Exogenous Dopamine Alleviates Combined High Temperature and Drought Stress in Loquat [Eriobotrya japonica (Thunb.) Lindl.] Seedlings: Improvements in Photosynthetic Efficiency, Oxidative Damage and Osmotic Regulation
Abstract
1. Introduction
2. Results
2.1. Plant Phenotypes and Root Morphological Parameters of Loquat Seedlings
2.2. TWC and RWC of Loquat Seedlings
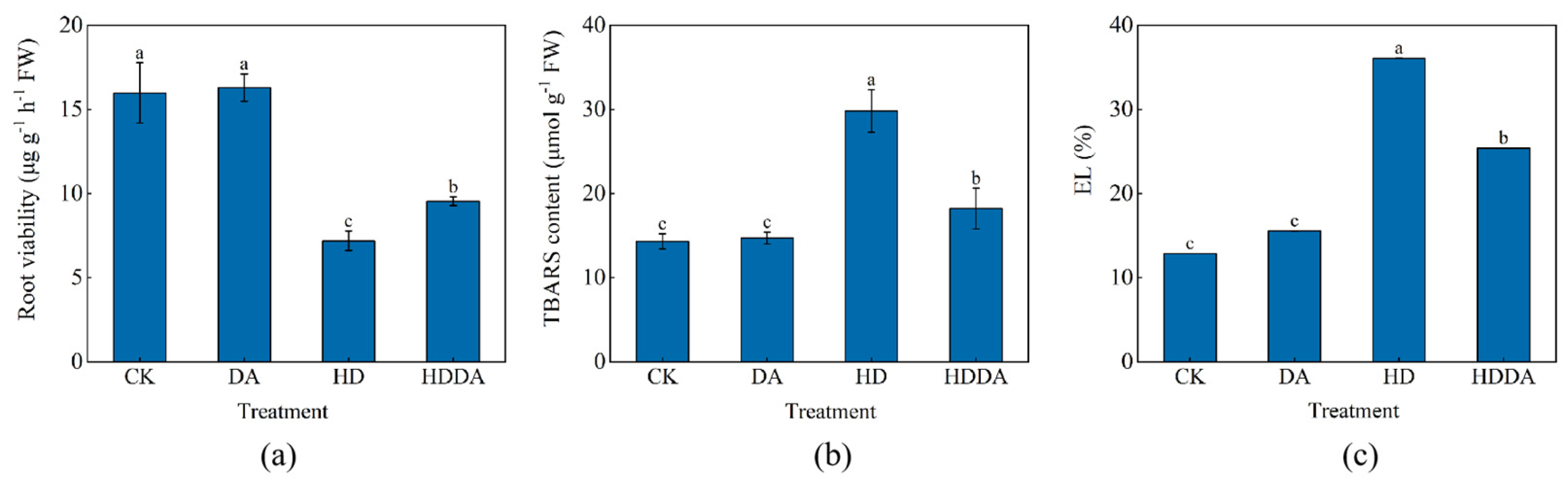
2.3. Root Viability, TBARS Content and EL of Loquat Seedlings
2.4. Photosynthetic Pigment Content and Photosynthetic Parameters of Loquat Seedlings
2.5. Antioxidant System of Loquat Seedlings
2.5.1. Superoxide Anion Production Rate and H2O2 Accumulation
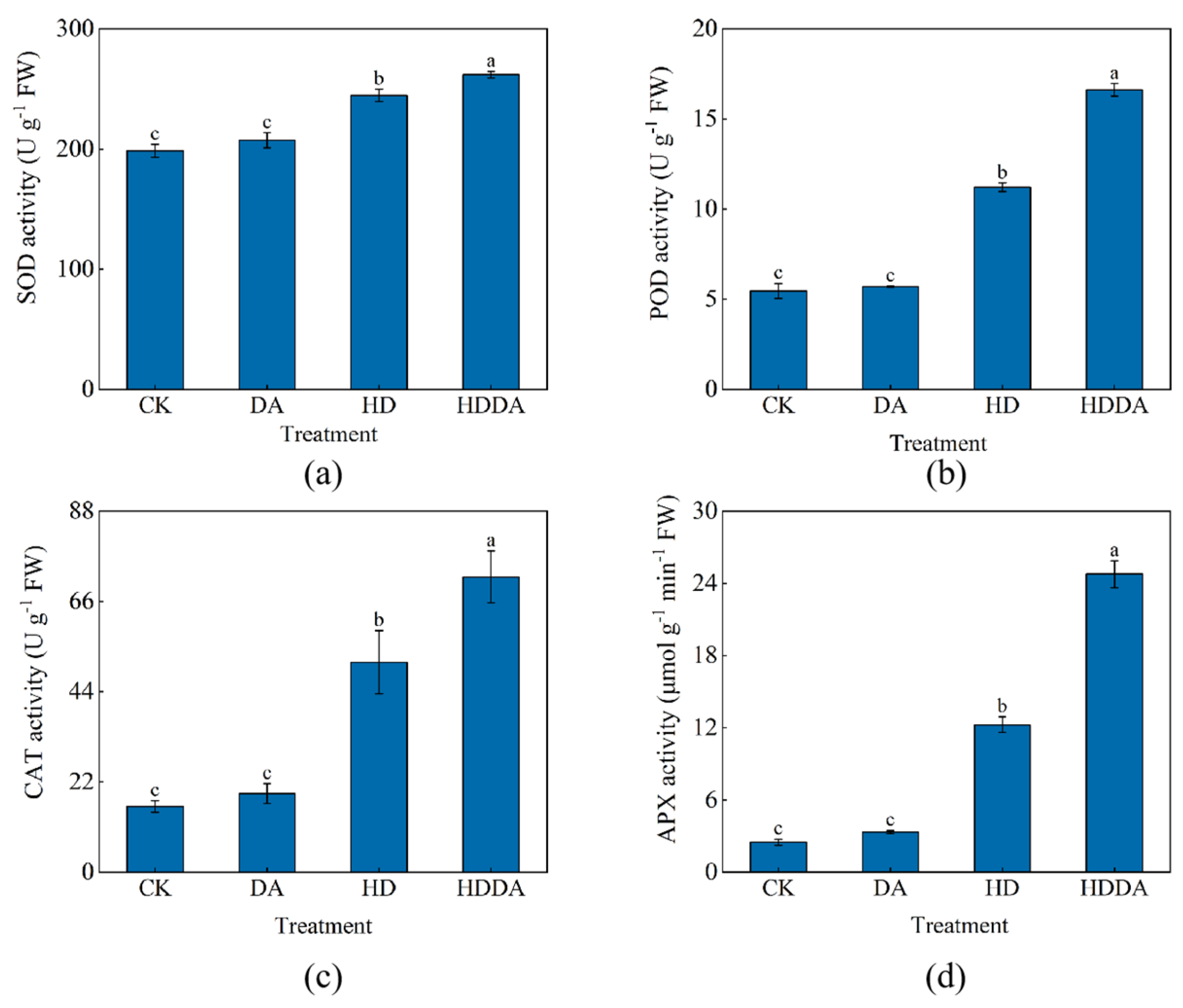
2.5.2. Antioxidant Enzyme Activities
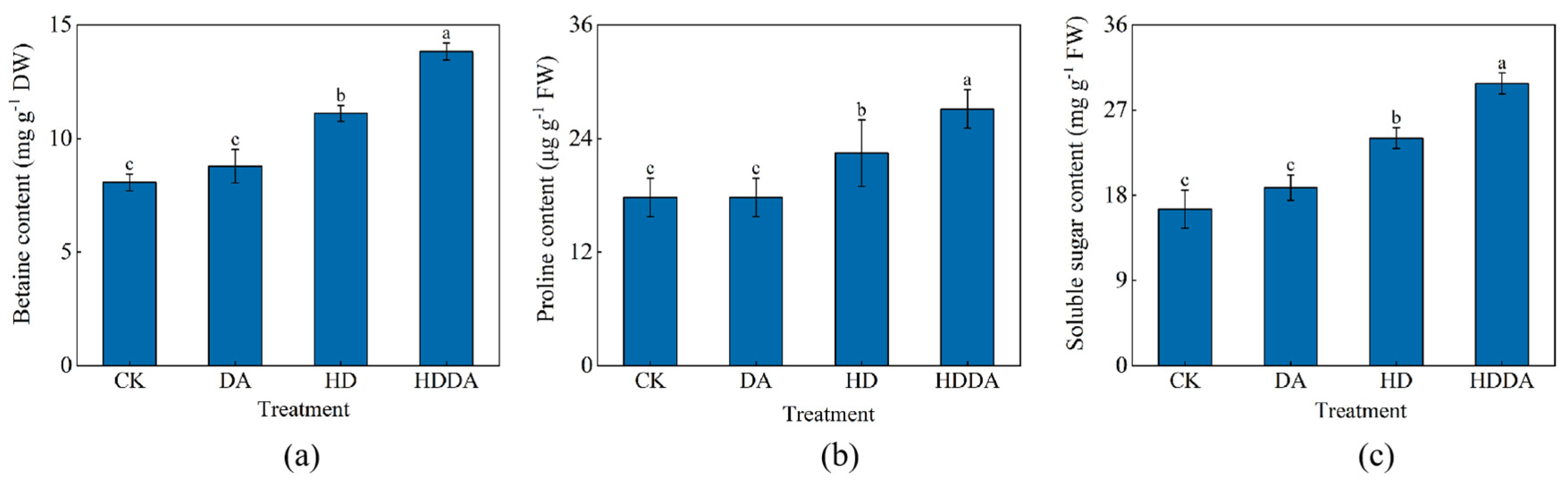
2.6. Osmotic Regulating Substance Accumulation of Loquat Seedlings
3. Discussion
3.1. Dopamine Alleviates Combined High Temperature and Drought Stress in Loquat Seedlings
3.2. Physiological Mechanism of Dopamine’s Alleviation of Combined High Temperature and Drought Stress in Loquat Seedlings
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.3. Determination of Morphological Parameters
4.4. Determination of Tissue Water Content and Relative Water Content
4.5. Determination of Photosynthetic Parameters, Photosynthetic Pigment Content, and Root Vitality
extraction volume/(fresh weight × incubation time)
4.6. Evaluation of Membrane Damage
4.7. Determination of ROS
endogenous nitrite control) × extraction volume/(fresh weight × hydroxylamine incubation time)
4.8. Determination of Antioxidant Enzyme Activities
volume]/[0.5 × absorbance of light-exposed control × fresh weight × volume of enzyme extract in reaction]
enzyme extract in reaction)
reaction time × volume of enzyme extract in reaction)
cuvette path length × reaction time × volume of enzyme extract in reaction)
4.9. Analysis of Osmotic Regulating Substances
volume of toluene used for extraction)/fresh weight
(volume of extract assayed × fresh weight)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DA | Dopamine |
| ROS | Reactive oxygen species |
| TBARS | Thiobarbituric acid-reactive substances |
| EL | Electrolyte leakage |
| RH | Relative humidity |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| APX | Ascorbate peroxidase |
| HD | High temperature and drought |
| HDDA | Dopamine was applied under high temperature and drought stress |
| Pn | Net photosynthetic rate |
| Tr | Transpiration rate |
| Gs | Stomatal conductance |
| Ci | Intercellular CO2 concentration |
| Pro | Proline |
| SS | Soluble sugar |
| SPS | Sucrose phosphate synthase |
| MDH | Malate dehydrogenase |
| RWC | Relative water content |
| PAR | Photosynthetically active radiation |
References
- Yang, M.X.; Mou, Y.L.; Meng, Y.R.; Liu, S.; Peng, C.H.; Zhou, X.L. Modeling the effects of precipitation and temperature patterns on agricultural drought in China from 1949 to 2015. Sci. Total Environ. 2020, 711, 135139. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Signal transduction networks during stress combination. J. Exp. Bot. 2020, 71, 1734–1741. [Google Scholar] [CrossRef]
- Habti, E.A.; Fleury, D.; Jewell, N.; Garnett, T.; Tricker, P.J. Tolerance of combined drought and heat stress is associated with transpiration maintenance and water soluble carbohydrates in wheat grains. Front. Plant Sci. 2020, 11, 568693. [Google Scholar] [CrossRef]
- Ostmeyer, T.; Parker, N.; Jaenisch, B.; Alkotami, L.; Bustamante, C.; Jagadish, S.V.K. Impacts of heat, drought, and their interaction with nutrients on physiology, grain yield, and quality in field crops. Plant Physiol. Rep. 2020, 25, 549–568. [Google Scholar] [CrossRef]
- China Meteorological Administration Climate Change Centre. Blue Book on Climate Change in China (2023); Science Press: Beijing, China, 2023; pp. 33–40. (In Chinese)
- Pawłowska, A.M.; Żurek, N.; Kapusta, I.; Leo, M.D.; Braca, A. Antioxidant and antiproliferative activities of phenolic extracts of Eriobotrya japonica (Thunb.) Lindl. fruits and leaves. Plants 2023, 12, 3221. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Zhang, Y.; Qie, H.; Wang, H. Identification of two new S-RNases and molecular S -genotyping of twenty loquat cutivars [Eriobotrya japonica (Thunb.) Lindl.]. Sci. Hortic. 2017, 218, 48–55. [Google Scholar] [CrossRef]
- Wang, P. Practical Techniques for High-Quality and Efficient Cultivation of Loquat; China Agriculture Press: Beijing, China, 2008; pp. 26–30. (In Chinese) [Google Scholar]
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wang, S.; Jing, D.; Liang, G. Physiological and transcription analyses reveal the regulatory mechanism of melatonin in inducing drought resistance in loquat (Eriobotrya japonica Lindl.) seedlings. Environ. Exp. Bot. 2021, 181, 104291. [Google Scholar] [CrossRef]
- Ji, W.; Luo, H.; Song, Y.; Hong, E.; Li, Z.; Lin, B.; Fan, C.; Wang, H.; Song, X.; Jin, S.; et al. Changes in Photosynthetic Characteristics of Paeonia suffruticosa under High Temperature Stress. Agronomy 2022, 12, 1203. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Song, T. Heat and drought priming induce tolerance to subsequent heat and drought stress by regulating leaf photosynthesis, root morphology, and antioxidant defense in maize seedlings. Environ. Exp. Bot. 2022, 202, 105010. [Google Scholar] [CrossRef]
- Hlahla, J.M.; Mafa, M.S.; Merwe, R.V.D.; Moloi, M.J. Tolerance to combined drought and heat stress in edamame is associated with enhanced antioxidative responses and cell wall modifications. Physiol. Plant. 2025, 177, e70187. [Google Scholar] [CrossRef]
- Berto, S.D.D.C.; Bezerra, F.S.; Morais, J.E.F.D.; Leal, L.Y.D.C.; Paulino, M.K.S.S.; Araújo, C.A.F.; Gonçalves, W.J.F.; Nascimento, C.W.A.D.; Souza, E.R.D. Silicon mitigates the effects of water deficit in ratoon sugarcane and enhances leaf water potential, osmotic adjustment, and biomass production. J. Plant Growth Regul. 2025, in press. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.; He, Y.; Yin, X.; Jiang, A. Effect of short-time high-temperature treatment on the photosynthetic performance of different heat-tolerant grapevine cultivars. Photochem. Photobiol. 2021, 97, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, H.; Yan, L.P.; Gao, L.; Zhai, S.; Xu, Y. Physiological, photosynthetic and stomatal ultrastructural responses of Quercus acutissima seedlings to drought stress and rewatering. Forests 2024, 15, 71. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Zhu, L.; Zhang, P.; Qi, H.; Zhang, K.; Sun, H.; Zhang, Y.; Lei, X.; Li, A.; et al. Leaf hydraulic decline coordinates stomatal and photosynthetic limitations through anatomical adjustments under drought stress in cotton. Front. Plant Sci. 2025, 16, 1622308. [Google Scholar] [CrossRef] [PubMed]
- Gugliuzza, G.; Talluto, G.; Martinelli, F.; Farina, V.; Bianco, R.L. Water deficit affects the growth and leaf metabolite composition of young loquat plants. Plants 2020, 9, 274. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, C.; Xu, Q.; Chen, X.; Jiang, F.; Zhang, Y.; Hu, W.; Zheng, S.; Su, W.; Jiang, J. Integrated analysis of the metabolome, transcriptome and miRNome reveals crucial roles of auxin and heat shock proteins in the heat stress response of loquat fruit. Sci. Hortic. 2022, 294, 110764. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X. Dopamine-induced abiotic stress tolerance in horticultural plants. Sci. Hortic. 2023, 307, 111506. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Zhang, X.; Liu, C.; Liang, W.; Li, C.; Ma, F.; Li, C. Exogenous dopamine application promotes alkali tolerance of apple seedlings. Plants 2019, 8, 580. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Luo, X.; Luo, Y.; Lin, L.; Huang, K.; Zhang, H.; Deng, Q. Effects of exogenous dopamine on photosynthesis characteristics and antioxidant system of loquat seedlings under drought stress. J. Northwest AF Univ. (Nat. Sci. Ed.) 2025, 53, 109–116. (In Chinese) [Google Scholar]
- Gao, T.; Zhang, Z.; Liu, X.; Wu, Q.; Chen, Q.; Liu, Q.; Nocker, S.; Ma, F.; Li, C. Physiological and transcriptome analyses of the effects of exogenous dopamine on drought tolerance in apple. Plant Physiol. Bioch. 2020, 148, 260–272. [Google Scholar] [CrossRef]
- Raza, A.; Salehi, H.; Rahman, M.A.; Zahid, Z.; Haghjou, M.M.; Najafi-Kakavand, S.; Charagh, S.; Osman, H.S.; Albaqami, M.; Zhuang, Y.; et al. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front. Plant Sci. 2022, 13, 961872. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Shang, Y.K.; Liu, S.Y.; Jiang, Y.Y.; Wang, L.; Liao, J.Q.; Yang, R.W.; Zhang, L. Effects on antioxidant enzymes, lipid peroxidation and photosynthetic of burdock (Arctium lappa L.) under water stress. Biol. Bull. 2022, 49, 114–123. [Google Scholar] [CrossRef]
- Liu, H.; Bao, G.; Dou, Z.; Liu, H.; Bai, J.; Chen, Y.; Yuan, Y.; Zhang, X.; Xi, J. Response characteristics of highland barley under freeze-thaw, drought and artemisinin stresses. BMC Plant Biol. 2022, 22, 126. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Wang, X.; Shi, M.; Zhang, R.; Wang, Y.; Zhang, W.; Qin, S.; Kang, Y. Dynamics of physiological and biochemical effects of heat, drought and combined stress on potato seedlings. Chem. Biol. Technol. Agric. 2024, 11, 109. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, Z.; Jing, G.; Gao, S.; Liu, C.; Ai, S.; Liu, Y.; Liu, Q.; Li, C.; Ma, F. Dopamine confers cadmium tolerance in apples by improving growth, reducing reactive oxygen species, and changing secondary metabolite levels. Environ. Exp. Bot. 2023, 208, 105264. [Google Scholar] [CrossRef]
- Parkash, V.; Snider, J.L.; Awori, K.J.; Pilon, C.; Brown, N.; Almeida, I.B.; Tishchenko, V. Peanut (Arachis hypogaea L.) growth and photosynthetic response to high and low temperature extremes. Plant Physiol. Bioch. 2025, 220, 109479. [Google Scholar] [CrossRef] [PubMed]
- Fiutak, G.; Mohammadi, X.; Filipczak-Fiutak, M.; Jarzebski, M.; Sterczynska, M.; Pratap-Singh, A. Pre-harvest applications of pulsed light increases vitamin C, chlorophyll, carotenoids and proteins in alfalfa sprouts. Sci. Hortic. 2024, 332, 113200. [Google Scholar] [CrossRef]
- Chen, J.H.; Tang, M.T.; Jin, X.Q.; Li, H.; Chen, L.S.; Wang, Q.L.; Sun, A.Z.; Yi, Y. Regulation of Calvin–Benson cycle enzymes under high temperature stress. aBIOTECH 2022, 3, 65–77. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, T.; Chang, A.; Fan, Z.; Li, X.; Li, C.; Zheng, M.; Sun, Y.; Wan, X.; Meng, J.; et al. Miscanthus sinensis ‘Gracillimus’ shows strong submergence tolerance implying its potential utilization in construction of ecological ditches. Agronomy 2025, 15, 109. [Google Scholar] [CrossRef]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophys. 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Ma, C.; Feng, Y.; Wang, J.; Zheng, B.; Wang, X.; Jiao, N. Integrative physiological, transcriptome, and proteome analyses provide insights into the photosynthetic changes in maize in a maize-peanut intercropping system. Plants 2024, 13, 65. [Google Scholar] [CrossRef]
- Lu, Z.; Ren, T.; Li, Y.; Cakmak, I.; Lu, J. Nutrient limitations on photosynthesis: From individual to combinational stresses. Trends Plant Sci. 2025, 25, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Gao, T.T.; Zhang, Z.J.; Tan, K.X.; Jin, Y.B.; Zhao, Y.J.; Ma, F.W.; Li, C. The mitigation effects of exogenous dopamine on low nitrogen stress in Malus hupehensis. J. Integr. Agric. 2020, 19, 2709–2724. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, Z.; Han, Y.; Sun, Y. Exogenous dopamine promotes photosynthesis and carbohydrate metabolism of downy mildew-infected cucumber. Sci. Hortic. 2022, 295, 110842. [Google Scholar] [CrossRef]
- Kanazawa, K.; Sakakibara, H. High content of dopamine, a strong antioxidant, in Cavendish banana. J. Agric. Food Chem. 2000, 48, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Du, P.; Zhang, J.; Ji, J.; Xu, J.; Liang, B. Dopamine alleviates cadmium stress in apple trees by recruiting beneficial microorganisms to enhance the physiological resilience revealed by high-throughput sequencing and soil metabolomics. Hortic. Res. 2023, 10, uhad112. [Google Scholar] [CrossRef]
- Khan, T.A.; Ahmad, A.; Saeed, T.; Yusuf, M.; Faisal, M.; Alatar, A.A. Investigating the influence of selenium and epibrassinolide on antioxidant activity, proline accumulation, and protein expression profiles in wheat plants experiencing heat and drought stress. Front. Plant Sci. 2024, 15, 1441483. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Tang, T.; Kong, W.; Su, Y.; Wang, Y.; Cheng, H.; Yang, Y.; Zhao, X. Response of watermelon to drought stress and its drought-resistance evaluation. Plants 2025, 14, 1289. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Y.; Liu, Y.; Ma, M.; Li, X.; Zhang, D.; Ding, K.; Li, C.; Zhou, Y.; Ma, F. Overexpression of tyrosine decarboxylase (MdTYDC) enhances drought tolerance in Malus domestica. Sci. Hortic. 2021, 289, 110425. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Cao, H.; Tian, X.; Liu, S.; Liu, C.; Zhang, Z.; Mao, K.; Ma, F.; Li, C. Dopamine regulates its own synthesis via MdORG2 to improve low-nitrogen tolerance in apple plants. Plant Cell Environ. 2025, 48, 470–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gong, S.; Fu, L.; Hu, G.; Li, G.; Hu, S.; Yang, J. The involvement of antioxidant enzyme system, nitrogen metabolism and osmoregulatory substances in alleviating salt stress in inbred maize lines and hormone regulation mechanisms. Plants 2022, 11, 1547. [Google Scholar] [CrossRef]
- Wen, Y.; Zhou, L.J.; Xu, Y.J.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Growth performance and osmolyte regulation of drought-stressed walnut plants are improved by mycorrhiza. Agriculture 2024, 14, 367. [Google Scholar] [CrossRef]
- Chen, R.; Hu, X.; Chen, D.; Wang, W.; Zhen, J. Photosynthetic, antioxidant activities, and osmoregulatory responses in winter wheat differ during the stress and recovery periods under heat, drought, and combined stress. Plant Sci. 2023, 327, 111557. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Zhang, H.; Luo, W.; Chen, A. Effects of drought stress on chloroplast ultrastructure and physiological characteristics of Seriphidiumtran siliense seedlings. Pratac. Sci. 2025, 42, 1403–1413. (In Chinese) [Google Scholar]
- Niu, X.; Deqing, C.; Liang, D. Effects of exogenous melatonin and abscisic acid on osmotic adjustment substances of ‘Summer Black’ grape under drought stress. IOP Conf. Ser. Earth Environ. Sci. 2019, 295, 012012. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, C.; Wei, B.; Zhang, J.; An, Y.; Wang, L. Exogenous 5-aminolevulinic acid promotes osmotic stress tolerance of walnuts by modulating photosynthesis, osmotic adjustment and antioxidant systems. Forests 2023, 14, 1789. [Google Scholar] [CrossRef]
- Dai, Z.; Yang, J.; Li, D.; Chen, Y.; Shao, Z.; Wang, Y. The impact of dopamine on the photosynthetic performance and quality of Shine Muscat grape in the Hexi Corridor of Gansu province. J. Fruit Sci. 2025, 42, 94–111. (In Chinese) [Google Scholar]
- Ju, Y.L.; Yue, X.F.; Zhao, X.F.; Zhao, H.; Fang, Y.L. Physiological, micro-morphological and metabolomic analysis of grapevine (Vitis vinifera L.) leaf of plants under water stress. Plant Physiol. Bioch. 2018, 130, 501–510. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Clemensson-Lindell, A. Triphenyltetrazolium chloride as an indicator of fine-root vitality and environmental stress in coniferous forest stands: Applications and limitations. Plant Soil 1994, 159, 297–300. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1986, 125, 189–198. [Google Scholar] [CrossRef]
- Jia, Y.; Yue, P.; Li, K.; Xie, Y.; Li, T.; Pu, Y.; Xu, X.; Wang, G.; Zhang, S.; Li, Y.; et al. Mechanisms of cadmium tolerance and detoxification in two ornamental plants. Agronomy 2023, 13, 2039. [Google Scholar] [CrossRef]
- Yuan, Y.X. The production rate and malondialdehyde content of different tissue superoxide anions in wetland Phrgmintes australis. Sci. Technol. Eng. 2019, 19, 40–43. (In Chinese) [Google Scholar]
- Liu, T.; Jiao, X.; Yang, S.; Zhang, Z.; Ye, X.; Li, J.; Qi, H.; Hu, X. Crosstalk between GABA and ALA to improve antioxidation and cell expansion of tomato seedling under cold stress. Environ. Exp. Bot. 2020, 180, 104228. [Google Scholar] [CrossRef]
- Tian, Z.M.; He, J.D.; Wang, Z.Y.; Zhang, Z.; Quinet, M.; Meng, Y. Exogenous melatonin enhances drought tolerance and germination in common buckwheat seeds through the coordinated effects of antioxidant and osmotic regulation. BMC Plant Biol. 2025, 25, 613. [Google Scholar] [CrossRef]
- Lee, M.R.; Kim, C.S.; Park, T.; Choi, Y.S.; Lee, K.H. Optimization of the ninhydrin reaction and development of a multiwell plate-based high-throughput proline detection assay. Anal. Biochem. 2018, 556, 57–62. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, S.; Ma, Y.; Liu, Z.; Tu, H.; Wang, H.; Zhang, J.; Chen, Q.; He, W.; Li, M.; et al. Soluble sugar and organic acid composition and flavor evaluation of Chinese cherry fruits. Food Chem. X 2023, 20, 100953. [Google Scholar] [CrossRef]


| Treatment | Length (cm) | Surface Area (cm2) | Volume (cm3) | Mean Diameter (mm) | Number of Tips |
|---|---|---|---|---|---|
| CK | 1257.432 ± 84.211 ab | 712.033 ± 24.115 a | 70.036 ± 5.004 a | 1.925 ± 0.157 a | 214.775 ± 14.467 a |
| DA | 1311.926 ± 55.772 a | 728.285 ± 23.368 a | 72.069 ± 4.806 a | 2.084 ± 0.104 a | 225.817 ± 12.445 a |
| HD | 801.002 ± 67.605 c | 500.624 ± 26.007 b | 41.992 ± 8.427 c | 1.346 ± 0.112 c | 140.582 ± 6.404 c |
| HDDA | 1164.553 ± 57.623 b | 544.464 ± 45.018 b | 54.955 ± 4.720 b | 1.647 ± 0.153 b | 163.231 ± 4.940 b |
| Treatment | TWC (%) | RWC (%) | ||
|---|---|---|---|---|
| Leaf | Root | Leaf | Root | |
| CK | 75.152 ± 1.193 a | 70.963 ± 3.795 a | 82.253 ± 3.613 a | 88.078 ± 2.668 a |
| DA | 79.910 ± 5.591 a | 73.195 ± 5.264 a | 84.482 ± 2.958 a | 86.825 ± 4.764 a |
| HD | 46.584 ± 4.701 c | 57.375 ± 3.073 c | 56.921 ± 6.430 c | 65.515 ± 2.173 c |
| HDDA | 61.558 ± 5.589 b | 65.320 ± 2.882 b | 67.440 ± 2.351 b | 77.424 ± 4.753 b |
| Treatment | Carotenoid Content (mg g−1 FW) | Chlorophyll Content (mg g−1 FW) | Pn (μmol m−2 s−1) | Tr (mmol m−2 s−1) | Gs (mol m−2 s−1) | Ci (μmol mol−1) |
|---|---|---|---|---|---|---|
| CK | 0.083 ± 0.009 b | 0.662 ± 0.014 b | 14.681 ± 0.847 a | 3.900 ± 0.390 a | 0.134 ± 0.013 a | 204.858 ± 8.882 c |
| DA | 0.081 ± 0.003 b | 0.704 ± 0.005 a | 15.766 ± 0.725 a | 4.201 ± 0.333 a | 0.159 ± 0.009 a | 213.073 ± 1.123 c |
| HD | 0.067 ± 0.004 c | 0.499 ± 0.021 d | 3.770 ± 0.234 c | 1.343 ± 0.110 c | 0.030 ± 0.011 c | 251.606 ± 8.018 a |
| HDDA | 0.099 ± 0.007 a | 0.612 ± 0.024 c | 8.353 ± 0.240 b | 3.147 ± 0.376 b | 0.081 ± 0.019 b | 232.442 ± 1.375 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Luo, Y.; Wang, X.-L.; Kong, X.-M.; Zhang, H.-F.; Lin, L.-J.; Li, Y.-X.; Huang, K.-W.; Deng, Q.-X.; Jia, Y.-X. Exogenous Dopamine Alleviates Combined High Temperature and Drought Stress in Loquat [Eriobotrya japonica (Thunb.) Lindl.] Seedlings: Improvements in Photosynthetic Efficiency, Oxidative Damage and Osmotic Regulation. Plants 2025, 14, 2650. https://doi.org/10.3390/plants14172650
Luo X, Luo Y, Wang X-L, Kong X-M, Zhang H-F, Lin L-J, Li Y-X, Huang K-W, Deng Q-X, Jia Y-X. Exogenous Dopamine Alleviates Combined High Temperature and Drought Stress in Loquat [Eriobotrya japonica (Thunb.) Lindl.] Seedlings: Improvements in Photosynthetic Efficiency, Oxidative Damage and Osmotic Regulation. Plants. 2025; 14(17):2650. https://doi.org/10.3390/plants14172650
Chicago/Turabian StyleLuo, Xian, Ya Luo, Xiao-Li Wang, Xiao-Mei Kong, Hui-Fen Zhang, Li-Jin Lin, Yu-Xing Li, Ke-Wen Huang, Qun-Xian Deng, and Yong-Xia Jia. 2025. "Exogenous Dopamine Alleviates Combined High Temperature and Drought Stress in Loquat [Eriobotrya japonica (Thunb.) Lindl.] Seedlings: Improvements in Photosynthetic Efficiency, Oxidative Damage and Osmotic Regulation" Plants 14, no. 17: 2650. https://doi.org/10.3390/plants14172650
APA StyleLuo, X., Luo, Y., Wang, X.-L., Kong, X.-M., Zhang, H.-F., Lin, L.-J., Li, Y.-X., Huang, K.-W., Deng, Q.-X., & Jia, Y.-X. (2025). Exogenous Dopamine Alleviates Combined High Temperature and Drought Stress in Loquat [Eriobotrya japonica (Thunb.) Lindl.] Seedlings: Improvements in Photosynthetic Efficiency, Oxidative Damage and Osmotic Regulation. Plants, 14(17), 2650. https://doi.org/10.3390/plants14172650





