Spatiotemporal Dynamics of Potential Distribution Patterns of Nitraria tangutorum Bobr. Under Climate Change and Anthropogenic Disturbances
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Overview
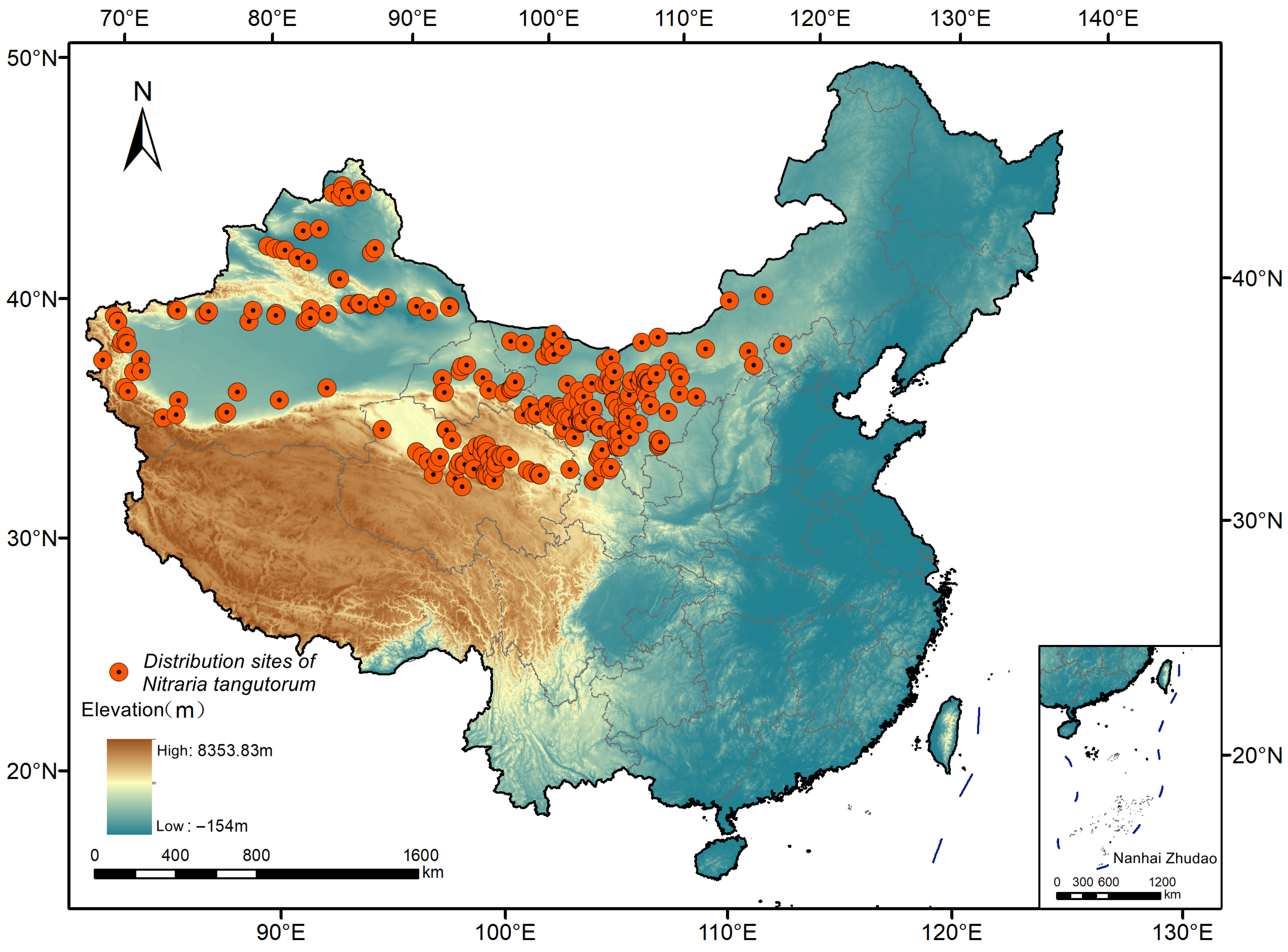
2.2. Distribution Data
2.3. Environmental Factors
- SSP1-2.6 (sustainable development, ≤2 °C warming by 2100);
- SSP3-7.0 (regional rivalry, ≤4.1 °C warming);
- SSP5-8.5 (fossil-fueled development, ≤5 °C warming) [40].
2.4. Model Construction and Evaluation
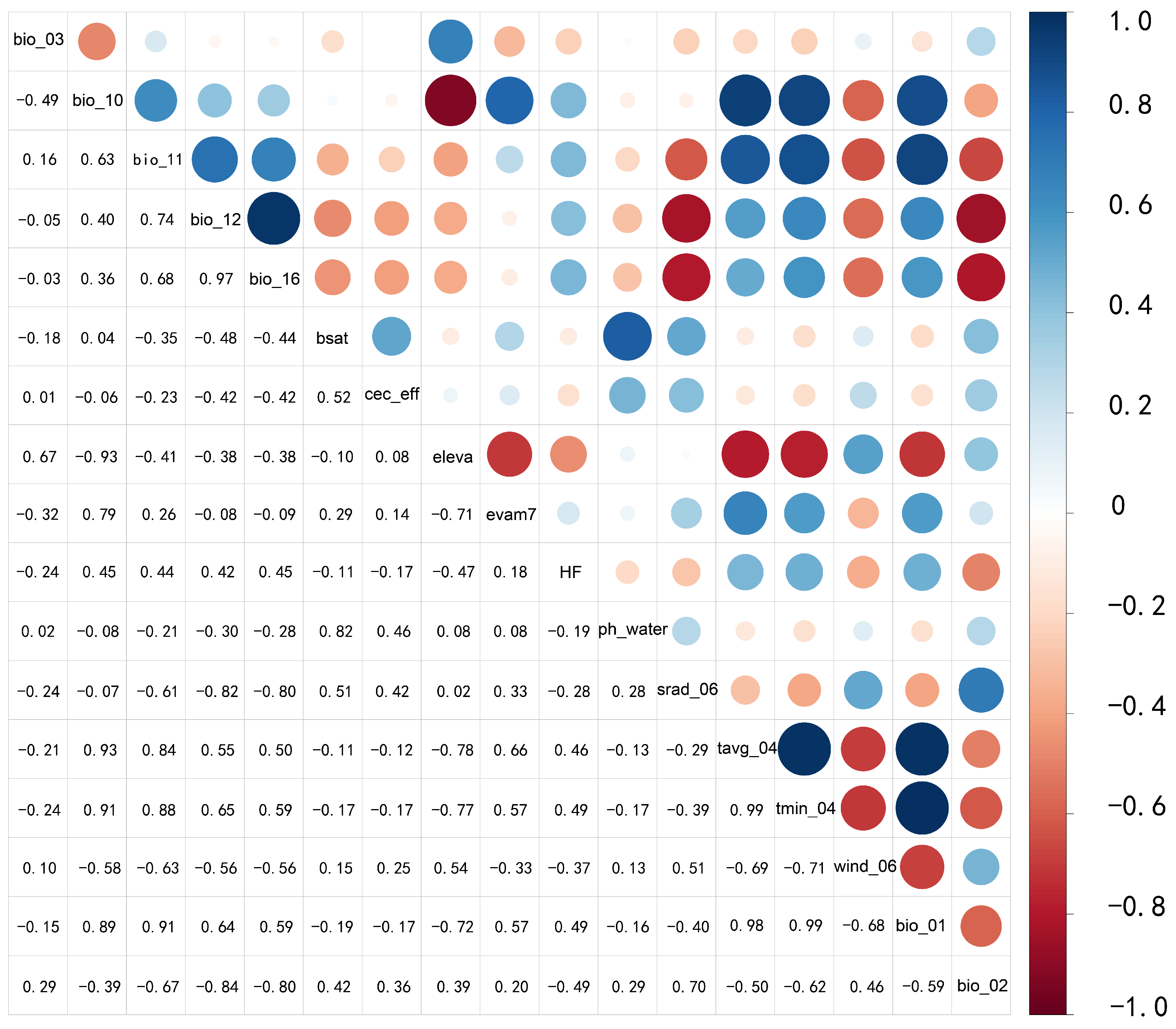
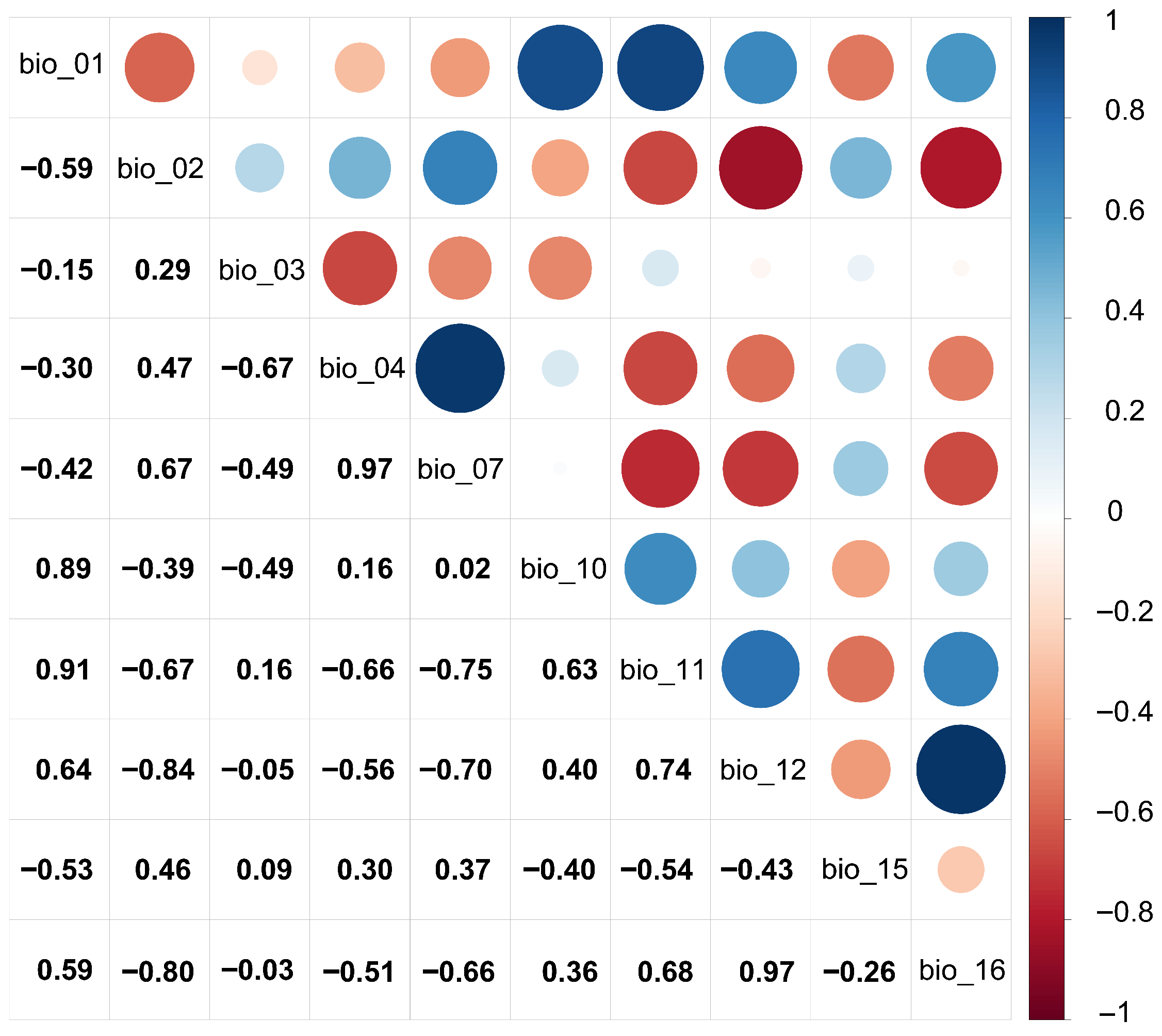
2.4.1. Environmental Factor Screening
2.4.2. MaxEnt Model Settings
- 0.9–1.0: Excellent predictive performance;
- 0.8–0.9: Good predictive performance;
- 0.7–0.8: Moderate predictive performance;
- 0.6–0.7: Poor predictive performance;
- 0.5–0.6: Failed predictive performance [47].
2.4.3. Potential Suitable Habitat Classification
- Low suitability (MTSPS value ≤ p < 0.4);
- Moderate suitability (MZ) (0.4 ≤ p < 0.6);
- High suitability (HZ) (suitability ≥0.6).
2.4.4. Multivariate Environmental Similarity Surfaces (MESS) and Least Similar Variables Analysis
2.4.5. Optimal Planting Zone Delineation
3. Results
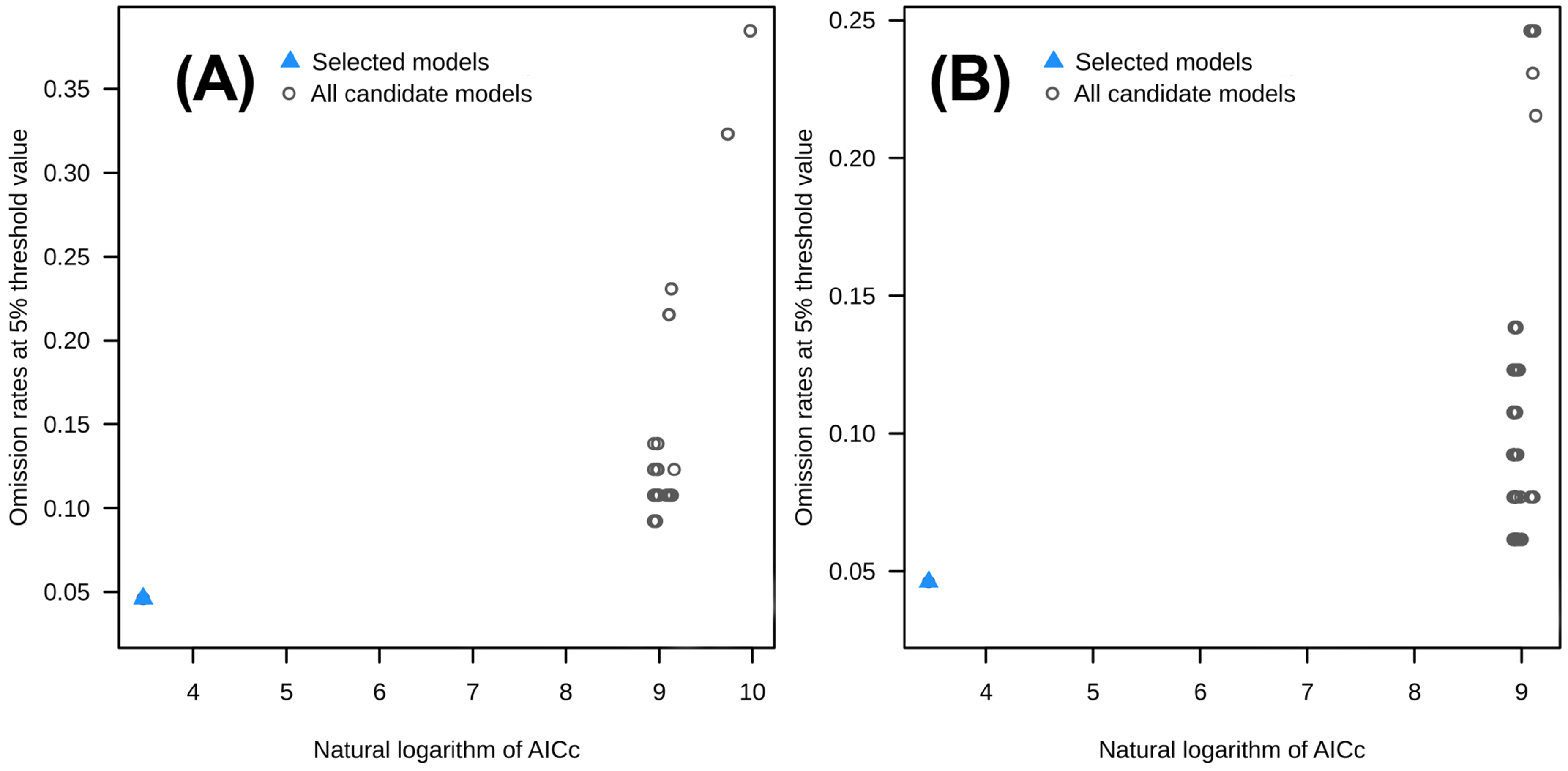
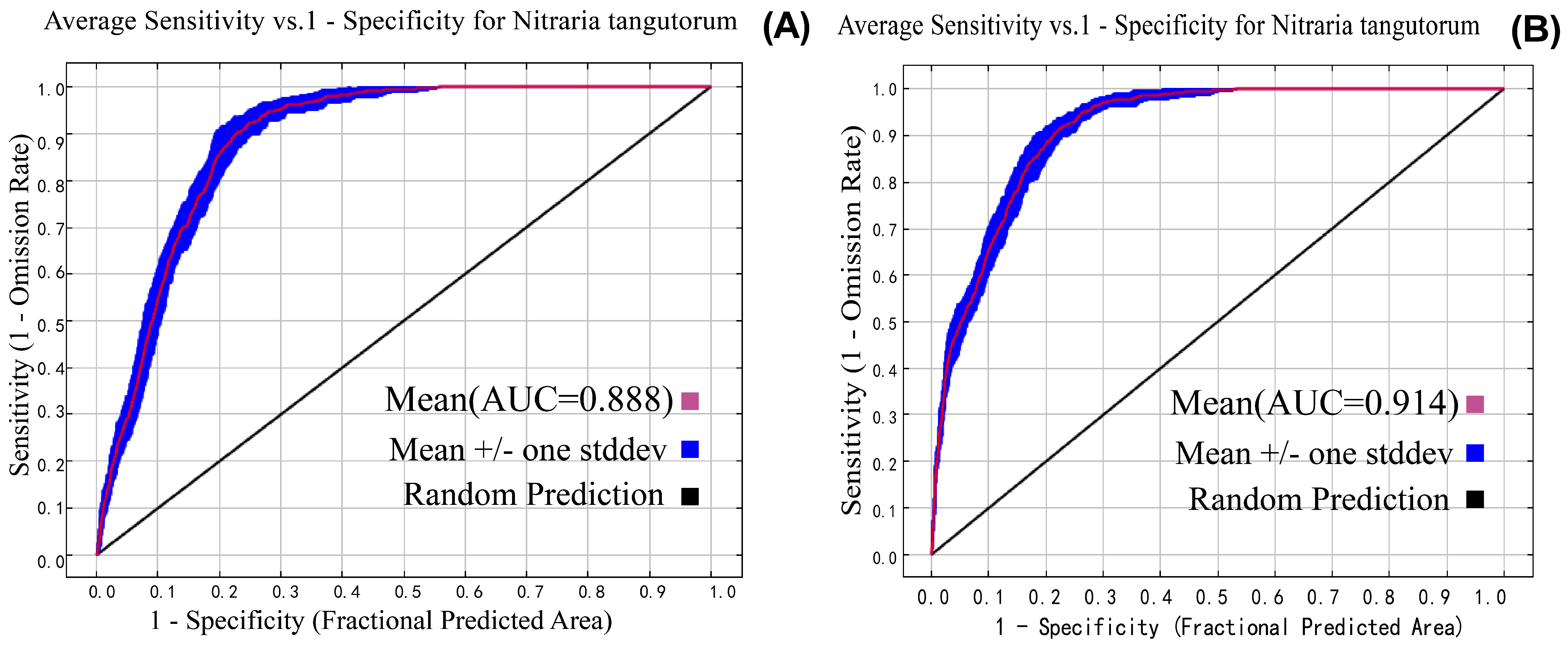
3.1. Model Optimization and Accuracy Evaluation
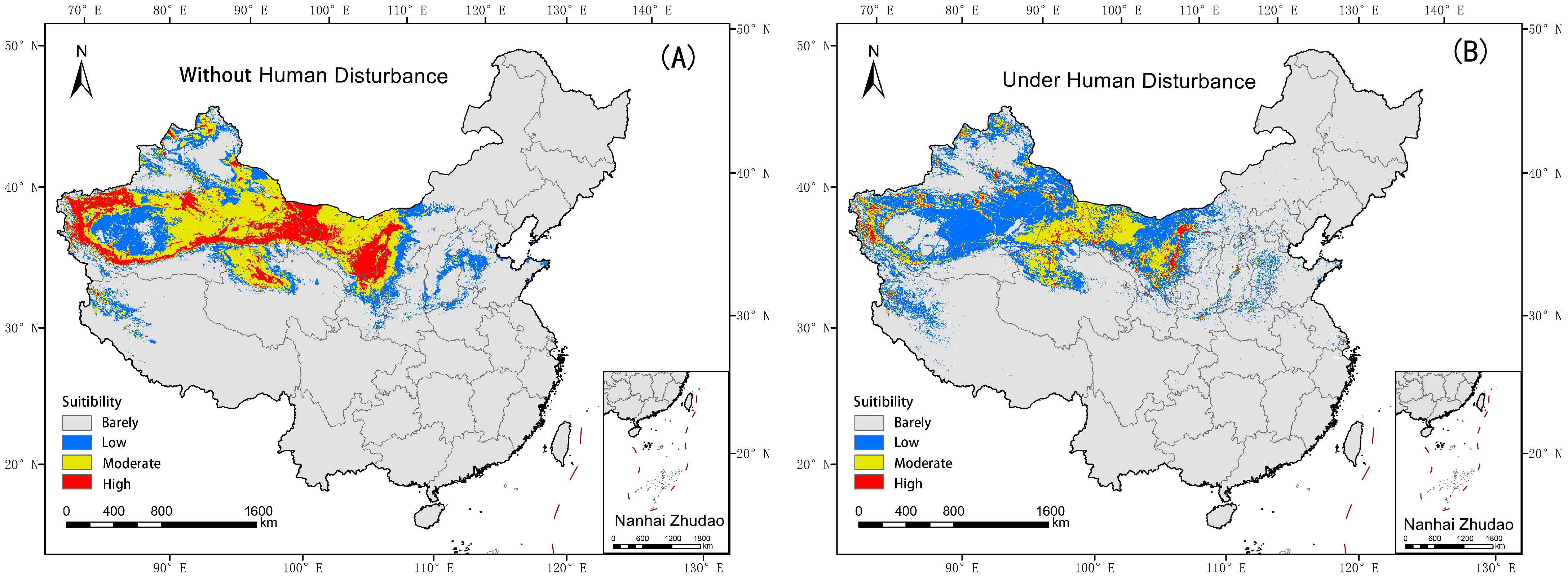
3.2. The Suitable Habitat Distribution of Nitraria tangutorum in Modern Times
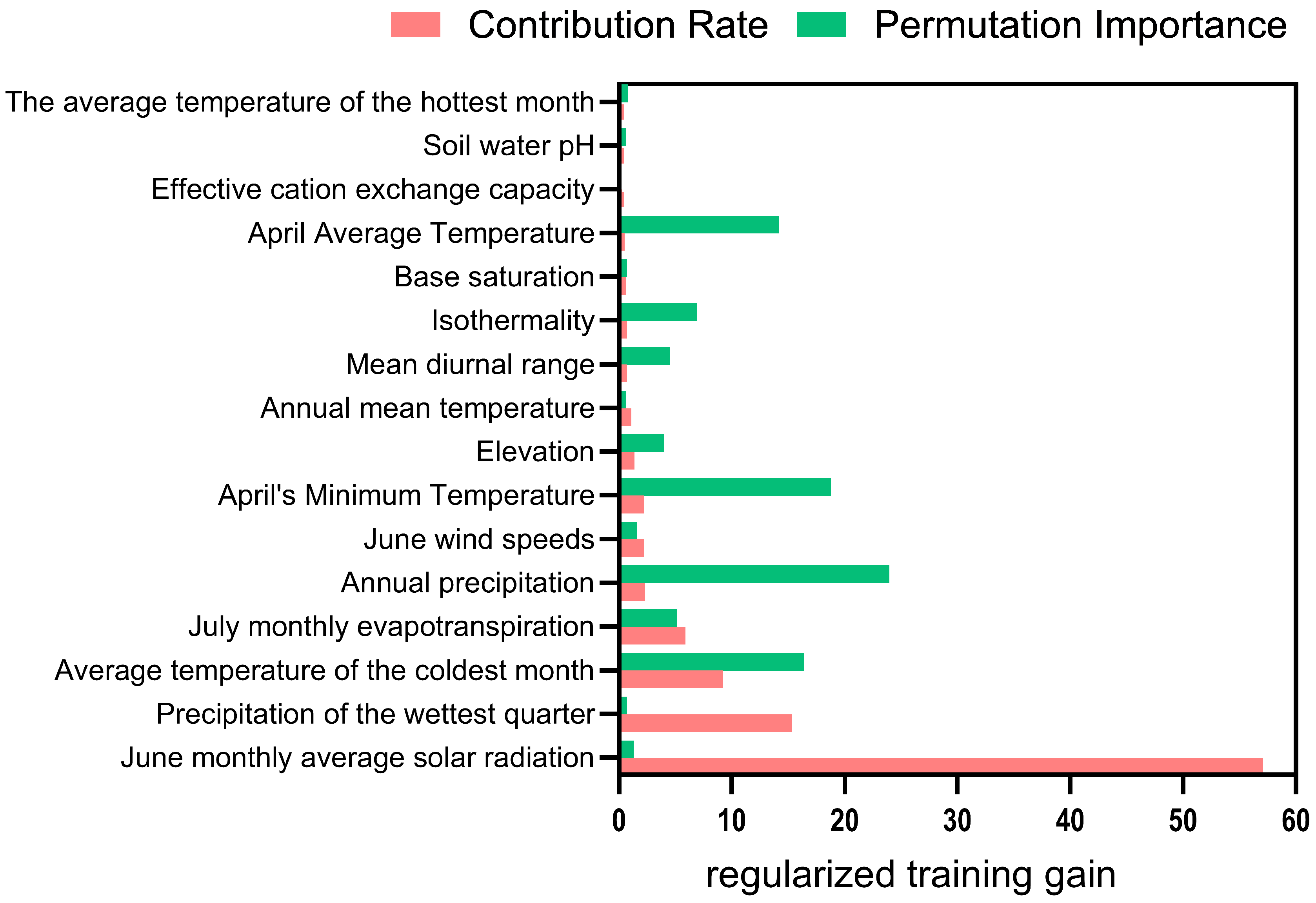
3.3. Environmental Factors Influencing the Potential Suitable Habitat Distribution of Nitraria tangutorum
- June average solar radiation: >23,696 kJ/(m2·day) , with an optimal value at 25,067 kJ/(m2·day) ;
- Wettest month precipitation: 3.7–83.7 mm, with an optimal value at 3.7 mm;
- Mean temperature of the coldest month: −34.7–−7.7 °C, with an optimal value at −29.5 °C;
- Annual precipitation: 6.2–144.7 mm, with an optimal value at 6.2 mm;
- April minimum temperature: 1.5–27.7 °C, with an optimal value at 23.0 °C;
- April mean temperature: 9.2–31.1 °C, with an optimal value at 26.4 °C.
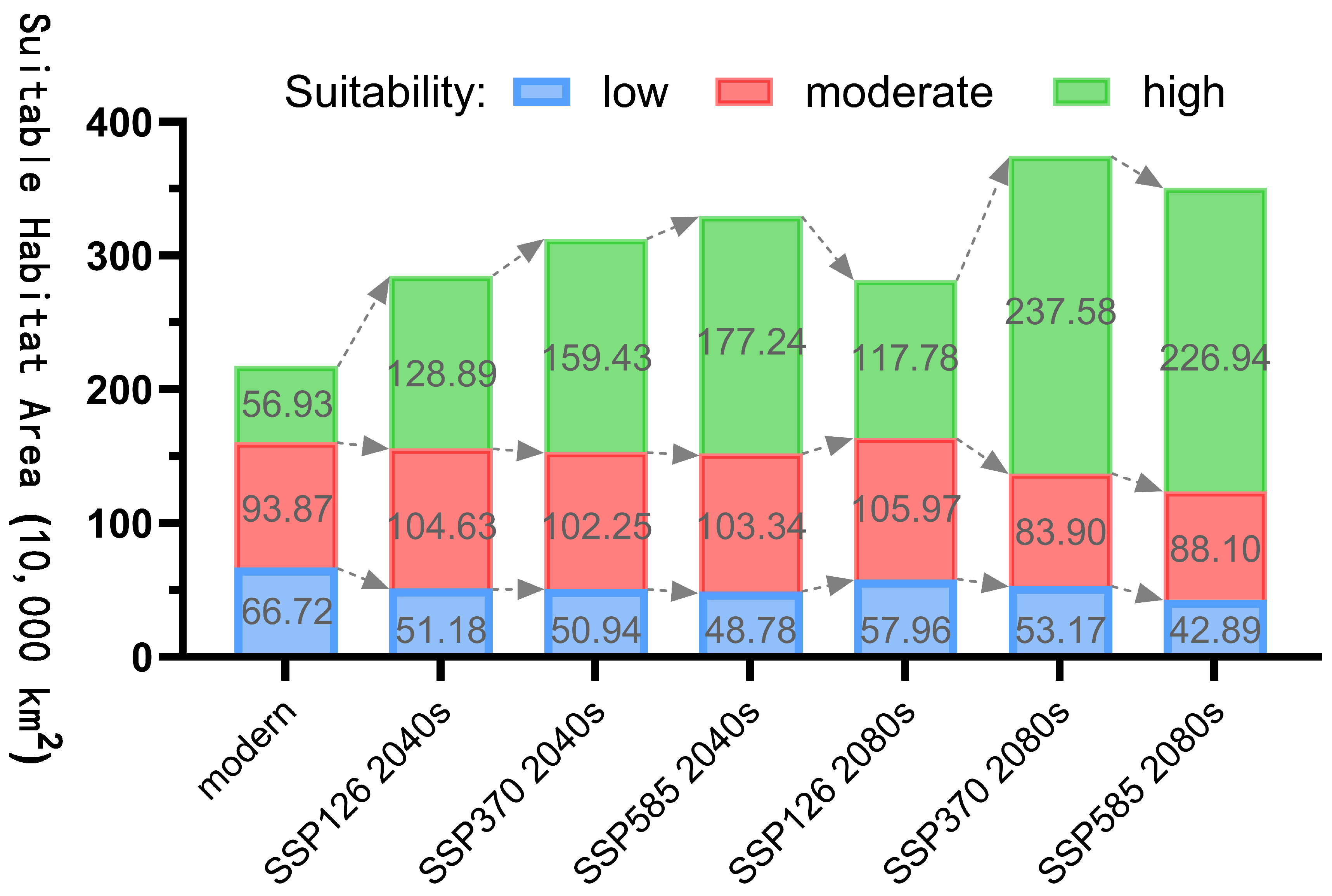
3.4. Changes in Suitable Habitats of Nitraria tangutorum Under CMIP6 Climate Scenarios
3.5. Multivariate Environmental Similarity Surfaces and Least Similar Variables Analysis
4. Discussion
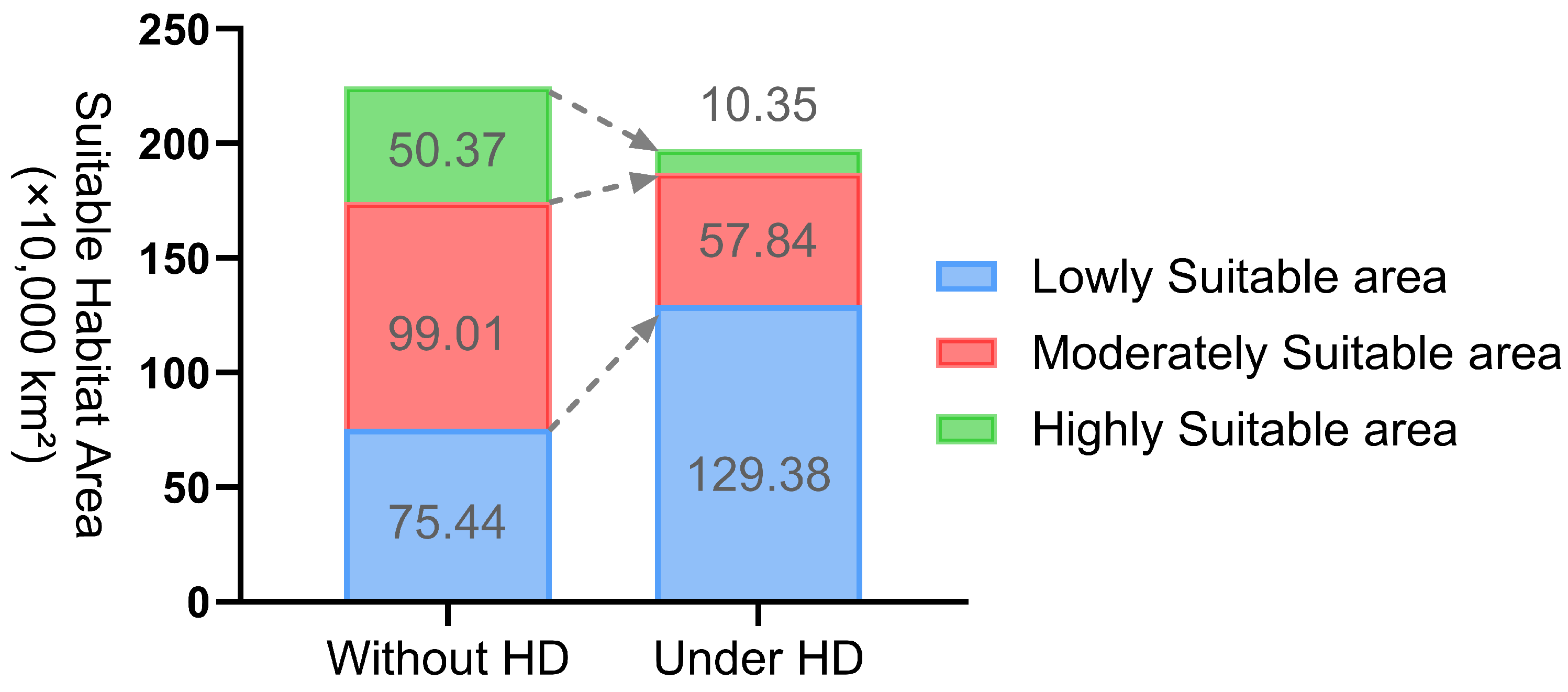
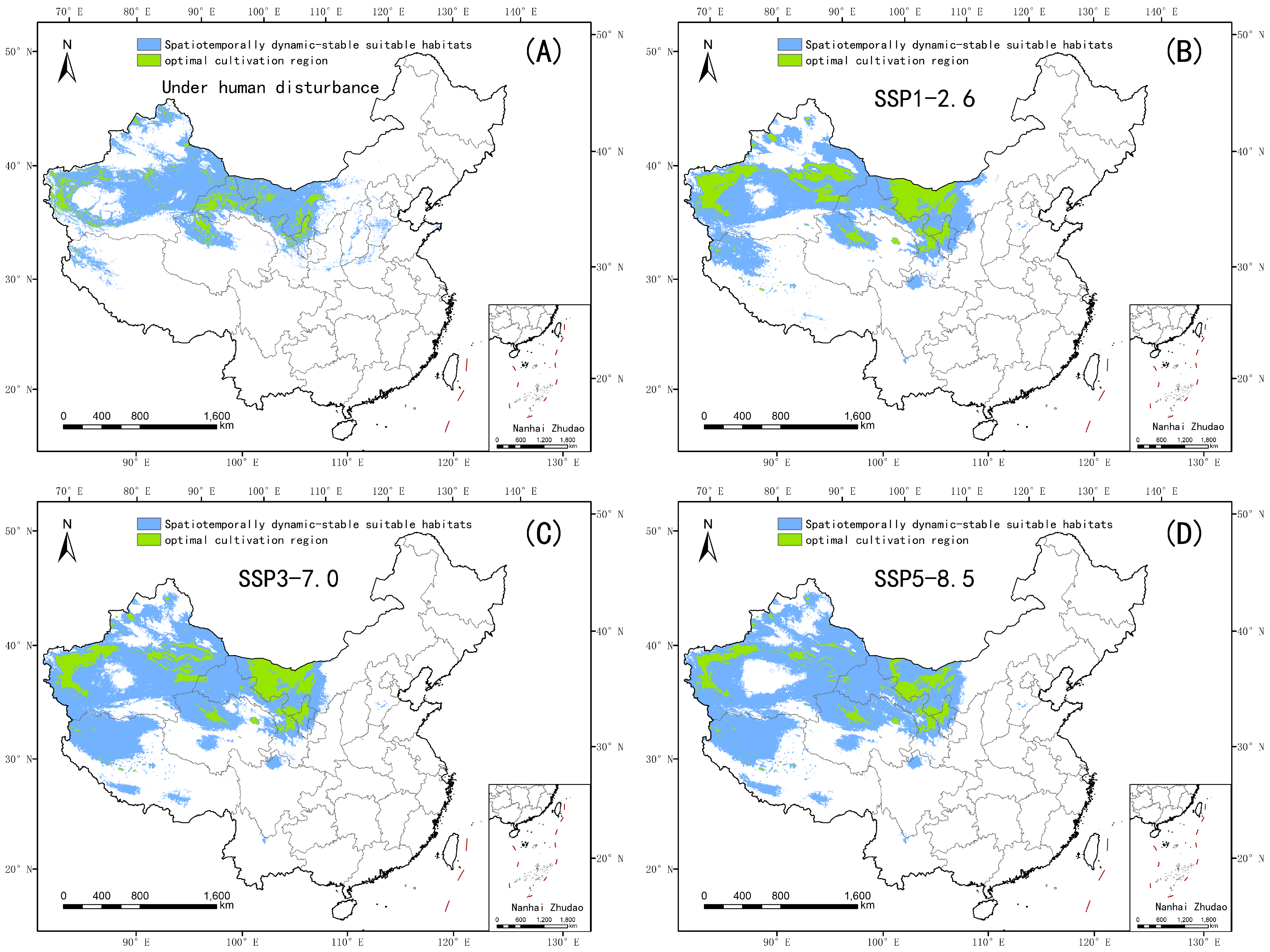
4.1. Changes in the Suitable Habitat of Nitraria tangutorum Under the Current Climate Baseline Period
4.2. Impacts of Future Climate Scenario Change on the Suitable Habitat of Nitraria tangutorum
4.3. Spatial Response Patterns of Suitable Habitats to Climate Scenario Variations
4.4. Influencing Factors: Environmental Variables Determining Species Distribution Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RM | Regularization Multiplier |
| FC | Feature Combination |
| ROC | Receiver Operating Characteristic Curve |
| AUC | Area Under The Receiver Operating Characteristic Curve |
| MTSPS | Maximum Test Sensitivity and Specificity |
| HSIs | Habitat Suitability Indices |
| MESS | Multivariate Environmental Similarity Surfaces |
| MOD | Most Dissimilar Variables Analysis |
| HD | Human Disturbance |
| HF | Human Footprint Index |
References
- Zhao, Y.L.; Zhang, L.J. Research on Quantitative Evaluation Methods for Vulnerable Ecological Environments. Adv. Geogr. Sci. 1998, 17, 67–72. [Google Scholar]
- Wang, G.X.; Cheng, G.D.; Xu, Z.M. Water resource utilization and its ecological and environmental issues in the arid region of northwest China. J. Nat. Resour. 1999, 2, 14–21. [Google Scholar]
- Tuerxun, M. Analysis and Research on the Suitable Habitat of Xanthium italicum in Xinjiang. Master’s Thesis, Xinjiang University, Urumqi, China, 2017. [Google Scholar]
- Wang, Y.; Sun, Y.; Hu, T.; Qin, D.H.; Song, L.C. Attribution of Temperature Changes in Western China. Int. J. Climatol. 2018, 38, 742–750. [Google Scholar] [CrossRef]
- Wang, Y.G.; Yang, X.H.; Yu, C.T.; Hu, Z.S. Current status, ecological functions, and conservation strategies of Nitraria plants. J. Soil Water Conserv. Res. 2007, 3, 74–79. [Google Scholar]
- Duan, Y.Z.; Zhu, G.X.; Du, Z.Y.; Li, Y.; Lu, K.; Shi, J.G. Simulated analysis of suitable habitats for Nitraria Plants Arid Reg. Northwestern China. Arid Zone Resour. Environ. 2021, 35, 124–129. [Google Scholar]
- Yu, S.F.; Mai, M.T.; Aysilemiti, A.S. A review on research of Nitraria plants in Xinjiang. J. Agric. Sci. 2016, 6, 52–54. [Google Scholar]
- Zhu, L.M.; Wu, J.X.; Li, M.J.; Fang, H.; Zhang, J.B.; Chen, Y.C.; Chen, J.H.; Cheng, T.L. Genome-Wide Discovery of CBL Genes in Nitraria tangutorum Bobr. and Functional Analysis of NtCBL1-1 under Drought and Salt Stress. For. Res. 2023, 3, 28. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.C.; Zhang, P. Review on Nitraria L. Species Research. J. Agric. Sci. Res. 2006, 4, 61–64. [Google Scholar]
- Chinese Academy of Sciences, Editorial Committee of the Flora of China. Flora of China; Science Press: Beijing, China, 2004; Volume 43, pp. 5–6. [Google Scholar]
- Li, S.F.; Zhang, Q.C.; Zhang, Q.C.; Zong, C.W.; Tian, X.F. Research progress on Nitraria plants. J. Beihua Univ. (Natural Sci. Ed.) 2005, 4, 78–81. [Google Scholar]
- Jiang, X. Correlation Between Geographical Distribution of Multiple Plant Species and Climate in Arid Northwestern China and Prediction of Potential Distributions. Master’s Thesis, Graduate University of the Chinese Academy of Sciences, Beijing, China, 2003. [Google Scholar]
- Li, X.Z. Impact of Climate Change on Growth and Development of Dominant Forage Grasses in Inner Mongolia Grasslands. Master’s Thesis, Inner Mongolia Agricultural University, Inner Mongolia, China, 2014. [Google Scholar]
- Ren, L.C. Introduction and Drought-Cold Tolerance Study of 13 Woody Plant Species in Baiyunebo Mining Area. Master’s Thesis, Beijing Forestry University, Beijing, China, 2011. [Google Scholar]
- Du, J.H.; Yan, P.; Dong, Y.X. Phenological Response of Nitraria tangutorum Bobr. to Climate Change in Minqin County, Gansu Province, Northwest China. Int. J. Biometeorol. 2010, 54, 583–593. [Google Scholar] [CrossRef]
- Yu, S.F.; Ai, S.D.; Li, M.T. Correlation analysis of biomass in Nitraria tangutorum Bobr. Shrubs Its Predict. Model. West. For. Sci. 2015, 44, 31–36+44. [Google Scholar]
- Jiang, F.Z.; Wang, J.; Zhang, Y.P. Survey and comprehensive utilization of wild Nitraria resources in the Qaidam Basin. Qinghai Sci. Technol. 2005, 1, 15–17. [Google Scholar]
- Ji, D.J.; Zhang, D.F.; Yu, Q. Investigation on germplasm resources of Nitraria in the Qaidam Basin and their utilization prospects. Qinghai Agric. For. Sci. Technol. 2022, 1, 38–41. [Google Scholar]
- Bai, M.S.; Li, G.Q.; Chen, Y.Y. Regional study on medicinal active ingredient content in Nitraria. J. Northwest For. Univ. 2008, 6, 147–150. [Google Scholar]
- Zhang, P.; Ha, S.; Yue, X.L.; Zhuang, Y.M. Morphology and sedimentary characteristics of Nitraria Shrub Sand Dunes. J. Arid Land Geogr. 2008, 31, 926–932. [Google Scholar]
- Zhang, H.C.; Li, S.H.; Mason, J.A.; Yizhaq, H.; Gui, D.W.; Xu, Z.W. Biogeomorphological niche of a landform: Machine learning approaches reveal controls on the geographical distribution of Nitraria tangutorum Bobr. Nebkhas. Earth Surf. Process. Landforms 2024, 49, 1515–1529. [Google Scholar] [CrossRef]
- He, Y.H.; Tian, Y.L.; Ye, D.M.; Qin, J.Q.; Guo, L.S. Relationships between aboveground biomass and leaf area in Nitraria plants. Chin. Desert 2005, 20, 541–546. [Google Scholar]
- Qiu, G.Y.; Li, C.; Yan, C. Characteristics of soil evaporation, plant transpiration, and water budget in Nitraria dunes in arid northwest China. Gricultural For. Meteorol. 2015, 203, 107–117. [Google Scholar] [CrossRef]
- Li, X.L.; Dang, X.H.; Zhai, B.; Wei, Y.J.; Chi, X.; Wu, H.M. Architectural Characteristics and Biomass Allocation Patterns of Adventitious Roots in Nitraria tangutorum Shrubland. Chin. J. Deserts 2022, 42, 172. [Google Scholar]
- Xu, S.Q.; Ji, X.B.; Jin, B.W.; Zhang, J.B. Root distribution of three dominant desert shrubs and their water uptake dynamics. J. Plant Ecol. 2016, 10, 780–790. [Google Scholar] [CrossRef]
- Zhu, L.H.; Fang, Z.K.; Suo, Y.R. Characteristics and development prospects of Nitraria tangutorum Qaidam Basin. Qinghai Sci. Technol. 2005, 6, 12–15. [Google Scholar]
- Gao, H.; Suo, Y.R. Amino acid contents and nutritional evaluation of Nitraria sibirica and Nitraria tangutorum Qaidam Basin. Amino Acids Biol. Resour. 2002, 4, 4–7. [Google Scholar]
- Ma, H. Study on Bioactive Components in Nitraria tangutorum Bobr. Doctoral Dissertation, Nanjing University of Science and Technology, Nanjing, China, 2014. [Google Scholar]
- Soberón, J.M. Niche and area of distribution modeling: A population ecology perspective. Ecography 2010, 33, 159–167. [Google Scholar] [CrossRef]
- Zhu, G.P.; Liu, G.Q.; Bu, W.J.; Gao, Y.B. Fundamental principles of ecological niche models and their applications in biodiversity conservation. Biodiversity 2013, 21, 90–98. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudίk, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Chilimbi, T.; Hirzel, M. Dynamic hot data stream prefetching for general-purpose pro-grams. ACM Sigplan Not. 2002, 37, 199–209. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: How to compute habitat-suitability maps without absence data? ECOLOGY 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Hirzel, A.; Guisan, A. Which is the optimal sampling strategy for habitat suitability modelling. Ecol. Model. 2002, 157, 331–341. [Google Scholar] [CrossRef]
- Lu, K.; Liu, M.; Feng, Q.; Liu, W.; Zhu, M.; Duan, Y.Z. Pdicting the Global Distribution of Nitraria L. Under Climate Change Based on Optimized MaxEnt Modeling. Plants 2025, 14, 67. [Google Scholar] [CrossRef]
- Halimujiang, M. Geographical Distribution Patterns and Study on Potential Suitable Habitats of four Nitraria Species. Master’s Thesis, Shihezi University, Shihezi, China, 2021. [Google Scholar]
- Stanton, J.C.; Pearson, R.G.; Horning, N.; Ersts, P.; Akcakaya, H.R. Combining static and dynamic variables in species distribution models under climate change. Methods Ecol. Evol. 2012, 3, 349–357. [Google Scholar] [CrossRef]
- Dang, R.L.; Xiao, L.P.; Xue, F.G. Phytogeographical Analysis of Plant Genera in the Arid Deserts of Northwestern China. Guangxi Plants 2002, 24, 121–128. [Google Scholar]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. ECOGRAPHY 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, S.T.; Gao, Y.; Yang, L.; Yu, H. Prediction of global potential suitable habitats of Nicotiana alata Link et Otto based on MaxEnt model. Sci. Rep. 2023, 13, 4851. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.W.; Li, X.C.; Wen, Y.N.; Huang, J.X.; Du, P.J.; Su, W.; Miao, S.X.; Geng, M.Q. A global record of annual terrestrial Human Footprint dataset from 2000 to 2018. Sci. Data 2022, 9, 176. [Google Scholar] [CrossRef]
- Zhu, G.P.; Qiao, H.J. Impact of MaxEnt model complexity on predicting potential distribution ranges of species. Biodiversity 2016, 24, 1189–1196. [Google Scholar]
- Yang, J.T.; Huang, Y.; Jiang, X.; Chen, H.; Liu, M.; Wang, R.L. Potential geographical distribution of the edangred plant Isoetes under human activities using MaxEnt and GARP. Glob. Ecol. Conserv. 2022, 38, e02186. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tu, Z.H.; Zhang, Y.L.; Zhong, W.P.; Xia, H.; Hao, Z.Y.; Zhang, C.G.; Li, H.G. Predicting the impact of climate change on the distribution of two relict Liriodendron species by coupling the MaxEnt model and actual physiological indicators in relation to stress tolerance. J. Environ. Manag. 2022, 322, 116024. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudίk, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. ECOGRAPHY 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Urbani, F.; D’Alessandro, P.; Frasca, R.; Biondi, M. Maximum entropy modeling of geographic distributions of the flea beetle species endemic in Italy (Coleoptera: Chrysomelidae: Galerucinae: Alticini). Zool. Anz. 2015, 258, 99–109. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. MMaximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Nolan, V.; Kaky, E.D.; Alatawi, A.S.; Gilbert, F. Mapping the Indian crested porcupine across Iraq: The benefits of species distribution modelling when species data are scarce. Mamm. Biol. 2022, 102, 1851–1866. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Fang, Y. esponse of spatial distribution patterns of Quercus chenii to climate change since the Last Glacial Maximum. J. Plant Ecol. 2016, 40, 1164–1178. [Google Scholar]
- Zhang, X.Q. Geographical Distribution and Climate Suitability of Typical Ecologically and Economically Important Tree Species in Arid Northwest China. Doctoral Dissertation, University of Chinese Academy of Sciences, Beijing, China, 2018. [Google Scholar]
- Jiang, S.X.; Hu, J.; Wang, F.L. Analysis of population dynamics of Nitraria tangutorum China Using MaxEnt Model. Chin. Wild Plant Resour. 2024, 43, 122–128. [Google Scholar]
- Yin, H.; Tian, C.; Ma, Q.Q.; Lv, G.H.; Zeng, F.J. Spatiotemporal dynamics of potential distribution patterns of Alhagi sparsifolia under climate change and human disturbance. Acta Ecol. Sin. 2022, 42, 7349–7361. [Google Scholar]
- Anderson, R.P.; Peterson, A.T.; Gómez-Laverde, M. Using niche-based GIS modeling to test geographic predictions of competitive exclusion and competitive release in South American pocket mice. OIKOS 2002, 98, 3–16. [Google Scholar] [CrossRef]
- Pan, S.Y.; Zhu, Z.H.; Yao, T.H.; Wang, Y.X.; Zhou, P. Spatial distribution prediction of Polygonum multiflorum under climate change in China. J. Northwest A&F Univ. (Natural Sci. Ed.) 2016, 44, 192–198. [Google Scholar]
- Planillo, A.; Malo, G.E. Infrastructure features outperform environmental variables explaining rabbit abundance around motorways. Ecol. Evol. 2018, 8, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.X.; Liang, Y.; Li, Z.Y.; Liu, B.; Wu, M.M.; Lau, M.K.; Feng, Y. Projected effects of climate change and urban expansion on species-level biodiversity of plants in main city clusters of Northern China. Front. Ecol. Evol. 2023, 11, 1153448. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Liu, L.Y.; Shi, P.J.; Zhang, G.M.; Qu, Z.Q.; Tang, Y.; Lei, J.; Wen, H.M.; Xiong, Y.Y.; Wang, J.P.; et al. Morphology, spatial pattern and sediment of Nitraria tangutorum Nebkhas Barchans Interdune Areas Southeast Margin Badain Jaran Desert, China. Geomorphology 2015, 232, 182–192. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists: Statistical explanation of MaxEnt. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- He, M.Y.; Ma, F.Y.; Ding, J.J.; Niu, P.X.; Luo, C.K.; Wang, M.; Jiang, P. Analysis of Spatial Suitable Habitats of Four Subspecies of Hippophae rhamnoides China Based MaxEnt Model. Plants 2025, 14, 1682. [Google Scholar] [CrossRef]
- Zhu, N.; Guo, Y.L. Study on the distribution of suitable habitats for Haloxylon ammodendron (C.A.Mey) Bunge based on four commonly used machine learning algorithms. Ecol. Sci. 2023, 42, 190–199. [Google Scholar]
- Wu, J.G.; Lv, J.J.; Zhou, Q.F. Potential impacts of climate change on the distribution of six desert plant species. Acta Bot. Sin. 2010, 45, 723–738. [Google Scholar]
- Zhang, Y.L.; Zhang, P.; Gu, X.C.; Long, A.H. Projections of temperature and precipitation changes in Xinjiang from 2021 to 2050 based on the CMIP6 model. PLoS ONE 2024, 19, e0307911. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.B.; Zhao, W.Z.; Jin, B.; Liu, J.; Xu, F.N.; Zhou, H. Seasonal variations in energy exchange and evapotranspiration of an oasis-desert ecotone in an arid region. Hydrol. Process. 2021, 35, e14364. [Google Scholar] [CrossRef]
- Xu, S.Q.; Yu, Z.B.; Ji, X.B.; Edward, A.S. Comparing three models to estimate transpiration of desert shrubs. J. Hydrol. 2017, 550, 603–615. [Google Scholar] [CrossRef]
- Chun, L.; Na, R.S.; Zhao, S.Z.; Hao, Y.Z.; Hu, Z.M.; Ma, Y.H. Resources and utilization of Nitraria Species. Chin. J. Wild Plant Resour. 2016, 35, 58–60+63. [Google Scholar]
- Yang, H. Plant Diversity and Influencing Factors in the Kumtag Desert. Doctoral Dissertation, Beijing Forestry University, Beijing, China, 2021. [Google Scholar]
- Qin, J.; Si, J.H.; Jia, B.; Zhao, C.Y.; Zhou, D.M.; He, X.H.; Wang, C.L.; Zhu, X.L. Water use strategies of Nitraria tangutorum in the lake-basin region of the Badain Jaran Desert. Front. Plant Sci. 2023, 12, e17830. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C. Geographical Distribution Patterns of Major Desert Plants in Temperate China. Doctoral Dissertation, Shihezi University, Shihezi, China, 2018. [Google Scholar]


| Variable Type | Variable Name | Variable Description | Unit | Original Spatial Resolution |
|---|---|---|---|---|
| Temperature | bio1 | Annual mean temperature | °C | 30 arc-s |
| bio2 | Monthly mean diurnal temperature range | °C | ||
| bio3 | Isothermality | |||
| bio10 | Mean temperature of warmest month | °C | ||
| bio11 | Mean temperature of coldest month | °C | ||
| tavg4 | April mean temperature | °C | ||
| tmin4 | April minimum temperature | °C | ||
| Precipitation | bio12 | Annual precipitation | mm | 30 arc-s |
| bio16 | Wettest month precipitation | mm | ||
| Evaporation | evam7 | July evapotranspiration | mm/month | 30 arc-s |
| Radiation | srad6 | June mean solar radiation | kJ/(m2·day) | 30 arc-s |
| Wind | wind6 | June mean wind speed | m/s | 30 arc-s |
| Soil | bsat | Base saturation | % | 30 arc-s |
| ph_water | Water pH | −log H+ | ||
| cec_eff | Effective cation exchange capacity | cmol/kg | ||
| Topography | eleva | Elevation | m | 30 arc-s |
| Human Impact | HF | Human footprint | 30 arc-s |
| Variable Name | Variable Description | Unit |
|---|---|---|
| bio1 | Annual mean temperature | °C |
| bio2 | Monthly mean diurnal temperature range | °C |
| bio3 | Isothermality | |
| bio4 | Temperature variation coefficient | |
| bio7 | Temperature annual range | °C |
| bio10 | Mean temperature of warmest month | °C |
| bio11 | Mean temperature of coldest month | °C |
| bio12 | Annual precipitation | mm |
| bio15 | Precipitation seasonality | |
| bio16 | Wettest month precipitation | mm |
| Category | Area (×104 km2) | Change Under HD (×104 km2) | Change Rate | |
|---|---|---|---|---|
| Without HD | Under HD | |||
| Suitable Area | 224.82 | 197.58 | −27.24 | −12.12% |
| Lowly Suitable Area | 75.44 | 129.38 | 53.94 | 71.49% |
| Moderately Suitable Area | 99.01 | 57.84 | −41.17 | −41.58% |
| Highly Suitable Area | 50.37 | 10.35 | −40.02 | −79.44% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, Y.; Cao, J.; Fang, H.; Feng, B.; Zhu, L.; Chu, X.; Lu, Y.; Han, C.; Lu, L.; Zhang, J.; et al. Spatiotemporal Dynamics of Potential Distribution Patterns of Nitraria tangutorum Bobr. Under Climate Change and Anthropogenic Disturbances. Plants 2025, 14, 2706. https://doi.org/10.3390/plants14172706
Weng Y, Cao J, Fang H, Feng B, Zhu L, Chu X, Lu Y, Han C, Lu L, Zhang J, et al. Spatiotemporal Dynamics of Potential Distribution Patterns of Nitraria tangutorum Bobr. Under Climate Change and Anthropogenic Disturbances. Plants. 2025; 14(17):2706. https://doi.org/10.3390/plants14172706
Chicago/Turabian StyleWeng, Yutao, Jun Cao, Hao Fang, Binjian Feng, Liming Zhu, Xueyi Chu, Yajing Lu, Chunxia Han, Lu Lu, Jingbo Zhang, and et al. 2025. "Spatiotemporal Dynamics of Potential Distribution Patterns of Nitraria tangutorum Bobr. Under Climate Change and Anthropogenic Disturbances" Plants 14, no. 17: 2706. https://doi.org/10.3390/plants14172706
APA StyleWeng, Y., Cao, J., Fang, H., Feng, B., Zhu, L., Chu, X., Lu, Y., Han, C., Lu, L., Zhang, J., & Cheng, T. (2025). Spatiotemporal Dynamics of Potential Distribution Patterns of Nitraria tangutorum Bobr. Under Climate Change and Anthropogenic Disturbances. Plants, 14(17), 2706. https://doi.org/10.3390/plants14172706





