Effect of Mosses and Long-Term N Addition on δ13C and δ18O Values of Respired CO2 Under a Temperate Forest Floor
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Study Site
2.2. Layout of Field Experiment
2.3. Measurements of δ13C and δ18O Values of Respired CO2 as Well as CO2 and CH4 Fluxes
2.4. Measurements of Soil Properties
2.5. Calculation and Statistical Analysis
3. Results
3.1. Changes in Soil CO2 and CH4 Fluxes as Well as δ13C and δ18O Values of Respired CO2 Prior to and After the Removal of Moss Blankets
3.2. Effect of Long-Term N Addition on Soil Properties Under Moss-Covered Forest Floors
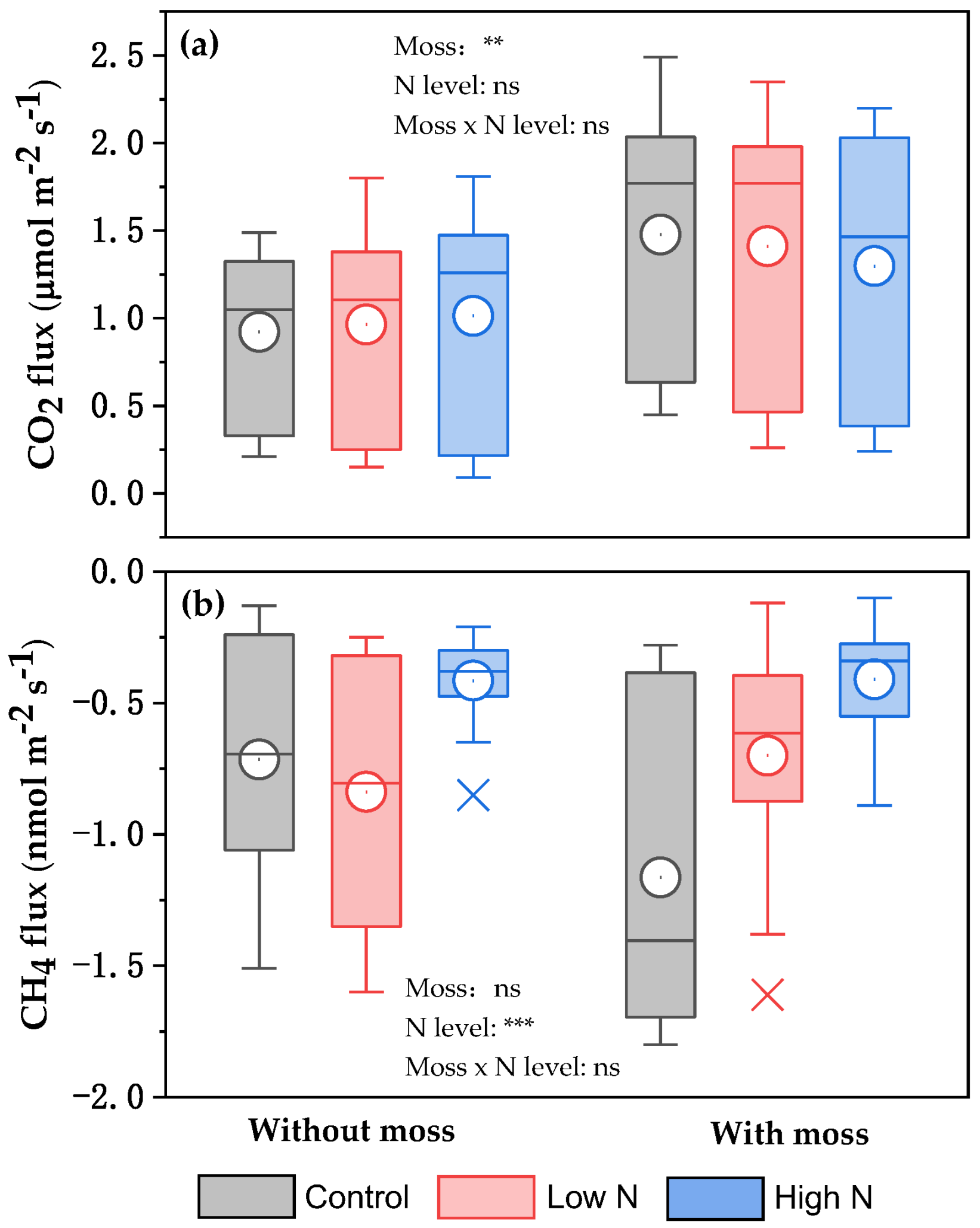
3.3. Effects of Mosses and Long-Term N Addition on the δ13C and δ18O Values of Respired CO2 as Well as CO2 and CH4 Fluxes
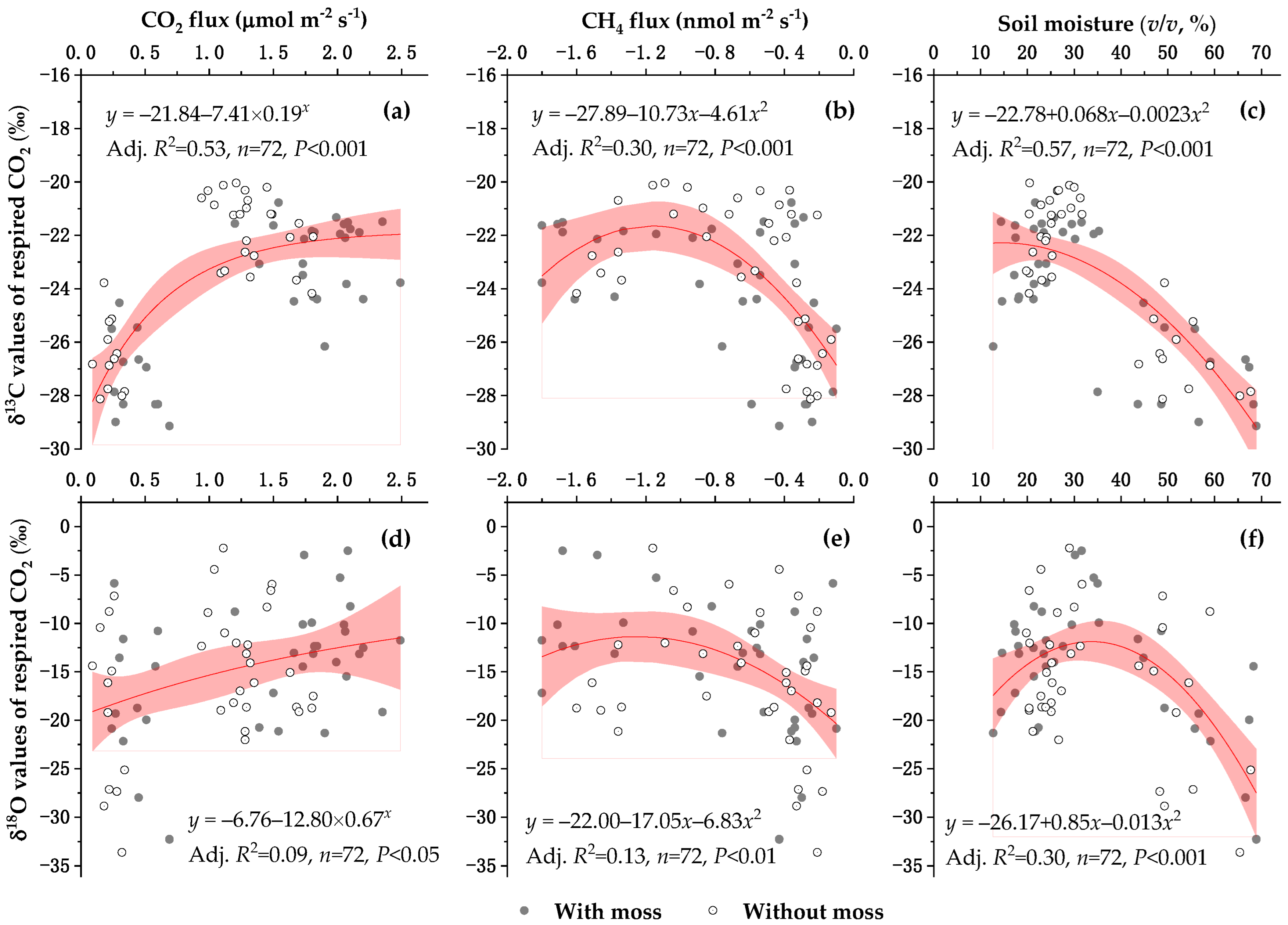
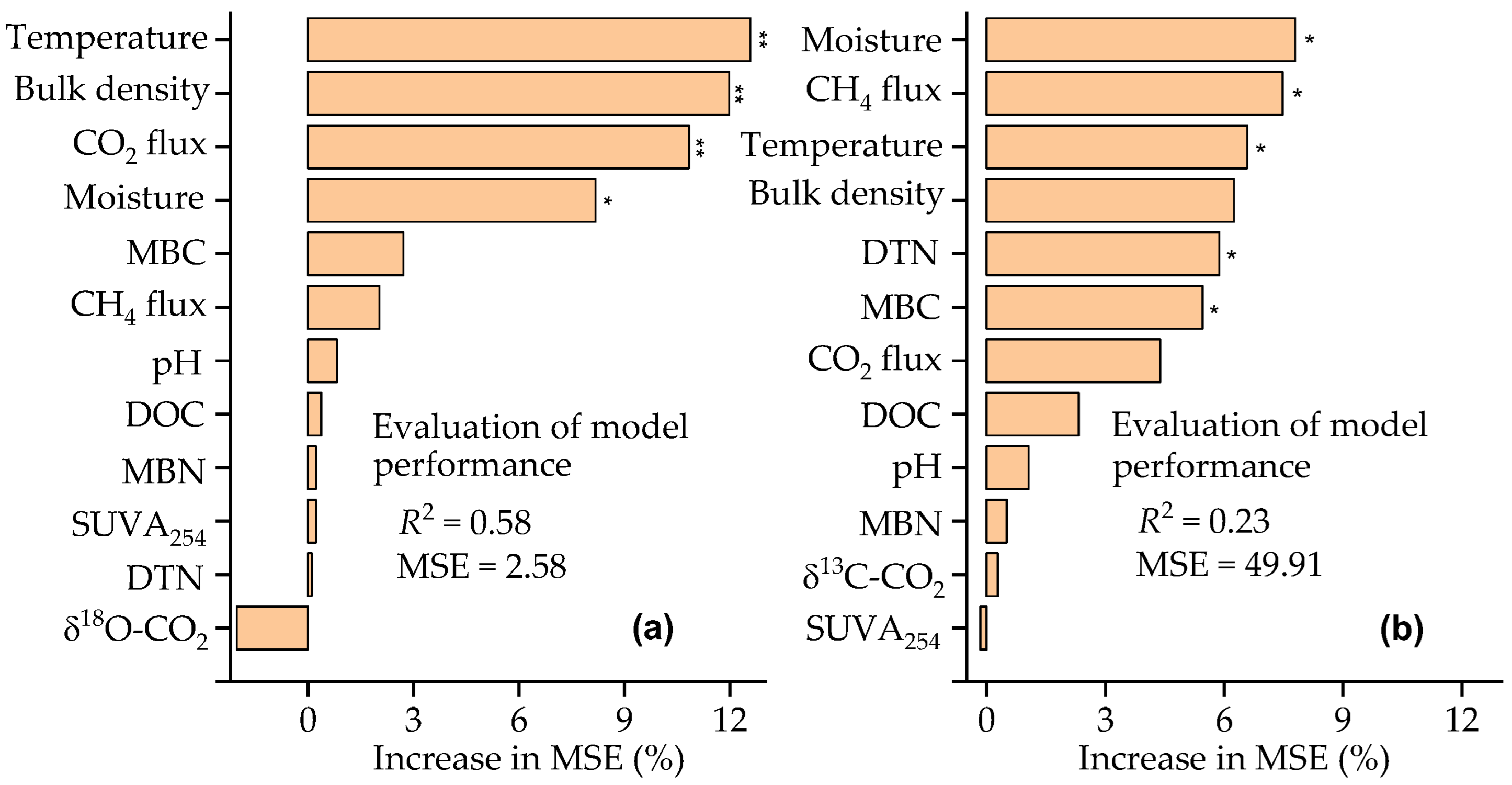
3.4. Factors Affecting the δ13C and δ18O Values of Respired CO2 Under Well-Drained Forest Floors
4. Discussion
4.1. In Situ Measurements of δ13C and δ18O Values of Respired CO2
4.2. Effect of Mosses and Long-Term N Addition on the δ13C Values of Respired CO2
4.3. Effect of Mosses and Long-Term N Addition on the δ18O Values of Respired CO2
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farquhar, G.D.; Lloyd, J.; Taylor, J.A.; Flanagan, L.B.; Syversten, J.P.; Hubick, K.T.; Wong, S.C.; Ehleringer, J.R. Vegetation effects on the isotopic composition of oxygen in atmospheric CO2. Nature 1993, 363, 439–443. [Google Scholar] [CrossRef]
- Ciais, P.; Tans, P.P.; Trolier, M.; White, J.W.C.; Francey, R.J. A large northern hemisphere terrestrial CO2 sink indicated by the 13C/12C ratio of atmospheric CO2. Science 1995, 269, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Ehleringer, J.R.; Bowling, D.R.; Flanagan, L.B.; Fessenden, J.; Helliker, B.; Martinelli, L.A.; Ometto, J.P. Stable isotopes and carbon cycle processes in forests and grasslands. Plant Biol. 2002, 4, 181–189. [Google Scholar] [CrossRef]
- Pataki, D.E.; Ehleringer, J.R.; Flanagan, L.B.; Yakir, D.; Bowling, D.R.; Still, C.J.; Buchmann, N.; Kaplan, J.O.; Berry, J.A. The application and interpretation of Keeling plots in terrestrial carbon cycle research. Glob. Biogeochem. Cycles 2003, 17, 1022. [Google Scholar] [CrossRef]
- McDowell, N.G.; Bowling, D.R.; Schauer, A.; Irvine, J.; Bond, B.J.; Law, B.E.; Ehleringer, J.R. Associations between carbon isotope ratios of ecosystem respiration, water availability and canopy conductance. Glob. Change Biol. 2004, 10, 1767–1784. [Google Scholar] [CrossRef]
- Ometto, J.P.H.; Flanagan, L.B.; Martinelli, L.A.; Ehleringer, J.R. Oxygen isotope ratios of waters and respired CO2 in Amozonian forest and pasture ecosystems. Ecol. Appl. 2005, 15, 58–70. [Google Scholar] [CrossRef][Green Version]
- Barbour, M.M.; Hunt, J.E.; Kodama, N.; Laubach, J.; McSeveny, T.M.; Rogers, G.N.D.; Tcherkez, G.; Wingate, L. Rapid changes in δ13C of ecosystem-respired CO2 after sunset are consistent with transient 13C enrichment of leaf respired CO2. New Phytol. 2011, 190, 990–1002. [Google Scholar] [CrossRef]
- Mauritz, M.; Celis, G.; Ebert, C.; Hutchings, J.; Ledman, J.; Natali, S.M.; Pegoraro, E.; Salmon, V.G.; Schädel, C.; Taylor, M.; et al. Using stable carbon isotopes of seasonal ecosystem respiration to determine permafrost carbon loss. JGR Biogeosci. 2019, 124, 46–60. [Google Scholar] [CrossRef]
- Diao, H.Y.; Wang, A.Z.; Yuan, F.H.; Guan, D.X.; Dai, G.H.; Wu, J.B. Environmental effects on carbon isotope discrimination from assimilation to respiration in a coniferous and broad-leaved mixed forest of Northeast China. Forests 2020, 11, 1156. [Google Scholar] [CrossRef]
- Xu, X.K. Effect of changes in throughfall on soil respiration in global forest ecosystems: A meta-analysis. Forests 2023, 14, 1037. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Brooks, J.R.; Varney, G.T.; Ehleringer, J.R. Discrimination against C18O16O during photosynthesis and the oxygen isotope ratio of respired CO2 in boreal forest ecosystems. Glob. Biogeochem. Cycles 1997, 11, 83–98. [Google Scholar] [CrossRef]
- Riley, W.J.; Still, C.J.; Helliker, B.R.; Ribas-Carbo, M.; Berry, J.A. 18O composition of CO2 and H2O ecosystem pools and fluxes in a tallgrass prairie: Simulations and comparisons to measurements. Glob. Change Biol. 2003, 9, 1567–1581. [Google Scholar] [CrossRef]
- Moyes, A.B.; Gaines, S.J.; Siegwolf, R.T.W.; Bowling, D.R. Diffusive fractionation complicates isotopic partitioning of autotrophic and heterotrophic sources of soil respiration. Plant Cell Environ. 2010, 33, 1804–1819. [Google Scholar] [CrossRef]
- Goffin, S.; Aubinet, M.; Maier, M.; Plain, C.; Schack-kirchner, H.; Longdoz, B. Characterization of the soil CO2 production and its carbon isotope composition in forest soil layers using the flux-gradient approach. Agric. For. Meteorol. 2014, 188, 45–57. [Google Scholar] [CrossRef]
- Williams, T.G.; Flanagan, L.B. Effect of changes in water content on photosynthesis, transpiration and discrimination against 13CO2 and C18O16O in Pleurozium and Sphagnum. Oecologia 1996, 108, 38–46. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Kubien, D.S.; Ehleringer, J.R. Spatial and temporal variation in the carbon and oxygen stable isotope ratio of respired CO2 in a boreal forest ecosystem. Tellus B Chem. Phys. Meteorol. 1999, 51, 367–384. [Google Scholar] [CrossRef]
- Min, K.; Lehmeier, C.A.; Ballantyne, F.; Billings, S.A. Carbon availability modifies temperature responses of heterotrophic microbial respiration, carbon uptake affinity, and stable carbon isotope discrimination. Front. Microbiol. 2016, 7, 2083. [Google Scholar] [CrossRef]
- Wingate, L.; Ogée, J.; Cuntz, M.; Genty, B.; Reiter, L.; Seibt, U.; Yakir, D.; Maseyk, K.; Pendall, E.G.; Barbour, M.M.; et al. The impact of soil microorganisms on the global budget of δ18O in atmospheric CO2. Proc. Natl. Acad. Sci. USA 2009, 106, 22411–22415. [Google Scholar] [CrossRef]
- Wingate, L.; Ogée, J.; Burlett, R.; Bosc, A. Strong seasonal disequilibrium measured between the oxygen isotope signals of leaf and soil CO2 exchange. Glob. Change Biol. 2010, 16, 3048–3064. [Google Scholar] [CrossRef]
- Goulden, M.L.; Crill, P.M. Automated measurements of CO2 exchange at the moss surface of a black spruce forest. Tree Physiol. 1997, 17, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Delucia, E.H.; Turnbull, M.H.; Walcroft, A.S.; Griffin, K.L.; Tissue, D.T.; Glenny, D.; McSeveny, T.M.; Whitehead, D. The contribution of bryophytes to the carbon exchange for a temperate rainforest. Glob. Change Biol. 2003, 9, 1158–1170. [Google Scholar] [CrossRef]
- Silvola, J. Combined effects of varying water content and CO2 concentration on photosynthesis in Sphagnum fuscum. Holarct. Ecol. 1990, 13, 224–228. Available online: https://www.jstor.org/stable/3682506 (accessed on 2 July 2025).
- Rice, S.K.; Giles, L. The influence of water content and leaf anatomy on carbon isotope discrimination and photosynthesis in Sphagnum. Plant Cell Environ. 1996, 19, 118–124. [Google Scholar] [CrossRef]
- Fessenden, J.E.; Ehleringer, J.R. Temporal variation in δ13C of ecosystem respiration in the Pacific Northwest: Links to moisture stress. Oecologia 2003, 136, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Coxson, D.S.; Mcintyre, D.D.; Vogel, H.J. Pulse release of sugars and polyols from canopy bryophytes in tropical montane rain forest Guadeloupe French West Indies. Biotropica 1992, 24, 121–133. [Google Scholar] [CrossRef]
- Wilson, J.A.; Coxson, D.S. Carbon flux in a subalpine spruce-fir forest: Pulse release from Hylocomium splendens feather-moss mats. Can. J. Bot. 1999, 77, 564–569. [Google Scholar] [CrossRef]
- Deluca, T.H.; Zackrisson, O.; Nilsson, M.C.; Sellstedt, A. Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 2002, 419, 917–920. [Google Scholar] [CrossRef]
- Sun, S.Q.; Liu, T.; Wu, Y.H.; Wang, G.X.; Zhu, B.; DeLuca, T.H.; Wang, Y.Q.; Luo, J. Ground bryophytes regulate net soil carbon efflux: Evidence from two subalpine ecosystems on the east edge of the Tibet Plateau. Plant Soil 2017, 417, 363–375. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.C.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Leppänen, S.M.; Salemaa, M.; Smolander, A.; Mäkipää, R.; Tiirola, M. Nitrogen fixation and methanotrophy in forest mosses along a N deposition gradient. Environ. Exp. Bot. 2013, 90, 62–69. [Google Scholar] [CrossRef]
- Saiz, E.; Sgouridis, F.; Drijfhout, F.P.; Peichl, M.; Nilsson, M.B.; Ullah, S. Chronic atmospheric reactive nitrogen deposition suppress biological nitrogen fixation in peatlands. Environ. Sci. Technol. 2021, 55, 1310–1318. [Google Scholar] [CrossRef]
- Mäkipää, R. Sensitivity of forest floor bryophytees in boreal forests to sulphur and nitrogen deposition. Water Air Soil Pollut. 1995, 85, 1239–1244. [Google Scholar] [CrossRef]
- Gunnarsson, U.; Rydin, H. Nitrogen fertilization reduces Sphagnum production in bog communities. New Phytol. 2000, 147, 527–537. [Google Scholar] [CrossRef]
- Salemaa, M.; Mäkipää, R.; Oksanen, J. Differences in the growth response of three bryophyte species to nitrogen. Environ. Pollut. 2008, 152, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Limpens, J.; Granath, G.; Gunnarsson, U.; Aerts, R.; Bayley, S.; Bragazza, L.; Bubier, J.; Buttler, A.; van den Berg, L.J.L.; Francez, A.J.; et al. Climatic modifiers of the response to nitrogen deposition in peatforming Sphagnum mosses: A meta-analysis. New Phytol. 2011, 191, 496–507. [Google Scholar] [CrossRef]
- Gundale, M.J.; Deluca, T.H.; Nordin, A. Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Glob. Change Biol. 2011, 17, 2743–2753. [Google Scholar] [CrossRef]
- Lu, F.; Yi, B.L.; Qin, K.; Bu, Z.J. Long-term nitrogen addition eliminates the cooling effect on climate in a temperate peatland. Plants 2025, 14, 1183. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.S.; Niu, S.L. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Yu, M.X.; Wang, Y.P.; Baldock, J.A.; Jiang, J.; Mo, J.M.; Zhou, G.Y.; Yan, J.H. Divergent responses of soil organic carbon accumulation to 14 years of nitrogen addition in two typical subtropical forests. Sci. Total Environ. 2020, 707, 136104. [Google Scholar] [CrossRef]
- Xu, X.K.; Han, L.; Luo, X.B.; Han, S.J. Synergistic effects of nitrogen amendments and ethylene on atmospheric methane uptake under a temperate old-growth forest. Adv. Atmos. Sci. 2011, 28, 843–854. [Google Scholar] [CrossRef]
- Xia, N.; Du, E.Z.; Wu, X.H.; Tang, Y.; Wang, Y.; de Vries, W. Effects of nitrogen addition on soil methane uptake in global forest biomes. Environ. Pollut. 2020, 264, 114751. [Google Scholar] [CrossRef]
- Jordan, S.F.A.; Schloemer, S.; Krüger, M.; Heffner, T.; Horn, M.A.; Blumenberg, M. Interferences caused by the biogeochemical methane cycle in peats during the assessment of abandoned oil wells. Biogeosciences 2025, 22, 809–830. [Google Scholar] [CrossRef]
- Xu, X.K.; Inubushi, K. Responses of ethylene and methane consumption to temperature and pH in temperate volcanic forest soils. Eur. J. Soil Sci. 2009, 60, 489–498. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Q.J.; Xu, Z.Z.; Du, W.X.; Yu, J.; Meng, S.W.; Zhou, H.; Qi, L.H.; Shah, S. How can the shade intolerant Korean pine survive under dense deciduous canopy? For. Ecol. Manag. 2020, 457, 117735. [Google Scholar] [CrossRef]
- Kammer, A.; Tuzson, B.; Emmenegger, L.; Knohl, A.; Mohn, J.; Hagedorn, F. Application of a quantum cascade laser-based spectrometer in a closed chamber system for real-time δ13C and δ18O measurements of soil-respired CO2. Agric. For. Meteorol. 2011, 151, 39–48. [Google Scholar] [CrossRef]
- Keeling, C.D. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochim. Cosmochim. Acta 1958, 13, 322–334. [Google Scholar] [CrossRef]
- Keeling, C.D. The concentration and isotopic abundances of carbon dioxide in rural and marine air. Geochim. Cosmochim. Acta 1961, 24, 277–298. [Google Scholar] [CrossRef]
- Xu, X.K.; Wu, H.H.; Yue, J.; Tang, S.R.; Cheng, W.G. Effects of snow cover on carbon dioxide emissions and their δ13C values of temperate forest soils with and without litter. Forests 2023, 14, 1384. [Google Scholar] [CrossRef]
- Al-Shammary, A.A.G.; Kouzani, A.Z.; Kaynak, A.; Khoo, S.Y.; Norton, M.; Gates, W. Soil bulk density estimation methods: A review. Pedosphere 2018, 28, 581–596. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookesa, P.C.; Jenkinsona, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C: An automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Brookes, P.C.; Powlson, D.S. Measuring soil microbial biomass. Soil Biol. Biochem. 2004, 36, 5–7. [Google Scholar] [CrossRef]
- Xu, X.K.; Luo, X.B.; Jiang, S.H.; Xu, Z.J. Biodegradation of dissolved organic carbon in soil extracts and leachates from a temperate forest stand and its relationship to ultraviolet absorbance. Chin. Sci. Bull. 2012, 57, 912–920. [Google Scholar] [CrossRef][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Xu, X.K.; Duan, C.T.; Wu, H.H.; Luo, X.B.; Han, L. Effects of changes in throughfall on soil GHG fluxes under a mature temperate forest, northeastern China. J. Environ. Manag. 2021, 294, 112950. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic Stoichiometry and Ecological Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef]
- Fu, Y.W.; Li, Y.F.; Yu, Z.C. Last millennium hydroclimate and atmospheric circulation change in Northeast China: A dual δ13C and δ18O approach from a mountaintop Sphagnum bog. Quat. Sci. Rev. 2022, 295, 107781. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Varney, G.T. Influence of vegetation and soil CO2 exchange on the concentration and stable oxygen isotope ratio of atmospheric CO2 within a Pinus resinosa canopy. Oecologia 1995, 101, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Tans, P.P. Oxygen isotopic equilibrium between carbon dioxide and water in soils. Tellus B Chem. Phys. Meteorol. 1998, 50, 163–178. [Google Scholar] [CrossRef]
- Hesterberg, R.; Siegenthaler, U. Production and stable isotopic composition of CO2 in a soil near Bern, Switzerland. Tellus B Chem. Phys. Meteorol. 1991, 43, 197–205. [Google Scholar] [CrossRef]
- Kylin, H.; Bouwman, H. Hydration state of the moss hylocomium splendens and the lichen cladina stellaris governs uptake and revolatilization of airborne α and γ-hexachlorocyclohexane. Environ. Sci. Technol. 2012, 46, 10982–10989. [Google Scholar] [CrossRef] [PubMed]
- Knohl, A.; Werner, R.A.; Brand, W.A.; Buchmann, N. Short-term variations in δ13C of ecosystem respiration reveals link between assimilation and respiration in a deciduous forest. Oecologia 2005, 142, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Gavrichkova, O.; Proietti, S.; Moscatello, S.; Portarena, S.; Battistelli, A.; Matteucci, G.; Brugnoli, E. Short-term natural δ13C and δ18O variations in pools and fluxes in a beech forest: The transfer of isotopic signal from recent photosynthates to soil respired CO2. Biogeosciences 2011, 8, 2833–2846. [Google Scholar] [CrossRef]
- Mironov, V.L. Threshold behavior hidden in the growth response of peat moss Sphagnum riparium to temperature. Plants 2024, 13, 3241. [Google Scholar] [CrossRef] [PubMed]
- Gillon, J.; Yakir, D. Influence of carbonic anhydrase activity in terrestrial vegetation on the 18O content of atmospheric CO2. Science 2001, 291, 2584–2587. [Google Scholar] [CrossRef]
- Gimeno, T.E.; Ogée, J.; Royles, J.; Gibon, Y.; West, J.B.; Burlett, R.; Jones, S.P.; Sauze, J.; Wohl, S.; Benard, C.; et al. Bryophyte gas-exchange dynamics along varying hydration status reveal a significant carbonyl sulphide (COS) sink in the dark and COS source in the light. New Phytol. 2017, 215, 965–976. [Google Scholar] [CrossRef]
- Ryhti, K.; Schiestl-Aalto, P.; Tang, Y.; Rinne-Garmston, K.T.; Ding, Y.Y.; Pumpanen, J.; Biaso, C.; Saurer, M.; Baeck, J.; Kulmala, L. Effects of variable temperature and moisture conditions on respiration and nonstructural carbohydrate dynamics of tree roots. Agric. For. Meteorol. 2022, 323, 109040. [Google Scholar] [CrossRef]
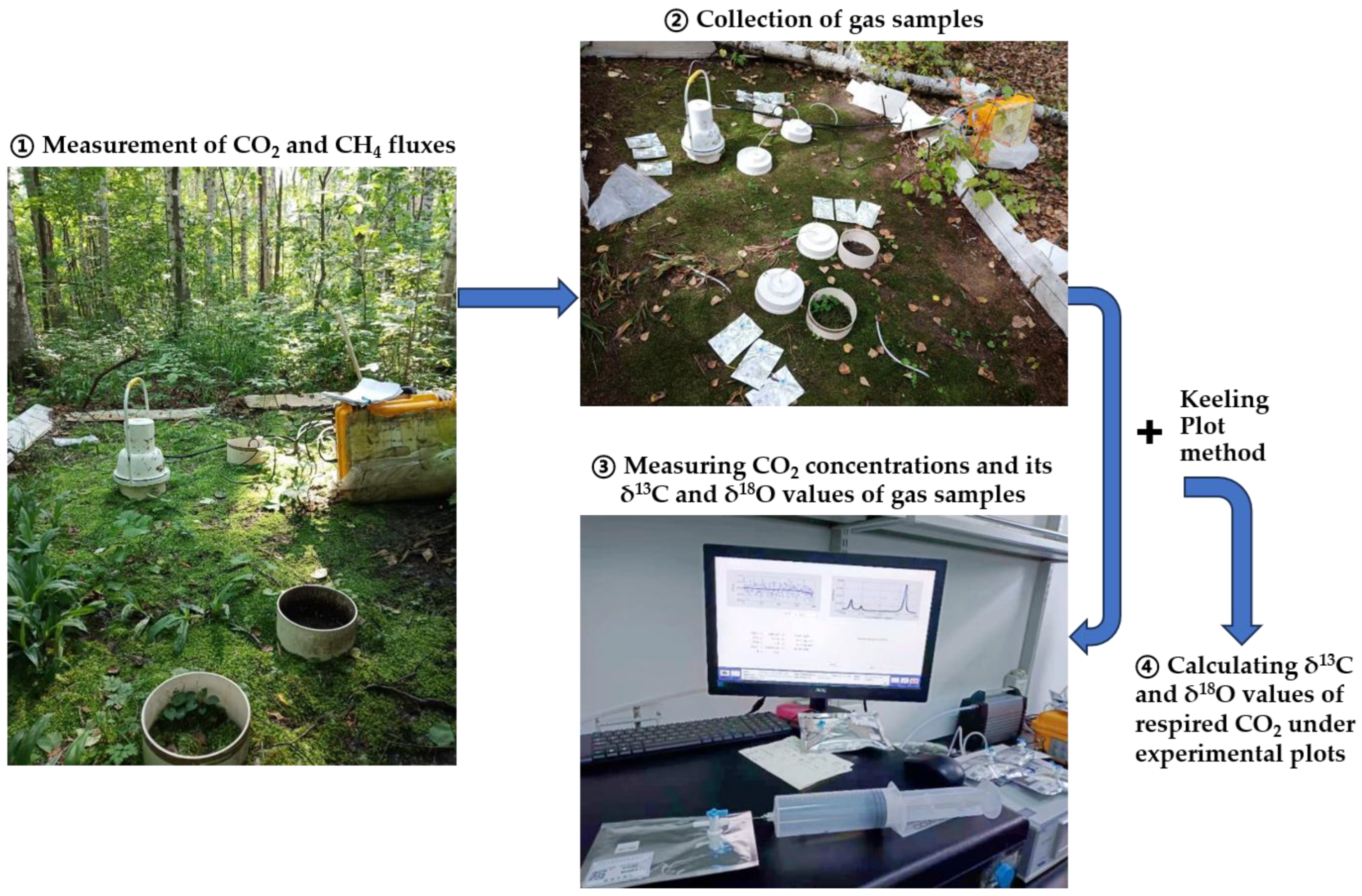
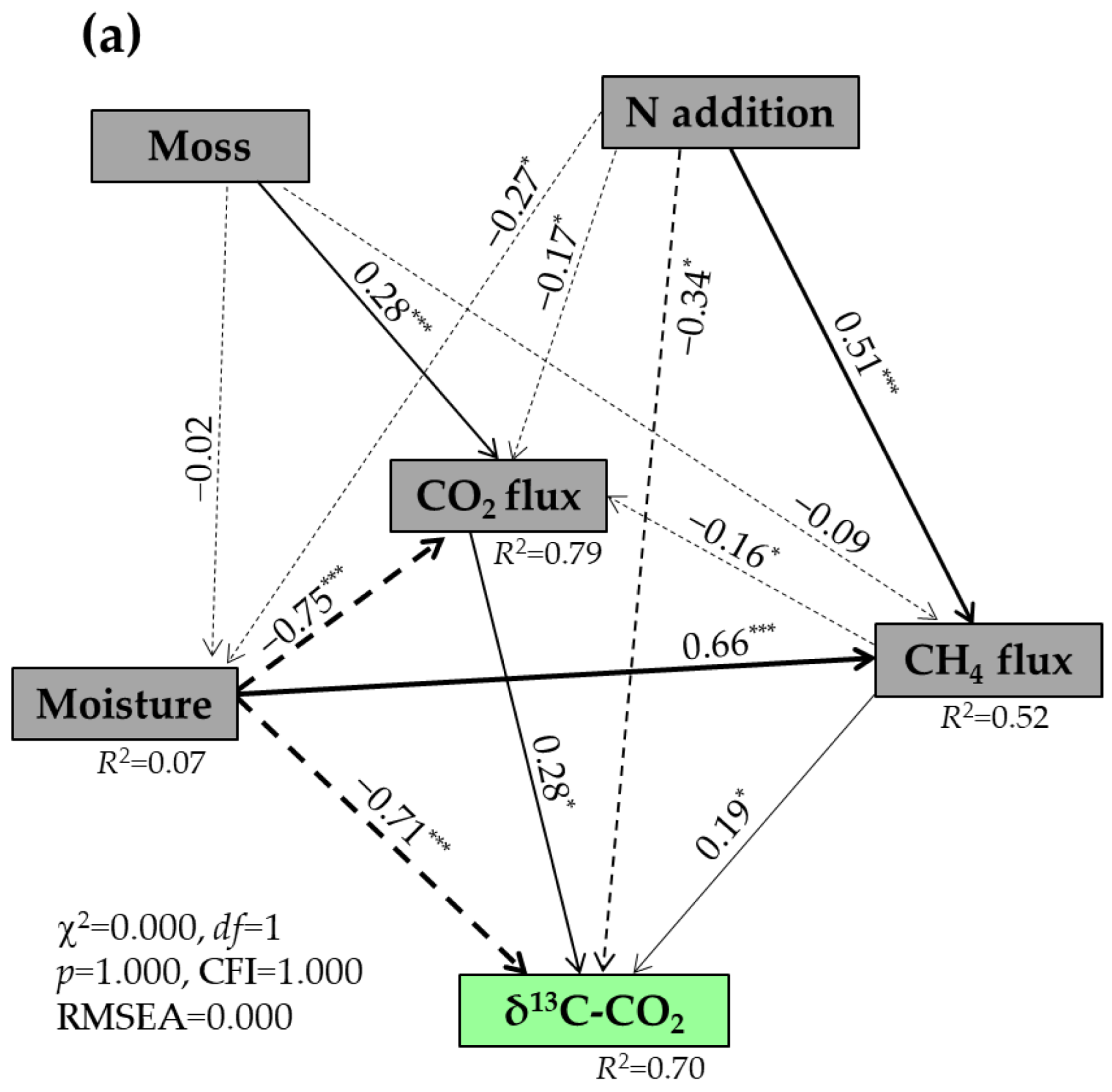

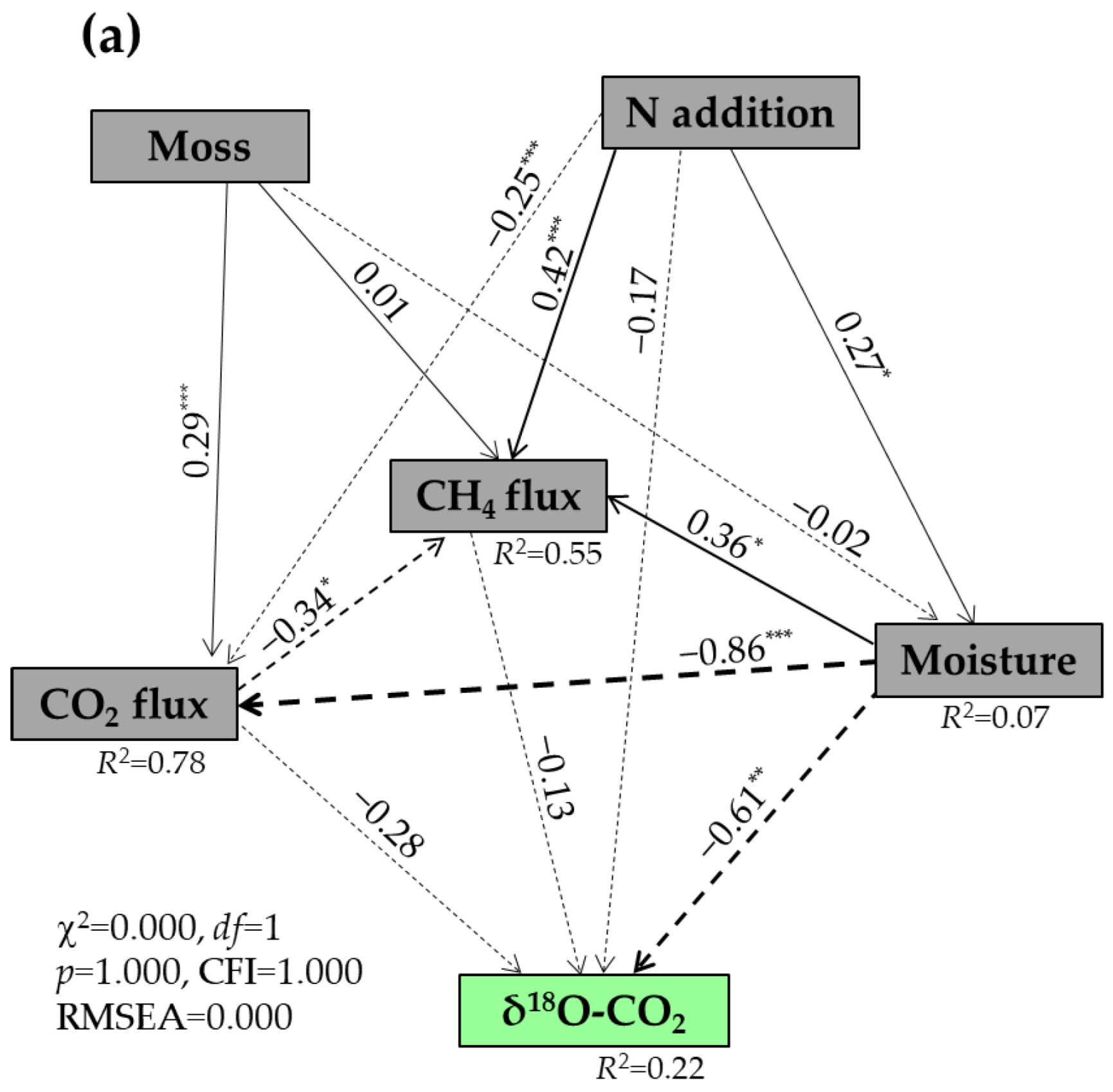
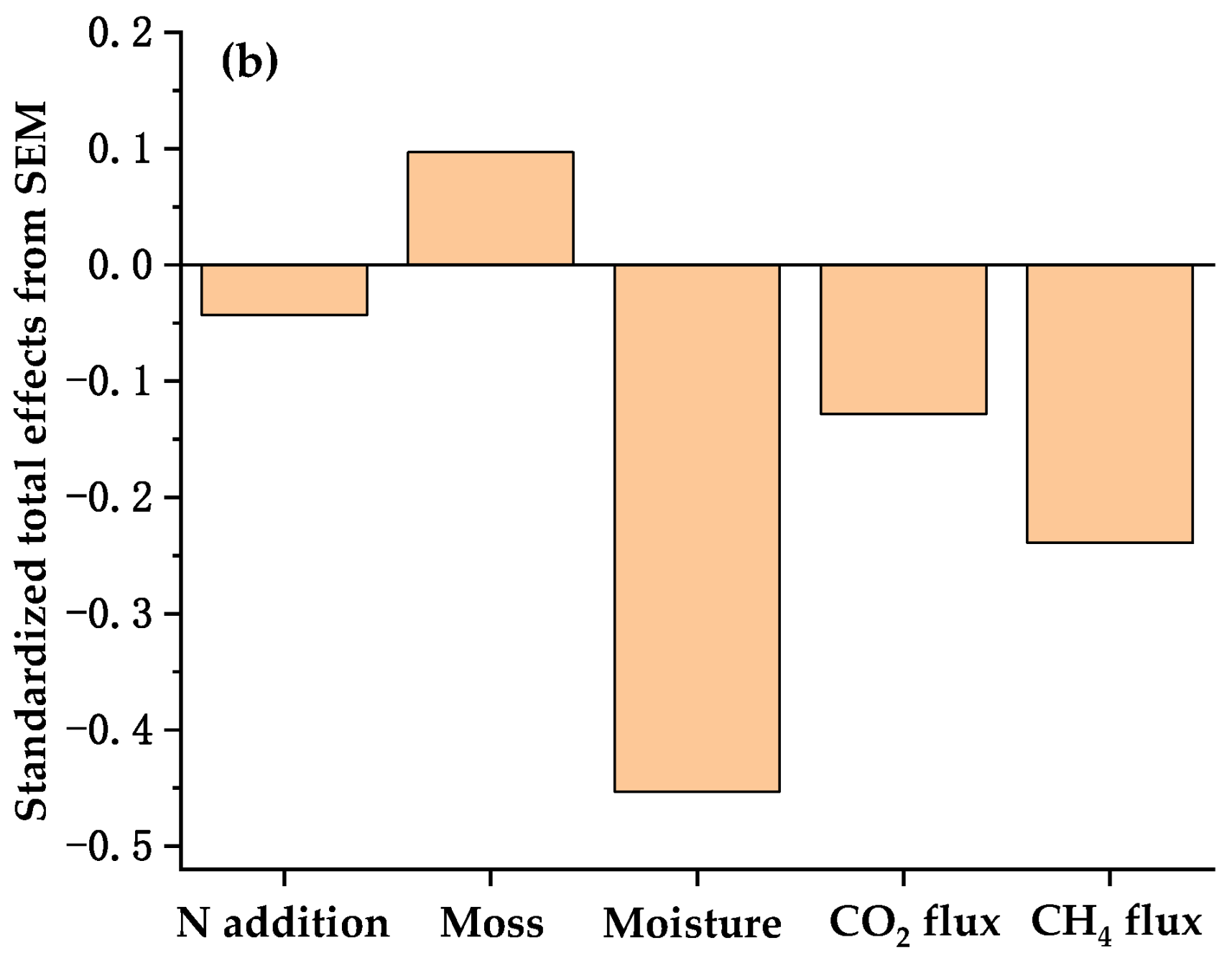
| Treatments | DOC (μg C g−1) | DTN (μg N g−1) | SUVA254 (L mg−1 C m−1) | MBC (μg C g−1) | MBN (μg N g−1) | pH (Water) | Bulk Density (g cm−3) |
|---|---|---|---|---|---|---|---|
| Control | 90.2 (72.5–107.5) | 14.8 (9.4–22.5) | 1.92 (1.49–2.25) | 604.1 (390.1–996.7) | 140.5 (80.8–192.90) | 5.84 (5.49–6.10) | 0.95 (0.49–1.30) |
| Low N | 95.1 (67.8–141.7) | 15.4 (9.0–23.4) | 2.22 (1.56–2.87) | 527.5 (305.9–897.6) | 113.7 (79.7–156.7) | 5.45 (5.02–6.00) | 1.01 (0.56–1.31) |
| High N | 103.8 (80.2–139.2) | 27.8 (17.1–52.9) | 2.31 (1.85–3.45) | 734.5 (432.3–1226.2) | 168.2 (137.3–191.7) | 5.17 (4.95–5.55) | 0.90 (0.50–1.18) |
| ANOVA with treatment as a fixed factor and year as a random factor (p value) | |||||||
| Treatment | 0.265 | 0.015 | 0.003 | 0.120 | 0.066 | 0.004 | 0.012 |
| Year | 0.511 | 0.049 | 0.340 | 0.012 | 0.517 | 0.020 | 0.000 |
| Treatment × Year | 0.315 | 0.173 | 0.975 | 0.067 | 0.102 | 0.061 | 0.810 |
| Treatments | δ13C Values of Respired CO2 (‰) | δ18O Values of Respired CO2 (‰) |
|---|---|---|
| With mosses | ||
| Control | −23.95 (−29.14 to −21.51) | −13.89 (−32.27 to −2.49) |
| Low N | −24.76 (−28.32 to −21.49) | −12.50 (−21.32 to −5.87) |
| High N | −23.88 (−28.99 to −20.77) | −16.78 (−22.16 to −8.79) |
| Without mosses | ||
| Control | −22.92 (−28.01 to −20.12) | −15.38 (−33.60 to −2.22) |
| Low N | −23.70 (−28.13 to −20.04) | −14.27 (−27.14 to −4.43) |
| High N | −23.28 (−27.75 to −20.31) | −17.48 (−28.86 to −8.77) |
| ANOVA with moss and treatment as fixed factors and year as a random factor (p value) | ||
| Treatment | 0.480 | 0.715 |
| Moss | 0.025 | 0.003 |
| Year | 0.002 | 0.331 |
| Treatment × Moss | 0.312 | 0.935 |
| Treatment × Year | 0.007 | 0.029 |
| Moss × Year | 0.330 | 0.997 |
| Treatment × Moss × Year | 0.933 | 0.367 |
| CO2 Flux | CH4 Flux | Moisture | Temperature | DOC | DTN | pH | Bulk Density | MBC | MBN | SUVA254 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| δ13C | 0.65 ** | −0.44 ** | −0.74 ** | 0.76 ** | −0.21 | −0.39 * | −0.34 * | 0.79 ** | −0.61 ** | 0.32 | 0.03 |
| δ18O | 0.34 ** | −0.34 ** | −0.41 ** | 0.30 ** | −0.25 | −0.40 ** | −0.09 | 0.41 * | −0.33 | 0.18 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Kong, Y.; Feng, E.; Yue, J.; Cheng, W.; Khoroshaev, D.; Kivalov, S. Effect of Mosses and Long-Term N Addition on δ13C and δ18O Values of Respired CO2 Under a Temperate Forest Floor. Plants 2025, 14, 2707. https://doi.org/10.3390/plants14172707
Xu X, Kong Y, Feng E, Yue J, Cheng W, Khoroshaev D, Kivalov S. Effect of Mosses and Long-Term N Addition on δ13C and δ18O Values of Respired CO2 Under a Temperate Forest Floor. Plants. 2025; 14(17):2707. https://doi.org/10.3390/plants14172707
Chicago/Turabian StyleXu, Xingkai, Yuhua Kong, Erpeng Feng, Jin Yue, Weiguo Cheng, Dmitriy Khoroshaev, and Sergey Kivalov. 2025. "Effect of Mosses and Long-Term N Addition on δ13C and δ18O Values of Respired CO2 Under a Temperate Forest Floor" Plants 14, no. 17: 2707. https://doi.org/10.3390/plants14172707
APA StyleXu, X., Kong, Y., Feng, E., Yue, J., Cheng, W., Khoroshaev, D., & Kivalov, S. (2025). Effect of Mosses and Long-Term N Addition on δ13C and δ18O Values of Respired CO2 Under a Temperate Forest Floor. Plants, 14(17), 2707. https://doi.org/10.3390/plants14172707








