Abstract
Daphne odora is an evergreen shrub belonging to the Thymelaeaceae family that is widely cultivated as an ornamental garden plant. Its roots, leaves, and flowers have traditionally been used in Chinese medicine to treat pain, skin diseases, and rheumatism. While previous phytochemical studies have reported the presence of phenols, coumarins, biflavonoids, lignans, and daphnane diterpenoids in D. odora, its flowers remain largely unexplored. In the present study, the first comprehensive investigation of daphnane diterpenoids contained in the flower buds and blooming flowers of D. odora was conducted using ultra-high-performance liquid chromatography coupled with Q-Exactive-Orbitrap high-resolution mass spectrometry (UHPLC-Q-Exactive-Orbitrap MS). A total of 30 daphnane diterpenoids were identified, including 12 previously unreported compounds, through detailed analysis of their retention times and MS/MS fragmentation patterns. Comparative profiling revealed that flower buds contained a higher abundance and greater diversity of daphnane diterpenoids than flowers. Furthermore, LC–MS-guided isolation enabled the purification of a novel compound, daphneodorin I (16), and its structure was elucidated through extensive physicochemical and spectroscopic analyses. Compound 16 represents the first daphnane diterpenoid with a Z-configured phenolic acyl moiety isolated from plants of the Thymelaeaceae family.
1. Introduction
Daphne odora Thunb. is an evergreen shrub belonging to the Thymelaeaceae family. Its roots, leaves, and flowers have been traditionally used in Chinese medicine for the treatment of pain, skin disorders, and rheumatism [1,2]. Today, this plant is widely cultivated as an ornamental species worldwide and is commonly known as “winter daphne” because of its fragrant pinkish-white flowers that bloom in early spring. Previous phytochemical studies on D. odora have reported the isolation of phenols, coumarins, biflavonoids, lignans, and daphnane diterpenoids [3,4,5,6,7,8,9]. Furthermore, daphnane diterpenoids were isolated from the leaves and stems of D. odora in our previous study [10,11]. Daphnane diterpenoids are a characteristic class of diterpenoids found in plants of the Thymelaeaceae family [12]. Their chemical structures are highly diverse, featuring a trans-fused 5/7/6 tricyclic ring system with various oxygenated functional groups. These diterpenoids have been extensively studied for their wide range of biological activities, including anticancer, anti-HIV, and analgesic effects, and are considered potential lead compounds for drug development. In particular, structural variations, such as those in the orthoester moiety and acyl substituents, have been reported to markedly influence both bioactivity and toxicity [13,14,15]. Therefore, chemical investigation of daphnane diterpenoids present in plants is essential to understand their pharmacological potential and safety profiles.
The flowers of D. odora progress through several distinct stages, beginning with the swelling of pink buds in late winter, which then open into clusters of fragrant, four-lobed flowers, typically pink or white (Figure 1) [1]. Moreover, floral secondary metabolite profiles vary significantly across different developmental stages [16,17]. Although the flowers of D. odora have been used in traditional Chinese medicines, their chemical constituents remain largely unexplored. D. genkwa, a medicinal plant in the same genus Daphne, has yielded numerous daphnane diterpenoids from its flower buds [18,19]. Inspired by these findings, the present study focused on the daphnane diterpenoids present in the flower buds and blooming flowers of D. odora. Herein, we report the identification of daphnane diterpenoids in the flowers of D. odora using an ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry (UHPLC-Q-Exactive-Orbitrap MS), as well as LC-MS-guided isolation and structural elucidation of a previously undescribed daphnane diterpenoid (16).

Figure 1.
Flower buds before blooming (A) and fully opened flowers (B) of D. odora.
2. Results and Discussion
2.1. Detection of Daphnane Diterpenoids in the Flower Buds and Flowers of D. odora Using LC-MS/MS
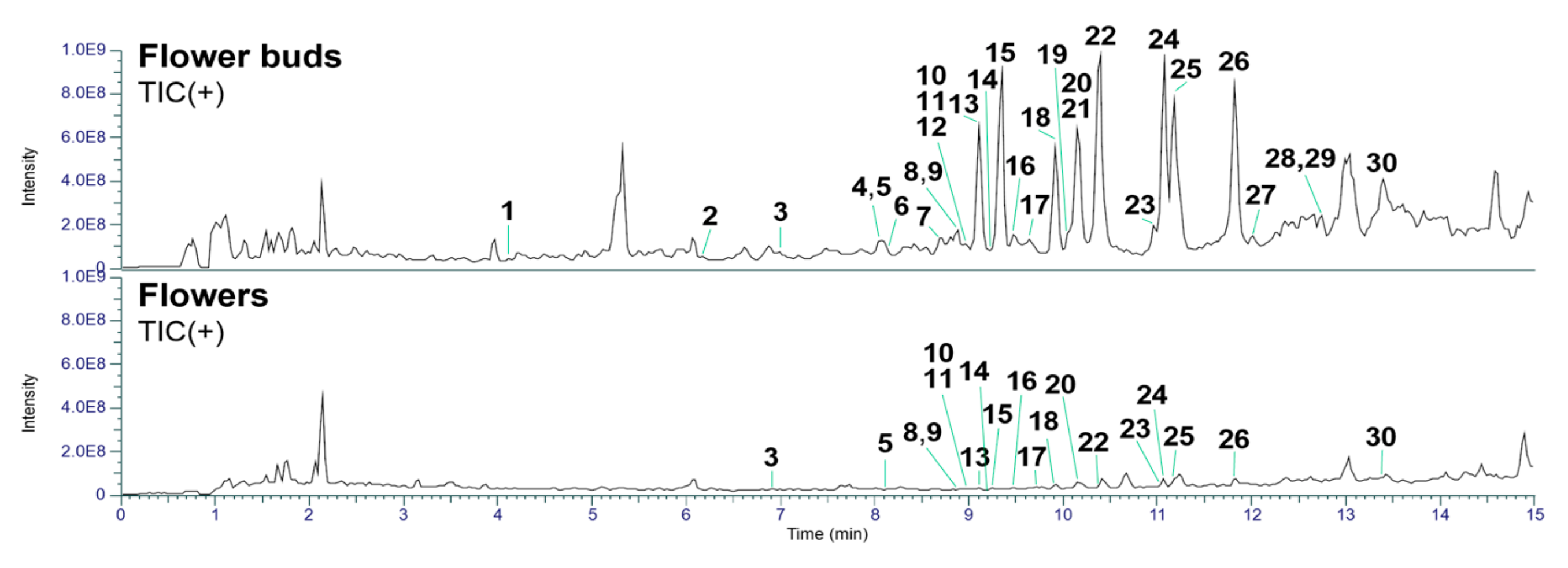
In the present study, we separately collected flower buds prior to blooming and fully opened flowers for LC-MS analysis. The collected samples were extracted with MeOH and subsequently partitioned between EtOAc and H2O. The EtOAc-soluble fractions were further subjected to pretreatment using a Sep-Pak tC18 Plus Long cartridge and analyzed by UHPLC-Q-Exactive-Orbitrap MS. The acquired raw data were preprocessed using MZmine 2 software [20] and subsequently analyzed with the in-house developed CNPs-MFSA application [21] for the recognition of daphnane diterpenoids. The above qualitative analysis revealed that the flower buds contained a higher abundance of daphnane diterpenoids and more previously undescribed compounds than the flowers (Figure 2 and Table S1). Furthermore, detailed structural identification of the detected daphnane diterpenoids was performed following the same protocol described in our previous studies [22]. As summarized in Table 1, several daphnane diterpenoids were commonly identified in both flowers and buds, with 19 compounds detected in the flowers and 30 compounds detected in the buds.
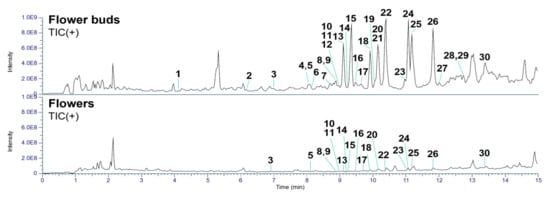
Figure 2.
Total ion chromatograms in positive ion mode of the flower buds and flowers of D. odora.
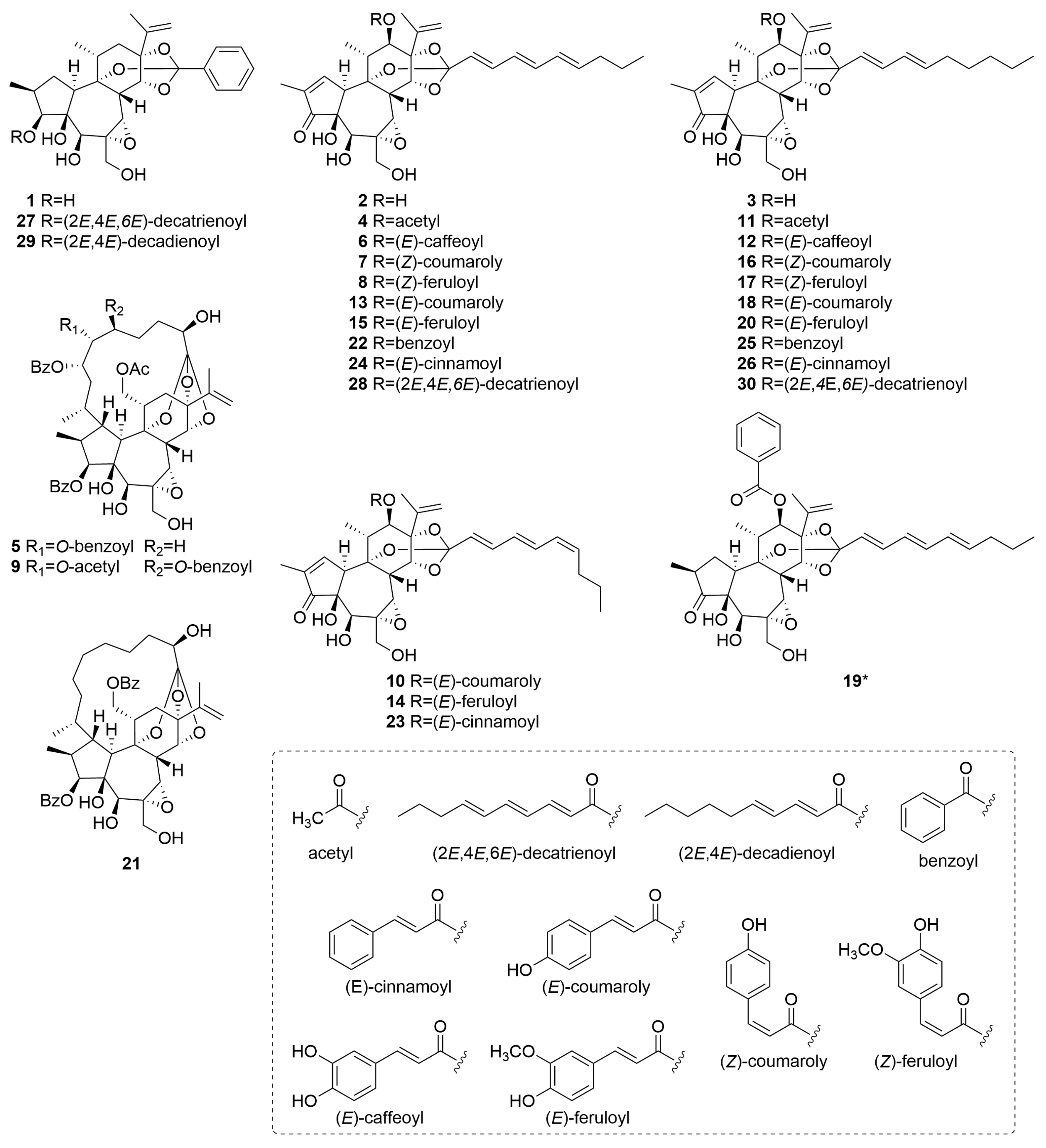
The identified daphnane diterpenoids were classified as two types, daphnane orthoesters (1–4, 6–8, 10–20, and 22–30) and macrocyclic daphnane orthoesters (5, 9, and 21), based on their chemical structural characteristics [12]. Moreover, several previously unreported daphnane diterpenoids (6–8, 10, 12, 14, 16, 17, 19, 23, 28, and 30) have been identified in the buds of D. odora. Their structural elucidation based on MS/MS fragmentation analysis is described below.

Table 1.
Tentative identification of daphnane diterpenoids in the flower buds and flowers of D. odora.
Table 1.
Tentative identification of daphnane diterpenoids in the flower buds and flowers of D. odora.
| No. | tR, min (+/−) | Precursor Ion, m/z (Error, ppm) | Molecular Formula | Tentative Identification | Reference | |
|---|---|---|---|---|---|---|
| [M + H]+ a | [M–HCOO]− b | |||||
| 1 | 4.21/4.19 | 487.2325 (−0.33) | 485.2189 (1.74) | C27H34O8 | 3-deoxo-1,2-dihydro-3-hydroxydaphnetoxin | [23,24] |
| 2 | 6.09/6.08 | 543.2587 (−0.24) | 587.2507 (1.51) | C30H38O9 | peddiea factor A1 | [25] |
| 3 | 6.93/6.95 | 545.2746 (0.16) | 589.2662 (1.25) | C30H40O9 | 12-hydroxyexcoecariatoxin | [26] |
| 4 | 8.10/8.08 | 585.2686 (−1.38) | 629.2609 (0.91) | C32H40O10 | peddiea factor V2 | [25] |
| 5 c | 8.10/8.11 | 1028.4264 (−1.02) | 1055.3934 (1.53) | C55H62O18 | daphneodrin B | [10] |
| 6 e | 8.13/8.14 | 705.2899 (−0.90) | 703.2770 (1.39) | C39H44O12 | 12-O-(E)-caffeoyl-9,13,14-ortho-(2E,4E,6E)-decatrienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | - |
| 7 e | 8.68/8.70 | 689.2951 (−0.77) | 687.2814 (0.42) | C39H44O11 | 12-O-(Z)-coumaroyl-9,13,14-ortho-(2E,4E,6E)-decatrienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | - |
| 8 e | 8.88/8.87 | 719.3056 (−0.83) | 717.2920 (0.48) | C40H46O12 | 12-O-(Z)-feruloyl-9,13,14-ortho-(2E,4E,6E)-decatrienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | - |
| 9 c | 8.88/8.89 | 970.4219 (−0.09) | 997.3874 (1.05) | C53H60O16 | daphneodorin A | [10] |
| 10 e | 8.93/8.95 | 689.2952 (−0.60) | 687.2819 (1.13) | C39H44O11 | 12-O-(E)-coumaroyl-9,13,14-ortho-(2E,4E,6Z)-decatrienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | - |
| 11 | 8.97/8.95 | 587.2850 (−0.17) | 631.2769 (1.36) | C32H42O10 | yuanhuadin | [27] |
| 12 e | 8.97/8.95 | 707.3051 (−1.63) | 705.2924 (1.09) | C39H46O12 | 12-O-(E)-caffeoyl-9,13,14-ortho-(2E,4E)-decadienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | - |
| 13 c | 9.10/9.12 | 689.2953 (−0.42) | 687.2814 (0.42) | C39H44O11 | daphneodorin E | [11] |
| 14 e | 9.18/9.17 | 719.3061 (−0.16) | 763.2982 (1.35) | C40H46O12 | 12-O-(E)-feruloyl-9,13,14-ortho-(2E,4E,6Z)-decatrienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | - |
| 15 c | 9.35/9.34 | 719.3051 (−1.60) | 763.2970 (−0.17) | C40H46O12 | actilobin C | [28] |
| 16 e,f | 9.47/9.49 | 691.3111 (−0.28) | 689.2972 (0.65) | C39H46O11 | 12-O-(Z)-coumaroyl-9,13,14-ortho-(2E,4E)-decadienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | - |
| 17 e | 9.67/9.68 | 721.3220 (0.15) | 719.3081 (1.12) | C40H48O12 | 12-O-(Z)-feruloyl-9,13,14-ortho-(2E,4E)-decadienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | - |
| 18 c | 9.91/9.92 | 691.3109 (−0.54) | 689.2968 (0.11) | C39H46O11 | daphneodorin D | [11] |
| 19 d | 10.05/10.03 | 649.3000 (−1.04) | 693.2921 (0.58) | C37H44O10 | 1,2-dihydroyuanhuajine | [24] |
| 20 c | 10.15/10.16 | 721.3212 (−0.95) | 765.3127 (−0.05) | C40H48O12 | actilobin D | [28] |
| 21 c | 10.15/10.16 | 775.3693 (0.67) | 819.3587 (−1.25) | C44H54O12 | gnidimacrin | [29] |
| 22 c | 10.36/10.35 | 647.2845 (−0.91) | 691.2759 (−0.09) | C37H42O10 | yuanhuajine | [7] |
| 23 e | 10.93/10.91 | 673.3004 (−0.46) | 717.2928 (1.58) | C39H44O10 | 12-O-(E)-cinnamoyl-9,13,14-ortho-(2E,4E,6Z)-decatrienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | - |
| 24 c | 11.07/11.06 | 673.2999 (−1.27) | 717.2918 (0.22) | C39H44O10 | 12-O-(E)-cinnamoyl-9,13,14-ortho-(2E,4E,6E)-decatrienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | [7] |
| 25 c | 11.18/11.16 | 649.2998 (−1.42) | 693.2917 (0.14) | C37H44O10 | yuanhuacine/odoracin | [30] |
| 26 c | 11.81/11.82 | 675.3157 (−0.95) | 719.3074 (0.10) | C39H46O10 | 12-O-(E)-cinnamoyl-9,13,14-ortho-(2E,4E)-decadienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | [7] |
| 27 | 12.01/12.00 | 635.3215 (0.02) | 679.3135 (1.70) | C37H46O9 | actilobin F | [29] |
| 28 e | 12.74/12.73 | 691.3474 (−0.38) | 735.3394 (1.03) | C40H50O10 | 12-O-(2E,4E,6E)-decatrienoyl-9,13,14-ortho-(2E,4E,6E)-decatrienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | - |
| 29 | 12.77/12.76 | 637.3369 (−0.31) | 681.3286 (0.76) | C37H48O9 | wikstroemia factor M1 | [27] |
| 30 d | 13.39/13.38 | 693.3630 (−0.50) | 737.3547 (0.66) | C40H52O10 | 12-O-(2E,4E,6E)-decatrienoyl-9,13,14-ortho-(2E,4E)-decadienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | [24] |
a Detected as [M + NH4]+ ion for compounds 5 and 9. b Detected as [M–H]− ion for compounds 1, 6–8, 10, 12, 13, and 16–18. c Identifications were confirmed by comparison of retention times and product ion spectra with authentic standards. d Compounds previously identified in other Daphne species using LC-MS analysis. e Previously unreported compounds. f Compounds were isolated in the present study.
2.2. Identification of Previously Unreported Daphnane Diterpenoids by MS/MS Fragmentation Analysis
Compounds 6, 12, and 28 exhibited a series of characteristic product ions in the positive ion mode at m/z 359 (C20H23O6+) → m/z 341 (C20H21O5+) → m/z 269 (C17H17O3+), indicating that they share a typical daphnane skeleton bearing a 1,2-en-3-oxo-4,5-dihydroxy-6,7-epoxy moiety with a substituent at C-12 (Figures S1–S3). In the negative ion mode, the observation of [M−RCOOH−CH2O−H]− ions is useful for identifying the acyl moiety attached via orthoester linkages on the C-ring [22]. For compounds 6 and 28, a neutral loss of C10H14O2 from the deprotonated molecular ion suggested the presence of a 2,4,6-decatrienoyl moiety linked to the C-ring through an orthoester bond. In contrast, compound 12 exhibited a neutral loss of C10H16O2, indicating the presence of a 2,4-decadienoyl moiety at the same position. Structural information on the acyl moiety at C-12 was further obtained by analyzing product ions below m/z 250. Compounds 6 and 12 exhibited common characteristic ions corresponding to C9H7O3+ and C9H7O4−. Given that numerous daphnane diterpenoids isolated from D. odora possess phenylpropanoid-derived substituents, such as cinnamoyl, coumaroyl, and feruloyl groups biosynthesized from phenylalanine, these diagnostic ions were attributed to the caffeoyl group [11]. Therefore, the substituent at C-12 in compounds 6 and 12 was assigned as a caffeoyl group. Compound 28 showed only diagnostic ions corresponding to C10H13O+, C7H7O+, and C10H13O2−, indicating that a 2,4,6-decatrienoyl moiety was attached to C-12. Thus, these findings demonstrate that compounds 6, 12, and 28 are previously unreported compounds. Their proposed structures are illustrated in Figure 3.
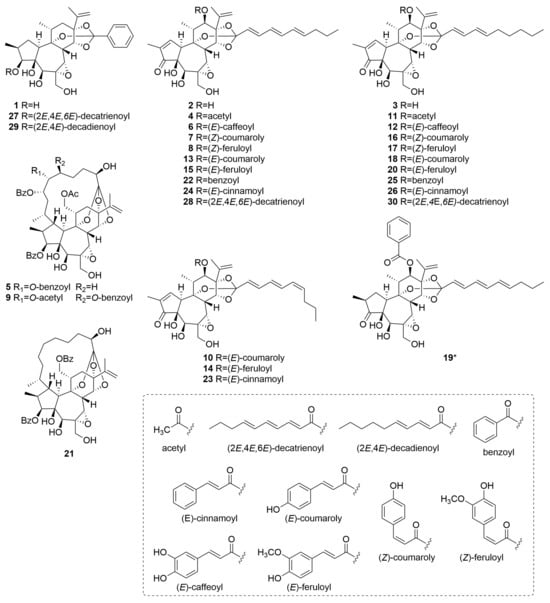
Figure 3.
Structures of daphnane diterpenoids 1–30 identified in D. odora. Asterisks indicate previously unreported compounds.
Based on the product ion spectra obtained in both positive and negative ion modes, compounds 7, 8, 10, 14, 16, 17, and 23 were suggested to possess a daphnane skeleton with a substituent at C-12. Detailed comparisons of the retention behavior, predicted molecular formulas, and MS/MS fragmentation patterns with those of authentic standards revealed that these compounds are novel geometric isomers of the known compounds 13, 15, 18, 20, and 24, respectively (Figure S4). Specifically, compounds 7 (tR = 8.68 min) and 10 (tR = 8.93 min) correspond to isomers of daphneodorin E (13, tR = 9.10 min) (Figures S5 and S6); compounds 8 (tR = 8.88 min) and 14 (tR = 9.18 min) to actilobin C (15, tR = 9.35 min) (Figures S7 and S8); compound 16 (tR = 9.47 min) to daphneodorin D (18, tR = 9.91 min) (Figure S9); compound 17 (tR = 9.67 min) to actilobin D (20, tR = 10.15 min) (Figure S10); and compound 23 (tR = 10.93 min) to 12-O-(E)-cinnamoyl-9,13,14-ortho-(2E,4E,6E)-decatrienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide (24, tR = 11.07 min) (Figure S11). These geometric isomers are presumed to arise from differences in the E/Z configuration of double bonds present in either the aliphatic or phenolic acyl moieties. Our previous study demonstrated that, under consistent LC conditions, isomers bearing a (2E,4E,6Z)-decatrienoyl moiety eluted 0.15 min earlier than their (2E,4E,6Z) counterparts [24]. Based on this finding, compounds 10, 14, and 23, which eluted approximately 0.15 min earlier than compounds 13, 15, and 24, respectively, were suggested to possess a (2E,4E,6Z)-decatrienoyl moiety attached to the C-ring via an orthoester linkage.
In contrast to the previously discussed isomers, compounds 7, 8, 16, and 17 eluted approximately 0.45 min earlier than their known analogs, a more pronounced shift that is likely attributable to the presence of a Z-configured double bond in the aromatic acyl substituent. Specifically, compounds 7 and 16 were presumed to possess a (Z)-coumaroyl moiety at C-12, whereas compounds 8 and 17 were suggested to possess a (Z)-feruloyl moiety at C-12. Nevertheless, the structural assignment of these compounds could not be conclusively determined from MS/MS fragmentation and retention time data alone. To clarify their structures, LC–MS-guided isolation was performed on the flower buds, leading to the successful purification of compound 16 for detailed spectroscopic analysis.
2.3. LC-MS Guided Isolation and Structural Elucidation of Compound 16
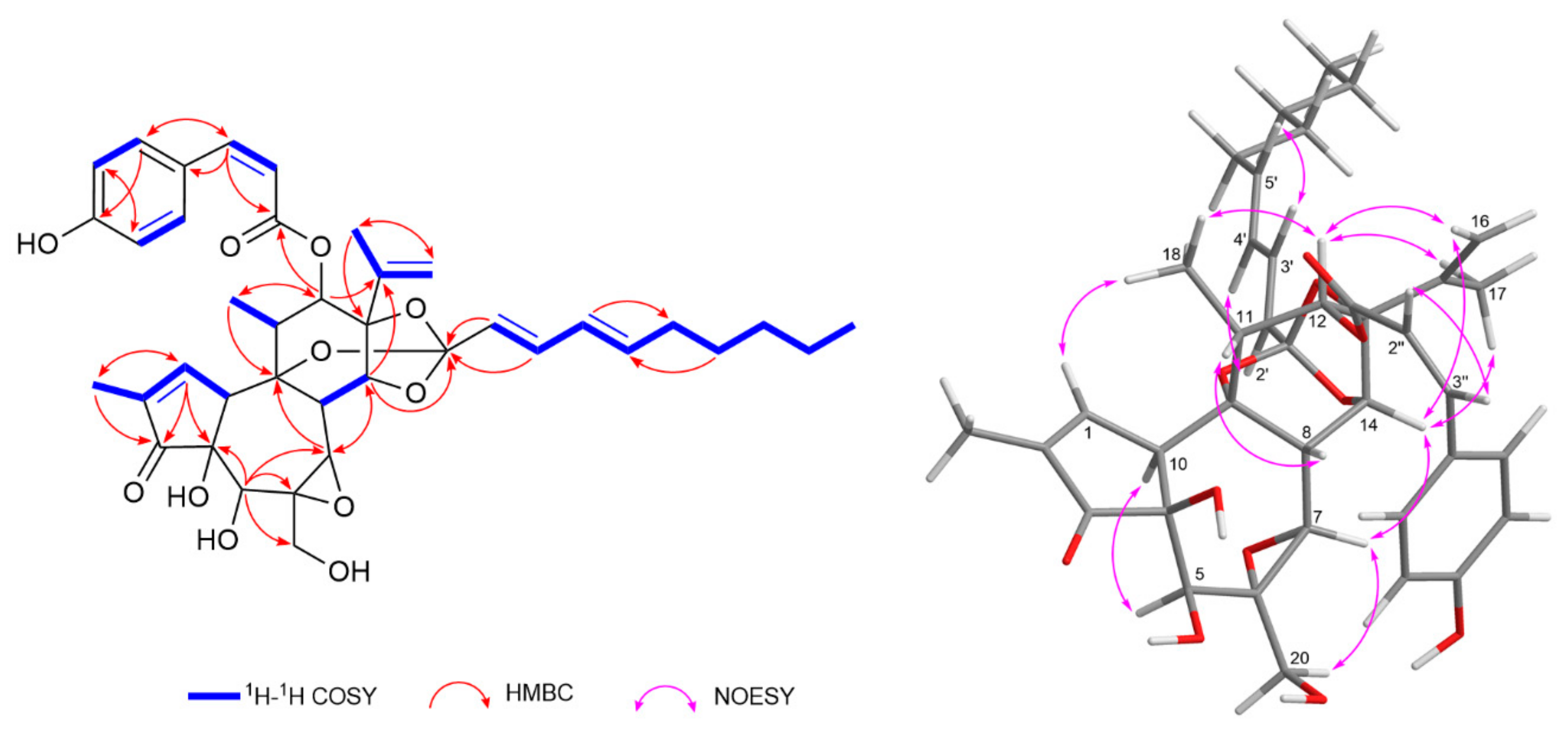
Compound 16 was isolated as a colorless solid, [α]23D + 16.4 (c 0.07, MeOH). Its molecular formula was determined as C39H46O11 from the negative HRESIMS data, showing a deprotonated molecular ion at m/z 689.2967 [M−H]− (calcd for C39H45O11, 689.2967). The 1H- and 13C-NMR resonances of compound 16 were virtually identical to those of daphneodorin D (18), except for resonances assignable to the phenolic acyl moiety at C-12 (Table 2). A detailed analysis revealed characteristic resonances for a daphnane orthoester, including an isopropenyl moiety at δH 4.74 (Ha-16), 4.92 (Hb-16), 1.69 (H3-17), δC 142.7 (C-15), 113.7 (C-16), and 18.8 (C-18); an α,β-unsaturated carbonyl group at δH 7.57 (H-1), δC 161.3 (C-1), 136.6 (C-2), and 209.6 (C-3); an epoxy group at δH 3.23 (H-7), δC 58.6 (C-6), and 66.3 (C-7); as well as a quaternary carbon at δC 116.6 (C-1′) consistent with the orthoester group. The presence of a 2,4-decadienylidyne moiety was indicated by the proton resonances for a conjugated diene at δH 5.56 (d, J = 15.4 Hz, H-2′), 6.57 (dd, J = 15.4, 10.9 Hz, H-3′), 5.99 (dd, J = 15.2, 10.9 Hz, H-4′), and 5.80 (dt, J = 15.2, 7.2 Hz, H-5′), a n-pentyl moiety with four methylenes at δH 2.06 (H-6′), 1.35 (H-7′), 1.25 (H-8′), and 1.28 (H-9′), and a terminal methyl group at δH 0.86 (t, J = 7.0 Hz, H3-10′). The large coupling constants between H-2′/H-3′ and H-4′/H-5′ (J = 15.4 and 15.2 Hz, respectively) and the NOESY correlations between H-2′/H-4′ and H-3′/H-5′ indicated a 2E,4E geometry of the conjugated diene (Figure 4). The position of the decadienylidyne moiety was confirmed from the HMBC correlations from H-14, H-2′, and H-3′ to C-1′. Furthermore, compound 16 exhibited proton resonances consistent with a p-coumaroyl moiety, including olefinic protons at δH 5.78 (d, J = 12.2 Hz, H-2″) and 7.05 (d, J = 12.2 Hz, H-3″), as well as aromatic protons at δH 6.89 (d, J = 8.4 Hz, H-6″,8″) and 7.15 (d, J = 8.4 Hz, H-5″,9″). The relatively small coupling constant between H-2″ and H-3″ (J = 12.2 Hz) indicated a Z-configured p-coumaroyl moiety. The HMBC correlation from H-12 to the ester carbonyl carbon at δC 165.1 (C-1″) confirmed that the (Z)-coumaroyl moiety was attached at C-12 (Figure 4). Thus, the structure of compound 16 was determined as 12-O-(Z)-coumaroyl-9,13,14-ortho-(2E,4E)-decadienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide, a geometric isomer of daphneodorin D (18), and was named daphneodorin I.

Table 2.
1H-NMR (500 MHz) and 13C-NMR (125 MHz) spectroscopic data for compound 16 (CDCl3).
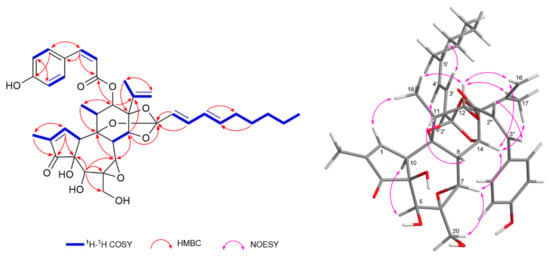
Figure 4.
Key 1H–1H COSY, HMBC, and NOESY correlations for compound 16.
3. Materials and Methods
3.1. General Experimental Producers
Optical rotation, UV, ECD, IR, NMR, and HRESI-MS measurements were performed using the instruments and conditions described in the literature [31]. Column chromatography was carried out on Diaion HP-20 (Mitsubishi Chemical Corporation, Tokyo, Japan), as well as ODS silica gel, silica gel, and preparative HPLC, following the procedures described in the literature [31].
3.2. Plant Materials
Plants of D. odora were cultivated at the Toho University Medicinal Plant Garden in Chiba, Japan. Blooming flowers were collected in late February 2020 and flower buds were collected in early February 2021. The plant materials were identified by one of the authors (W.L.). Voucher specimens THMPG-7 (blooming flowers) and THMPG-8 (flower buds) were deposited at the Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, Toho University, Japan.
3.3. Extraction and Isolation
Air-dried flower buds (756 g) and flowers (956 g) of D. odora were extracted four times with methanol (MeOH, 5 L for flower buds and 8 L for flowers, 1 h each) using ultrasonic extraction at room temperature. The resulting extracts were filtered and concentrated under reduced pressure at a temperature not exceeding 40 °C. The MeOH extracts of the flower buds (515 g) and flowers (434 g) were each suspended in H2O and partitioned with ethyl acetate (EtOAc). The EtOAc fractions of the flower buds (55.1 g) and flowers (64.3 g) were collected for LC-MS analysis and subsequent isolation.
For the isolation process, the EtOAc fraction of the flower buds (55.1 g) was subjected to Diaion HP-20 column chromatography and eluted with a gradient of MeOH/H2O (5:5 to 10:0, v/v) to yield four fractions (E1 to E4). Fraction E3 (8.39 g) was further separated by ODS column chromatography using a stepwise gradient of MeOH−H2O (from 7:3 to 10:0, v/v), affording 13 fractions (E3-1 to E3-13). Combined fractions E3-6 and E3-7 (2.28 g) were subjected to silica gel column chromatography and eluted with EtOAc−MeOH (1:1, v/v) followed by MeOH−HCOOH (95:5, v/v) to give two subfractions (E3-6-1 and E3-6-2). Finally, fraction E3-6-1 (754 mg) was repeatedly purified by RP-HPLC (70% CH3CN) to afford compound 16 (0.7 mg).
Daphneodorin I (16)
Colorless solid; [α]23D +16.4 (c 0.07, MeOH); UV (MeOH) λmax nm (log ε) 230 (4.68), 314 (4.24); ECD (MeOH) [θ]25 (nm): 17,222 (206), −55,825 (228), 21,744 (246), −11,284 (309), 4892 (351); IR (KBr) cm−1: 3429, 2955, 2927, 2856, 1703, 1631, 1605, 1514, 1456, 1381, 1363, 1312, 1281, 1153, 1109, 1085, 1057, 1040, 1010; 1H- and 13C-NMR spectroscopic data, see Table 2; HRESI-MS (negative) m/z: 689.2967 [M−H]− (calcd for C39H45O11: 689.2967).
3.4. Qualitative Analysis of Flower Buds and Flower of D. odora
3.4.1. Sample Preparation
The EtOAc fractions of the flower buds and flowers (10.0 mg each) were dissolved in 50% MeOH (1.0 mL) and loaded onto a conditioned Sep-Pak tC18 Plus Long Cartridge (Waters, Milford, MA, USA), washed with 50% MeOH (10 mL), and subsequently eluted with 100% MeOH (10 mL). Finally, the 100% MeOH eluates were then filtered through a 0.20 mm membrane filter (Merck Millipore, Burlington, MA, USA) prior to qualitative analysis.
3.4.2. LC-MS/MS Conditions
LC–MS/MS analysis was performed using an ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry (UHPLC-Q-Exactive-Orbitrap MS, Thermo FisherScientific, Waltham, MA, USA) equipped with a heated electrospray ionization (HESI) source, under conditions consistent with a previous study [23]. Chromatographic separation was carried out on a YMC-Triart C18 column (150 mm × 2.1 mm, 1.9 μm) at a flow rate of 0.4 mL/min with the column oven maintained at 40 °C. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), using a linear gradient from 50% to 100% B over 15 min. Mass spectrometric data were acquired in profile mode over an m/z range of 300–1500, with a resolution of 35,000 (m/z 200) in full MS mode and 17,500 (m/z 200) in the dd-MS2 mode. Fragmentation was carried out using higher-energy collisional dissociation (HCD) with a normalized collision energy of 15–20 eV in positive ion mode and 10 eV in negative ion mode [24].
3.4.3. Data Analysis
All mass data were acquired using the Thermo Scientific Xcalibur 4.1 software (Thermo Fisher Scientific, Waltham, MA, USA). The raw data were processed using MZmine 2.5.3 [20] and subsequently analyzed for qualitative identification with the in-house developed CNPs-MFSA application [21]. The detailed parameters used for data processing have been described in the literature [21]. Furthermore, to perform a detailed structural analysis of the detected daphnane diterpenoids, additional data processing was conducted using the Thermo Scientific FreeStyle 1.6 software. The elemental compositions of the detected peaks were calculated within a mass tolerance of ±5 ppm. The identified daphnane diterpenoids were further examined using the CAS SciFinder Discovery Platform (Chemical Abstracts Service, Columbus, OH, USA) to determine whether they corresponded to any known compounds.
4. Conclusions
In the present study, phytochemical analysis using UHPLC-Q-Exactive-Orbitrap MS led to the identification of 30 daphnane diterpenoids, including 12 previously unreported compounds, from the flower buds and blooming flowers of D. odora. Moreover, a novel daphnane diterpenoid, daphneodorin I (16), was successfully obtained via LC–MS-guided isolation, and its structure was elucidated using extensive physicochemical and spectroscopic analyses. Although daphnane diterpenoids have been previously isolated from various parts of D. odora, this is the first study to demonstrate their presence in flowers. Notably, the flower buds were found to contain a higher abundance of daphnane diterpenoids than the flowers. Furthermore, this study provides the first evidence of the occurrence of daphnane diterpenoids bearing a Z-configured phenolic acyl moiety in Thymelaeaceae plants [32]. Given the extremely low yield of daphneodorin I (16) isolated from the flower buds of D. odora, it is likely that the phenolic acyl moieties in this species predominantly exist in the E-configuration. The formation of Z-isomers may result from the photoinduced geometric isomerization of the corresponding E-isomers upon exposure to ultraviolet radiation in plant tissues [33,34]. Because the Z-isomer adopts a three-dimensional structure distinct from that of the E-isomer, such configurational differences may influence intermolecular interactions with biological targets and, consequently, affect bioactivity [32,35]. Collectively, these findings highlight the flower buds of D. odora as a rich source of structurally diverse daphnane diterpenoids and underscore their potential for further chemical and biological investigations.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants14172616/s1, Table S1: Comparison of peak areas (positive ion mode) for 30 daphnane diterpenoids identified in the flower buds and flowers of D. odora; Figures S1–S3: HCD product ion spectra of compounds 6, 12, and 28; Figure S4: Extracted ion chromatograms (XICs) in positive ion mode for compounds 7, 8, 10, 14–18, 23, and 24; Figures S5–S11: HCD product ion spectra of compounds 7, 8, 10, 14–18, 23, and 24; Figures S12–S18: 1D and 2D NMR spectra of daphneodorin I (16); Figure S19: UV spectrum of daphneodorin I (16); Figure S20: ECD spectrum of daphneodorin I (16); Figure S21: HRESIMS data of daphneodorin I (16); Figure S22: IR spectrum of daphneodorin I (16).
Author Contributions
Conceptualization, K.O. and W.L.; investigation, K.O., K.M., M.G., M.Z., T.K., and W.L.; writing—original draft preparation, K.O.; writing—review and editing, M.Z. and W.L.; project administration, K.O. and W.L.; funding acquisition, K.O. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science KAKENHI 24K09867 (W.L.) and 24K09881 (K.O.).
Data Availability Statement
All new research data were presented in this contribution.
Acknowledgments
The authors are grateful to Tsuyoshi Kawakami (Faculty of Pharmaceutical Sciences, Toho University) for the plant material collection. All authors express their appreciation to the editorial board and reviewer for their suggestions and efforts in reviewing this paper. The authors also appreciate the editor for his assistance during the review process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, Z.; Peter, H.R.; Hong, D. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden: St. Louis, MO, USA, 1994; Volume 13, p. 238. [Google Scholar]
- Shirai, K. Tyuyakudaiziten; Shanghai Scientific & Technical Publishers: Shanghai, China; Shougakukan, Inc.: Tokyo, Japan, 1985; Volume 2. [Google Scholar]
- Kogiso, S.; Hosozawa, S.; Wada, K.; Munakata, K. Daphneolone in roots of Daphne odora. Phytochemistry 1974, 13, 2332–2334. [Google Scholar] [CrossRef]
- Satô, M.; Hasegawa, M. Conversion of daphnin to daphnetin-8-glucoside in Daphne odora. Phytochemistry 1969, 8, 1211–1214. [Google Scholar] [CrossRef]
- Baba, K.; Takeuchi, K.; Hamasaki, F.; Kozawa, M. Chemical studies on the constituents of the Thymelaeaceous plants. I.: Structures of two new flavans from Daphne odora Thunb. Chem. Pharm. Bull. 1986, 34, 595–602. [Google Scholar] [CrossRef]
- Baba, K.; Yoshikawa, M.; Taniguchi, M.; Kozawa, M. Biflavonoids from Daphne odora. Phytochemistry 1995, 38, 1021–1026. [Google Scholar] [CrossRef]
- Okunishi, T.; Umezawa, T.; Shimada, M. Isolation and enzymatic formation of lignans of Daphne genkwa and Daphne odora. J. Wood Sci. 2001, 47, 383–388. [Google Scholar] [CrossRef]
- Ohigashi, H.; Hirota, M.; Ohtsuka, T.; Koshimizu, K.; Fujiki, H.; Suganuma, M.; Tamaizumi, Z.; Sugimura, T. Resiniferonol-related diterpene esters from Daphne odora Thunb. and their ornithine decarboxylase-inducing activity in mouse skin. Agric. Biol. Chem. 1982, 46, 2605–2608. [Google Scholar] [CrossRef]
- Shigemori, H.; Nakasone, R.; Kurisu, M.; Onodera, M.; Miyamae, Y.; Matsuura, D.; Kanatani, H.; Yano, S. Promoting effects on hepatocyte growth factor production of daphnane diterpenoids from Daphne odora. Heterocycles 2013, 87, 1087. [Google Scholar] [CrossRef]
- Otsuki, K.; Li, W.; Asada, Y.; Chen, C.H.; Lee, K.H.; Koike, K. Daphneodorins A–C, anti-HIV gnidimacrin related macrocyclic daphnane orthoesters from Daphne odora. Org. Lett. 2020, 22, 11–15. [Google Scholar] [CrossRef]
- Otsuki, K.; Li, W.; Miura, K.; Asada, Y.; Huang, L.; Chen, C.H.; Lee, K.H.; Koike, K. Isolation, structural elucidation, and anti-HIV activity of daphnane diterpenoids from Daphne odora. J. Nat. Prod. 2020, 83, 3270–3277. [Google Scholar] [CrossRef]
- Otsuki, K.; Li, W. Tigliane and daphnane diterpenoids from Thymelaeaceae family: Chemistry, biological activity, and potential in drug discovery. J. Nat. Med. 2023, 77, 625–643. [Google Scholar] [CrossRef]
- Adolf, W.; Sorg, B.; Hergenhahn, M.; Hecker, E. Structure-activity relations of polyfunctional diterpenes of the daphnane type I. Revised structure for resiniferatoxin and structure-activity relations of resiniferonol and some of its esters. J. Nat. Prod. 1982, 45, 347–354. [Google Scholar] [CrossRef]
- Hou, Z.L.; Yao, G.D.; Song, S.J. Daphnane-type diterpenes from genus Daphne and their anti-tumor activity. Chin. Herb. Med. 2021, 13, 145–156. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, A.H.H.; Eguchi, K.; Kishimoto, N.; Asano, T.; Kato, H.; Hitora, Y.; Kotani, S.; Nakamura, T.; Tsuchiya, S.; Kawahara, T.; et al. Isolation, synthesis, and structure-activity relationship study on daphnane and tigliane diterpenes as HIV latency-reversing agents. J. Med. Chem. 2022, 65, 3460–3472. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E.; Casal, S. Phytochemical characterization of Borago officinalis L. and Centaurea cyanus L. during flower development. Food Res. Int. 2019, 123, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Önder, S.; Tonguç, M.; Erbaş, S.; Önder, D.; Mutlucan, M. Investigation of phenological, primary and secondary metabolites changes during flower developmental of Rosa damascena. Plant Physiol. Biochem. 2022, 192, 20–34. [Google Scholar] [CrossRef]
- Zhao, H.D.; Lu, Y.; Yan, M.; Chen, C.H.; Morris-Natschke, S.L.; Lee, K.H.; Chen, D.F. Rapid recognition and targeted isolation of anti-HIV daphnane diterpenes from Daphne genkwa guided by UPLC-MSn. J. Nat. Prod. 2020, 83, 134–141. [Google Scholar] [CrossRef]
- Pan, R.R.; Zhang, C.Y.; Li, Y.; Zhang, B.B.; Zhao, L.; Ye, Y.; Song, Y.N.; Zhang, M.; Tie, H.Y.; Zhang, H.; et al. Daphnane diterpenoids from Daphne genkwa inhibit PI3K/Akt/mTOR signaling and induce cell cycle arrest and apoptosis in human colon cancer cells. J. Nat. Prod. 2020, 83, 1238–1248. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Zhang, M.; Otsuki, K.; Tan, L.J.; Kikuchi, T.; Li, N.; Li, W. Automated annotation of complex natural products using a Modular Fragmentation-based Structure Assembly (MFSA) strategy. Sci. Adv. 2025, 11, eadw4693. [Google Scholar] [CrossRef]
- Otsuki, K.; Zhang, M.; Tan, L.; Komaki, M.; Shimada, A.; Kikuchi, T.; Zhou, D.; Li, N.; Li, W. Isomer differentiation by UHPLC-Q-Exactive-Orbitrap MS led to enhanced identification of daphnane diterpenoids in Daphne tangutica. Phytochem. Anal. 2025, 36, 1053–1062. [Google Scholar] [CrossRef]
- Wu, D.; Sorg, B.; Adolf, W.; Seip, E.H.; Hecker, E. Oligo- and macrocyclic diterpenes in Thymelaeaceae and Euphorbiaceae occurring and utilized in Yunnan (Southwest China) 3. Two new daphnane type 9,13,14-orthoesters from Wikstroemia mekongenia. Phytother. Res. 1993, 7, 72–75. [Google Scholar] [CrossRef]
- Li, L.Z.; Gao, P.Y.; Wang, L.H.; Song, S.J. A novel daphnane-type diterpene from the flower bud of Daphne genkwa. Chem. Nat. Compd. 2010, 46, 380–382. [Google Scholar] [CrossRef]
- Adolf, W.; Dossaji, S.F.; Seip, E.H.; Hecker, E. Skin irritant diterpene orthoesters of the daphnane type from Peddiea africana and P. volkensii. Phytochemistry 1985, 24, 2047–2049. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Zhang, F.; Yang, P.; Gao, X.; Song, Q. Preparation of yuanhuacine and relative daphne diterpene esters from Daphne genkwa and structure-activity relationship of potent inhibitory activity against DNA topoisomerase I. Bioorg. Med. Chem. 2006, 14, 3888–3895. [Google Scholar] [CrossRef]
- Adolf, W.; Seip, E.H.; Hecker, E.; Dossaji, S.F. Irritant principles of the mezereon family (Thymelaeaceae), V. New skin irritants and tumor promoters of the daphnane and 1 alpha-alkyldaphnane type from Synaptolepis kirkii and Synaptolepis retusa. J. Nat. Prod. 1988, 51, 662–674. [Google Scholar] [CrossRef]
- Huang, S.Z.; Zhang, X.J.; Li, X.Y.; Kong, L.M.; Jiang, H.Z.; Ma, Q.Y.; Liu, Y.Q.; Hu, J.M.; Zheng, Y.T.; Li, Y.; et al. Daphnane-type diterpene esters with cytotoxic and anti-HIV-1 activities from Daphne acutiloba Rehd. Phytochemistry 2012, 75, 99–107. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Shizuri, Y.; Murae, T.; Swenny, J.G.; Haynes, H.R.; Shen, M.S.; Barrick, J.C.; Bryan, A.F.; vander Helm, D.; Wu, K.K. Letter: Gnidimacrin and gnidimacrin 20-palmitate, novel macrocyclic antileukemic diterpenoid esters from Gnidia subcordata. J. Am. Chem. Soc. 1976, 98, 5719–5720. [Google Scholar] [CrossRef]
- Kogiso, S.; Wada, K.; Munakata, K. Odoracin, a nematicidal constituent from Daphne odora. Agric. Biol. Chem. 1976, 40, 2119–2120. [Google Scholar] [CrossRef]
- Otsuki, K.; Zhang, M.; Kikuchi, T.; Tsuji, M.; Tejima, M.; Bai, Z.S.; Zhou, D.; Huang, L.; Chen, C.H.; Lee, K.H.; et al. Identification of anti-HIV macrocyclic daphnane orthoesters from Wikstroemia ligustrina by LC-MS analysis and phytochemical investigation. J. Nat. Med. 2021, 75, 1058–1066. [Google Scholar] [CrossRef]
- Liu, F.; Yang, X.; Liang, Y.; Dong, B.; Su, G.; Tuerhong, M.; Jin, D.Q.; Xu, J.; Guo, Y. Daphnane diterpenoids with nitric oxide inhibitory activities and interactions with iNOS from the leaves of Trigonostemon thyrsoideus. Phytochemistry 2018, 147, 57–67. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kirkpatrick, J.; Kuhnert, N.; Roozendaal, H.; Salgado, P.R. LC–MSn analysis of the cis isomers of chlorogenic acids. Food Chem. 2008, 106, 379–385. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Castejón, M.L.; Rubio-Senent, F.; Fernández-Prior, Á.; Rodríguez-Gutiérrez, G.; Fernández-Bolaños, J. Isolation and structural determination of cis- and trans-p-coumaroyl-secologanoside (comselogoside) from olive oil waste (alperujo). Photoisomerization with ultraviolet irradiation and antioxidant activities. Food Chem. 2024, 432, 137233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).