Antagonistic Interaction Between Microplastics and Herbivory on the Growth of Native and Invasive Plants
Abstract
1. Introduction
2. Results
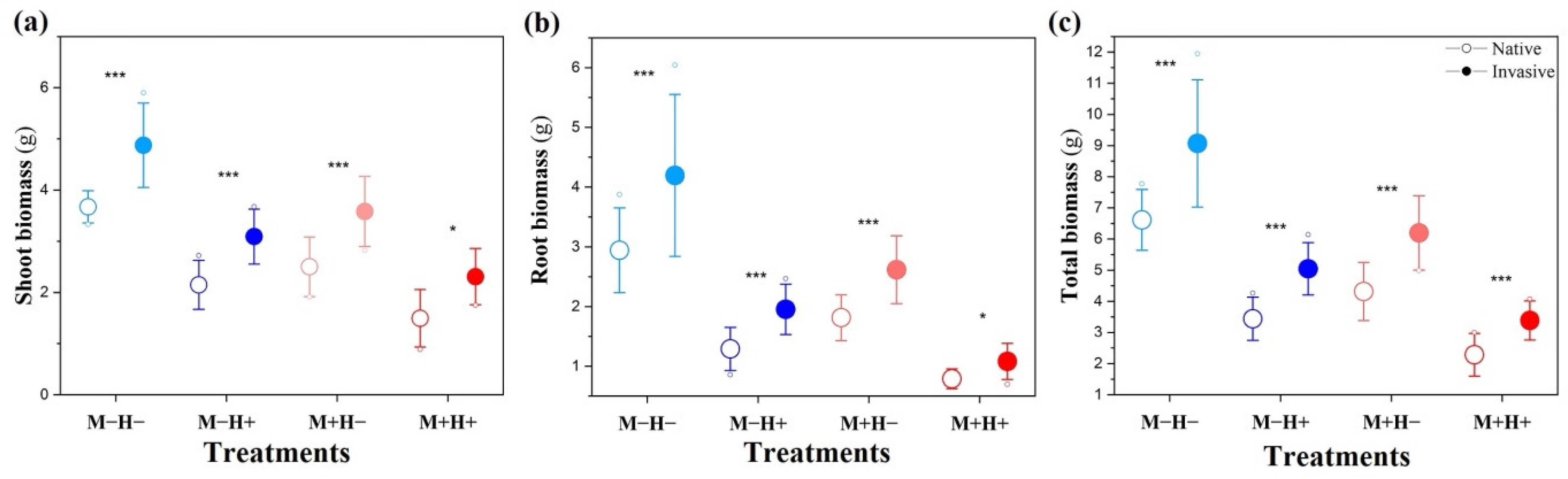
2.1. Effect on the Biomass
2.2. Deleterious Effects (DEs) of PE-MPs and Herbivory
2.3. Effect on Biomass Allocation
3. Discussion
4. Materials and Methods
4.1. Study Site and Plant Species Selection
4.2. Experimental Design
4.3. Seedlings Preparation
4.4. Microplastic Contamination Treatment
4.5. Plant Transplanting
4.6. Herbivory Treatment
4.7. Harvest and Measurement
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PE-MPs | Polyethylene microplastics |
| DE | Deleterious effect |
References
- Sa’adu, I.; Farsang, A. Plastic contamination in agricultural soils: A review. Environ. Sci. Eur. 2023, 35, 13. [Google Scholar] [CrossRef]
- Shi, X.; Shi, R.; Fu, X.; Zhao, Y.; Ge, Y.; Liu, J.; Chen, C.; Liu, W. Impact of microplastics on plant physiology: A meta-analysis of dose, particle size, and crop type interactions in agricultural ecosystems. Sci. Total Environ. 2024, 955, 177245. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xiong, X.; Zhang, Y.; Wu, C.; Xu, X.; Sun, C.; Shi, H. Global transportation of plastics and microplastics: A critical review of pathways and influences. Sci. Total Environ. 2022, 831, 154884. [Google Scholar] [CrossRef]
- Ullah, R.; Tsui, M.T.K.; Chen, H.; Chow, A.; Williams, C.; Ligaba-Osena, A. Microplastics interaction with terrestrial plants and their impacts on agriculture. J. Environ. Qual. 2021, 50, 1024–1041. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Y.; Tan, W.; Zhang, Z. Microplastics as an emerging environmental pollutant in agricultural soils: Effects on ecosystems and human health. Front. Environ. Sci. 2022, 10, 855292. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; de Souza Machado, A.A.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef]
- Zhai, Y.; Bai, J.; Chang, P.; Liu, Z.; Wang, Y.; Liu, G.; Cui, B.; Peijnenburg, W.; Vijver, M.G. Microplastics in terrestrial ecosystem: Exploring the menace to the soil-plant-microbe interactions. Trends Anal. Chem. 2024, 174, 117667. [Google Scholar] [CrossRef]
- Gentili, R.; Quaglini, L.; Cardarelli, E.; Caronni, S.; Montagnani, C.; Citterio, S. Toxic impact of soil microplastics (PVC) on two weeds: Changes in growth, phenology and photosynthesis efficiency. Agronomy 2022, 12, 1219. [Google Scholar] [CrossRef]
- Fu, Y.; van Kleunen, M.; Ma, K.; Liu, Y. The more microplastic types pollute the soil, the stronger the growth suppression of invasive alien and native plants. J. Ecol. 2024, 112, 1444–1457. [Google Scholar] [CrossRef]
- Shi, X.; Yang, G.; Zheng, Y. Effects of microplastics, fertilization and pesticides on alien and native plants. Plants 2024, 13, 2947. [Google Scholar] [CrossRef]
- Meng, Z.; Mo, X.; Meng, W.; Hu, B.; Liu, B.; Li, H.; Liu, J.; Xu, M.; Hou, Q.; Lu, X. Microplastics could alter invasive plant community performance and the dominance of Amaranthus palmeri. Sci. Total Environ. 2024, 912, 169275. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Rillig, M.C. Effects of microplastic fibers and drought on plant communities. Environ. Sci. Technol. 2020, 54, 6166–6173. [Google Scholar] [CrossRef]
- Heinen, R.; Thakur, M.P.; Hiddes De Fries, J.R.; Steinauer, K.; Vandenbrande, S.; Jongen, R.; Bezemer, T.M. Foliar herbivory on plants creates soil legacy effects that impact future insect herbivore growth via changes in plant community biomass allocation. Funct. Ecol. 2022, 36, 1047–1062. [Google Scholar] [CrossRef]
- Geppert, C.; Boscutti, F.; La Bella, G.; De Marchi, V.; Corcos, D.; Filippi, A.; Marini, L. Contrasting response of native and non-native plants to disturbance and herbivory in mountain environments. J. Biogeogr. 2021, 48, 1594–1605. [Google Scholar] [CrossRef]
- Sakata, Y.; Craig, T.P. An exotic herbivore reinforces competition between exotic and native plants. J. Ecol. 2021, 109, 2740–2753. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, C.; Gu, Y.; Shi, Y.; Gao, X. Microplastics in plant-soil ecosystems: A meta-analysis. Environ. Pollut. 2022, 308, 119718. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, Y.; Deng, M.; Zhang, Y.; Luo, J.; Han, R.; Xu, L. Microplastics and nanoplastics alter the physicochemical properties of willow trees and lead to mortality in leaf beetle larvae. Plant Cell Environ. 2024, 48, 2895–2909. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, Q.; Chen, L.; Zhu, X.; Zhao, S.; Duan, C.; Zhang, X.; Song, D.; Fang, L.A. critical review of microplastics in the soil-plant system: Distribution, uptake, phytotoxicity and prevention. J. Hazard. Mater. 2022, 424, 127750. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef]
- Iqbal, B.; Zhao, T.; Yin, W.; Zhao, X.; Xie, Q.; Khan, K.Y.; Zhao, X.; Nazar, M.; Li, G.; Du, D. Impacts of soil microplastics on crops: A review. Appl. Soil Ecol. 2023, 181, 104680. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, L.; Zhou, W.; Wang, Q.; Yu, D. Effect of microfibers combined with UV-B and drought on plant community. Chemosphere 2022, 288, 132413. [Google Scholar] [CrossRef]
- Li, G.; Tang, Y.; Lou, J.; Wang, Y.; Yin, S.; Li, L.; Iqbal, B.; Lozano, Y.M.; Zhao, T.; Du, D. The promoting effects of soil microplastics on alien plant invasion depend on microplastic shape and concentration. Sci. Total Environ. 2024, 926, 172089. [Google Scholar] [CrossRef]
- Oduor, A.M. Invasive plant species that experience lower herbivory pressure may evolve lower diversities of chemical defense compounds in the exotic range. Am. J. Bot. 2022, 109, 1382–1393. [Google Scholar] [CrossRef]
- Wu, S.; Chen, L.; Zhou, Y.; Xiao, F.; Liu, D.; Wang, Y. Invasive plants have higher resistance to native generalist herbivores than exotic noninvasive congeners. Environ. Entomol. 2023, 52, 81–87. [Google Scholar] [CrossRef]
- Rondoni, G.; Chierici, E.; Agnelli, A.; Conti, E. Microplastics alter behavioural responses of an insect herbivore to a plant-soil system. Sci. Total Environ. 2021, 787, 147716. [Google Scholar] [CrossRef]
- Atkinson, J.; Gallagher, R.; Czyżewski, S.; Kerr, M.; Trepel, J.; Buitenwerf, R.; Svenning, J.C. Integrating functional traits into trophic rewilding science. J. Ecol. 2024, 112, 936–953. [Google Scholar] [CrossRef]
- Agrawal, A.A. A scale-dependent framework for trade-offs, syndromes, and specialization in organismal biology. Ecology 2020, 101, e02924. [Google Scholar] [CrossRef] [PubMed]
- Gols, R. Tolerance to insect herbivory increases with progressing plant development. Plant Biol. 2025, 27, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Rieu, I.; Mariani, C.; van Dam, N.M. How plants handle multiple stresses: Hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol. Biol. 2016, 91, 727–740. [Google Scholar] [CrossRef]
- Shan, L.; Oduor, A.M.; Liu, Y. Herbivory and elevated levels of CO2 and nutrients separately, rather than synergistically, impacted biomass production and allocation in invasive and native plant species. Glob. Change Biol. 2023, 29, 6741–6755. [Google Scholar] [CrossRef]
- Ben Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- He, F.; Sun, J.; Wan, J.S.; Nawaz, M.; Javed, Q.; Pan, L.; Khattak, W.A.; Bo, Y.; Xiang, Y.; Ren, G. Microplastics and cadmium affect invasion success by altering complementarity and selection effects in native community. Sci. Total Environ. 2024, 921, 171135. [Google Scholar] [CrossRef]
- Monson, R.K.; Trowbridge, A.M.; Lindroth, R.L.; Lerdau, M.T. Coordinated resource allocation to plant growth–defense tradeoffs. New Phytol. 2022, 233, 1051–1066. [Google Scholar] [CrossRef]
- He, Z.; Webster, S.; He, S.Y. Growth–defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, D.; Flory, S.L. Populations of a widespread invader and co-occurring native species vary in phenotypic plasticity. New Phytol. 2020, 225, 584–594. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, M.; Shahbaz, M.; Hu, Z.e.; Zhu, Z.; Lu, S.; Yu, Y.; Yao, H.; Chen, J.; Ge, T. Microplastics in soil can increase nutrient uptake by wheat. J. Hazard. Mater. 2022, 438, 129547. [Google Scholar] [CrossRef]
- Rathee, S.; Ahmad, M.; Sharma, P.; Singh, H.P.; Batish, D.R.; Kaur, S.; Kaur, A.; Yadav, S.S.; Kohli, R.K. Biomass allocation and phenotypic plasticity are key elements of successful invasion of Parthenium hysterophorus at high elevation. Environ. Exp. Bot. 2021, 184, 104392. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Alsharbaty, M.H.M.; Elsamahy, T.; El-Sapagh, S.; Lim, J.W.; Sun, J. Microplastics and their ecotoxicological impacts: Remediation approaches, challenges and future perspectives—A review. J. Clean. Prod. 2024, 452, 142153. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Landt, L.; Rillig, M.C. Plastic particles and their additives promote plant invasion through physicochemical mechanisms on seed germination. J. Ecol. 2025, 113, 275–288. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.; Ma, J.; Li, H.; Yan, J. The Checklist of the Naturalized Plants in China; Shanghai Scientific and Technical Publishers: Shanghai, China, 2019. [Google Scholar]
- Yan, X.; Liu, Q.; Shou, H.; Zeng, X.; Zhang, Y.; Chen, L.; Liu, Y.; Ma, H.; Qi, S.; Ma, J. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodivers. Sci. 2014, 22, 667. [Google Scholar] [CrossRef]
- Li, J.; Jin, Z.; Song, W. Do native parasitic plants cause more damage to exotic invasive hosts than native non-invasive hosts? An implication for biocontrol. PLoS ONE 2012, 7, e34577. [Google Scholar] [CrossRef] [PubMed][Green Version]
- R Core Team. R: A language and environment for statistical computing. R Found. Stat. Comput. 2016, 1, 409. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Maintainer, R. Package ‘nlme’. Linear Nonlinear Mix. Eff. Models Version 2017, 3, 274. [Google Scholar]


| Variable | Shoot Biomass | Root Biomass | Total Biomass | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Initial height | 25.313 | <0.001 | 25.313 | <0.001 | 40.450 | <0.001 |
| Plant status (S) | 119.652 | <0.001 | 119.652 | <0.001 | 94.536 | <0.001 |
| Species (nested within status) | 25.665 | <0.001 | 25.665 | <0.001 | 27.273 | <0.001 |
| PE-MPs (M) | 177.389 | <0.001 | 177.389 | <0.001 | 285.106 | <0.001 |
| Herbivory (H) | 85.679 | <0.001 | 85.679 | <0.001 | 125.359 | <0.001 |
| S × M | 4.699 | 0.031 | 4.699 | 0.031 | 0.732 | 0.393 |
| S × H | 2.338 | 0.128 | 2.338 | 0.128 | 2.111 | 0.148 |
| M × H | 6.165 | 0.014 | 6.165 | 0.014 | 11.195 | 0.001 |
| S × M × H | 0.119 | 0.731 | 0.119 | 0.731 | 0.376 | 0.540 |
| Variable | DE of PE-MPs | De of Herbivory | ||
|---|---|---|---|---|
| F | p | F | p | |
| Initial height | 0.933 | 0.336 | 0.255 | 0.615 |
| Plant status (S) | 14.147 | <0.001 | 1.756 | 0.188 |
| Species (nested within status) | 3.446 | 0.001 | 1.013 | 0.438 |
| Herbivory (H) | 0.223 | 0.638 | / | / |
| PE-MPs (M) | / | / | 0.013 | 0.910 |
| S × H | 0.274 | 0.602 | / | / |
| S × M | / | / | 0.152 | 0.698 |
| Variable | Root Mass Fraction | Shoot Mass Fraction | Root-Shoot Ratio | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Initial height | 11.786 | 0.001 | 10.882 | 0.001 | 6.756 | 0.010 |
| Plant status (S) | 0.030 | 0.862 | 0.007 | 0.933 | 0.025 | 0.874 |
| Species (nested within status) | 7.201 | <0.001 | 7.246 | <0.001 | 6.290 | <0.001 |
| Microplastics (M) | 27.274 | <0.001 | 25.277 | <0.001 | 16.003 | <0.001 |
| Herbivore (H) | 9.894 | 0.002 | 8.888 | 0.001 | 5.232 | 0.023 |
| S × M | 3.104 | 0.080 | 3.360 | 0.068 | 4.104 | 0.044 |
| S × H | 0.066 | 0.798 | 0.503 | 0.852 | 0.023 | 0.880 |
| M × H | 0.455 | 0.503 | 0.167 | 0.683 | 1.107 | 0.294 |
| S × M × H | 1.061 | 0.304 | 1.055 | 0.306 | 0.810 | 0.369 |
| Species | Genus | Family | Number of Chinese Provinces | Status in China | Region of Origin | Characteristics | Mode of Reproduction |
|---|---|---|---|---|---|---|---|
| Alternanthera sessilis | Alternanthera | Amaranthaceae | 14+ | Native | Tropical Asia to America | Perennial, herb | Asexual |
| Alternanthera philoxeroides | Alternanthera | 18 | Alien-2 | South America | Perennial, herb | Asexual | |
| Achyranthes bidentata | Achyranthes | 14 | Native | Eastern and Tropical Asia | Perennial, herb | Asexual | |
| Solidago canadensis | Solidago | Asteraceae | 10+ | Alien-3 | North America | Perennial, herb | Sexual |
| Wedelia chinensis | Wedelia | 5+ | Native | Asia | Perennial, herb | Asexual | |
| Wedelia trilobata | Wedelia | 5+ | Alien-2 | Central America | Perennial, herb | Asexual | |
| Commelina communis | Commelina | Commelinaceae | All except Qinghai/Xinjiang/Tibet | Native | Temperate Asia | Perennial, herb | Sexual |
| Crotalaria pallida | Crotalaria | Fabaceae | 19 | Alien-3 | Tropical Africa and Tropical Asia | Annual, herb | Sexual |
| Lolium perenne | Lolium | Poaceae | North, South, Southeast China | Native | Europe | Perennial, grass | Sexual |
| Paspalum notatum | Paspalum | Widespread flowering in China | Alien | South America | Perennial, grass | Sexual | |
| Hydrocotyl vulgaris | Hydrocotyle | Araceae | Yangtze River provinces (e.g., Zhejiang, Hubei) | Alien | Europe | Perennial, herb | Sexual |
| Solanum lyratum | Solanum | Solanaceae | Widespread in forests/disturbed sites | Native | East Asia | Perennial, herb | Sexual |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okundi, J.; Yuan, L.; Li, G.; Du, D.; Li, J. Antagonistic Interaction Between Microplastics and Herbivory on the Growth of Native and Invasive Plants. Plants 2025, 14, 2692. https://doi.org/10.3390/plants14172692
Okundi J, Yuan L, Li G, Du D, Li J. Antagonistic Interaction Between Microplastics and Herbivory on the Growth of Native and Invasive Plants. Plants. 2025; 14(17):2692. https://doi.org/10.3390/plants14172692
Chicago/Turabian StyleOkundi, Jeffrey, Ling Yuan, Guanlin Li, Daolin Du, and Junmin Li. 2025. "Antagonistic Interaction Between Microplastics and Herbivory on the Growth of Native and Invasive Plants" Plants 14, no. 17: 2692. https://doi.org/10.3390/plants14172692
APA StyleOkundi, J., Yuan, L., Li, G., Du, D., & Li, J. (2025). Antagonistic Interaction Between Microplastics and Herbivory on the Growth of Native and Invasive Plants. Plants, 14(17), 2692. https://doi.org/10.3390/plants14172692









