Biotechnological Test of Plant Growth-Promoting Bacteria Strains for Synthesis of Valorized Wastewater as Biofertilizer for Silvicultural Production of Holm Oak (Quercus ilex L.)
Abstract
1. Introduction
2. Results
2.1. Biometry
2.2. Nutritional Analysis
Major Components (PCA), by Treatment
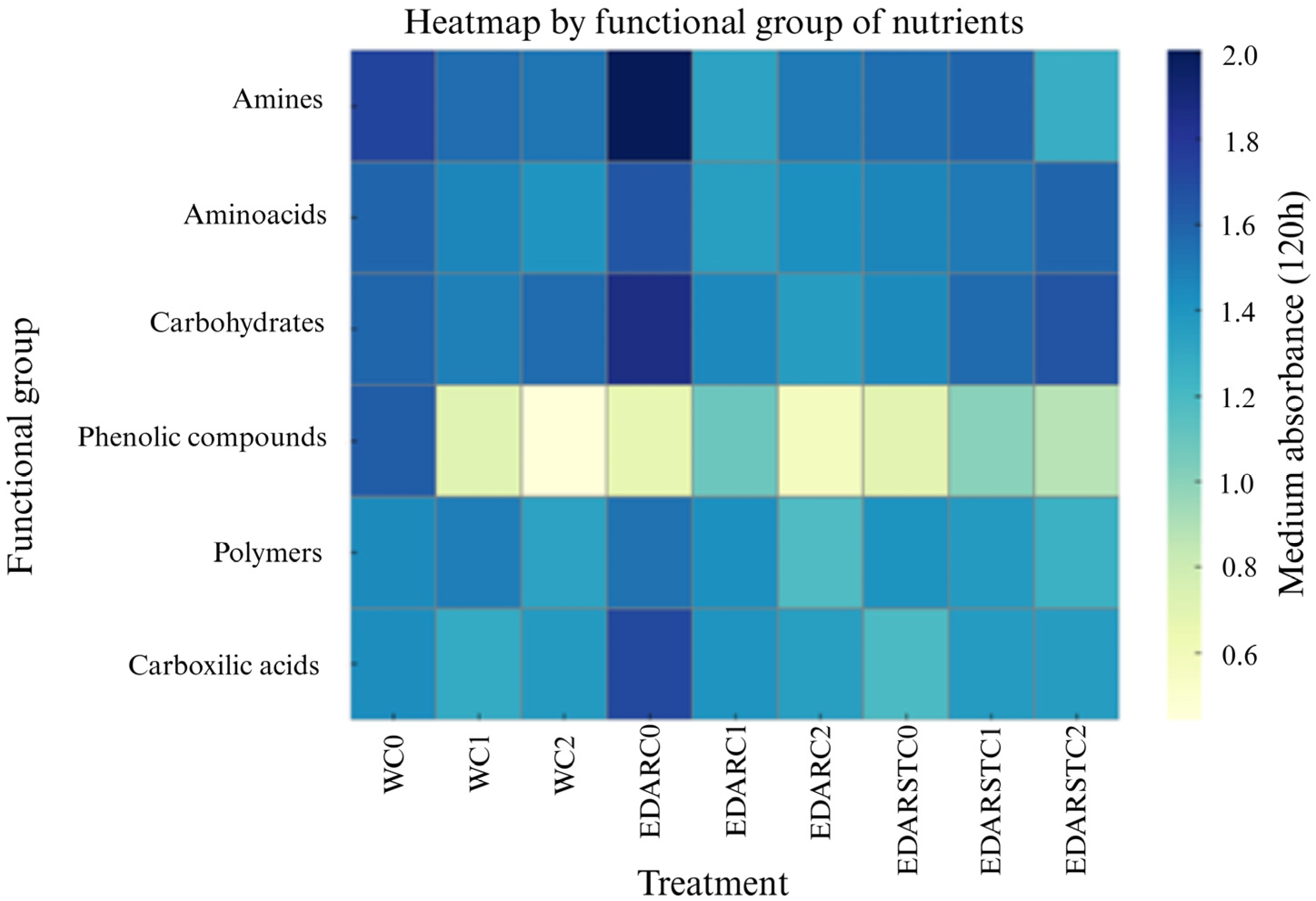
2.3. Functional Diversity: Biolog Ecoplate

2.4. Cenoantibiogram
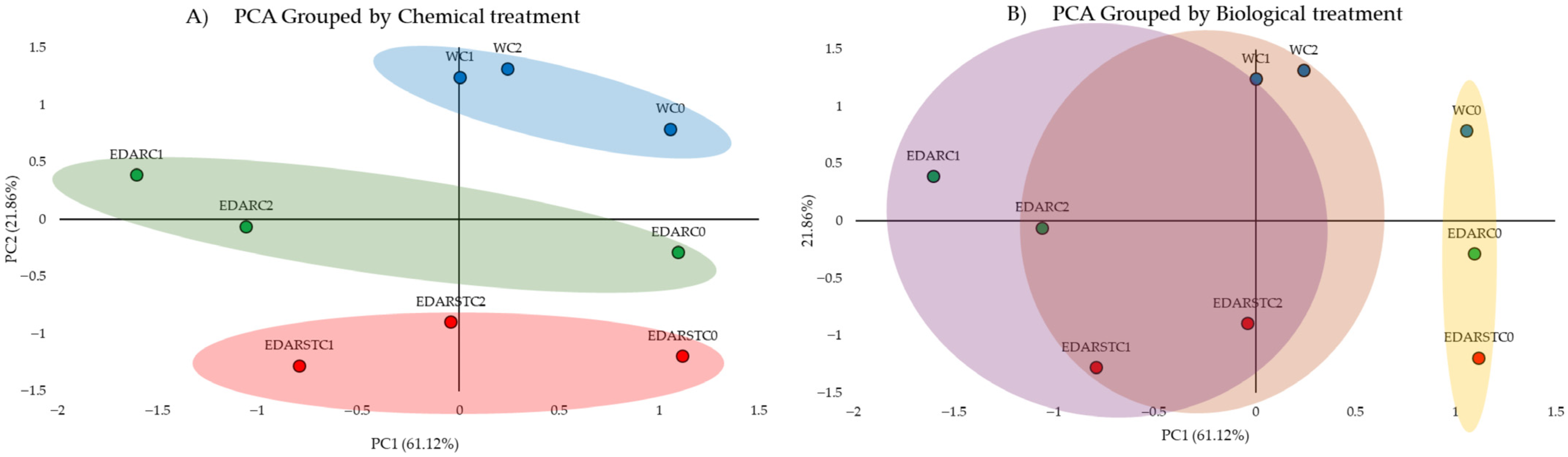
2.4.1. PCA
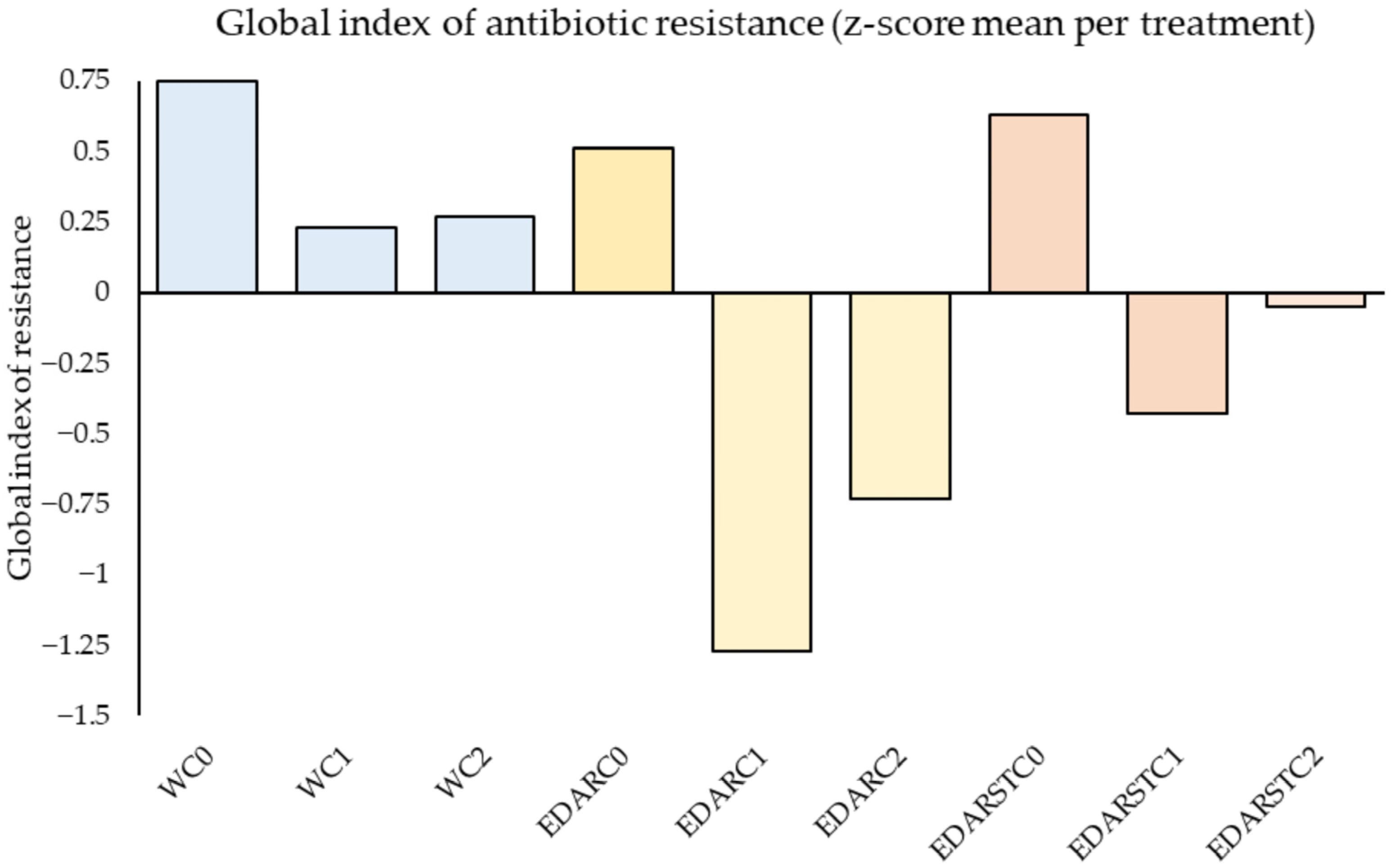
2.4.2. Resistance Index
2.5. Taxonomic Diversity (Alpha Diversity)
2.6. Beta Diversity
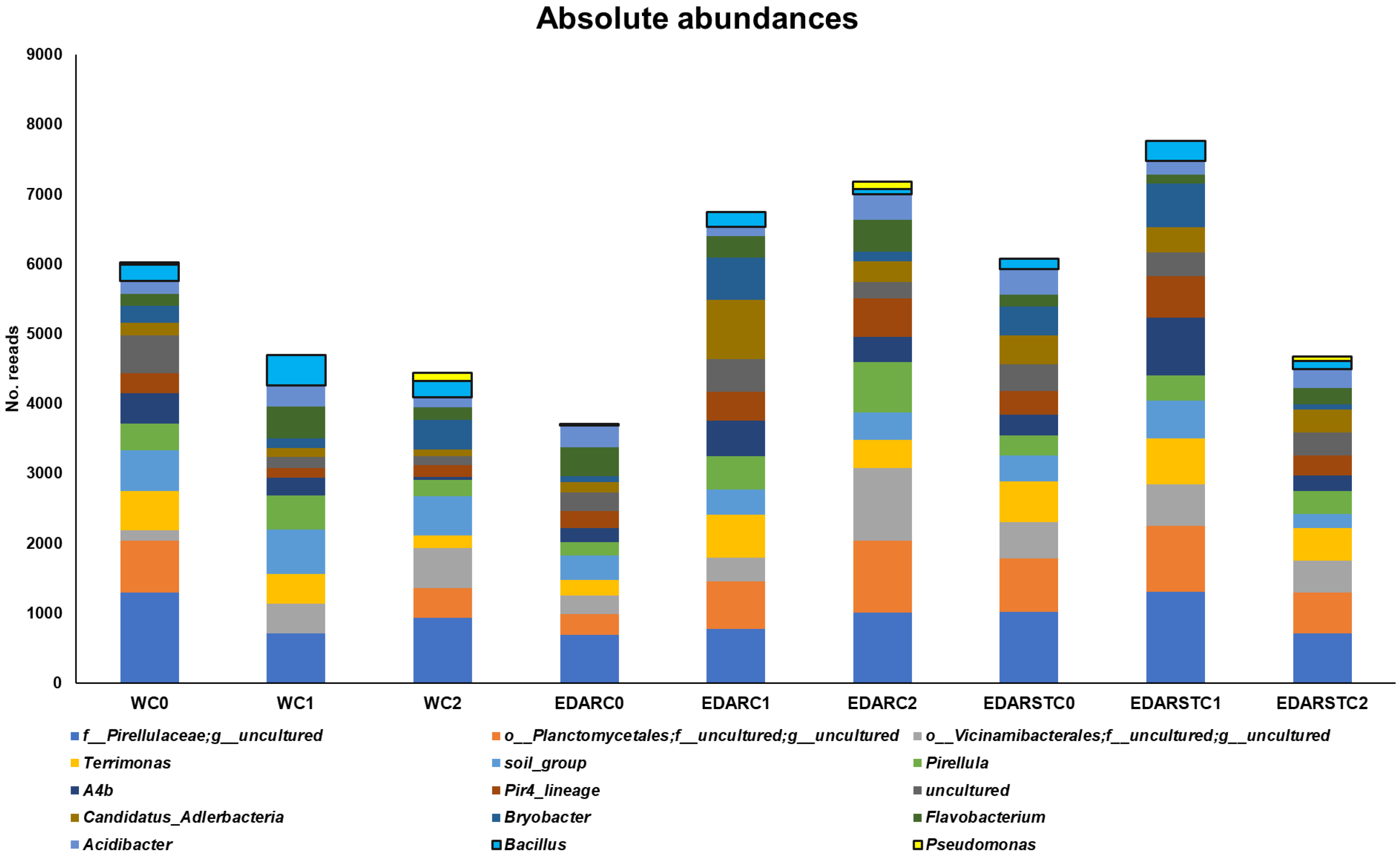
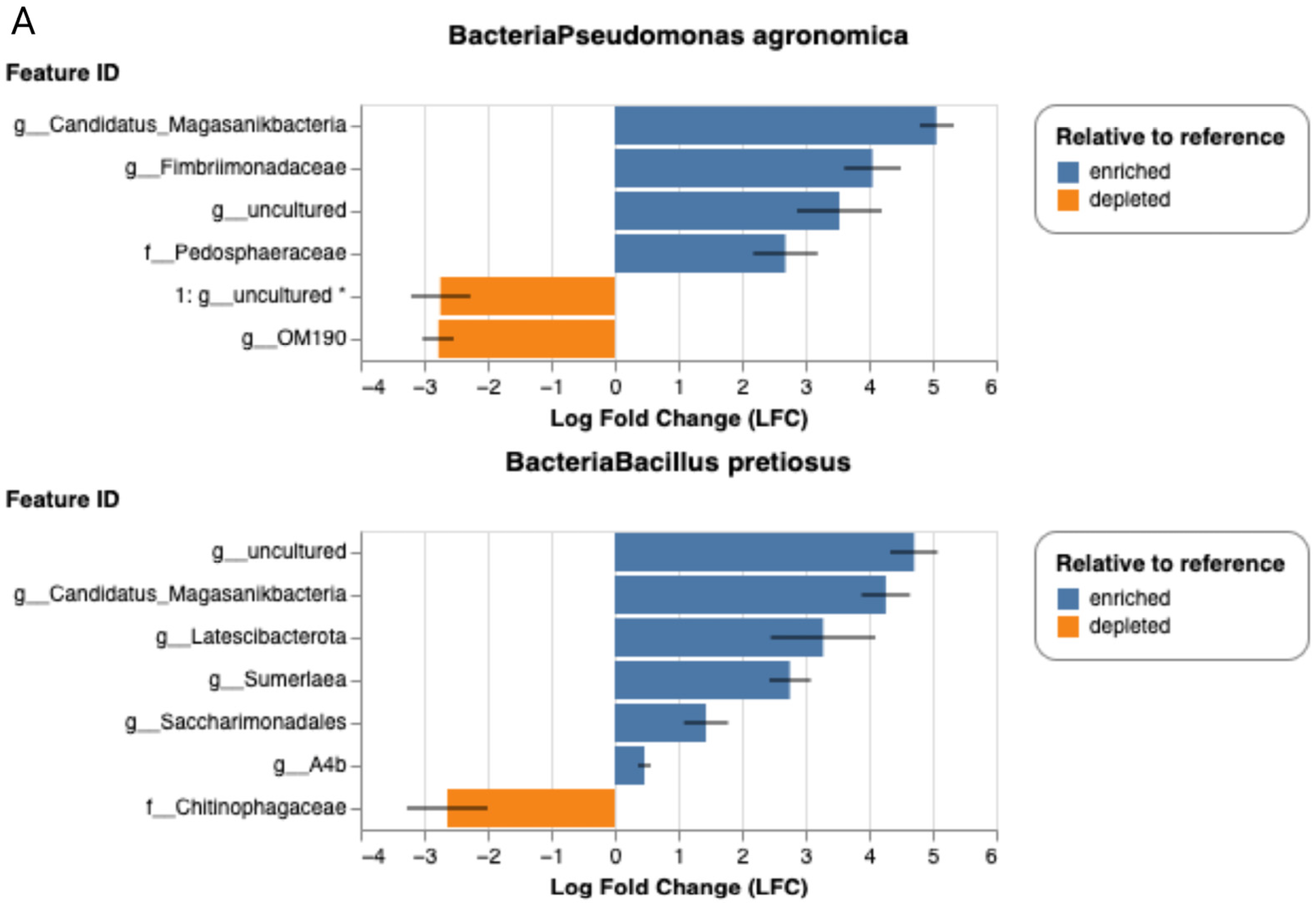
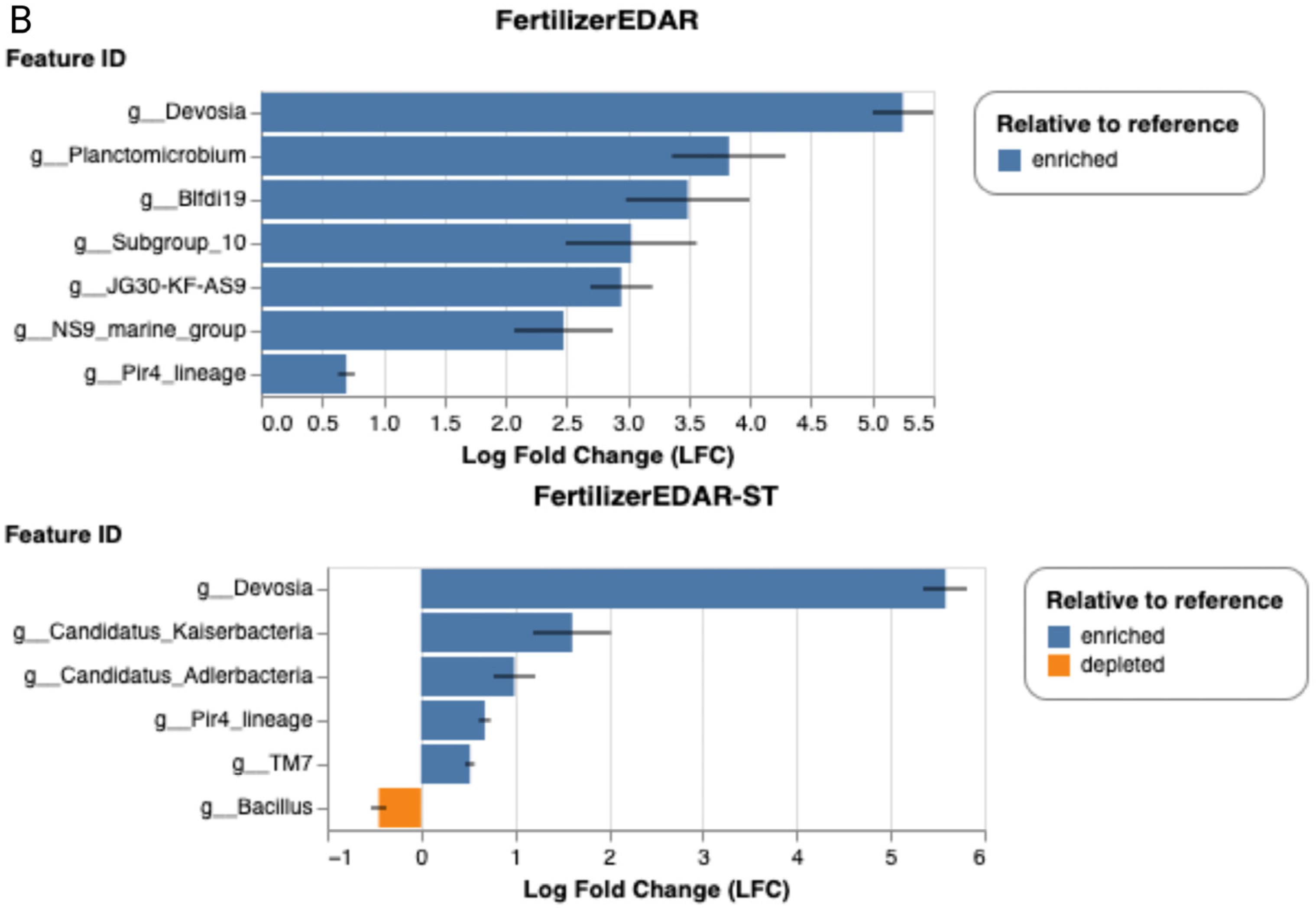
2.7. Relative Abundances at Genus Level
2.8. Gene Prediction
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Experimental Design: Irrigation Matrices (Chemical Treatment) and Biological Treatment (Strains)
4.3. Preparation of Bacterial Suspensions and Biofertilizers
4.3.1. Preparation of Bacterial Suspensions
4.3.2. Preparation of Biofertilizers
4.3.3. Plant Growth Conditions and Irrigation Regime
4.3.4. Harvesting, Total Biomass Measurement and Nutritional Analysis
4.4. Extraction of Soil Microbial Communities
4.5. Antibiotic Susceptibility Test—Cenoantibiogram
4.6. Study of the Functional Diversity of the Microbial Community
4.7. Nutritional and Biometric Analysis
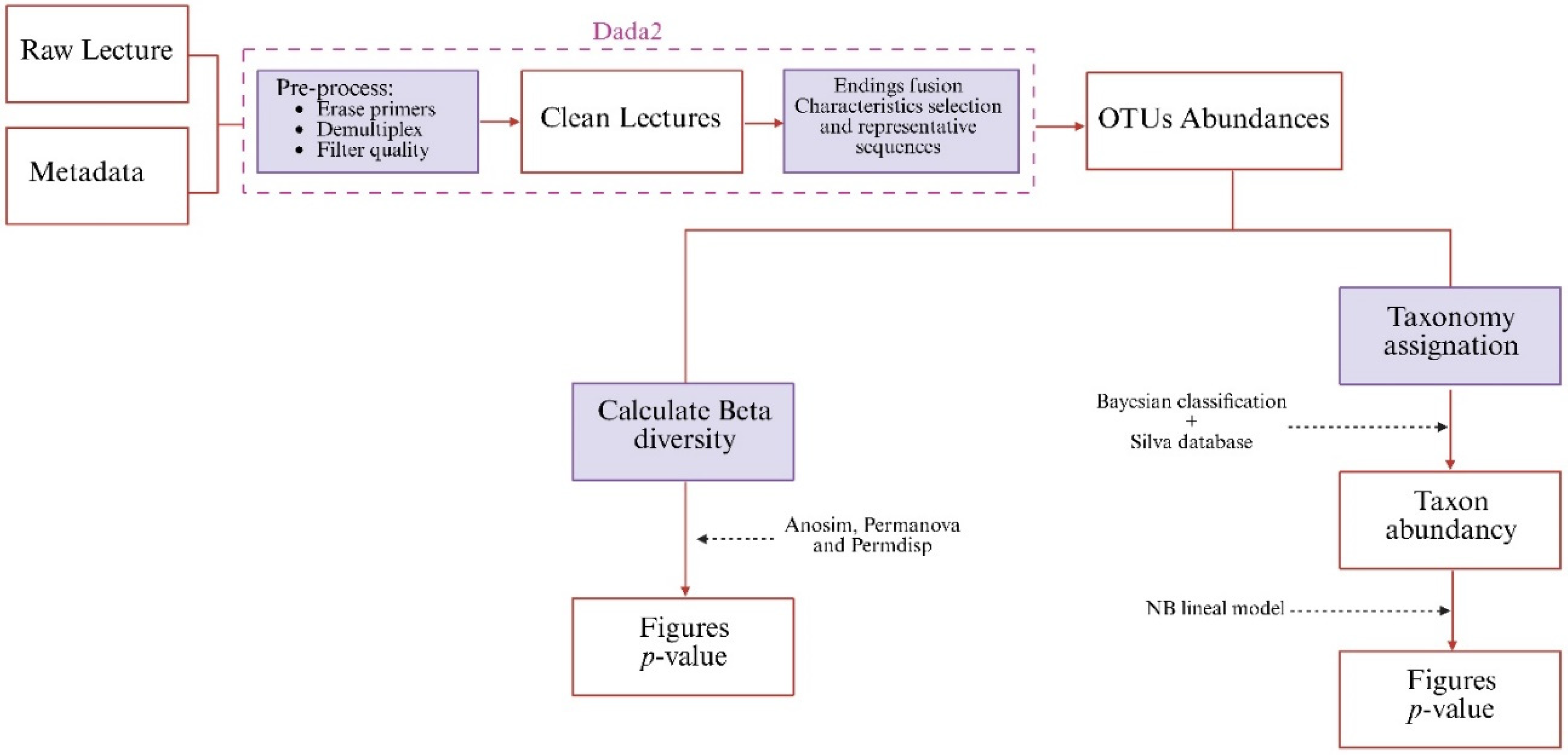
4.8. Metagenomics: Bioinformatic Processing and Statistical Analysis
4.8.1. Metagenomics
4.8.2. Bioinformatics Processing and Statistical Analysis
4.8.3. Gene Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morillas, L.; Leiva, M.J.; Gandullo, J.; Pérez-Ramos, I.M.; Cambrollé, J.; Matías, L. Consistent geographical gradient of water use efficiency evidences local adaptations to drought across the complete latitudinal distribution of Quercus suber. Plant Stress 2024, 12, 100432. [Google Scholar] [CrossRef]
- Campos, P.; Díaz, M.; Pulido, F.J. Las dehesas arboladas: Un equilibrio necesario entre explotación y conservación. Quercus 1998, 147, 31–35. [Google Scholar]
- Vicente, E.; Vilagrosa, A.; Ruiz-Yanetti, S.; Manrique-Alba, À.; González-Sanchís, M.; Moutahir, H.; Chirino, E.; Del Campo, A.; Bellot, J. Water Balance of Mediterranean Quercus ilex L. and Pinus halepensis Mill. Forests in Semiarid Climates: A Review in A Climate Change Context. Forests 2018, 9, 426. [Google Scholar] [CrossRef]
- Gasmi-Boubaker, A.; Losada, R.M.; Abdouli, H.; Rigueiro, A. Importance of Mediterranean forest products as food resource of domestic herbivores: The case of oak acorn. In New Trends for Innovation in the Mediterranean Animal Production; Wageningen Academic Publishers: Wageningen, The Netherlands, 2012; pp. 123–126. [Google Scholar]
- García-Valdés, R.; Svenning, J.; Zavala, M.A.; Purves, D.W.; Araújo, M.B. Evaluating the combined effects of climate and land-use change on tree species distributions. J. Appl. Ecol. 2015, 52, 902–912. [Google Scholar] [CrossRef]
- Ogaya, R.; Peñuelas, J. Climate Change Effects in a Mediterranean Forest Following 21 Consecutive Years of Experimental Drought. Forests 2021, 12, 306. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Harden, J.; Georgiou, K.; Hemes, K.S.; Malhotra, A.; Nolan, C.J.; Jackson, R.B. Fire effects on the persistence of soil organic matter and long-term carbon storage. Nat. Geosci. 2022, 15, 5–13. [Google Scholar] [CrossRef]
- Aponte, H.; Galindo-Castañeda, T.; Yáñez, C.; Hartmann, M.; Rojas, C. Microbial Community-Level Physiological Profiles and Genetic Prokaryotic Structure of Burned Soils Under Mediterranean Sclerophyll Forests in Central Chile. Front. Microbiol. 2022, 13, 824813. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, S.; Du, J.; Pan, H.; Lu, X.; Liu, Y.; Yang, L. Variations in the Diversity and Biomass of Soil Bacteria and Fungi under Different Fire Disturbances in the Taiga Forests of Northeastern China. Forests 2023, 14, 2063. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Liu, X.; Li, B. Microenvironment heterogeneity affected by anthropogenic wildfire-perturbed soil mediates bacterial community in Pinus tabulaeformis forests. Front. Microbiol. 2024, 15, 1415726. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Lasa, A.V.; Cobo-Díaz, J.F.; Villadas, P.J.; Pérez-Luque, A.J.; García-Rodríguez, F.M.; Tringe, S.G.; Fernández-López, M. Long-Term Persistence of Three Microbial Wildfire Biomarkers in Forest Soils. Forests 2023, 14, 1383. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. The soil microbiome—From metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Robas Mora, M.; Fernández Pastrana, V.M.; Oliva, L.L.; Lobo, A.P.; Jiménez Gómez, P.A. Plant growth promotion of the forage plant Lupinus albus Var. Orden Dorado using Pseudomonas agronomica sp. nov. and Bacillus pretiosus sp. nov. added over a valorized agricultural biowaste. Front. Microbiol. 2023, 13, 1046201. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Ministerio para la Transición Ecológica y el Reto Demográfico. Lodos de Depuración de Aguas Residuales. Available online: https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/temas/prevencion-y-gestion-residuos (accessed on 26 June 2025).
- Food and Agriculture Organization of the United Nations (FAO). The One Health in Action Approach: Guidelines for National Implementation [Internet]; FAO: Rome, Italy, 2023; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/ecb51a59-ac4d-407a-80de-c7d6c3e15fcc/content (accessed on 22 April 2025).
- Martínez, J.L.; Coque, T.M.; Baquero, F. What is a resistance gene? Ranking risk in resistomes. Nat. Rev. Microbiol. 2015, 13, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.E.; Gaudin, A.C.M. What is the agronomic potential of biofertilizers for maize? A meta-analysis. FEMS Microbiol. Ecol. 2018, 94, 7. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. One Health. 2023. Available online: https://www.who.int/es/news-room/fact-sheets/detail/one-health (accessed on 20 April 2025).
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; A van der Heijden, M.G. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Nerini, M.; Russo, A.; Decorosi, F.; Meriggi, N.; Viti, C.; Cavalieri, D.; Marvasi, M. A Microbial Phenomics Approach to Determine Metabolic Signatures to Enhance Seabream Sparus aurata Traceability, Differentiating between Wild-Caught and Farmed. Foods 2024, 13, 2726. [Google Scholar] [CrossRef]
- Laliberté, E.; Schweiger, A.K.; Legendre, P. Partitioning plant spectral diversity into alpha and beta components. Ecol. Lett. 2020, 23, 370–380. [Google Scholar] [CrossRef]
- Cordier, T.; Alonso-Sáez, L.; Apothéloz-Perret-Gentil, L.; Aylagas, E.; Bohan, D.A.; Bouchez, A.; Chariton, A.; Creer, S.; Frühe, L.; Keck, F.; et al. Ecosystems monitoring powered by environmental genomics: A review of current strategies with an implementation roadmap. Mol. Ecol. 2021, 30, 2937–2958. [Google Scholar] [CrossRef]
- Abadi, V.A.J.M.; Sepehri, M.; Rahmani, H.A.; Zarei, M.; Ronaghi, A.; Taghavi, S.M.; Shamshiripour, M. Role of Dominant Phyllosphere Bacteria with Plant Growth–Promoting Characteristics on Growth and Nutrition of Maize (Zea mays L.). J. Soil Sci. Plant Nutr. 2020, 20, 2348–2363. [Google Scholar] [CrossRef]
- Comeau, D.; Balthazar, C.; Novinscak, A.; Bouhamdani, N.; Joly, D.L.; Filion, M. Interactions Between Bacillus Spp., Pseudomonas Spp. and Cannabis sativa Promote Plant Growth. Front. Microbiol. 2021, 12, 715758. [Google Scholar] [CrossRef]
- Ferreira, E.T.; Barrochelo, S.C.; de Melo Sde, P.; Araujo, T.; Xavier, A.C.C.; Cechin, I.; da Silva, G.H.R.; Ali, S. Biofertilizers from wastewater treatment as a potential source of mineral nutrients for growth of amaranth plants. PLoS ONE 2023, 18, e0295624. [Google Scholar] [CrossRef]
- Sumathy, V.J.H. Biofertilizer production from agro-wastes. World J. Pharm. Res. 2017, 6, 1112–1124. [Google Scholar] [CrossRef]
- Ferreira, E.T.; Caetano, L.E.S.; Candido, J.M.B.; Cechin, I.; da Silva, G.H.R. Enhancing Plant Growth and Photosynthesis with Biofertilizers from Sewage Treatment. Agronomy 2025, 15, 610. [Google Scholar] [CrossRef]
- Nazarov, A.; Chetverikov, S.; Chetverikova, D.; Tuktarova, I.; Ivanov, R.; Urazgildin, R.; Garankov, I.; Kudoyarova, G. Microbial Preparations Combined with Humic Substances Improve the Quality of Tree Planting Material Needed for Reforestation to Increase Carbon Sequestration. Sustainability 2023, 15, 7709. [Google Scholar] [CrossRef]
- Garcia-Villaraco, A.; Ramos Solano, B.; Gutierrez-Mañero, F.J.; Lucas, J.A. Deciphering the Structural and Functional Diversity of Rhizobacteria from Stone Pine Inoculated with Plant Growth Promoting Rhizobacteria (PGPR) before and after Transplanted into Degraded Agricultural Soil. Soil Syst. 2024, 8, 39. [Google Scholar] [CrossRef]
- Du, S.; Li, X.Q.; Feng, J.; Huang, Q.; Liu, Y.R. Soil core microbiota drive community resistance to mercury stress and maintain functional stability. Sci. Total Environ. 2023, 894, 165056. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Yuan, M.; Duan, W.; Xia, J.; Zhang, X.; Zhao, Y.; Wang, J. Effect of soil microbial community on ecosystem multifunctionality in an alpine grassland. CATENA 2025, 249, 108714. [Google Scholar] [CrossRef]
- Liswadiratanakul, S.; Yamamoto, K.; Matsutani, M.; Wattanadatsaree, V.; Kihara, S.; Shiwa, Y.; Shiwachi, H. Replacement of water yam (Dioscorea alata L.) indigenous root endophytes and rhizosphere bacterial communities via inoculation with a synthetic bacterial community of dominant nitrogen-fixing bacteria. Front. Microbiol. 2023, 6, 14. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Han, X.; Han, G.; Xi, J.; Liu, Y.; Zhang, Y.; Xue, Q.; Guo, Q.; Lai, H. Actinobacterial biofertilizer improves the yields of different plants and alters the assembly processes of rhizosphere microbial communities. Appl. Soil Ecol. 2022, 171, 104345. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Kulkova, I.; Jakubowska, Z.; Wróbel, B. Non-native PGPB consortium consisting of Pseudomonas sp. G31 and Azotobacter sp. PBC2 promoted winter wheat growth and slightly altered the native bacterial community. Sci. Rep. 2025, 15, 3248. [Google Scholar] [CrossRef] [PubMed]
- Sierra-García, I.N.; Ferreira, M.J.; Torres-Ballesteros, A.; Louvado, A.; Gomes, N.; Cunha, A. Brevibacterium EB3 inoculation enhances rhizobacterial community interactions leading to improved growth of Salicornia europaea. Appl. Soil Ecol. 2024, 196, 105306. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Veríssimo, A.C.S.; Pinto, D.C.G.A.; Sierra-Garcia, I.N.; Granada, C.E.; Cremades, J.; Silva, H.; Cunha, Â. Engineering the Rhizosphere Microbiome with Plant Growth Promoting Bacteria for Modulation of the Plant Metabolome. Plants 2024, 13, 2309. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Liao, C.; Tian, Q.; Liu, F. Nitrogen availability regulates deep soil priming effect by changing microbial metabolic efficiency in a subtropical forest. J. For. Res. 2021, 32, 713–723. [Google Scholar] [CrossRef]
- Lin, X.; Zheng, X.; Wen, Y.; Yu, C.; Li, D.; Zhang, H. Microbial-induced carbon dioxide (CO2) mineralization: Investigating the bio-mineralization chemistry process and the potential of storage in sandstone reservoir. Appl. Energy 2025, 377, 124268. [Google Scholar] [CrossRef]
- Heuer, H.; Schmitt, H.; Smalla, K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr. Opin. Microbiol. 2011, 14, 236–243. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hijri, M.; Sobeh, M. Antibiotic resistance in plant growth promoting bacteria: A comprehensive review and future perspectives to mitigate potential gene invasion risks. Front. Microbiol. 2022, 13, 999988. [Google Scholar] [CrossRef] [PubMed]
- González-Reguero, D.; Robas-Mora, M.; Fernández-Pastrana, V.M.; Probanza-Lobo, A.; Jiménez-Gómez, P.A. Reduced Antibiotic Resistance in the Rhizosphere of Lupinus albus in Mercury-Contaminated Soil Mediated by the Addition of PGPB. Biology 2023, 12, 801. [Google Scholar] [CrossRef]
- van Belkum, A.; Bachmann, T.T.; Lüdke, G.; Lisby, J.G.; Kahlmeter, G.; Mohess, A.; Becker, K.; Hays, J.P.; Woodford, N.; Mitsakakis, K.; et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2019, 17, 51–62. [Google Scholar] [CrossRef]
- Freeland, G.; Hettiarachchy, N.; Atungulu, G.G.; Apple, J.; Mukherjee, S. Strategies to Combat Antimicrobial Resistance from Farm to Table. Food Rev. Int. 2023, 39, 27–40. [Google Scholar] [CrossRef]
- Razmilic, V.; Nouioui, I.; Karlyshev, A.; Jawad, R.; Trujillo, M.E.; Igual, J.M.; Andrews, B.A.; Asenjo, J.A.; Carro, L.; Goodfellow, M. Micromonospora parastrephiae sp. nov. and Micromonospora tarensis sp. nov., isolated from the rhizosphere of a Parastrephia quadrangularis plant growing in the Salar de Tara region of the Central Andes in Chile. Int. J. Syst. Evol. Microbiol. 2023, 73, 12. [Google Scholar] [CrossRef]
- González-Reguero, D.; Robas-Mora, M.; Alonso, M.R.; Fernández-Pastrana, V.M.; Lobo, A.P.; Gómez, P.A.J. Induction of phytoextraction, phytoprotection and growth promotion activities in Lupinus albus under mercury abiotic stress conditions by Peribacillus frigoritolerans subsp., mercuritolerans subsp. nov. Ecotoxicol. Environ. Saf. 2024, 285, 117139. [Google Scholar] [CrossRef] [PubMed]
- Nasuelli, M.; Novello, G.; Gamalero, E.; Massa, N.; Gorrasi, S.; Sudiro, C.; Hochart, M.; Altissimo, A.; Vuolo, F.; Bona, E. PGPB and/or AM Fungi Consortia Affect Tomato Native Rhizosphere Microbiota. Microorganisms 2023, 11, 1891. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.C. Perspectives and Challenges of Microbial Application for Crop Improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner Plant Values: Diversity, Colonization and Benefits from Endophytic Bacteria. Front. Microbiol. 2017, 8, 19. [Google Scholar] [CrossRef]
- García-Villaraco Velasco, A.; Probanza, A.; Gutierrez Mañero, F.; Ramos, B.; Lucas García, J. Functional diversity of rhizosphere microorganisms from different genotypes of Arabidopsis thaliana. Community Ecol. 2009, 10, 111–119. [Google Scholar] [CrossRef]
- Garland, J.L. Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol. Biochem. 1996, 28, 213–221. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naìїve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer Nature: London, UK, 2002. [Google Scholar]



| WC0 | WC1 | WC2 | EDARC0 | EDARC1 | EDARC2 | EDARSTC0 | EDARSTC1 | EDARSTC2 | |
|---|---|---|---|---|---|---|---|---|---|
| Planted | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| Survival after 1 year | 21 | 18 | 21 | 22 | 22 | 24 | 23 | 19 | 13 |
| Mortality rate | 40% | 49% | 40% | 37% | 37% | 31% | 34% | 46% | 63% |
| Facet | Group A (See Facet) | Group B (See Facet) | A (Median) | B (Median) | Test Statistic | p-Value | q-Value |
|---|---|---|---|---|---|---|---|
| Bacteria | C1 | C2 | 7.77 | 7.19 | 7 | 0.4 | 0.80 |
| Bacteria | C1 | C0 | 7.77 | 7.57 | 7 | 0.4 | 0.80 |
| Bacteria | C2 | C0 | 7.19 | 7.57 | 3 | 0.7 | 0.84 |
| Fertilizer | EDAR | EDARST | 7.67 | 7.57 | 4 | 1.00 | 1.00 |
| Fertilizer | EDAR | Water | 7.67 | 6.95 | 6 | 0.70 | 0.84 |
| Fertilizer | EDARST | Water | 7.57 | 6.95 | 7 | 0.40 | 0.80 |
| Identification (WGS) | IAA (µg.mL) | ACCd (p/a) | Siderophores(p/a) |
|---|---|---|---|
| Bacillus pretiosus | 5.61 ± 0.03 | − | + |
| Pseudomonas agronomica | 5.85 ± 0.09 | + | + |
| Parameters | Methods | Results | Units | |
|---|---|---|---|---|
| Physicochemical characteristics | Conductivity at 20 °C | A-F-PE-0015 Electrometry | 1454 | µS/cm |
| Conductivity at 25 °C | A-F-PE-0015 Electrometry | 1612 | µS/cm | |
| Biochemical Oxygen Demand (BOD5) | A-F-PE-0002 Gauge method | 3200 | mgO2/L | |
| Chemical Oxygen Demand | A-F-PE-0003 Digestion—Colorimetry | 6720 | mgO2/L | |
| Chemical demand for decanted oxygen | A-F-PE-0003 Digestion—Colorimetry | 4220 | mg/L | |
| Nitrates | A-F-PE-0010 Digestion | <0.05 | mg/L | |
| Kjeldhal Nitrogen | A-F-PE-0007 Kjeldhal | 296.7 | mg/L | |
| pH | A-F-PE-0010 Electrometry | 6.5 | U.pH. | |
| Suspended solids | A-F-PE-0006 Gravimetry | 3228 | mg/L | |
| Toxicity | PIT-F/0012 Bioluminescence assay with Vibrio fisheri | 14 | U.T. | |
| Majority cations | Potassium | A-D-PE-0025-ICP-OES | 60.2 | mg/L |
| Anions | Nitrates | A-BV-PE-0001 HPLC—Conductivity | <2.5 | mg/L |
| Orthophosphates | Ca-R-PE-0011 Spectrometry | 74.32 | mg PO4/L | |
| Sulfates | A-BV-PE-0001 HPLC—Conductivity | 89.0 | mg/L | |
| Sulfites | A-F-PE-0040 Volumetry | 4.5 | mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Pastrana, V.M.; González-Reguero, D.; Robas-Mora, M.; Penalba-Iglesias, D.; Alonso-Torreiro, P.; Probanza, A.; Jiménez-Gómez, P.A. Biotechnological Test of Plant Growth-Promoting Bacteria Strains for Synthesis of Valorized Wastewater as Biofertilizer for Silvicultural Production of Holm Oak (Quercus ilex L.). Plants 2025, 14, 2654. https://doi.org/10.3390/plants14172654
Fernández-Pastrana VM, González-Reguero D, Robas-Mora M, Penalba-Iglesias D, Alonso-Torreiro P, Probanza A, Jiménez-Gómez PA. Biotechnological Test of Plant Growth-Promoting Bacteria Strains for Synthesis of Valorized Wastewater as Biofertilizer for Silvicultural Production of Holm Oak (Quercus ilex L.). Plants. 2025; 14(17):2654. https://doi.org/10.3390/plants14172654
Chicago/Turabian StyleFernández-Pastrana, Vanesa M., Daniel González-Reguero, Marina Robas-Mora, Diana Penalba-Iglesias, Pablo Alonso-Torreiro, Agustín Probanza, and Pedro A. Jiménez-Gómez. 2025. "Biotechnological Test of Plant Growth-Promoting Bacteria Strains for Synthesis of Valorized Wastewater as Biofertilizer for Silvicultural Production of Holm Oak (Quercus ilex L.)" Plants 14, no. 17: 2654. https://doi.org/10.3390/plants14172654
APA StyleFernández-Pastrana, V. M., González-Reguero, D., Robas-Mora, M., Penalba-Iglesias, D., Alonso-Torreiro, P., Probanza, A., & Jiménez-Gómez, P. A. (2025). Biotechnological Test of Plant Growth-Promoting Bacteria Strains for Synthesis of Valorized Wastewater as Biofertilizer for Silvicultural Production of Holm Oak (Quercus ilex L.). Plants, 14(17), 2654. https://doi.org/10.3390/plants14172654









