Leaf Nutrient Resorption Efficiency Aligns with the Leaf but Not Root Economic Spectrum in a Tropical Mangrove Forest
Abstract
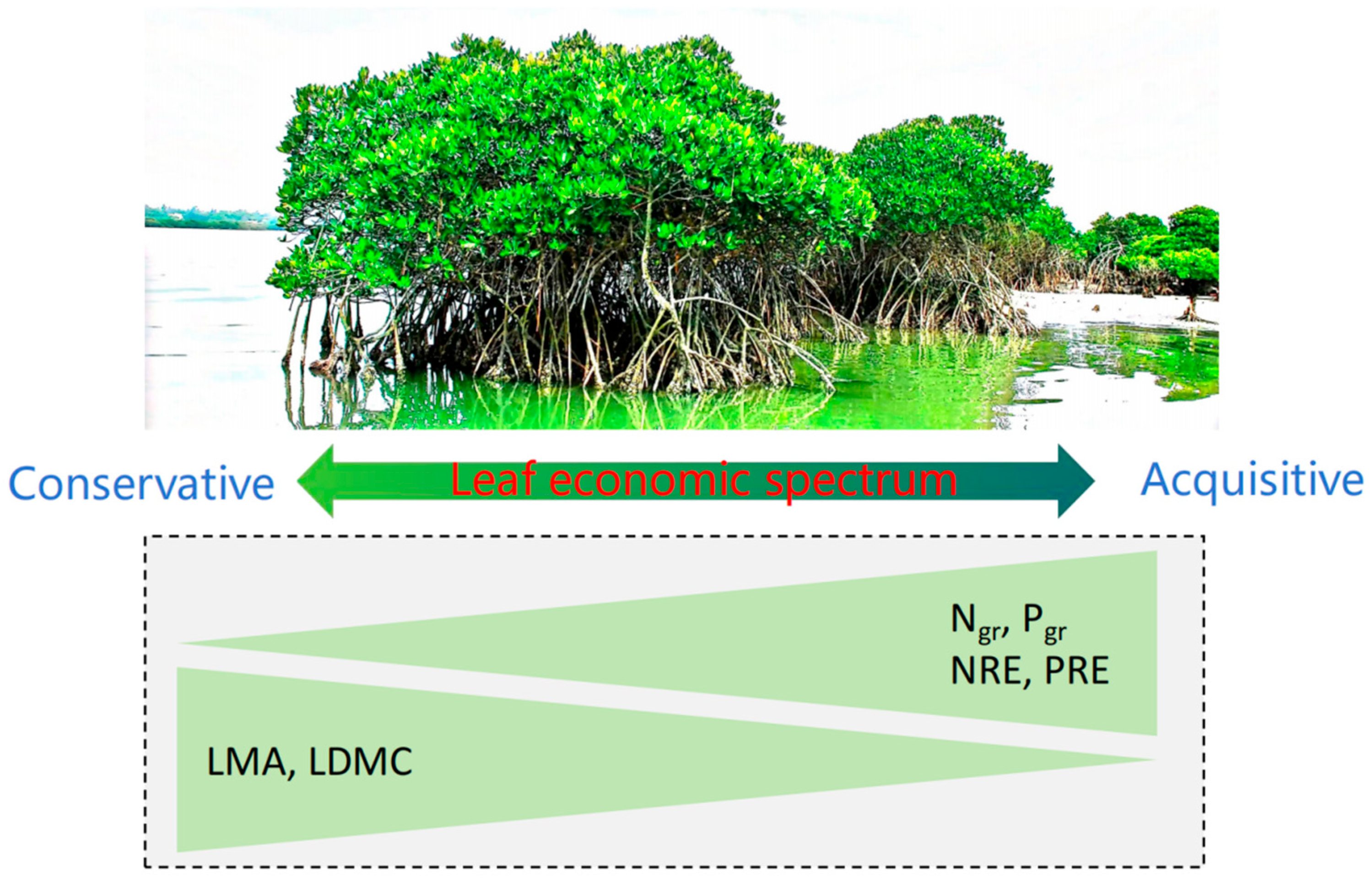
1. Introduction
- 1.
- Increased leaf nutrient contents (Ngr and Pgr) reduce NRE and PRE due to decreased selective pressure for internal nutrient conservation, as nutrient-rich species may rely more on soil nutrient uptake than on internal recycling.
- 2.
- Species with higher LMA and LDMC are expected to exhibit higher NRE and PRE values, given that these traits are associated with conservative resource-use strategies and an extended leaf lifespan, which promote efficient nutrient retention.
- 3.
- Species with higher SRL, Nroot, and RD are likely to have lower NRE and PRE, as these traits indicate an acquisitive strategy focused on nutrient acquisition from the soil rather than conservation through leaf resorption.
2. Materials and Methods
2.1. Study Site
2.2. Leaf and Fine Root Sampling
2.3. Leaf Structural Traits
2.4. Leaf Nutrient Contents and Resorption Efficiency
2.5. Root Economic Traits
2.6. Statistical Analyses
3. Results
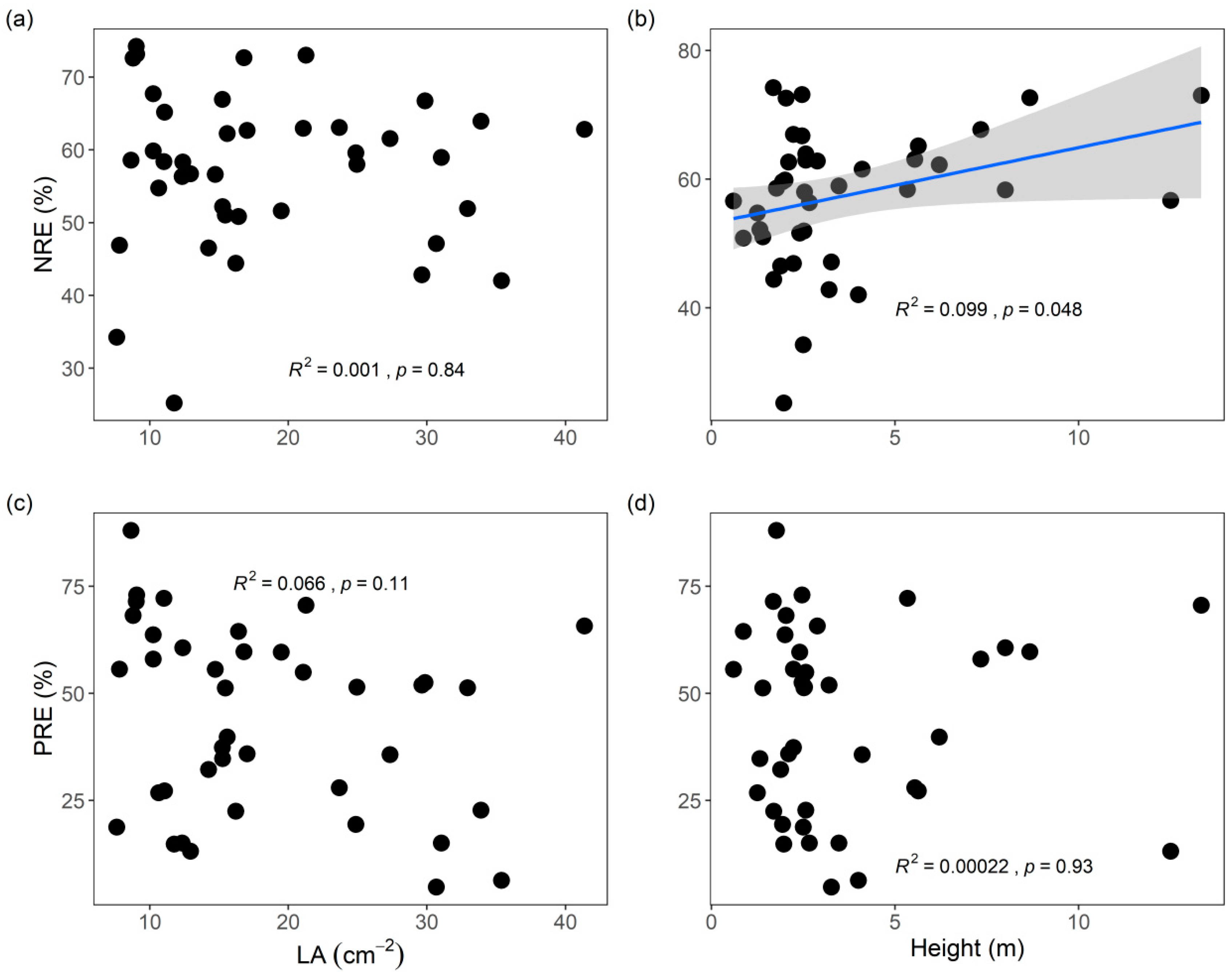
3.1. Trait Variation Across Mangrove Species
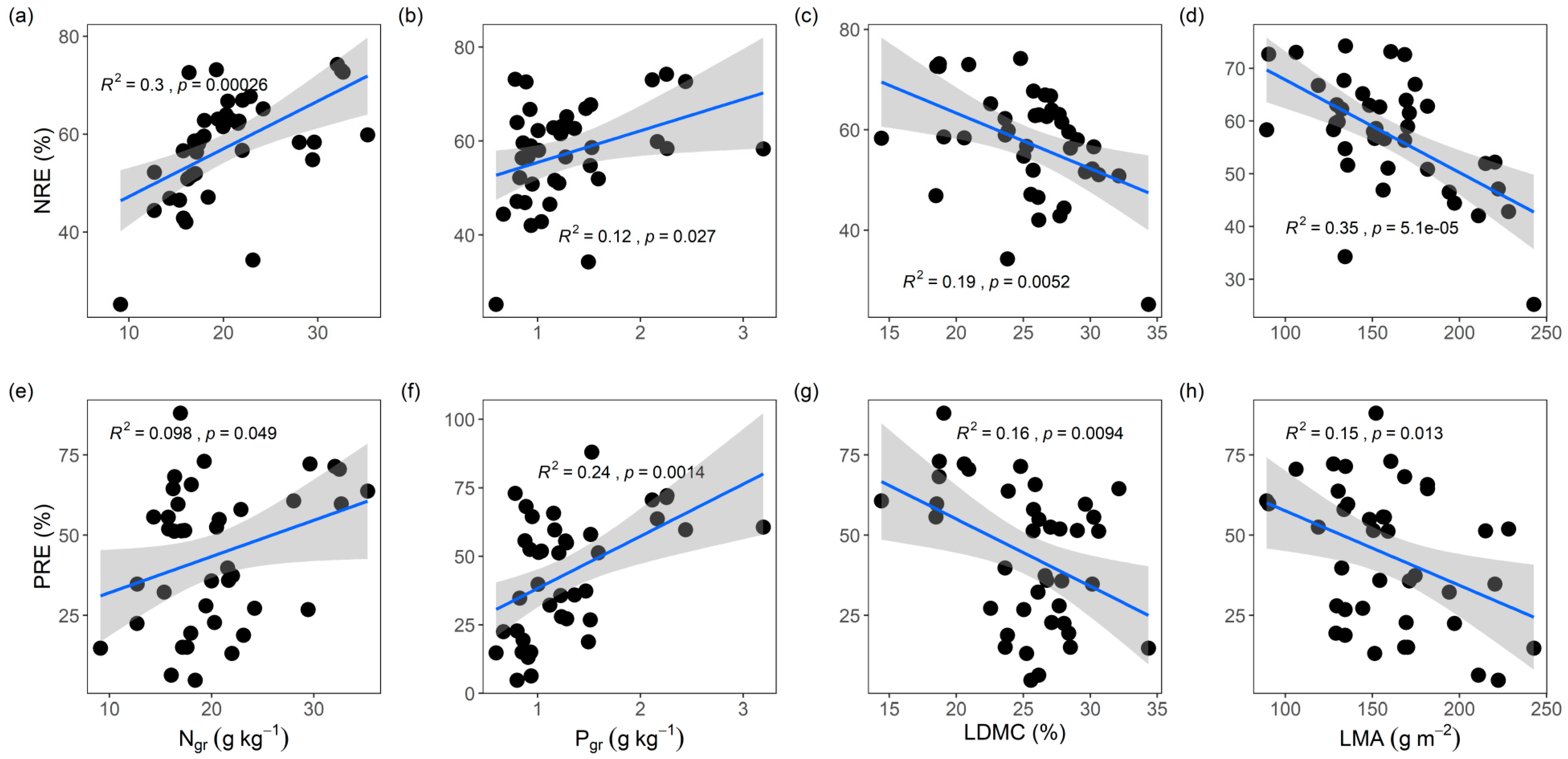
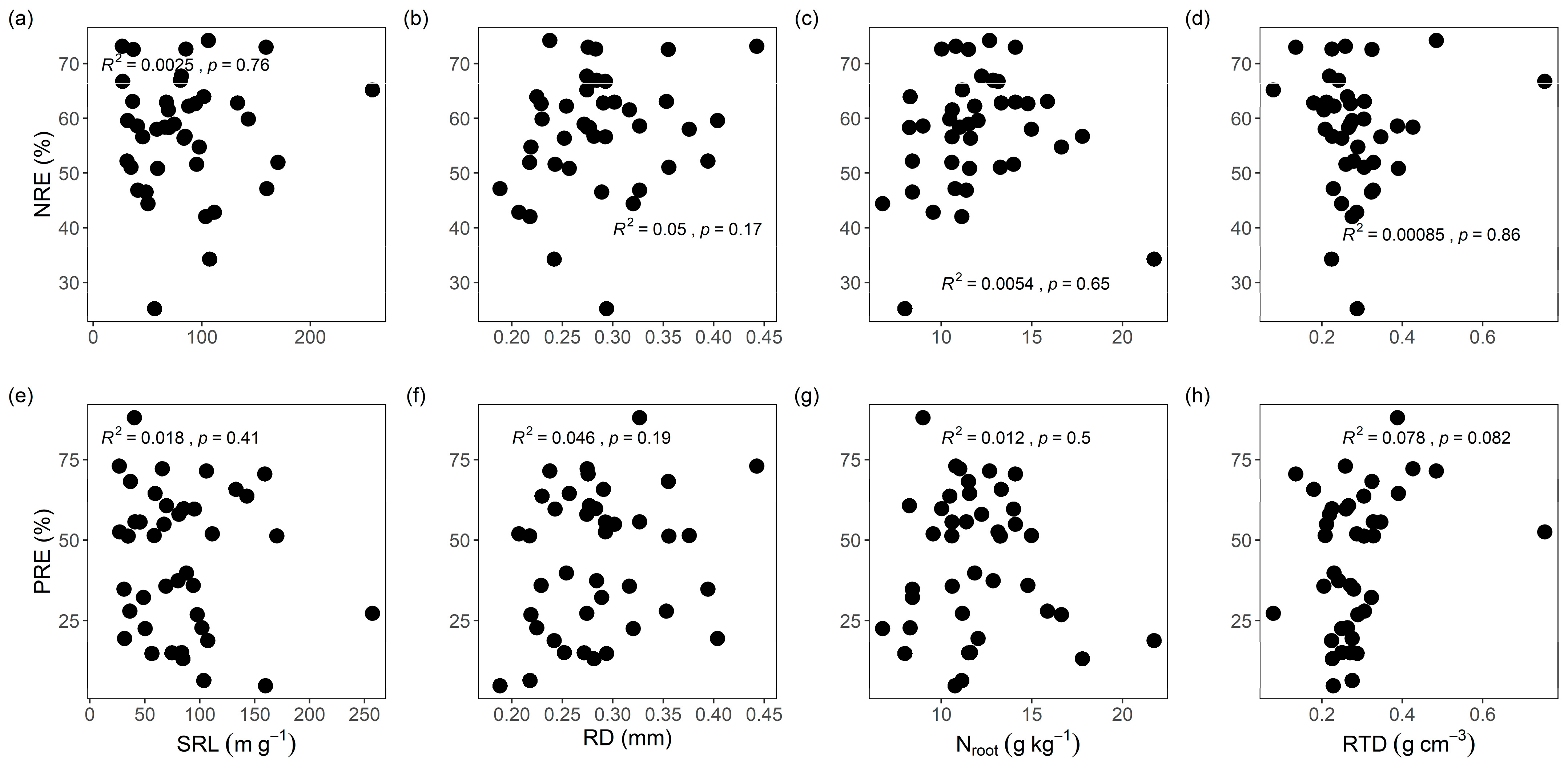
3.2. Leaf NuRE Links to Economic Traits and Resource-Use Strategies
4. Discussion
4.1. NRE and PRE Are Mediated by Leaf Economic Traits
4.2. Root Economic Traits Had No Effect on NRE and PRE
4.3. Limitations and Future Directions in Root Trait–Resorption Studies
4.4. Integrating Above- and Belowground Strategies for Sustainable Mangrove Management
- In nitrogen-deficient soils, Sonneratia caseolaris is recommended because of its high NRE (65.60%) and low LMA (103.33 g m−2). Its trait profile is especially advantageous in tidal areas where sediment nitrogen availability is naturally reduced.
- For phosphorus-limited sites, Lumnitzera racemosa is an optimal choice given its high PRE (71.24%), thick-rooted morphology (RD = 0.36 mm), and reliance on mycorrhizal symbiosis, which are traits that are well suited for nutrient acquisition in compacted sediments.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NRE | Nitrogen resorption efficiency |
| PRE | Phosphorus resorption efficiency |
| Ngr | Green leaf nitrogen content |
| Pgr | Green leaf phosphorus content |
| LMA | Leaf mass per area |
| LDMC | Leaf dry mass content |
| LA | Leaf area |
| Height | Plant height |
| SRL | Specific root length |
| RD | Root diameter |
| Nroot | Root nitrogen content |
| RTD | Root tissue density |
Appendix A
| Species | Growth Forms | NRE | PRE | Ngr | Pgr | LMA | LDMC | LA | Height | SRL | RD | Nroot | RTD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avicennia marina | Shrub | 55.79 ± 8.27 | 45.21 ± 13.12 | 29.96 ± 2.57 | 1.86 ± 0.41 | 133.37 ± 2.01 | 24.38 ± 0.63 | 9.38 ± 1.36 | 1.86 ± 0.26 | 113.43 ± 20.09 | 0.23 ± 0.01 | 15.38 ± 4.94 | 0.33 ± 0.11 |
| Kandelia obovata | Shrub | 62.24 ± 2.19 | 35.83 ± 8.15 | 20.39 ± 1.11 | 1.24 ± 0.27 | 161.38 ± 12.23 | 27.01 ± 1.03 | 16.41 ± 3.66 | 2.39 ± 0.13 | 81.31 ± 10.96 | 0.27 ± 0.03 | 13.34 ± 1.39 | 0.24 ± 0.02 |
| Aegiceras corniculatum | Shrub | 52.54 ± 1.37 | 57.77 ± 2.82 | 16.25 ± 0.20 | 1.15 ± 0.14 | 158.31 ± 18.76 | 30.66 ± 1.06 | 16.51 ± 2.10 | 1.32 ± 0.40 | 58.68 ± 26.29 | 0.29 ± 0.05 | 12.35 ± 1.55 | 0.33 ± 0.06 |
| Sonneratia caseolaris | Tree | 65.60 ± 4.18 | 65.81 ± 3.25 | 30.70 ± 1.13 | 2.50 ± 0.48 | 103.33 ± 17.92 | 18.62 ± 2.99 | 15.36 ± 4.64 | 8.83 ± 1.66 | 94.96 ± 43.58 | 0.28 ± 0.01 | 10.85 ± 2.45 | 0.26 ± 0.12 |
| Sonneratia apetala | Tree | 62.96 ± 2.37 | 34.56 ± 9.52 | 22.65 ± 0.58 | 1.18 ± 0.28 | 140.43 ± 9.07 | 24.30 ± 1.47 | 12.46 ± 2.37 | 7.92 ± 1.57 | 127.66 ± 86.40 | 0.27 ± 0.01 | 13.25 ± 3.05 | 0.19 ± 0.07 |
| Ceriops tagal | Shrub | 42.10 ± 5.86 | 26.09 ± 4.59 | 12.47 ± 1.28 | 0.80 ± 0.23 | 213.57 ± 22.69 | 29.66 ± 3.54 | 14.36 ± 1.91 | 1.72 ± 0.14 | 46.60 ± 10.86 | 0.32 ± 0.05 | 7.90 ± 0.78 | 0.28 ± 0.03 |
| Lumnitzera racemosa | Shrub | 62.82 ± 6.28 | 71.24 ± 6.69 | 16.72 ± 1.02 | 1.02 ± 0.34 | 159.30 ± 7.04 | 18.75 ± 0.24 | 8.57 ± 0.54 | 2.13 ± 0.15 | 36.23 ± 6.62 | 0.36 ± 0.05 | 10.67 ± 1.16 | 0.32 ± 0.05 |
| Rhizophora stylosa | Tree | 46.00 ± 2.28 | 28.62 ± 13.3 | 16.81 ± 0.59 | 1.09 ± 0.35 | 219.07 ± 7.66 | 26.30 ± 0.99 | 32.15 ± 2.56 | 3.25 ± 0.30 | 136.25 ± 33.58 | 0.21 ± 0.01 | 10.51 ± 0.68 | 0.28 ± 0.04 |
| Bruguiera sexangula | Tree | 61.87 ± 1.94 | 37.87 ± 8.35 | 18.80 ± 0.70 | 1.01 ± 0.16 | 132.00 ± 13.28 | 28.03 ± 0.87 | 25.84 ± 2.74 | 3.12 ± 0.82 | 38.32 ± 13.98 | 0.36 ± 0.05 | 14.00 ± 1.74 | 0.39 ± 0.25 |
| Bruguiera gymnorrhiza | Tree | 61.82 ± 1.07 | 34.84 ± 11.16 | 18.96 ± 0.68 | 1.03 ± 0.20 | 173.13 ± 5.75 | 26.13 ± 1.84 | 33.41 ± 5.95 | 3.25 ± 0.34 | 94.6 ± 29.28 | 0.28 ± 0.04 | 10.93 ± 2.09 | 0.23 ± 0.04 |
| K | P | λ | P | |
|---|---|---|---|---|
| NRE | 0.08 | 0.36 | <0.0001 | 1.00 |
| PRE | 0.10 | 0.44 | 0.25 | 0.48 |
| Ngr | 0.04 | 0.48 | 0.53 | 0.38 |
| Pgr | 0.02 | 0.64 | 0.58 | 0.20 |
| LMA | 0.30 | 0.16 | 0.09 | 0.81 |
| LDMC | 0.03 | 0.60 | 0.24 | 0.64 |
| LA | 0.44 | 0.05 | 0.37 | 0.35 |
| Height | 0.03 | 0.64 | 0.73 | 0.07 |
| SRL | 0.12 | 0.19 | <0.0001 | 1.00 |
| RD | 0.28 | 0.08 | <0.0001 | 1.00 |
| Nroot | 0.07 | 0.36 | <0.0001 | 1.00 |
| RTD | 0.01 | 0.76 | 0.20 | 0.67 |
Appendix B

References
- Killingbeck, K.T. Nutrients in senesced leaves: Keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Zhang, S.-B.; Song, Y.; Wen, H.-D.; Chen, Y.-J. Leaf nitrogen and phosphorus resorption efficiencies are related to drought resistance across woody species in a Chinese savanna. Tree Physiol. 2024, 44, tpad149. [Google Scholar] [CrossRef]
- Estiarte, M.; Campioli, M.; Mayol, M.; Penuelas, J. Variability and limits of nitrogen and phosphorus resorption during foliar senescence. Plant Commun. 2023, 4, 100503. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Ge, J.; Zhao, C.; Xu, W.; Xu, K.; Xie, Z. Plant economics spectrum governs leaf nitrogen and phosphorus resorption in subtropical transitional forests. BMC Plant Biol. 2024, 24, 764. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Houlton, B.Z.; Smith, W.K.; Marklein, A.R.; Reed, S.C.; Parton, W.; Del Grosso, S.J.; Running, S.W. Patterns of new versus recycled primary production in the terrestrial biosphere. Proc. Natl. Acad. Sci. USA 2013, 110, 12733–12737. [Google Scholar] [CrossRef]
- Hu, Y.K.; Schollert, M.; Aerts, R.; van Logtestijn, R.S.P.; Weedon, J.T.; Cornelissen, J.H.C. Leaf functional traits predict timing of nutrient resorption and carbon depletion in deciduous subarctic plants. J. Ecol. 2024, 112, 2123–2134. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Li, R.S.; Wang, Y.; Yuan, C.Y.; Zhang, W.D.; Wang, Q.K.; Guan, X.; Chen, L.C.; Wang, S.L.; Han, J.M.; Yang, Q.P. Leaf economics spectrum prevails over nutrient resorption in regulating the temperature sensitivity of litter decomposition in a subtropical forest ecosystem. Biol. Fertil. Soils 2023, 59, 901–910. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, H.; Li, S.; Dai, X.; Meng, S.; Fu, X.; Yan, H.; Zheng, J.; Ma, N.; Kou, L. A ‘Get-Save-Return’ process continuum runs on phosphorus economy among subtropical tree species. J. Ecol. 2023, 111, 861–874. [Google Scholar] [CrossRef]
- Brant, A.N.; Chen, H.Y.H. Patterns and mechanisms of nutrient resorption in plants. Crit. Rev. Plant Sci. 2015, 34, 471–486. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhang, S.B.; Chen, Y.J.; Zhang, Y.P.; Poorter, L.; Bonser, S. Nutrient resorption is associated with leaf vein density and growth performance of dipterocarp tree species. J. Ecol. 2015, 103, 541–549. [Google Scholar] [CrossRef]
- Li, Q.M.; Bai, W.M.; Guo, Y.M.; Sheng, J.; Yuan, Y.J.; Zhang, W.H.; Zhou, M. The response of two nutrient acquisition strategies: Root traits and leaf nutrient resorption and their relationships to long-term mowing in a temperate steppe. Plant Soil 2023, 491, 191–203. [Google Scholar] [CrossRef]
- Shi, X.F.; Zhang, L.; Liu, C.; Zhang, Y.M.; Wang, M.; Wang, W.Q. Variable leaf nitrogen-phosphorus ratios and stable resorption strategies of Kandelia obovata along the southeastern coast of China. For. Ecol. Manag. 2024, 560, 121822. [Google Scholar] [CrossRef]
- Wood, T.E.; Lawrence, D.; Wells, J.A. Inter-specific variation in foliar nutrients and resorption of nine canopy-tree species in a secondary neotropical rain forest. Biotropica 2011, 43, 544–551. [Google Scholar] [CrossRef]
- Yu, L.; Huang, Z.D.; Li, Z.J.; Korpelainen, H.; Li, C.Y. Sex-specific strategies of nutrient resorption associated with leaf economics in Populus euphratica. J. Ecol. 2022, 110, 2062–2073. [Google Scholar] [CrossRef]
- Zhu, D.; Peng, S.; Wang, J.; Hui, D. Responses of nutrient resorption to human disturbances in Phoebe bournei forests. Forests 2022, 13, 905. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Kobe, R.K.; Lepczyk, C.A.; Iyer, M. Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 2005, 86, 2780–2792. [Google Scholar] [CrossRef]
- Jiang, D.; Nie, T.; He, Q.; Yan, J.; Feng, E.; Ye, Q. A trade-off between leaf carbon economics and plant size among mangrove species in Dongzhaigang, China. Ecol. Evol. 2024, 14, e70559. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Yan, E. Leaf nutrient concentration, nutrient resorption and litter decomposition in an evergreen broad-leaved forest in eastern China. For. Ecol. Manag. 2007, 239, 150–158. [Google Scholar] [CrossRef]
- Achat, D.L.; Pousse, N.; Nicolas, M.; Augusto, L. Nutrient remobilization in tree foliage as affected by soil nutrients and leaf life span. Ecol. Monogr. 2018, 88, 408–428. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Liu, X.-W.; Zhang, H.; Fan, H.-Q.; Lin, G.-H. Nutrient conservation strategies of a mangrove species Rhizophora stylosa under nutrient limitation. Plant Soil 2010, 326, 469–479. [Google Scholar] [CrossRef]
- Mueller, K.E.; Kray, J.A.; Blumenthal, D.M. Coordination of leaf, root, and seed traits shows the importance of whole plant economics in two semiarid grasslands. New Phytol. 2024, 241, 2410–2422. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Weigelt, A.; van Der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.R.; Valverde-Barrantes, O.J.; Bruelheide, H.; Freschet, G.T.; Iversen, C.M.; et al. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 2020, 6, eaba3756. [Google Scholar] [CrossRef]
- Weemstra, M.; Kiorapostolou, N.; van Ruijven, J.; Mommer, L.; de Vries, J.; Sterck, F. The role of fine-root mass, specific root length and life span in tree performance: A whole-tree exploration. Funct. Ecol. 2020, 34, 575–585. [Google Scholar] [CrossRef]
- Weigelt, A.; Mommer, L.; Andraczek, K.; Iversen, C.M.; Bergmann, J.; Bruelheide, H.; Fan, Y.; Freschet, G.T.; Guerrero-Ramírez, N.R.; Kattge, J.; et al. An integrated framework of plant form and function: The belowground perspective. New Phytol. 2021, 232, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, S.O.; Atkins, D.H.; Mount, H.E.; Laughlin, D.C. Traits associated with the conservation gradient are the strongest predictors of early-stage fine root decomposition rates. J. Ecol. 2024, 112, 2828–2842. [Google Scholar] [CrossRef]
- Peng, H.Y.; Yan, Z.B.; Chen, Y.H.; Zhao, X.J.; Han, W.X. Effects of body size and root to shoot ratio on foliar nutrient resorption efficiency in Amaranthus mangostanus. Am. J. Bot. 2019, 106, 363–370. [Google Scholar] [CrossRef]
- Kammann, S.; Hortua, D.A.S.; Kominoski, J.S.; Fett, T.M.; Gillis, L.G. Understanding how nutrient limitation and plant traits influence carbon in mangrove-seagrass coastal ecosystems. Limnol. Oceanogr. 2022, 67, S89–S103. [Google Scholar] [CrossRef]
- Reef, R.; Feller, I.C.; Lovelock, C.E. Nutrition of mangroves. Tree Physiol. 2010, 30, 1148–1160. [Google Scholar] [CrossRef]
- Twomey, A.J.; Lovelock, C.E. Variation in mangrove geometric traits among genera and climate zones. Estuaries Coasts 2025, 48, 55. [Google Scholar] [CrossRef]
- Wei, L.; Lin, F.; Gao, J.; Rugema, J.; Akram, W.; Wang, Y.-s. Complexity of leaf trait covariation for mangrove species. npj Biodivers. 2025, 4, 6. [Google Scholar] [CrossRef]
- Li, W.; Guan, W.; Chen, H.; Liao, B.W.; Hu, J.; Peng, C.H.; Rui, J.P.; Tian, J.Q.; Zhu, D.; He, Y.X. Archaeal communities in the sediments of different mangrove stands at Dongzhaigang, China. J. Soils Sediments 2016, 16, 1995–2004. [Google Scholar] [CrossRef]
- Lu, C.; Li, L.; Wang, Z.; Su, Y.; Su, Y.; Huang, Y.; Jia, M.; Mao, D. The national nature reserves in China: Are they effective in conserving mangroves? Ecol. Indic. 2022, 142, 109265. [Google Scholar] [CrossRef]
- Jiang, C.; Pauly, D.; Wang, W.; Du, J.; Cheng, J.; Wang, M. A preliminary model of the mangrove ecosystem of Dongzhaigang Bay, Hainan, (China) based on Ecopath and Ecospace. Front. Mar. Sci. 2023, 10, 1277226. [Google Scholar] [CrossRef]
- Bai, J.K.; Meng, Y.C.; Gou, R.K.; Lyu, J.C.; Dai, Z.; Diao, X.P.; Zhang, H.S.; Luo, Y.Q.; Zhu, X.S.; Lin, G.H. Mangrove diversity enhances plant biomass production and carbon storage in Hainan island, China. Funct. Ecol. 2021, 35, 774–786. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, M.; Sun, Z.; Wang, W.; Chen, Q. Changes in the leaf functional traits of mangrove plant assemblages along an intertidal gradient in typical mangrove wetlands in Hainan, China. Glob. Ecol. Conserv. 2023, 48, e02749. [Google Scholar] [CrossRef]
- Ahmed, S.; Sarker, S.K.; Friess, D.A.; Kamruzzaman, M.; Jacobs, M.; Sillanpää, M.; Naabeh, C.S.S.; Pretzsch, H. Mangrove tree growth is size-dependent across a large-scale salinity gradient. For. Ecol. Manag. 2023, 537, 120954. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Shi, X.; Li, X.; Deng, Y.; Wang, M.; Wang, W. A re-evaluation of the tidal sorting hypothesis of mangrove zonation: Propagule specific gravity matters. Front. Mar. Sci. 2024, 11, 1368156. [Google Scholar] [CrossRef]
- Twomey, A.; Lovelock, C. Global spatial dataset of mangrove genus distribution in seaward and riverine margins. Sci. Data 2024, 11, 306. [Google Scholar] [CrossRef]
- Pierick, K.; Leuschner, C.; Homeier, J. Topography as a factor driving small-scale variation in tree fine root traits and root functional diversity in a species-rich tropical montane forest. New Phytol. 2021, 230, 129–138. [Google Scholar] [CrossRef]
- Sun, L.; Ataka, M.; Han, M.; Han, Y.; Gan, D.; Xu, T.; Guo, Y.; Zhu, B. Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol. 2021, 229, 259–271. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.-S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial bio-sphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Hou, J.; McCormack, M.L.; Reich, P.B.; Sun, T.; Phillips, R.P.; Lambers, H.; Chen, H.Y.H.; Ding, Y.; Comas, L.H.; Valverde-Barrantes, O.J.; et al. Linking fine root lifespan to root chemical and morphological traits—A global analysis. Proc. Natl. Acad. Sci. USA 2024, 121, e2320623121. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Ge, H.; Pan, S.; Li, P.; Guo, L.; Yang, L.; Peng, Z.; Wang, B.; Wang, Z.; et al. Plant traits mediate foliar uptake of deposited nitrogen by mature woody plants. Plant Cell Environ. 2024, 47, 4870–4885. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M. Inferring the historical patterns of biological evolution. Nature 1999, 401, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, S.P.; Garland, T.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef] [PubMed]
- Campanella, M.V.; Bertiller, M.B. Is N-resorption efficiency related to secondary compounds and leaf longevity in coexisting plant species of the arid Patagonian Monte, Argentina? Austral. Ecol. 2011, 36, 395–402. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Tamm, Ü. Species differences in timing of leaf fall and foliage chemistry modify nutrient resorption efficiency in deciduous temperate forest stands. Tree Physiol. 2005, 25, 1001–1014. [Google Scholar] [CrossRef]
- Chapin, F.S. The mineral-nutrition of wild plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B.; Vose, J.M.; Gresham, C.; Volin, J.C.; Bowman, W.D. Generality of leaf trait relationships: A test across six biomes. Ecology 1999, 80, 1955–1969. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 1992, 62, 365–392. [Google Scholar] [CrossRef]
- Wright, I.J.; Westoby, M. Nutrient concentration, resorption and lifespan leaf traits of Australian sclerophyll species. Funct. Ecol. 2003, 17, 10–19. [Google Scholar] [CrossRef]
- Campanella, M.V.; Bertiller, M.B. Plant phenology, leaf traits and leaf litterfall of contrasting life forms in the arid Patagonian Monte, Argentina. J. Veg. Sci. 2008, 19, 75–85. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, K.; Wang, S.; Yang, Y. Concentrations and resorption patterns of 13 nutrients in different plant functional types in the karst region of south-western China. Ann. Bot. 2014, 113, 873–885. [Google Scholar] [CrossRef]
- Wang, W.Q.; Wang, M.; Lin, P. Seasonal changes in element contents in mangrove element retranslocation during leaf senescene. Plant Soil 2003, 252, 187–193. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Bongers, F. Contrasting nitrogen and phosphorus resorption efficiencies in trees and lianas from a tropical montane rain forest in Xishuangbanna, south-west China. J. Trop. Ecol. 2007, 23, 115–118. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Wang, J.; Guo, Z.; Wang, G.G.; Zeng, D.; Wu, T. Tree stoichiometry and nutrient resorption along a chronosequence of Metasequoia glyptostroboides forests in coastal China. For. Ecol. Manag. 2018, 430, 445–450. [Google Scholar] [CrossRef]
- Jiang, D.; Li, Q.; Geng, Q.; Zhang, M.; Xu, C.; Hu, G.; Shen, C.; Ruan, H.; Xu, X. Nutrient resorption and stoichiometric re-sponses of poplar (Populus deltoids) plantations to N addition in a coastal region of eastern China. J. Plant Ecol. 2021, 14, 591–604. [Google Scholar] [CrossRef]
- Shah, J.A.; Liu, W.; Ullah, S.; Duan, H.; Shen, F.; Liao, Y.; Huang, G.; Wu, J. Linkages among leaf nutrient concentration, resorption efficiency, litter decomposition and their stoichiometry to canopy nitrogen addition and understory removal in subtropical plantation. Ecol. Process. 2024, 13, 27. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef]
- Deng, M.; Liu, L.; Sun, Z.; Piao, S.; Ma, Y.; Chen, Y.; Wang, J.; Qiao, C.; Wang, X.; Li, P. Increased phosphate uptake but not resorption alleviates phosphorus deficiency induced by nitrogen deposition in temperate Larix principis-rupprechtii plantations. New Phytol. 2016, 212, 1019–1029. [Google Scholar] [CrossRef]
- Ding, J.; Ge, W.; Liu, Q.; Wang, Q.; Kong, D.; Yin, H. Temperature drives the coordination between above-ground nutrient conservation and below-ground nutrient acquisition in alpine coniferous forests. Funct. Ecol. 2023, 37, 1674–1687. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Yang, F.; Dai, X.; Meng, S.; Hu, M.; Kou, L.; Fu, X. Relationships between root exudation and root morphological and architectural traits vary with growing season. Tree Physiol. 2024, 44, tpad118. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, J.P.; Cantarel, A.A.M.; Simon, L.; Haichar, F.e.Z. Root exudation rate as functional trait involved in plant nutrient-use strategy classification. Ecol. Evol. 2018, 8, 8573–8581. [Google Scholar] [CrossRef]
- Deng, M.; Hu, S.; Guo, L.; Jiang, L.; Huang, Y.; Schmid, B.; Liu, C.; Chang, P.; Li, S.; Liu, X.; et al. Tree mycorrhizal association types control biodiversity-productivity relationship in a subtropical forest. Sci. Adv. 2023, 9, eadd4468. [Google Scholar] [CrossRef] [PubMed]
- Carmona, C.P.; Bueno, C.G.; Toussaint, A.; Träger, S.; Díaz, S.; Moora, M.; Munson, A.D.; Pärtel, M.; Zobel, M.; Tamme, R. Fine-root traits in the global spectrum of plant form and function. Nature 2021, 597, 683–687. [Google Scholar] [CrossRef]
- Joswig, J.S.; Wirth, C.; Schuman, M.C.; Kattge, J.; Reu, B.; Wright, I.J.; Sippel, S.D.; Rüger, N.; Richter, R.; Schaepman, M.E.; et al. Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nat. Ecol. Evol. 2022, 6, 36–50. [Google Scholar] [CrossRef]
- Weber, M.J.; Brown, M.L. Effects of resource pulse magnitude on nutrient availability, productivity, stability, and food web dynamics in experimental aquatic ecosystems. Hydrobiologia 2018, 814, 191–203. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H.; Shen, Q.; Tang, X.; Xiong, C.; Li, H.; Pang, J.; Ryan, M.H.; Lambers, H.; Shen, J. Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol. 2019, 223, 882–895. [Google Scholar] [CrossRef]
- Lambers, H.; Albornoz, F.; Kotula, L.; Laliberté, E.; Ranathunge, K.; Teste, F.P.; Zemunik, G. How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant Soil 2018, 424, 11–33. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Tron, S.; Laio, F.; Ridolfi, L. Effect of water table fluctuations on phreatophytic root distribution. J. Theor. Biol. 2014, 360, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Bucher, S.F.; Römermann, C. The timing of leaf senescence relates to flowering phenology and functional traits in 17 herba-ceous species along elevational gradients. J. Ecol. 2021, 109, 1537–1548. [Google Scholar] [CrossRef]

| Variables | Abbreviation | Mean | Min | Max | CV (%) |
|---|---|---|---|---|---|
| Leaf nitrogen resorption efficiency (%) | NRE | 57.37 | 25.24 | 74.23 | 18.74 |
| Leaf phosphorus resorption efficiency (%) | PRE | 43.79 | 4.80 | 88.05 | 49.59 |
| Green leaf nitrogen content (g kg−1) | Ngr | 20.37 | 9.12 | 35.25 | 29.59 |
| Green leaf phosphorus content (g kg−1) | Pgr | 1.29 | 0.60 | 3.20 | 43.25 |
| Leaf mass per area (g m−2) | LMA | 159.39 | 89.26 | 242.71 | 22.84 |
| Leaf dry mass content (%) | LDMC | 25.38 | 14.42 | 34.34 | 16.58 |
| Leaf area (cm2) | LA | 18.44 | 7.62 | 41.36 | 48.84 |
| Plant height (m) | Height | 3.58 | 0.60 | 13.33 | 80.62 |
| Specific root length (m g−1) | SRL | 82.80 | 26.69 | 257.19 | 57.14 |
| Root diameter (mm) | RD | 0.29 | 0.19 | 0.44 | 20.01 |
| Root nitrogen content (g kg−1) | Nroot | 11.92 | 6.77 | 21.73 | 24.41 |
| Root tissue density (g cm−3) | RTD | 0.28 | 0.08 | 0.75 | 37.14 |
| NRE | PRE | Ngr | Pgr | LDMC | LMA | LA | |
|---|---|---|---|---|---|---|---|
| PRE | 0.48 ** | ||||||
| Ngr | 0.55 *** | 0.31 * | |||||
| Pgr | 0.35 * | 0.49 ** | 0.80 *** | ||||
| LDMC | −0.43 ** | −0.41 ** | −0.51 *** | −0.55 *** | |||
| LMA | −0.59 *** | −0.39 * | −0.72 *** | −0.63 *** | 0.51 *** | ||
| LA | −0.03 | −0.26 | −0.23 | −0.26 | 0.29 | 0.32 * | |
| Height | 0.32 * | 0.01 | −0.01 | 0.40 * | −0.42 ** | −0.44 ** | −0.01 |
| NRE | PRE | SRL | RD | Nroot | |
|---|---|---|---|---|---|
| PRE | 0.48 ** | ||||
| SRL | 0.05 | −0.13 | |||
| RD | 0.22 | 0.21 | −0.63 *** | ||
| Nroot | 0.07 | −0.11 | 0.10 | −0.09 | |
| RTD | 0.03 | 0.28 | −0.45 ** | −0.004 | −0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Nie, T.; He, Q.; Xu, Z.; Chen, H.Y.H.; Feng, E.; Peñuelas, J. Leaf Nutrient Resorption Efficiency Aligns with the Leaf but Not Root Economic Spectrum in a Tropical Mangrove Forest. Plants 2025, 14, 2610. https://doi.org/10.3390/plants14172610
Jiang D, Nie T, He Q, Xu Z, Chen HYH, Feng E, Peñuelas J. Leaf Nutrient Resorption Efficiency Aligns with the Leaf but Not Root Economic Spectrum in a Tropical Mangrove Forest. Plants. 2025; 14(17):2610. https://doi.org/10.3390/plants14172610
Chicago/Turabian StyleJiang, Dalong, Tao Nie, Qiuyu He, Zuo Xu, Han Y. H. Chen, Erhui Feng, and Josep Peñuelas. 2025. "Leaf Nutrient Resorption Efficiency Aligns with the Leaf but Not Root Economic Spectrum in a Tropical Mangrove Forest" Plants 14, no. 17: 2610. https://doi.org/10.3390/plants14172610
APA StyleJiang, D., Nie, T., He, Q., Xu, Z., Chen, H. Y. H., Feng, E., & Peñuelas, J. (2025). Leaf Nutrient Resorption Efficiency Aligns with the Leaf but Not Root Economic Spectrum in a Tropical Mangrove Forest. Plants, 14(17), 2610. https://doi.org/10.3390/plants14172610






