in human placental choriocarcinoma cells through induction of oxidative stress. Cancer Lett. 2017, 399, 10–19. [Google Scholar] [CrossRef]
- Jo, S.; Ha, T.K.; Han, S.-H.; Kim, M.E.; Jung, I.; Lee, H.-W.; Bae, S.K.; Lee, J.S. Myricetin Induces Apoptosis of Human Anaplastic Thyroid Cancer Cells via Mitochondria Dysfunction. Anticancer Res. 2017, 37, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Hu, Q.; Wang, J.; Liu, Z.; Wu, D.; Lu, W.; Huang, J. Myricetin is a novel inhibitor of human inosine 5’-monophosphate dehydrogenase with anti-leukemia activity. Biochem. Biophys. Res. Commun. 2016, 477, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, J.; Chen, X.; Guo, W.; Du, Y.; Wang, Y.; Zang, W.; Zhang, S.; Zhao, G. Myricetin enhance chemosensitivity of 5-fluorouracil on esophageal carcinoma in vitro and in vivo. Cancer Cell Int. 2014, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, L.; Liu, H.; Zhao, G.; Ming, L. Enhancement of recombinant myricetin on the radiosensitivity of lung cancer A549 and H1299 cells. Diagn. Pathol. 2014, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Pinto, H.B.; Brust, F.R.; Macedo, A.J.; Trentin, D.S. The antivirulence compound myricetin possesses remarkable synergistic effect with antibacterials upon multidrug resistant Staphylococcus aureus. Microb. Pathog. 2020, 149, 104571. [Google Scholar] [CrossRef]
- Pasetto, S.; Pardi, V.; Murata, R.M. Anti-HIV-1 Activity of Flavonoid Myricetin on HIV-1 Infection in a Dual-Chamber In Vitro Model. PLoS ONE 2015, 9, e115323. [Google Scholar] [CrossRef]
- Griep, M.A.; Blood, S.; Larson, M.A.; Koepsell, S.A.; Hinrichs, S.H. Myricetin inhibits Escherichia coli DnaB helicase but not primase. Bioorg. Med. Chem. 2007, 15, 7203–7208. [Google Scholar] [CrossRef]
- Sun, H.; Niisato, N.; Nishio, K.; Hamilton, K.L.; Marunaka, Y. Distinct Action of Flavonoids, Myricetin and Quercetin, on Epithelial Cl− Secretion: Useful Tools as Regulators of Cl− Secretion. Biomed Res. Int. 2014, 2014, 902735. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Ji, P.; Chi, K.; Chen, X.; Zhou, Y.; Chen, S.; Cheng, Y.; Flaumenhaft, R.; Yuan, C.; Huang, M. Enhanced inhibition of protein disulfide isomerase and anti-thrombotic activity of a rutin derivative: Rutin: Zn complex. RSC Adv. 2023, 13, 11464–11471. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Tzeng, T.-F.; Liou, S.-S.; Chang, Y.-S.; Liu, I.-M. Myricetin Increases Hepatic Peroxisome Proliferator-Activated Receptor α Protein Expression and Decreases Plasma Lipids and Adiposity in Rats. Evidence-Based Complement. Altern. Med. 2012, 2012, 787152. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Z.; Li, B.; Peng, Y.; Yang, X.; Xiao, Y.; Ni, R.; Qi, X.-L. Myricetin ameliorates cognitive impairment in 3×Tg Alzheimer’s disease mice by regulating oxidative stress and tau hyperphosphorylation. Biomed. Pharmacother. 2024, 177, 116963. [Google Scholar] [CrossRef]
- Liu, M.; Guo, H.; Li, Z.; Zhang, C.; Zhang, X.; Cui, Q.; Tian, J. Molecular Level Insight Into the Benefit of Myricetin and Dihydromyricetin Uptake in Patients With Alzheimer’s Diseases. Front. Aging Neurosci. 2020, 12, 601603. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Yan, F.; Dong, M.; Ren, Y. Research progress of quercetin in cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1203713. [Google Scholar] [CrossRef]
- Mirza, M.A.; Mahmood, S.; Hilles, A.R.; Ali, A.; Khan, M.Z.; Zaidi, S.A.; Iqbal, Z.; Ge, Y. Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications—A Review. Pharmaceuticals 2023, 16, 1631. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Kannan, S.; Balakrishnan, J.; Govindasamy, A.; Arunagiri, R. New insights into the antibacterial mode of action of quercetin against uropathogen Serratia marcescens in-vivo and in-vitro. Sci. Rep. 2022, 12, 21912. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies2. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef]
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M.H.; Baghbadorani, P.Z.; Eskandari, N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: An update. Front. Immunol. 2023, 14, 1077531. [Google Scholar] [CrossRef] [PubMed]
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; Astudillo-de la Vega, H.; Hernández de la Cruz, O.N.; López-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wu, Y.; Li, J.; Yang, M.; Xu, H.; Li, Y.; Tong, P.; Shao, R.; Liu, Y.; Kong, X. Quercetin liposomes conjugated with hyaluronidase: An efficient drug delivery system to block pancreatic cancer. J. Control. Release 2025, 382, 113642. [Google Scholar] [CrossRef]
- Mirazimi, S.M.A.; Dashti, F.; Tobeiha, M.; Shahini, A.; Jafari, R.; Khoddami, M.; Sheida, A.H.; EsnaAshari, P.; Aflatoonian, A.H.; Elikaii, F.; et al. Application of Quercetin in the Treatment of Gastrointestinal Cancers. Front. Pharmacol. 2022, 13, 860209. [Google Scholar] [CrossRef] [PubMed]
- Dilek, B.; Meltem, Ö. Quercetin suppresses cell proliferation using the apoptosis pathways in MCF-7 and MDA-MB-231 human breast carcinoma cells in monolayer and spheroid model cultures. S. Afr. J. Bot. 2023, 162, 259–270. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 55. [Google Scholar] [CrossRef]
- Khorsandi, L.; Orazizadeh, M.; Niazvand, F.; Abbaspour, M.R.; Mansouri, E.; Khodadadi, A. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratisl. Lek. Listy 2017, 118, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Kyuichi, K.; Seiko, O.; Akari, I.; Yoshichika, K.; Zai-Si, J.; Hiroshi, T.; Terao, J. Effect of quercetin and its glucuronide metabolite upon 6-hydorxydopamine-induced oxidative damage in Neuro-2a cells. Free Radic. Res. 2012, 46, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Sudhakaran, P.R.; Helen, A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell. Immunol. 2016, 310, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Dagher, O.; Mury, P.; Thorin-Trescases, N.; Noly, P.E.; Thorin, E.; Carrier, M. Therapeutic Potential of Quercetin to Alleviate Endothelial Dysfunction in Age-Related Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 658400. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, T.; Wan, R.; Niu, W.; Bian, Y.; Li, J. Quercetin ameliorates atherosclerosis by inhibiting inflammation of vascular endothelial cells via Piezo1 channels. Phytomedicine 2024, 132, 155865. [Google Scholar] [CrossRef]
- Kuipers, E.N.; Dam, A.D.V.; Held, N.M.; Mol, I.M.; Houtkooper, R.H.; Rensen, P.C.N.; Boon, M.R. Quercetin Lowers Plasma Triglycerides Accompanied by White Adipose Tissue Browning in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 19, 1786. [Google Scholar] [CrossRef]
- Grzelak-Błaszczyk, K.; Milala, J.; Kosmala, M.; Kołodziejczyk, K.; Sójka, M.; Czarnecki, A.; Klewicki, R.; Juśkiewicz, J.; Fotschki, B.; Jurgoński, A. Onion quercetin monoglycosides alter microbial activity and increase antioxidant capacity. J. Nutr. Biochem. 2018, 56, 81–88. [Google Scholar] [CrossRef]
- Ramzan, N.; Butt, H.; Azeem, M.; Hanif, M.; Mahmood, K.; Rehman, S.; Shahwar, D.; Zeeshan, M.; Ahmad, Q.-A.; Jabeen, M. Therapeutic applications of quercetin-metallic complexes: A review. BioMetals 2025, 1–22. [Google Scholar] [CrossRef]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]
- Aggarwal, D.; Chaudhary, M.; Mandotra, S.K.; Tuli, H.S.; Chauhan, R.; Joshi, N.C.; Kaur, D.; Dufossé, L.; Chauhan, A. Anti-inflammatory potential of quercetin: From chemistry and mechanistic insight to nanoformulations. Curr. Res. Pharmacol. Drug Discov. 2025, 8, 100217. [Google Scholar] [CrossRef]
- Townsend, E.A.; Emala, C.W.S. Quercetin acutely relaxes airway smooth muscle and potentiates β-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCβ and PDE4. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L396–L403. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H.; Zare Javid, A.; Ahangarpour, A.; Zaman, F.; Hosseini, S.A.; Zohoori, V.; Aghamohammadi, V.; Yazdanfar, S.; Ghasemi Deh Cheshmeh, M. The effects of rutin supplement on blood pressure markers, some serum antioxidant enzymes, and quality of life in patients with type 2 diabetes mellitus compared with placebo. Front. Nutr. 2023, 10, 1214420. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Arima, H.; Ashida, H.; Danno, G. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Ahmad, I.; Khan, M.S.; Khan, R.A.; Khan, J.M. Rutin inhibits mono and multi-species biofilm formation by foodborne drug resistant Escherichia coli and Staphylococcus aureus. Food Control 2017, 79, 325–332. [Google Scholar] [CrossRef]
- Thakur, M.; Singh, M.; Kumar, S.; Dwivedi, V.P.; Dakal, T.C.; Yadav, V. A Reappraisal of the Antiviral Properties of and Immune Regulation through Dietary Phytochemicals. ACS Pharmacol. Transl. Sci. 2023, 6, 1600–1615. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Metwaly, A.M.; El-Fakharany, E.M.; Alsfouk, A.A.; Ibrahim, I.M.; Elkaeed, E.B.; Eissa, I.H. Integrated in Silico and in Vitro Studies of Rutin’s Potential against SARS-CoV-2 through the Inhibition of the RNA-dependent RNA Polymerase. Curr. Med. Chem. 2025, 32, 6353–6379. [Google Scholar] [CrossRef]
- Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFκB/MAPK pathway. Chem. Biol. Interact. 2015, 231, 98–107. [Google Scholar] [CrossRef]
- Hao, G.; Dong, Y.; Huo, R.; Wen, K.; Zhang, Y.; Liang, G. Rutin Inhibits Neuroinflammation and Provides Neuroprotection in an Experimental Rat Model of Subarachnoid Hemorrhage, Possibly Through Suppressing the RAGE-NF-κB Inflammatory Signaling Pathway. Neurochem. Res. 2016, 41, 1496–1504. [Google Scholar] [CrossRef]
- Perk, A.A.; Shatynska-Mytsyk, I.; Gerçek, Y.C.; Boztaş, K.; Yazgan, M.; Fayyaz, S.; Farooqi, A.A. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 2014, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, S. Modulatory effects of rutin on the expression of cytochrome P450s and antioxidant enzymes in human hepatoma cells. Acta Pharm. 2016, 66, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.F.L.; dos Santos, M.C.d.S.; Ginabreda, M.G.; Fortunato, R.S.; de Carvalho, D.P.; Freitas Ferreira, A.C. Correction: Flavonoid Rutin Increases Thyroid Iodide Uptake in Rats. PLoS ONE 2014, 9, 10–1371. [Google Scholar] [CrossRef]
- Chen, H.; Miao, Q.; Geng, M.; Liu, J.; Hu, Y.; Tian, L.; Pan, J.; Yang, Y. Anti-Tumor Effect of Rutin on Human Neuroblastoma Cell Lines through Inducing G2/M Cell Cycle Arrest and Promoting Apoptosis. Sci. World J. 2013, 2013, 269165. [Google Scholar] [CrossRef]
- Tian, R.; Yang, W.; Xue, Q.; Gao, L.; Huo, J.; Ren, D.; Chen, X. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur. J. Pharmacol. 2016, 771, 84–92. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Saklani, R.; Gupta, S.K.; Mohanty, I.R.; Kumar, B.; Srivastava, S.; Mathur, R. Cardioprotective effects of rutin via alteration in TNF-α, CRP, and BNP levels coupled with antioxidant effect in STZ-induced diabetic rats. Mol. Cell. Biochem. 2016, 420, 65–72. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Devi, S.; Salkini, M.A.; Alam, P. Rutin Improves Anxiety and Reserpine-Induced Depression in Rats. Molecules 2022, 27, 7313. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Lú-Chau, T.; Moreira, M.; Lema, J.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Oluranti, O.I.; Alabi, B.A.; Michael, O.S.; Ojo, A.O.; Fatokun, B.P. Rutin prevents cardiac oxidative stress and inflammation induced by bisphenol A and dibutyl phthalate exposure via NRF-2/NF-κB pathway. Life Sci. 2021, 284, 119878. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.C.; Gray, G.C. Pro-oxidant activity of flavonoids: Effects on glutathione and glutathione S-transferase in isolated rat liver nuclei. Cancer Lett. 1996, 104, 193–196. [Google Scholar] [CrossRef]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef]
- Hoe, S.-Z.; Lee, C.-N.; Mok, S.-L.; Kamaruddin, M.Y.; Lam, S.-K. Gynura procumbens Merr. decreases blood pressure in rats by vasodilatation via inhibition of calcium channels. Clinics 2011, 66, 143–150. [Google Scholar] [CrossRef]
- Poh, T.-F.; Ng, H.-K.; Hoe, S.-Z.; Lam, S.-K. Gynura procumbens causes vasodilation by inhibiting angiotensin II and enhancing bradykinin actions. J. Cardiovasc. Pharmacol. 2013, 61, 378–384. [Google Scholar] [CrossRef]
- Algariri, K.; Meng, K.Y.; Atangwho, I.J.; Asmawi, M.Z.; Sadikun, A.; Murugaiyah, V.; Ismail, N. Hypoglycemic and anti-hyperglycemic study of Gynura procumbens leaf extracts. Asian Pac. J. Trop. Biomed. 2013, 3, 358–366. [Google Scholar] [CrossRef]
- Lee, H.W.; Hakim, P.; Rabu, A.; Sani, H. Antidiabetic effect of Gynura procumbens leaves extracts involve modulation of hepatic carbohydrate metabolism in streptozotocin-induced diabetic rats. J. Med. Plant Res. 2012, 6, 796–812. [Google Scholar] [CrossRef]
- Lahrita, L.; Kato, E.; Kawabata, J. Uncovering potential of Indonesian medicinal plants on glucose uptake enhancement and lipid suppression in 3T3-L1 adipocytes. J. Ethnopharmacol. 2015, 168, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Aung, C.L.; Kawakami, F.; Imai, M.; Lwin, T.-T.; Kanzaki, M.; Mar, O.; Phyu, K.P.; Thwin, M.M.; Maruyama, H. Blood glucose-lowering effect of water and ethanolic extracts of Gynura procumbens (Lour.) Merr. Tradit. Kampo Med. 2021, 8, 138–147. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef]
- Hassan, Z.; Yam, M.F.; Ahmad, M.; Yusof, A.P.M. Antidiabetic properties and mechanism of action of Gynura procumbens water extract in streptozotocin-induced diabetic rats. Molecules 2010, 15, 9008–9023. [Google Scholar] [CrossRef]
- Guo, S.; Ouyang, H.; Du, W.; Li, J.; Liu, M.; Yang, S.; He, M.; Feng, Y. Exploring the protective effect of Gynura procumbens against type 2 diabetes mellitus by network pharmacology and validation in C57BL/KsJ db/db mice. Food Funct. 2021, 12, 1732–1744. [Google Scholar] [CrossRef]
- Sunarwidhi, A.L.; Sudarsono, S.; Nugroho, A.E. Hypoglycemic Effect of Combination of Azadirachta indica A. Juss. and Gynura procumbens (Lour.) Merr. Ethanolic Extracts Standardized by Rutin and Quercetin in Alloxan-induced Hyperglycemic Rats. Adv. Pharm. Bull. 2014, 4, 613–618. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxid. Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef]
- Jermnak, U.; Supsavhad, W.; Kunakornsawat, S.; Jaroensong, T.; Watcharasit, P.; Visitnonthachai, D.; Pairor, S.; Phaochoosak, N. Anti-cancer potentials of Gynura procumbens leaves extract against two canine mammary cancer cell lines. Vet. Med. Sci. 2022, 8, 69–84. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, J.W.; Fu, D.A.H.; Zhou, Y.; Cheng, W.Z.; Liu, Z.-L. Gynura procumbens ethanolic extract suppresses osteosarcoma cell proliferation and metastasis in vitro. Oncol. Lett. 2013, 6, 113–117. [Google Scholar] [CrossRef]
- Shwter, A.N.; Abdullah, N.A.; Alshawsh, M.A.; Alsalahi, A.; Hajrezaei, M.; Almaqrami, A.A.; Salem, S.D.; Abdulla, M.A. Chemoprevention of colonic aberrant crypt foci by Gynura procumbens in rats. J. Ethnopharmacol. 2014, 151, 1194–1201. [Google Scholar] [CrossRef]
- Ghofur, A.; Hamid, I.S.; Listyorini, D. Anti-carcinogenic activity of Gynura procumbens extract through cytochrome P450 and glutathione S-transferase. Int. J. PharmTech. Res. 2015, 8, 24–29. [Google Scholar]
- Liu, X.; Zhang, J.; Yi, T.; Li, H.; Tang, X.; Liu, D.; Wu, D.; Li, Y. Decoding tumor angiogenesis: Pathways, mechanisms, and future directions in anti-cancer strategies. Biomark. Res. 2025, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- ASHRAF, K. An updated phytochemical and pharmacological review on Gynura procumbens. Asian J. Pharm. Clin. Res. 2019, 9–14. [Google Scholar] [CrossRef]
- Vejanan, V.; Latip, J.; Chin, L.P.; Embi, N.; Sidek, H.M. In vitro and in vivo anti-plasmodial activities of Gynura procumbens. Sains Malaysiana 2012, 41, 1535–1542. [Google Scholar]
- Jarikasem, S.; Charuwichitratana, S.; Siritantikorn, S.; Chantratita, W.; Iskander, M.; Frahm, A.W.; Jiratchariyakul, W. Antiherpetic Effects of Gynura procumbens. Evid.-Based Complement. Altern. Med. 2013, 2013, 394865. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Maw, S.S.; Mon, M.M.; Oo, Z.K. Study on antioxidant and antitumor activities of some herbal extracts. World Acad. Sci. Eng. Technol. 2011, 75, 450–455. [Google Scholar]
- Hakim, P.; Sani, H.; Matnoor, M. Effects of Gynura procumbens Extract and Glibenclamide on Sperm Quality and Specific Activity of Testicular Lactate Dehydrogenase in Streptozotocin-induced Diabetic Rats. Malay J. Biochem. Mol. Biol. 2008, 16, 10–14. [Google Scholar]
- Kaur, P.; Bansal, M.P. Effect of selenium-induced oxidative stress on the cell kinetics in testis and reproductive ability of male mice. Nutrition 2005, 21, 351–357. [Google Scholar] [CrossRef]
- Li, T.-T.; Wen, L.-Y.; Meng, S.-S.; Li, Y.-S.; Tang, H.-B. Oral administration of ethanol extract from Gynura procumbens stems corrects kidney injury and renal anemia in chronic kidney disease. Front. Pharmacol. 2025, 15, 1476735. [Google Scholar] [CrossRef]
- Mahmood, A.A.; Mariod, A.A.; Al-Bayaty, F.; Abdel-Wahab, S.I. Anti-ulcerogenic activity of Gynura procumbens leaf extract against experimentally-induced gastric lesions in rats. J. Med. Plants Res. 2010, 4, 685–691. [Google Scholar]
- Kim, J.; Lee, C.-W.; Kim, E.K.; Lee, S.-J.; Park, N.-H.; Kim, H.-S.; Kim, H.-K.; Char, K.; Jang, Y.P.; Kim, J.-W. Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. J. Ethnopharmacol. 2011, 137, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-J.; Mu, Y.-M.; Li, T.-T.; Yang, Y.-L.; Zhang, M.-T.; Li, Y.-S.; Zhang, W.K.; Tang, H.-B.; Shang, H.-C. Gynura procumbens Reverses Acute and Chronic Ethanol-Induced Liver Steatosis through MAPK/SREBP-1c-Dependent and -Independent Pathways. J. Agric. Food Chem. 2015, 63, 8460–8471. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.A.J.; Alamri, Z.Z.; Abdulla, M.A.; Salehen, N.A.; Salim Amur Al Sinawi, Z.; Alfaifi, S.M. Hepatoprotective effects of Gynura procumbens against thioacetamide-induced cirrhosis in rats: Targeting inflammatory and oxidative stress signalling pathways. Heliyon 2023, 9, e19418. [Google Scholar] [CrossRef]
- Dwijayanti, D.R.; Rifa’i, M. Immunomodulator Testing on Ethanol Extract of Gynura procumbens Leaves to Mus musculus Adaptive Immune System: In Vitro Study. J. Exp. Life Sci. 2014, 4, 10–14. [Google Scholar] [CrossRef]
- Fowler, M.W. Plants, medicines and man. J. Sci. Food Agric. 2006, 86, 1797–1804. [Google Scholar] [CrossRef]
- Elbouzidi, A.; Taibi, M.; Addi, M. Exploring the Potential of Molecular Docking and In Silico Studies in Secondary Metabolite and Bioactive Compound Discovery for Plant Research. In Bioinformatics for Plant Research and Crop Breeding; Wiley: Hoboken, NJ, USA, 2024; pp. 413–434. ISBN 9781394209965. [Google Scholar]
- Zięba, A.; Piotr, S.; Dariusz, M.; Kaczor, A.A. What are the challenges with multi-targeted drug design for complex diseases? Expert Opin. Drug Discov. 2022, 17, 673–683. [Google Scholar] [CrossRef]
- Aarón, R.-H.; Sheila, C.-M.; Julio Emmanuel, G.-P.; Oscar, J.-G.; Aurelio, L.-M.; Jocksan Ismael, M.-C. In Silico strategies for drug discovery: Optimizing natural compounds from foods for therapeutic applications. Discov. Chem. 2025, 2, 133. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Kashyap, A.; Sarma, A.; Das, B.K.; Goswami, A.K. Rational Design of Natural Products for Drug Discovery. In Computational Methods for Rational Drug Design; John Wiley & Sons: Hoboken, NJ, USA, 2025; pp. 285–309. ISBN 9781394249190. [Google Scholar]
- Muhammad, N.; Hussain, I.; Fu, X.-A.; Ali, A.; Guo, D.; Noureen, L.; Subhani, Q.; Ahmad, N.; Zhu, Q.-F.; Cui, H.; et al. A Comprehensive Review of Instrumentation and Applications in Post-Column and In-Source Derivatization for LC-MS. Mass Spectrom. Rev. 2025. [Google Scholar] [CrossRef]
- Naithani, U.; Guleria, V. Integrative computational approaches for discovery and evaluation of lead compound for drug design. Front. Drug Discov. 2024, 4, 1362456. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chaudhary, P.; Patel, S. Genomics, Transcriptomics, Proteomics and Metabolomics Approaches BT—Fenugreek: Biology and Applications; Naeem, M., Aftab, T., Khan, M.M.A., Eds.; Springer: Singapore, 2021; pp. 355–373. ISBN 978-981-16-1197-1. [Google Scholar]
- Niu, Z.; Xiao, X.; Wu, W.; Cai, Q.; Jiang, Y.; Jin, W.; Wang, M.; Yang, G.; Kong, L.; Jin, X.; et al. PharmaBench: Enhancing ADMET benchmarks with large language models. Sci. Data 2024, 11, 985. [Google Scholar] [CrossRef]
- Adelusi, T.I.; Oyedele, A.-Q.K.; Boyenle, I.D.; Ogunlana, A.T.; Adeyemi, R.O.; Ukachi, C.D.; Idris, M.O.; Olaoba, O.T.; Adedotun, I.O.; Kolawole, O.E.; et al. Molecular modeling in drug discovery. Inform. Med. Unlocked 2022, 29, 100880. [Google Scholar] [CrossRef]
| Compound Class | Representative Compounds | Reported Activities | References |
|---|---|---|---|
| Flavonoids | Astragalin, Myricetin, Quercetin, kaempferol, and rutin | Antioxidant, antihypertensive, anti-inflammatory, and hepatoprotective activities | [17] |
| Phenolic acids | Caffeic acid, chlorogenic acid, ferulic acid, and gallic acid | Antioxidant, anti-diabetic, and antimicrobial activities | [25] |
| Saponins | Triterpenoid-type saponins | Immunomodulatory, hypolipidemic, and anticancer properties | [26] |
| Terpenoids and Sterols | β-Sitosterol, Stigmasterol, and Lupeol | Antidiabetic, anti-inflammatory, and cholesterol-lowering effects | [6] |
| Flavonoids | Metabolic Pathway Regulated | Therapeutic Effect | References |
|---|---|---|---|
| Astragalin | Inhibits α-glucosidase and modulates the AMPK signaling pathway | Hyperglycemic activity | [28] |
| Upregulates the expression and transcriptional activity of PGC1α, activates AMPK | Protective effect on mitochondrial quality control, and thus a promising drug candidate for the treatment of diabetic renal injury | [29] | |
| Inhibits the activity of CYP1B | Anti-tumor effect | [30] | |
| Inhibits HK2 through upregulating miR-125b | Inhibit the proliferation of hepatocellular carcinoma cells | [31] | |
| Downregulates the mRNA and protein expression of GLUT-1, LDH-A, and HK-2 in breast cancer cells | Anticancer potential in triple-negative breast cancer | [32] | |
| Inhibitory effect on PI3K/AKT signaling in gastric cancer | Anti-tumor effect | [33] | |
| Negatively modulates osteoclastogenesis via ROS and the MAPK signaling pathway | Preventive effect on inflammatory bone destruction | [34] | |
| Preserves blood-brain barrier integrity and inhibits neuroinflammation by modulating PK1/RIPK3/MLKL and mTOR/NF-κB pathways, thus alleviating LPS-induced depressive-like behaviors | Anti-inflammatory effect, potential to treat systemic inflammatory responses | [35] | |
| Kaempferol | Upregulates anti-inflammatory cytokines | Anti-inflammatory effect | [36] |
| Suppresses CD36 expression, mitochondrial membrane potential elevation, ROS production, MAPK/NF-κB expression, Ca2+ influx, and increases Nrf2/HO-1 levels in RAW264.7 | Reduces atherosclerotic plaque formation | [37] | |
| Suppresses hepatocyte ferroptosis via Nrf2 pathway activation | Organ-protective effect | [38] | |
| Regulates the balance of Th17/Treg Cells and secretion of IL-17 and FoxO signalling pathways | Therapeutic effect against gouty arthritis | [39] | |
| Reduces the action of tumor necrosis factor-α (TNF-α) by returning the levels of LXR-α to their basal levels in human hepatocarcinoma cells | Anti-cancer effect | [36] | |
| Inhibitory effect on the activation of NF-κB and Akt in LPS plus ATP-stimulated cardiac fibroblasts decrease the release of TNF-α, IL-1β, IL-6, and IL-18. | Anti-inflammatory and cardio-protective effect | [40] | |
| Myricetin | Inhibitory effect on matrix metalloproteinase 2 protein expression and enzyme activity | Anti-cancer effect | [41] |
| Downregulates the mRNA expressions of T-bet and GATA-3 in Delphian lymph nodes | Immunomodulatory effect in atopic dermatitis | [42] | |
| Activates Sirt1 to regulate the JNK/Smad3 pathway | Ameliorate airway inflammation | [43] | |
| Induces apoptosis and autophagy in human gastric cancer cells through inhibition of the PI3K/Akt/mTOR pathway | Anti-cancer effect | [44] | |
| Induces apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signaling | Anti-cancer effect in human colon cancer cells | [45] | |
| Quercetin | Inhibits LPS-induced cytokine storm by interacting with the AKT1-FoxO1 and Keap1-Nrf2 signaling pathway in macrophages | Anti-inflammatory effect | [46] |
| Attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway | Reduce the atherosclerotic plaque | [47] | |
| Potently inhibits LPS-induced ROS and NO production in microglial cells | Anti-inflammatory effect | [48] | |
| Prevents THP-1 macrophage pyroptosis by reducing the expression of NLRP3 and cleaved-caspase1, as well as IL-1β and N-GSDMD in a concentration-dependent manner. Also, suppresses NLRP3 inflammasome activation by inhibiting ROS overproduction. | Anti-inflammatory effect | [49] | |
| Promotes the expression of LC3-II and beclin 1 and suppresses the expression of p62. Upregulate mRNA levels of LC3-II, Atg5, Atg7, and Atg12. | Anti-cancer effect | [50] | |
| Modulates NMDA-R mediated downstream signaling and PI3K/AKT-Nrf2/ARE signaling pathways in the hippocampus | Protect from cadmium-induced cognitive deficits in rats | [51] | |
| Rutin | Downregulates p-AkT, p-ERK1/2, and p-mTOR | Promote growth arrest in A375 and C8161 melanoma cell lines | [52] |
| Downregulates the NF-kB pathway and reduces pathological tau levels, regulates tau hyperphosphorylation by increasing PP2A levels | Potential therapeutic effect in Alzheimer’s disease | [53] | |
| Reduces gut microbiota dysregulation, such as the ratio of Firmicutes to Bacteroidetes, by regulating the AMPK/SREBP1 pathway | Ameliorate nonalcoholic fatty liver disease in diabetic patients | [54] | |
| Alleviates EndMT by restoring autophagy through inhibiting HDAC1 via PI3K/AKT/mTOR pathway in diabetic kidney disease | Delay the onset of diabetic kidney disease | [55] | |
| Inhibits the NLRP3 Inflammasome signaling pathway | Anti-inflammatory and anti-oxidant effects in ulcerative colitis | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bose, P.A.; Sohag, M.M.H.; Rabbee, M.F.; Zamee, T.M.; Kona, J.-u.-n.; Elora, B.; Zaki, R.M.; Islam, K.; Baek, K.-H. Pharmacological Overview of Bioactive Natural Products from Gynura procumbens (Lour.) Merr. Plants 2025, 14, 2714. https://doi.org/10.3390/plants14172714
Bose PA, Sohag MMH, Rabbee MF, Zamee TM, Kona J-u-n, Elora B, Zaki RM, Islam K, Baek K-H. Pharmacological Overview of Bioactive Natural Products from Gynura procumbens (Lour.) Merr. Plants. 2025; 14(17):2714. https://doi.org/10.3390/plants14172714
Chicago/Turabian StyleBose, Ponkti Addrita, Md Mehadi Hasan Sohag, Muhammad Fazle Rabbee, Tareque Muzahid Zamee, Jab-un-nisha Kona, Bonhi Elora, Randa Mohammed Zaki, Kamrul Islam, and Kwang-Hyun Baek. 2025. "Pharmacological Overview of Bioactive Natural Products from Gynura procumbens (Lour.) Merr" Plants 14, no. 17: 2714. https://doi.org/10.3390/plants14172714
APA StyleBose, P. A., Sohag, M. M. H., Rabbee, M. F., Zamee, T. M., Kona, J.-u.-n., Elora, B., Zaki, R. M., Islam, K., & Baek, K.-H. (2025). Pharmacological Overview of Bioactive Natural Products from Gynura procumbens (Lour.) Merr. Plants, 14(17), 2714. https://doi.org/10.3390/plants14172714










