Antiproliferative and Anti-Migratory Activities of an Extract from Fridericia platyphylla Leaves and Its Molecular Profile
Abstract
1. Introduction
2. Results and Discussion
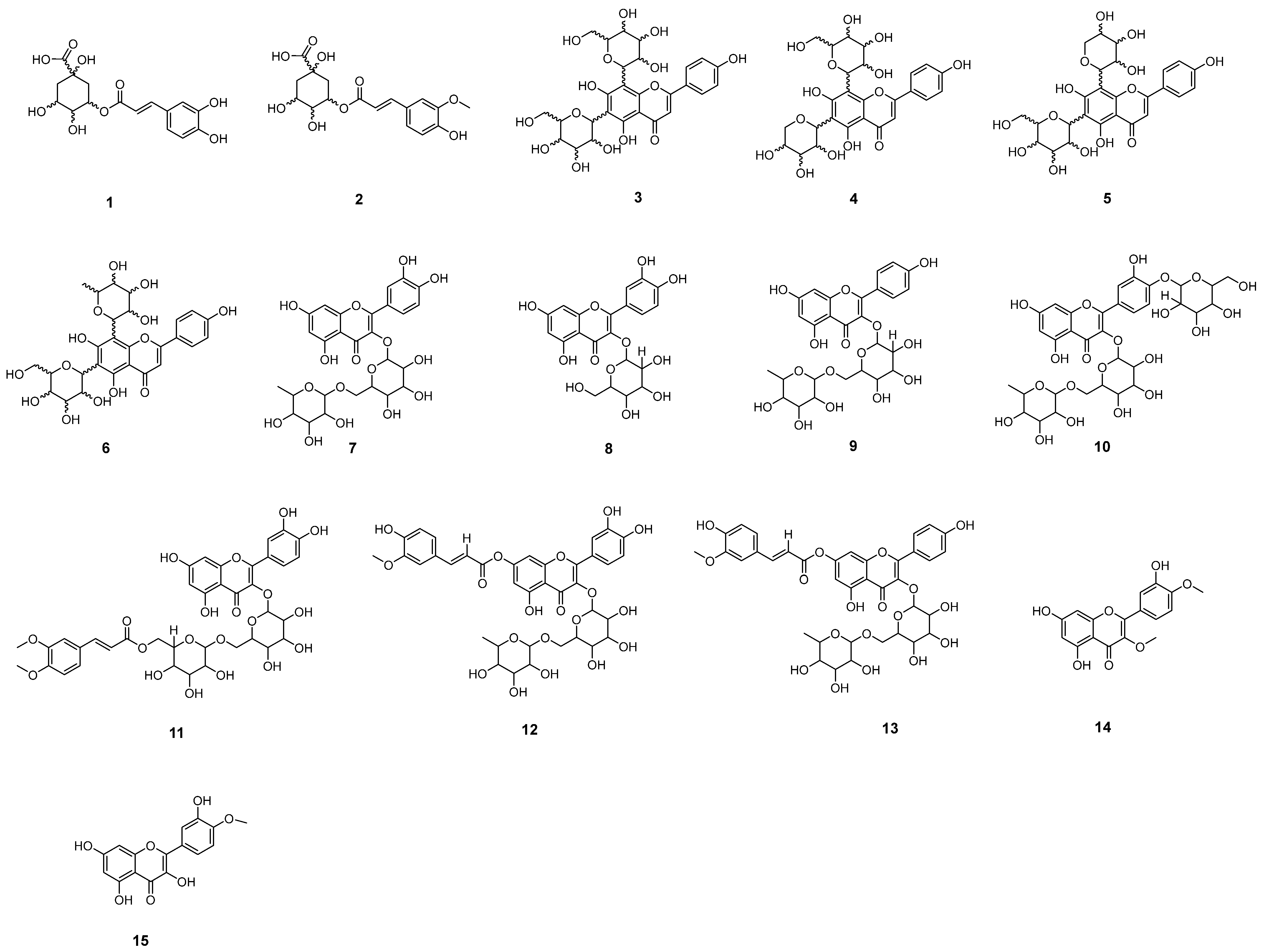
2.1. Chemical Profile of Crude Extract of Fridericia Platyphylla Leaves (FAB)
2.2. Chromatographic Peaks Annotation
2.2.1. Chlorogenic Acids
2.2.2. Glycosylated Flavones
2.2.3. Glycosylated Flavonols
2.2.4. Flavonol Aglycone
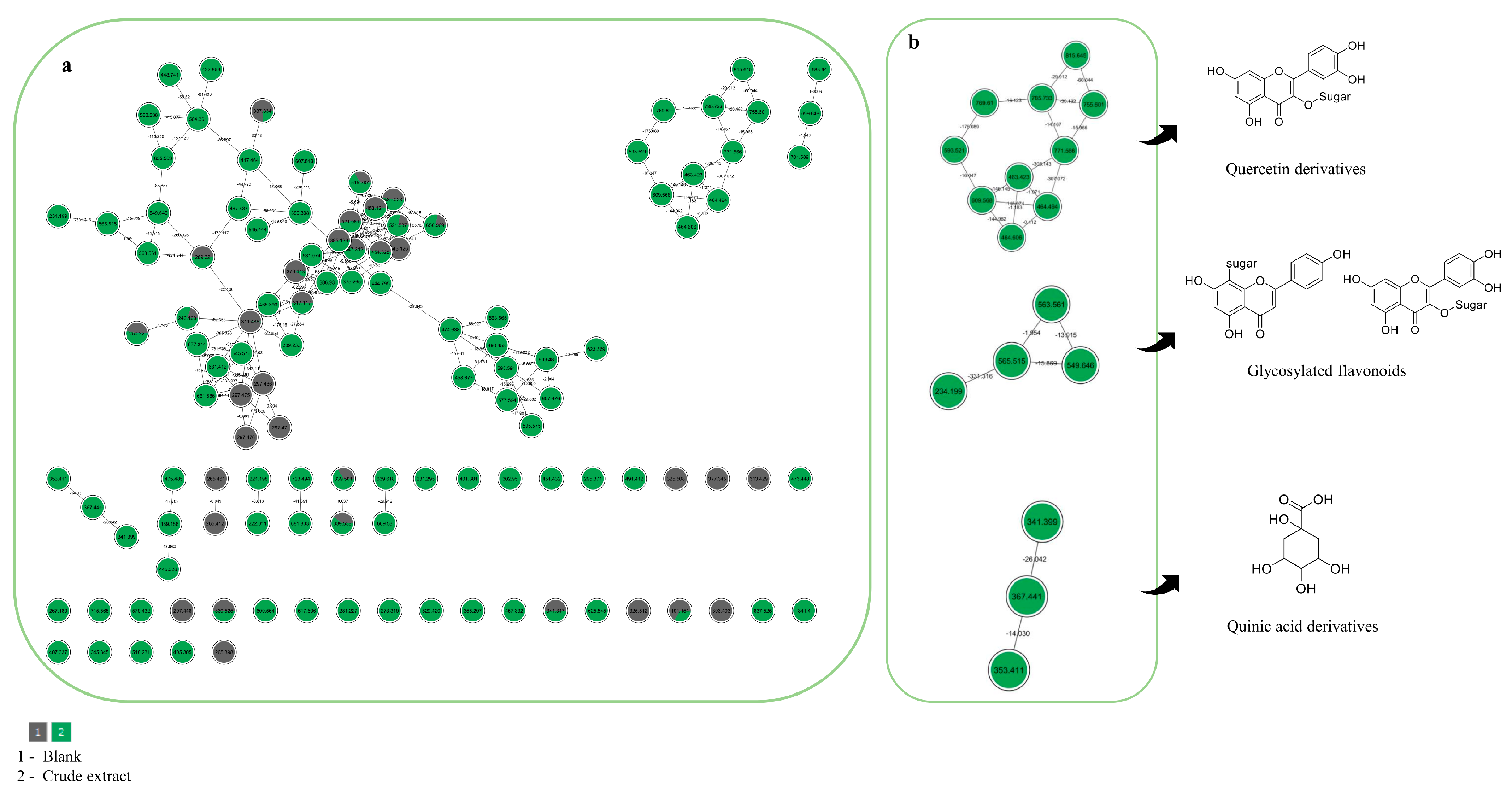
2.3. Global Natural Products Social Molecular Networking (GNPS)
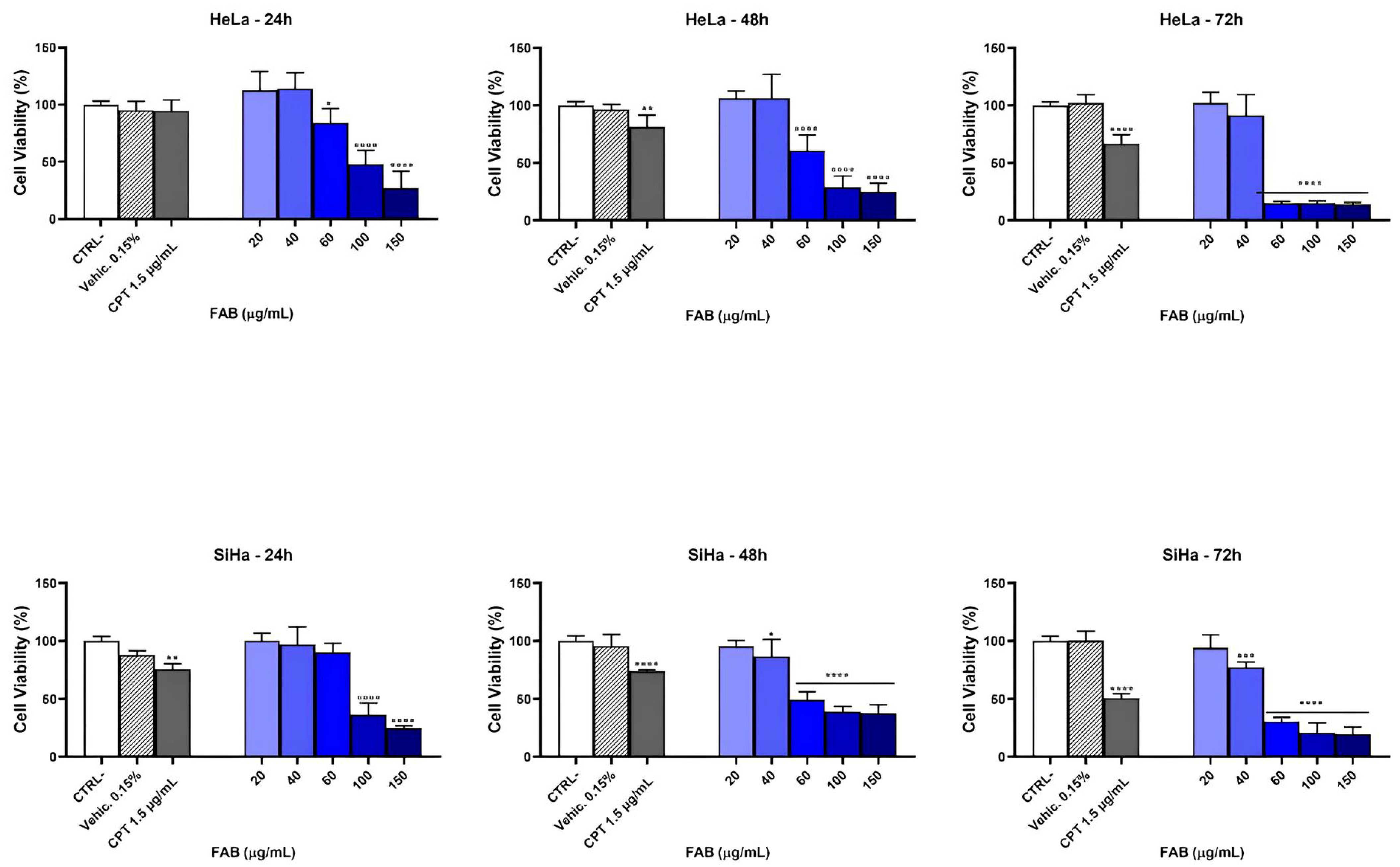
2.4. Cytotoxic Effect of FAB on HeLa and SiHa Cell Lines
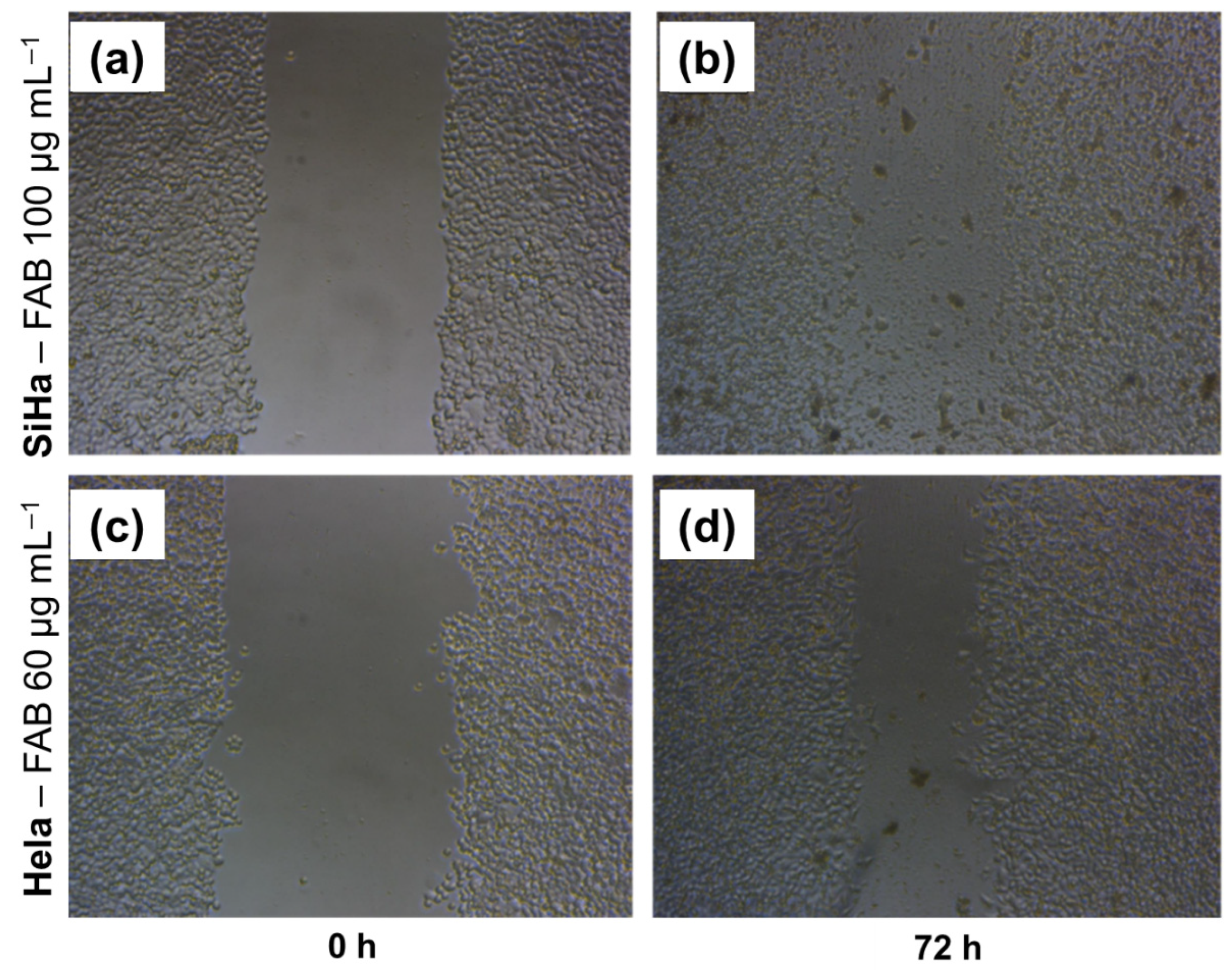
2.5. Wound Healing Model
3. Materials and Methods
3.1. Material Collection
3.2. Preparation of Ethanolic Extract
3.3. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis
3.4. MS/MS Data Processing and Classical Molecular Networking
3.5. Anticancer Assays
3.5.1. Cell Culture
3.5.2. MTT Cytotoxicity Assays
3.5.3. Wound-Healing Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, D.; Gupta, M.; Sarwat, M.; Siddique, H.R. Apigenin in Cancer Prevention and Therapy: A Systematic Review and Meta-analysis of Animal Models. Crit. Rev. Oncol. Hematol. 2022, 176, 103751. [Google Scholar] [CrossRef]
- Sobti, R.C.; Thakur, M.; Kaur, T. Molecular Biomarkers for Cancer Diagnosis and Therapy; Sobti, R.C., Sugimura, H., Sobti, A., Eds.; Springer Nature: Singapore, 2024. [Google Scholar] [CrossRef]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef]
- Shoaib, S.; Ansari, M.A.; Ghazwani, M.; Hani, U.; Jamous, Y.F.; Alali, Z.; Wahab, S.; Ahmad, W.; Weir, S.A.; Alomary, M.N.; et al. Prospective Epigenetic Actions of Organo-SulfurCompounds against Cancer: Perspective and Molecular Mechanisms. Cancers 2023, 15, 697. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, K.; Aktar, A.; Roy, P.; Biswas, B.; Hossain, M.E.; Sarkar, K.K.; Bachar, S.C.; Ahmed, F.; Monjur-Al-hossain, A.S.M.; Fukase, K. A Review on Mechanistic Insight of Plant Dericed Anticancer Bioactive Phytocompounds and Their Structure Activity Relationship. Molecules 2022, 27, 3036. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bisht, B.; Dutta, S.; Paul, M.K. Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy. Acta Pharmacol. Sin. 2022, 43, 2759. [Google Scholar] [CrossRef]
- Bozgeyik, E.; Bozgeyik, I. Unveiling the Therapeutic Potential of Natural-Based Anticancer Compounds Inducing Non-Canonical Cell Death Mechanisms. Pathol. Res. Pract. 2023, 248, 154693. [Google Scholar] [CrossRef]
- Han, S.; Cao, Y.; Guo, T.; Lin, Q.; Luo, F. Targeting IncRNA/Wnt axis by Flavonoids: A Promising Therapeutic Approach for Colorectal Cancer. Phytother. Res. 2022, 36, 4024. [Google Scholar] [CrossRef]
- Kasiri, N.; Rahmati, M.; Ahmadi, L.; Eskandari, N.; Motedayyen, H. Therapeutic Potential of Quercetin on Human Breast Cancer in Different Dimensions. Inflammopharmacology 2020, 28, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Brisdelli, F.; Di Francesco, L.; Giorgi, A.; Lizzi, A.R.; Luzi, C.; Mignogna, G.; Bozzi, A.; Schininà, M.E. Proteomic Analysis of Quercetin- Treated K562 Cells. Int. J. Mol. Sci. 2020, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Veeramuthu, D.; Raja, W.R.T.; Al-Dhabi, N.A.; Savarimuthu, I. Flavonoids: Anticancer Properties. In Flavonoids-From Biosynthesis to Human Health; Justino, G.C., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Kaehler, M. Fridericia in Flora e Funga do Brasil; Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB113396 (accessed on 10 August 2025).
- Bertanha, C.S.; Gimenez, V.M.M.; Furtado, R.A.; Tavares, D.C.; Cunha, W.R.; Silva, M.L.A.; Januario, A.H.; Borges, A.; Kawano, D.F.; Parreira, R.L.T.; et al. Isolation, in vitro and in sílico Evaluation of Phenylethanoid Glycoside from Arrabidaea brachypoda as Lipoxygenase Inhibitor. J. Braz. Chem. Soc. 2020, 31, 849–855. [Google Scholar] [CrossRef]
- Do Nascimento, J.R.; Miranda, J.A.M.; Vieira, F.C.; Rodrigues, D.P.; Vasconcelos, L.N.; Filho, J.L.P.; Lopes, A.C.C.B.; Tangerina, M.M.P.; Vilegas, W.; da Rocha, C.Q. A Review of the Phytochemistry and Pharmacological Properties of Gennus Arrabidaea. Pharmaceuticals 2022, 15, 658. [Google Scholar] [CrossRef] [PubMed]
- Rosário, M.S.D.; Mannochio-Russo, H.; Santos, A.L.D.; Pinheiro, A.A.; Vasconcelos, L.N.; Santos, A.P.S.; de Oliveira, L.T.; Martins, M.M.; Andrade, M.S.; Nascimento, M.D.S.B.; et al. Chemical Characterization and Evaluation of the Anti-Cancer Potential of Flowers from Fridericia platyphylla (Bignoniaceae). J. Braz. Chem. Soc. 2025, 36, e20240132. [Google Scholar] [CrossRef]
- Maciel-Silva, V.L.; da Rocha, C.Q.; Alencar, L.M.R.; Castelo-Branco, P.V.; Sousa, I.H.; Azevedo-Santos, A.P.; Vale, A.A.M.; Monteiro, S.G.; Soares, R.E.P.; Guimarães, S.J.A.; et al. Unusual Dimeric Flavonoids (Brachydins) Induce Ultrastructural Membrane Alterations Associated with Antitumor Activity in Cancer Cell Lines. Drug Chem. Toxicol. 2023, 46, 665–676. [Google Scholar] [CrossRef]
- Serpeloni, J.M.; Specian, A.F.L.; Ribeiro, D.L.; Benício, L.M.; Nunes, H.L.; Franchi, L.P.; Rocha, C.Q.; Vilegas, W.; Varanda, E.A.; Cólus, I.M.S. Fridericia platyphylla (Cham.) L.G. Lohmann Root Extract Exerts Cytotoxic and Antiproliferative Effects on Gastric Tumor Cells and Downregulates BCL-XL, BIRC5, and MET genes. Hum. Exp. Toxicol. 2020, 39, 338–354. [Google Scholar] [CrossRef]
- Nunes, H.L.; Tuttis, K.; Serpeloni, J.M.; Do Nascimento, J.R.; Da Rocha, C.Q.; Silva, V.A.O.; Lengert, A.V.H.; Reis, R.M.; De Syllos Cólus, I.M. Characterization of the in vitro Cytotoxic Effects of Brachydins Isolated from Fridericia platyphylla in a Prostate Cancer Cell Line. J. Toxicol. Environ. Health Part A 2020, 83, 547–558. [Google Scholar] [CrossRef]
- De Oliveira, L.C.B.; Ribeiro, D.L.; Nascimento, J.R.; da Rocha, C.Q.; Cólus, I.M.S.; Serpeloni, J.M. Anticancer Activities of Brachydin C in Human Prostate Tumor Cells (DU145) Grown in 2D and 3D Models: Stimulation of Cell Death and Downregulation of Metalloproteinases in Spheroids. Chem. Biol. Drug Des. 2022, 100, 747–762. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Identification of Hydroxycinnamoylquinic Acids of Arnica Flowers and Burdock Roots Using a Standardized LC-DAD-ESI/MS Profiling Method. J. Agric. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Vasil’Eva, A.G.; Gadimli, A.I.; Isaev, J.I.; Vennos, C. Caffeoylquinic Acids and Flavonoids of Fringed Sagewort (Artemisia frigida Willd.): HPLC-DAD-ESI-QQQ-MS Profile, HPLC-DAD Quantification, in Vitro Digestion Stability, and Antioxidant Capacity. Antioxidants 2019, 8, 307. [Google Scholar] [CrossRef]
- Liu, T.; Wu, X.; Dong, L.; Li, J.; Zhao, L.; Zhang, C.; Yang, R.; Song, G. Tracing of Gelsemium Elegans by UPLC-Q-TOF MS Fingerprint Analysis Technique. Microchem. J. 2024, 201, 110514. [Google Scholar] [CrossRef]
- Ahmed, M.H.O.; Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.H.; Mohamad, A.B.; Gaaz, T.S. Synthesis and characterization of a novel organic corrosion inhibitor for mild steel in 1 M hydrochloric acid. Results Phys. 2018, 9, 978–981. [Google Scholar] [CrossRef]
- Llorent-Martinez, E.J.; Spinola, V.; Gouveia, S.; Castilho, P.C. HPLC-ESI-MSn Characterization of Phenolic Compounds, Terpenoid Saponins, and Other Minor Compounds in Bituminaria bituminosa. Ind. Crops Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Artemisia annua L.: Essential Oil and Acetone Extract Composition and Antioxidant Capacity. Ind. Crops Prod. 2013, 45, 170–181. [Google Scholar] [CrossRef]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; Chaves, D.S.A.; Romaniuk, A.; Rybczynska, M.; Gryszczynska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L.; et al. Comparison of Bioactive Compounds Content in Leaf Extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro Cytotoxic Potential on Leukemia cell Lines. Rev. Bras. Farmacogn. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Dou, Y.; Shu, L.; Jia, X.; Yao, Y.; Chen, S.; Xu, Y.; Li, Y. Rapid Classification and Identification of Chemical Constituents in Leonurus japonicus Houtt Based on UPLC-Q-Orbitrap-MS Combined with Data Post-Processing Techniques. J. Mass Spectrom. 2023, 58, e4978. [Google Scholar] [CrossRef]
- Tiberti, L.A.; Yariwake, J.H.; Ndjoko, K.; Hostettmann, K. On-line LC/UV/MS Analysis of Flavonols in the Three Apple Varieties Most Widely Cultivated in Brazil. J. Braz. Chem. Soc. 2007, 18, 100–105. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Fu, X.M.; Zhuo, Y.; Yang, J.N.; Hao, N.; Wang, W.J.; Hu, Y.H.; Fang, J.S. Chemical Components and in vitro Anti-aging Activity of Yangxue Qingnao Wan based on UPLC-Q-TOF-MS/MS. China J. Chin. Mater. Medica 2024, 49, 4672–4686. [Google Scholar] [CrossRef]
- Patras, M.A.; Davalos, J.Z.; Kuhnert, N. Understanding the Fragmentation of Glucose in Mass Spectrometry. J. Mass Spectrom. 2023, 58, e4972. [Google Scholar] [CrossRef]
- Leichtweis, M.G.; Molina, A.K.; Pires, T.C.S.; Dias, M.I.; Calhelha, R.; Bachari, K.; Ziani, B.E.C.; Oliveira, M.B.P.P.; Pereira, C.; Barros, L. Biological Activity of Pumpkin Byproducts: Antimicrobial and Antioxidant Properties. Molecules 2022, 27, 8366. [Google Scholar] [CrossRef]
- Yang, P.; Xu, F.; Li, H.F.; Wang, Y.; Li, F.C.; Shang, M.Y.; Liu, G.X.; Wang, X.; Cai, S.Q. Detection of 191 Taxifolin Metabolites and Their Distribution in Rats Using HPLC-ESI-IT-TOF-MSⁿ. Molecules 2016, 21, 1209. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Lozano-Castellón, J.; Mardones, C.; Pérez, A.J.; Saéz, V.; Riquelme, S.; Von Baer, D.; Vallverdú-Queralt, A. Phenolic Profile of Grape Canes: Novel Compounds Identified by LC-ESI-LTQ-Orbitrap-MS. Molecules 2019, 24, 3763. [Google Scholar] [CrossRef]
- Ma, Q.; Xie, H.; Li, S.; Zhang, R.; Zhang, M.; Wei, X. Flavonoids from The Pericarps of Litchi chinensis. J. Agric. Food Chem. 2014, 62, 1073–1078. [Google Scholar] [CrossRef]
- Kuhnert, N.; Yassin, G.H.; Jaiswal, R.; Matei, M.F.; Grün, C.H. Differentiation of Prototropic Ions in Regioisomeric Caffeoyl Quinic Acids by Electrospray Ion Mobility Mass Spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 675–680. [Google Scholar] [CrossRef]
- Kachlicki, P.; Einhorn, J.; Muth, D.; Kerhoas, L.; Stobiecki, M. Evaluation of Glycosylation and Malonylation Patterns in Flavonoid Glycosides during LC/MS/MS Metabolite Profiling. J. Mass Spectrom. 2008, 43, 572–586. [Google Scholar] [CrossRef]
- Jiang, C.; Gates, P.J. Systematic Characterisation of the Fragmentation of Flavonoids Using High-Resolution Accurate Mass Electrospray Tandem Mass Spectrometry. Molecules 2024, 29, 5246. [Google Scholar] [CrossRef]
- Chua, L.S. A Review on Plant-based Rutin Extraction Methods and its Pharmacological Activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Ramos, S. Cancer Chemoprevention and Chemotherapy: Dietary Polyphenols and Signalling Pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef]
- Da Rocha, C.Q.; Faria, F.M.; Marcourt, L.; Ebrahimi, S.N.; Kitano, B.T.; Ghilardi, A.F.; Luiz Ferreira, A.; de Almeida, A.C.A.; Dunder, R.J.; Souza-Brito, A.R.M.; et al. Gastroprotective Effects of Hydroethanolic Root Extract of Arrabidaea Brachypoda: Evidences of Cytoprotection and Isolation of Unusual Glycosylated Polyphenols. Phytochemistry 2017, 135, 93–105. [Google Scholar] [CrossRef]
- Menezes, S.I.A.; da Rocha, C.Q.; Lima, V.A.S.; da Silva Cutrim, B.; Araújo, J.G.N.; Penha, M.S.C.; de Sá Sousa, J.C.; da Silva, C.C.; Paiva, P.M.G.; da Silva, L.C.N.; et al. Evaluation of the Antimicrobial and Antioxidant Activity of the Leaves of Fridericia Platyphylla Species. Rev. Contexto Saúde 2025, 25, e15190. [Google Scholar] [CrossRef]
- Butterweck, V.; Nahrstedt, A. What Is the Best Strategy for Preclinical Testing of Botanicals? A Critical Perspective. Planta Med. 2012, 78, 747–754. [Google Scholar] [CrossRef]
- Franconi, R.; Massa, S.; Paolini, F.; Vici, P.; Venuti, A. Plant-Derived Natural Compounds in Genetic Vaccination and Therapy for HPV-Associated Cancers. Cancers 2020, 12, 3101. [Google Scholar] [CrossRef]
- Kulmány, Á.E.; Frank, É.; Papp, D.; Szekeres, A.; Szebeni, G.J.; Zupkó, I. Biological Evaluation of Antiproliferative and Anti-Invasive Properties of an Androstadiene Derivative on Human Cervical Cancer Cell Lines. J. Steroid Biochem. Mol. Biol. 2021, 214, 105990. [Google Scholar] [CrossRef]
- Celegato, M.; Messa, L.; Goracci, L.; Mercorelli, B.; Bertagnin, C.; Spyrakis, F.; Suarez, I.; Cousido-Siah, A.; Travé, G.; Banks, L.; et al. A Novel Small-Molecule Inhibitor of the Human Papillomavirus E6-P53 Interaction That Reactivates P53 Function and Blocks Cancer Cells Growth. Cancer Lett. 2020, 470, 115–125. [Google Scholar] [CrossRef]
- Fatima, I.; Kanwal, S.; Mahmood, T. Natural Products Mediated Targeting of Virally Infected Cancer. Dose-Response 2019, 17, 1559325818813227. [Google Scholar] [CrossRef]
- Nwafor, E.O.; Lu, P.; Zhang, Y.; Liu, R.; Peng, H.; Xing, B.; Liu, Y.; Li, Z.; Zhang, K.; Zhang, Y.; et al. Chlorogenic Acid: Potential Source of Natural Drugs for the Therapeutics of Fibrosis and Cancer. Transl. Oncol. 2022, 15, 101294. [Google Scholar] [CrossRef]
- Xie, J.Z.; Xu, H.J.; Zhang, Q.; Wu, Z.; Wu, X.D.; Li, X.H.; Tan, Z.Y.; Xie, Y.F.; Qiu, L. Semi-Synthesis of Flavonoid Glycosides and Their Anti-Inflammatory and Antitumor Activities towards Triple Negative Breast Cancer. Chem. Biodivers 2023, 20, e202200899. [Google Scholar] [CrossRef]
- Rozatto, M.R. Determinação da Atividade Antimicrobiana In Vitro de Extratos, Frações e Compostos Isolados de Arrabidaea brachypoda. Master’s Thesis, Universidade Estadual Paulista (Júlio de Mesquita Filho” (UNESP), Araraquara, Brazil, 2012. [Google Scholar]
- Ribeiro, D.L.; Tuttis, K.; de Oliveira, L.C.B.; Serpeloni, J.M.; Gomes, I.N.F.; van Helvoort Lengert, A.; da Rocha, C.Q.; Reis, R.M.; de Syllos Cólus, I.M.; Antunes, L.M.G. The Antitumoral/Antimetastatic Action of the Flavonoid Brachydin A in Metastatic Prostate Tumor Spheroids In vitro Is Mediated by (Parthanatos) PARP-Related Cell Death. Pharmaceutics 2022, 14, 963. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Main, K.A.; Mikelis, C.M.; Doçi, C.L. In Vitro Wound Healing Assays to Investigate Epidermal Migration; Turksen, K., Ed.; Humana: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Sánchez-Carballido, M.A.; Delmas Suárez, C.; Gómez-Mora, J.A.; Bonneau, N. Effects of Flavonoids on Tongue Squamous Cell Carcinoma. Cell Biol. Int. 2020, 44, 686–720. [Google Scholar] [CrossRef]
- Sak, K. Characteristic Features of Cytotoxic Activity of Flavonoids on Human Cervical Cancer Cells. Asian Pac. J. Cancer Prev. 2014, 15, 8007–8018. [Google Scholar] [CrossRef]
- Meuris, F.; Gaudin, F.; Aknin, M.L.; Hémon, P.; Berrebi, D.; Bachelerie, F. Symptomatic Improvement in Human Papillomavirus-Induced Epithelial Neoplasia by Specific Targeting of the CXCR4 Chemokine Receptor. J. Investig. Dermatol. 2016, 136, 473–480. [Google Scholar] [CrossRef]
- Clemente-Soto, A.F.; Salas-Vidal, E.; Milan-Pacheco, C.; Sánchez-Carranza, J.N.; Peralta-Zaragoza, O.; González-Maya, L. Quercetin Induces G2 Phase Arrest and Apoptosis with the Activation of P53 in an E6 Expression-independent Manner in HPV-positive Human Cervical Cancer-derived Cells. Mol. Med. Rep. 2019, 19, 2097–2108. [Google Scholar] [CrossRef]
- Gomes, D.; Yaduvanshi, S.; Silvestre, S.; Duarte, A.P.; Santos, A.O.; Soares, C.P.; Kumar, V.; Passarinha, L.; Sousa, Â. Taxifolin and Lucidin as Potential E6 Protein Inhibitors: P53 Function Re-Establishment and Apoptosis Induction in Cervical Cancer Cells. Cancers 2022, 14, 2834. [Google Scholar] [CrossRef]
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Maleki Dizaj, S. Molecular Mechanisms of Anticancer Effect of Rutin. Phyther. Res. 2021, 35, 2500–2513. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, A.; Hamid, L.; Nisar, N.; Malik, J.A.; Ali, T.; Bader, G.N. Flavonoids as Promising Molecules in the Cancer Therapy: An Insight. Curr. Res. Pharmacol. Drug Discov. 2024, 6, 100167. [Google Scholar] [CrossRef]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological Basis and New Insights of Taxifolin: A Comprehensive Review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural Compound for Ovarian Cancer Treatment. J. Ovarian Res. 2019, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef]
- Kashafi, E.; Moradzadeh, M.; Mohamadkhani, A.; Erfanian, S. Kaempferol Increases Apoptosis in Human Cervical Cancer HeLa Cells via PI3K/AKT and Telomerase Pathways. Biomed. Pharmacother. 2017, 89, 573–577. [Google Scholar] [CrossRef]
- Tu, L.Y.; Bai, H.H.; Cai, J.Y.; Deng, S.P. The Mechanism of Kaempferol Induced Apoptosis and Inhibited Proliferation in Human Cervical Cancer SiHa Cell: From Macro to Nano. Scanning 2016, 38, 644–653. [Google Scholar] [CrossRef]
- Zheng, P.W.; Chiang, L.C.; Lin, C.C. Apigenin Induced Apoptosis Through p53-Dependent Pathway in Human Cervical Carcinoma Cells. Life Sci. 2005, 76, 1367–1379. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A Review on the Dietary Flavonoid Quercetin: Mini-Reviews. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Liang, Y.; Niu, H.; Limei, M.; Du, D.; Wen, L.; Xi, Q.; Huang, W. Eriodictyol 7-O-β-D-Glucopyranoside from Coreopsis tinctoria Nutt. Ameliorates Lipid Disorders via Protecting Mitochondrial Function and Suppressing Lipogenesis. Mol. Med. Rep. 2017, 16, 1298–1306. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R.; Ni, H. Eriodictyol Exerts Potent Anticancer Activity against A549 Human Lung Cancer Cell Line by Inducing Mitochondrial-Mediated Apoptosis, G2/M Cell Cycle Arrest and Inhibition of mTOR/PI3K/Akt Signalling Pathway. Arch. Med. Sci. 2020, 16, 446–452. [Google Scholar] [CrossRef]
- Minda, D.; Avram, S.; Pavel, I.Z.; Kis, B.; Ghitu, A.; Zupko, I.; Dehelean, C.; Buda, V.; DIaconeasa, Z.; Scurtu, A.; et al. An in vitro Evaluation of Apigenin and Apigenin-7-O-glucoside Against HeLa Human Cervical Cancer Cell Line. Rev. Chim. (Bucharest Rom.) 2020, 71, 140–144. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A Current Review on Its Beneficial Biological Activities. J. Food Biochem. 2017, 41, e12376. [Google Scholar] [CrossRef]
- Hu, L.; Luo, Y.; Yang, J.; Cheng, C. Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules 2025, 30, 1184. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Foshati, S. Bioavailability and Metabolism of Favonoids: A Review. Int. J. Nutr. Sci. 2017, 2, 180–184. [Google Scholar]
- Veleva, R.K.; Moskova-Doumanova, V.; Todorova, M.; Trendafilova, A.; Doumanov, J.; Topouzova-Hristova, T. Cytotoxicity of Flavonoid Glycosides, Flavonoids and Phenolic Acids from Inula oculus-christi on Mammalian Cell Lines. J. BioSci. Biotechnol. 2016, 5, 219–224. [Google Scholar]
- Data Analysis, Version 4.3; Bruker: Billerica, MA, USA, 2014.
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. Available online: https://ccms-ucsd.github.io/GNPSDocumentation/ (accessed on 1 April 2025). [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Global Natural Product Social Molecular Networking (GNPS). Available online: https://gnps.ucsd.edu/ProteoSAFe/static/gnps-splash.jsp?redirect=auth (accessed on 20 April 2025).
- Denizot, F.; Lang, R. Rapid Colorimetric Assay for Cell Growth and Survival: Modifications to the Tetrazolium dye Procedure Giving Improved Sensitivity and Reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef] [PubMed]
- GraphPad Prism, Version 7.0; GraphPad Software Inc.: San Diego, CA, USA, 2018.
- Minho, A.; Gaspar, E.; Domingues, R. Guia Prático para Determinação de Curva Dose-Resposta e Concentração Letal em Bioensaios com Extratos Vegetais. Comunicado Técnico, 93, Bagé, RS, Brazil, Abril 2016. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/172038/1/Comunicado-Tecnico-n-93.pdf (accessed on 20 April 2025).

| Peak | Retention Time (min) | Compound * | Stage | Fragments m/z (−) | Fragments m/z (+) | References |
|---|---|---|---|---|---|---|
| 1 | 11.6 | caffeoylquinic acid | MS2 | 353 ** [M − H]−; 191; 179 | - | [20,21,22] |
| 2 | 14.2 | feruloylquinic acid | MS2 | 369 ** [M − H]−; 193; 174; 134 | - | [23] |
| 3 | 15.7 | apigenin 6,8-C-dihexoside | MS2 | 593 [M − H]−; 473; 385; 355 | 595 [M + H]+; 577; 559; 529; 475; 457 | [24] |
| 4 | 16.8 | apigenin 6-C-pentosyl-8-C-hexoside | MS2 | 563 [M − H]−; 545; 473; 443; 385; 355 | 565 [M + H]+; 547; 529; 429; 411 | [24,25] |
| 5 | 16.9 | apigenin 6-C-hexosyl-8-C- pentoside | MS2 | 563 [M − H]−; 545; 473; 443; 385; 355 | 565 [M + H]+; 547; 529; 429; 411 | |
| 6 | 17.5 | apigenin 6-C-hexosyl-8-C-deoxihexoside | MS2 | 577 [M − H]−; 487; 457; 369; 339 | 579 [M + H]+; 561; 543; 443 | [26] |
| 7 | 18.9 | rutin | MS2 | 609 [M − H]−; 301 ** | 611 [M+ H]+; 303 ** | [27,28,29] |
| 8 | 19.1 | quercetin-O-hexoside | MS2 | 463 [M − H]−; 301 ** | - | [30] |
| 9 | 20.1 | Kaempferol-O- deoxyhexosyl-O-hexoside | MS2 | 593 [M − H]−; 285 ** | 595 [M + H]+; 549; 449; 287 | [31] |
| 10 | 20.5 | quercetin-O-deoxyhexosyl-O-dihexoside | MS2 | 771 [M − H]−; 609; 301 ** | - | [32] |
| 11 | 21.3 | quercetin-O- dimethoxycaffeoyl-O- hexosyl-O-hexoside | MS2 | 817 ** [M − H]−; 609; 591; 301 ** | - | [32] |
| 12 | 21.5 | quercetin-O-feruloyl-O-deoxyhexosyl-O- hexoside | MS2 | 785 [M − H]−; 609; 591; 301 ** | - | [32] |
| 13 | 22.6 | kaempferol-O-feruloyl-O-deoxyhexosyl-O- hexoside | MS2 | 769 [M − H]−; 593; 575; 285 ** | - | [33] |
| 14 | 23.8 | 3,4′-dimethoxy-quercetin | MS2 | 331 ** [M − H]−; 314; 299 | - | [34] |
| 15 | 25.5 | tamarixetin | MS2 | 315 ** [M − H]−; 300 | - | [34] |
| Cell Lines | 24 h | 48 h | 72 h |
|---|---|---|---|
| HeLa | 78.78 µg mL−1 | 59.52 µg mL−1 | 44.78 µg mL−1 |
| SiHa | 81.90 µg mL−1 | 50.01 µg mL−1 | 66.97 µg mL−1 |
| HeLa | % Closure [(T0 − TF/T0) × 100] | ||
|---|---|---|---|
| Mean ± SD | |||
| Extract (µg mL−1) | 24 h | 48 h | 72 h |
| CTRL− | 47.94 ± 1.57 | 75.02 ± 4.78 | 83.22 ± 5.65 |
| CPT 5 | 27.77 ± 9.57 | 35.80 ± 6.24 | 41.94 ± 2.88 |
| FAB 20 | 49.48 ± 6.54 | 75.93 ± 4.90 | 89.93 ± 8.12 |
| FAB 40 | 42.24 ± 6.90 | 62.77 ± 5.46 | 76.33 ± 5.23 |
| FAB 60 | 44.50 ± 3.81 | 50.74 ± 4.82 | 56.43 ± 10.78 |
| FAB 100 | 21.98 ± 7.35 | 22.28 ± 8.39 | 24.53 ± 9.42 |
| SiHa | % Closure [(T0 − TF/T0) × 100 | ||
| Mean ± SD | |||
| Extract (µg mL−1) | 24 h | 48 h | 72 h |
| CTRL− | 38.00 ± 2.52 | 68.00 ± 4.61 | 89.00 ± 10.42 |
| CPT 5 | 34.20 ± 1.10 | 60.40 ± 2.99 | 87.90 ± 6.16 |
| FAB 20 | 42.31 ± 3.23 | 73.04 ± 5.61 | 96.38 ± 3.19 |
| FAB 40 | 38.33 ± 3.21 | 45.10 ± 3.38 | 47.21 ± 3.74 |
| FAB 60 | 29.16 ± 4.15 | 30.75 ± 3.44 | 32.60 ± 3.55 |
| FAB 100 | 18.10 ± 2.84 | 19.19 ± 3.18 | 19.73 ± 2.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, J.A.R.; Miranda, A.d.J.A.; Lima, V.A.S.; Buna, S.d.S.S.; Rosário, M.S.d.; Lima, R.F.; Martins, M.M.; Andrade, M.S.d.; Nascimento, M.D.S.B.; Bolzani, V.d.S.; et al. Antiproliferative and Anti-Migratory Activities of an Extract from Fridericia platyphylla Leaves and Its Molecular Profile. Plants 2025, 14, 2693. https://doi.org/10.3390/plants14172693
Brito JAR, Miranda AdJA, Lima VAS, Buna SdSS, Rosário MSd, Lima RF, Martins MM, Andrade MSd, Nascimento MDSB, Bolzani VdS, et al. Antiproliferative and Anti-Migratory Activities of an Extract from Fridericia platyphylla Leaves and Its Molecular Profile. Plants. 2025; 14(17):2693. https://doi.org/10.3390/plants14172693
Chicago/Turabian StyleBrito, Jhonathas Aparecido R., Amanda de Jesus A. Miranda, Victor Antonio S. Lima, Samuel dos Santos S. Buna, Marcelino S. do Rosário, Rafael F. Lima, Monique M. Martins, Marcelo S. de Andrade, Maria D. S. B. Nascimento, Vanderlan da Silva Bolzani, and et al. 2025. "Antiproliferative and Anti-Migratory Activities of an Extract from Fridericia platyphylla Leaves and Its Molecular Profile" Plants 14, no. 17: 2693. https://doi.org/10.3390/plants14172693
APA StyleBrito, J. A. R., Miranda, A. d. J. A., Lima, V. A. S., Buna, S. d. S. S., Rosário, M. S. d., Lima, R. F., Martins, M. M., Andrade, M. S. d., Nascimento, M. D. S. B., Bolzani, V. d. S., Azevedo-Santos, A. P. S. d., Lima, J. A., Xavier, J. K. d. A. M., & Rocha, C. Q. d. (2025). Antiproliferative and Anti-Migratory Activities of an Extract from Fridericia platyphylla Leaves and Its Molecular Profile. Plants, 14(17), 2693. https://doi.org/10.3390/plants14172693









