Edible Flowers as Bioactive Food Ingredients with Antidiabetic Potential: A Study on Paeonia officinalis L., Forsythia × intermedia, Gomphrena globosa L., and Clitoria ternatea L.
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Studies of the Extracts
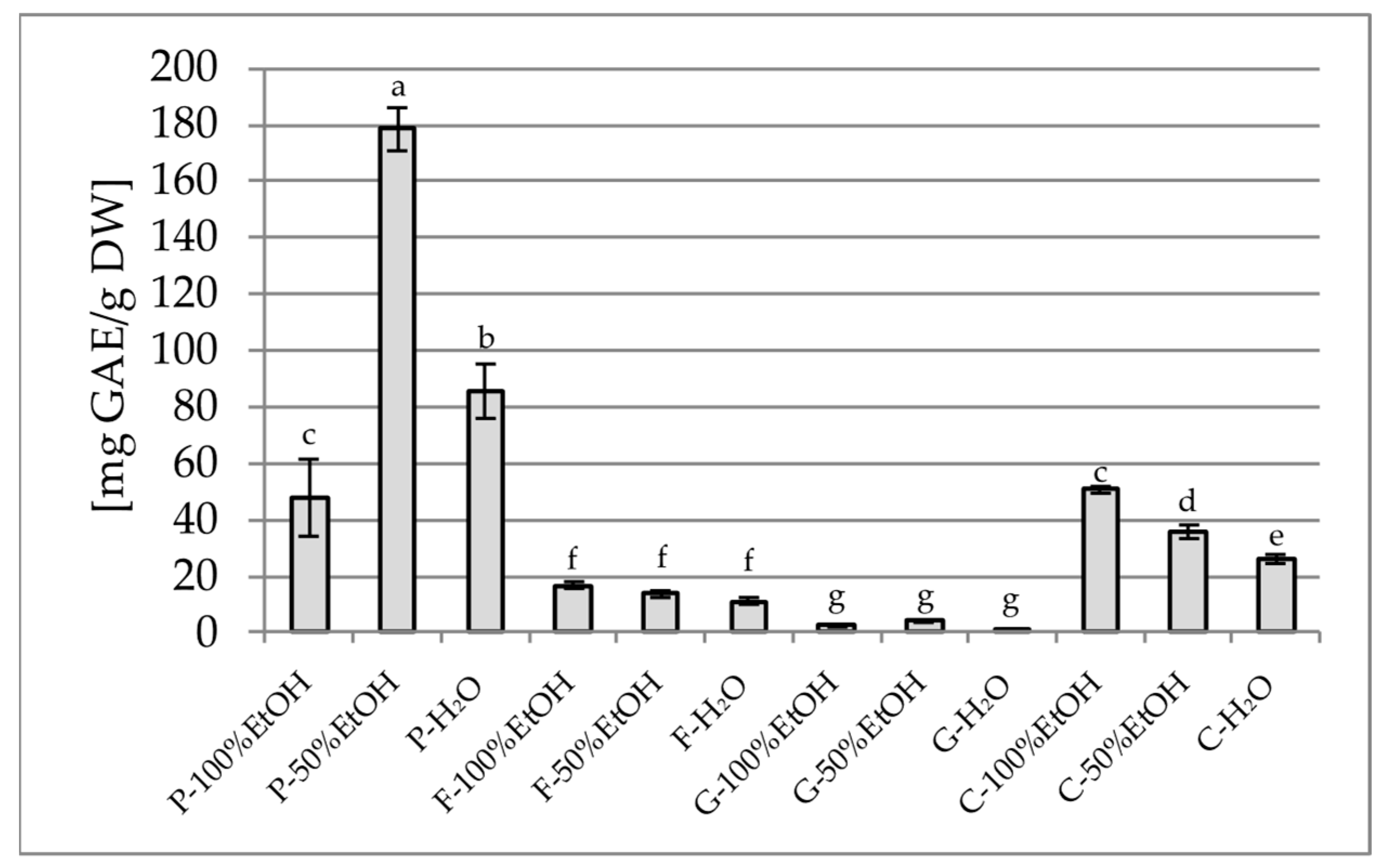
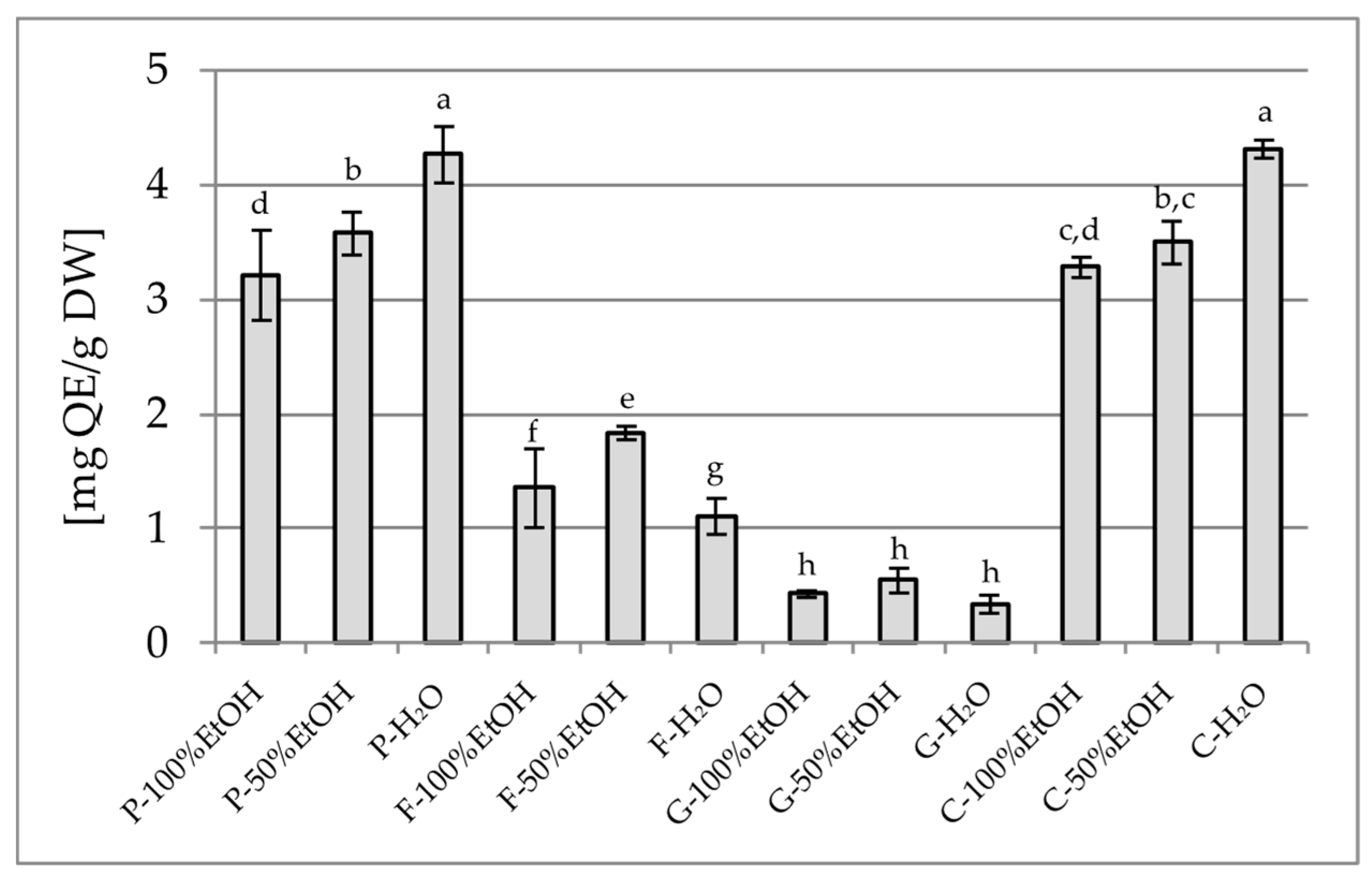
2.1.1. Total Polyphenol Content (TPC) and Total Flavonoid Content (TFC)
2.1.2. Standardisation of the Extract Using High-Performance Liquid Chromatography (HPLC)
2.1.3. GC-MS Analysis
2.2. Studies of the Biological Activity of Extracts
2.2.1. Antioxidant Activity Assay
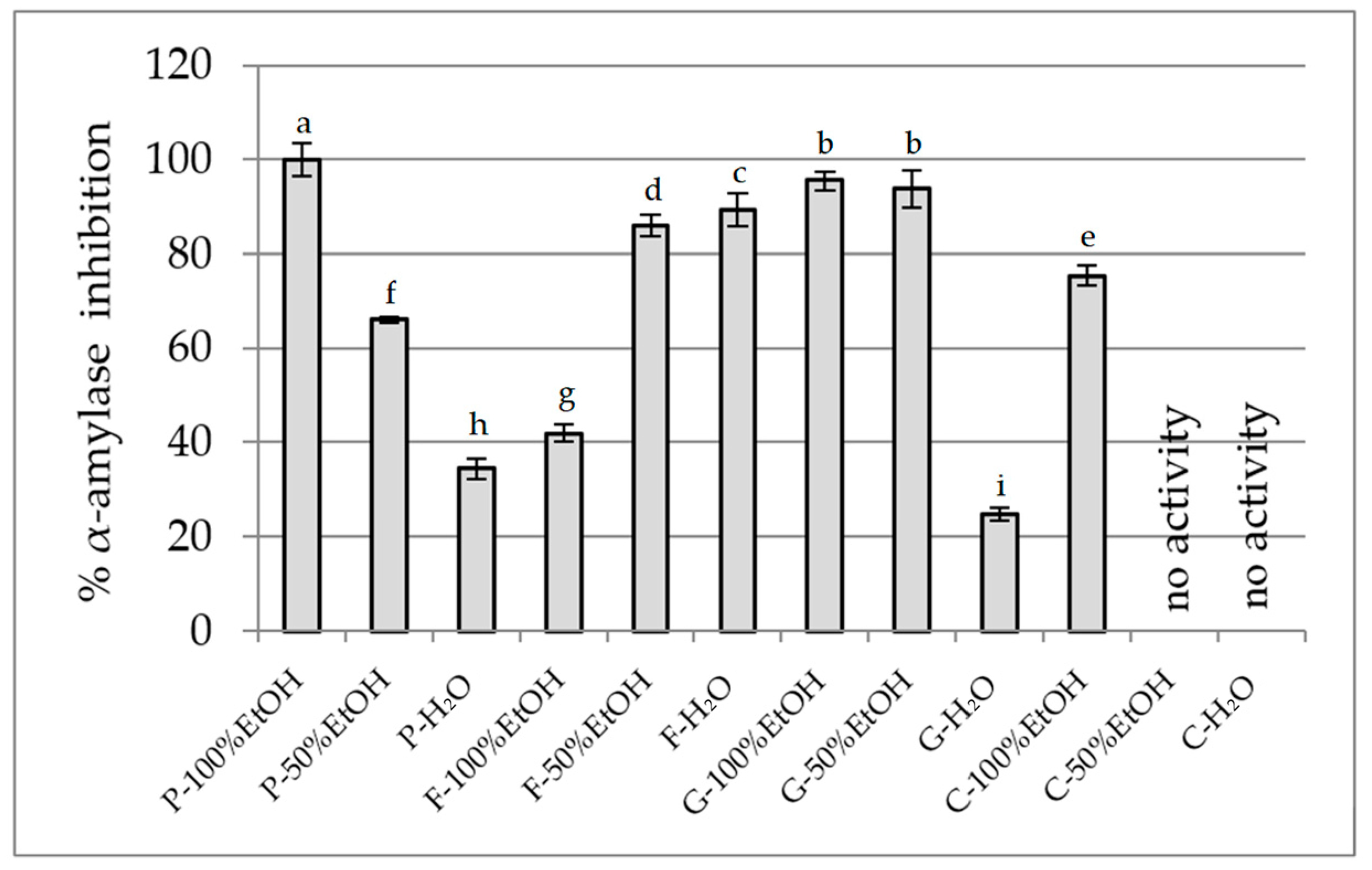
2.2.2. Inhibition of Carbohydrate-Hydrolysing Enzymes
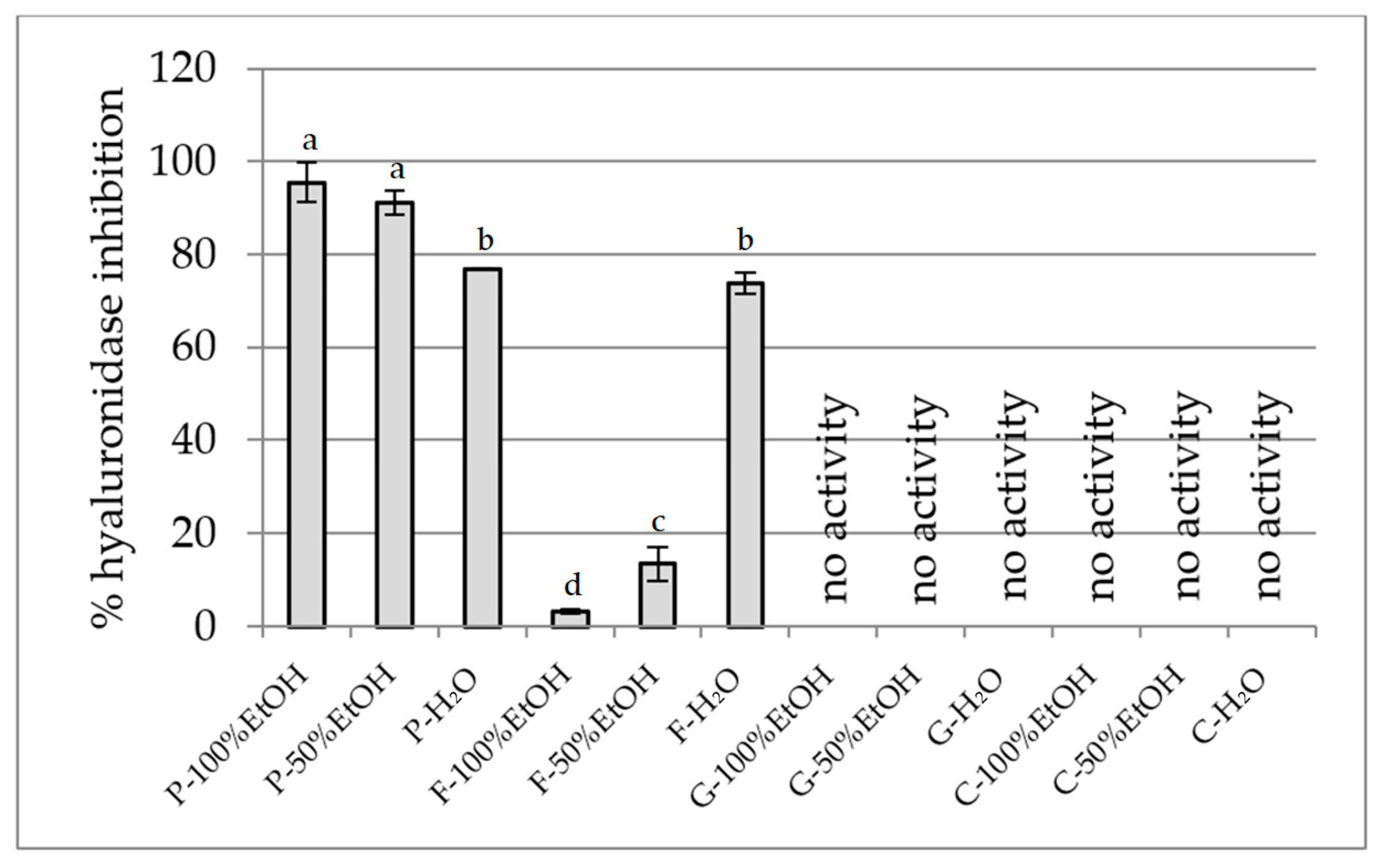
2.2.3. Anti-Inflammatory Activity Assay
Hyaluronidase Inhibition Assay
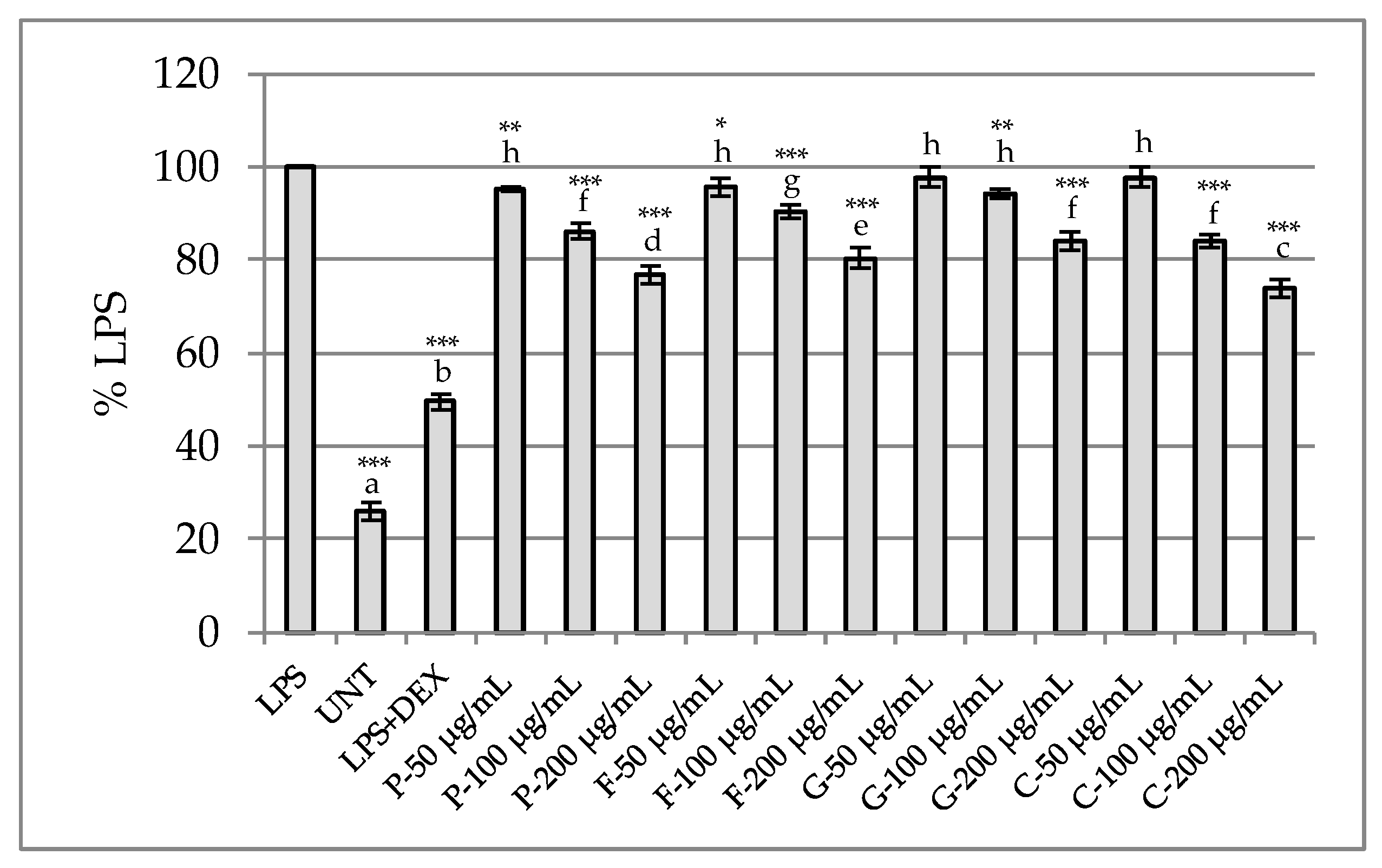
Determination of Anti-Inflammatory Potential in RAW 264.7 Model
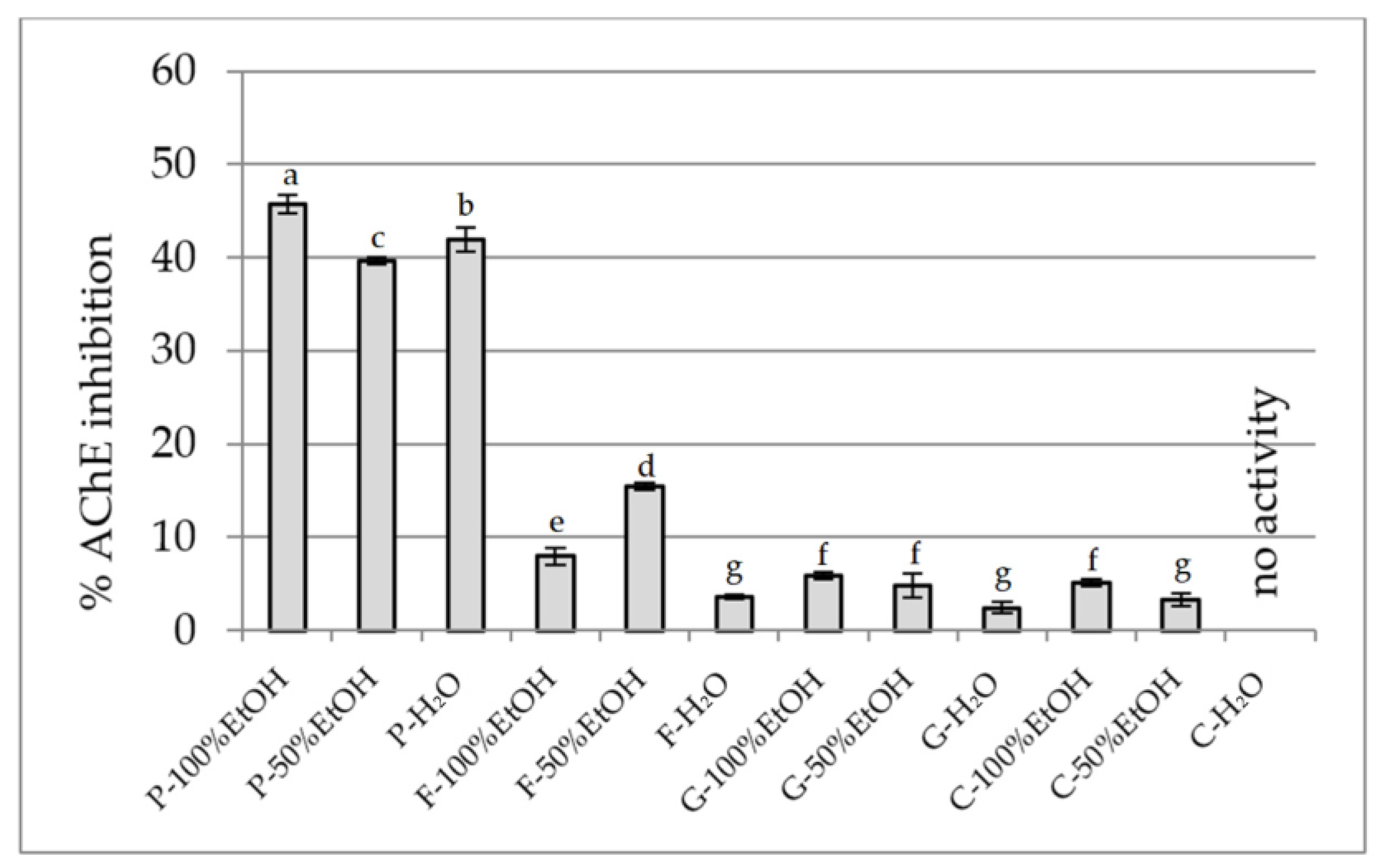
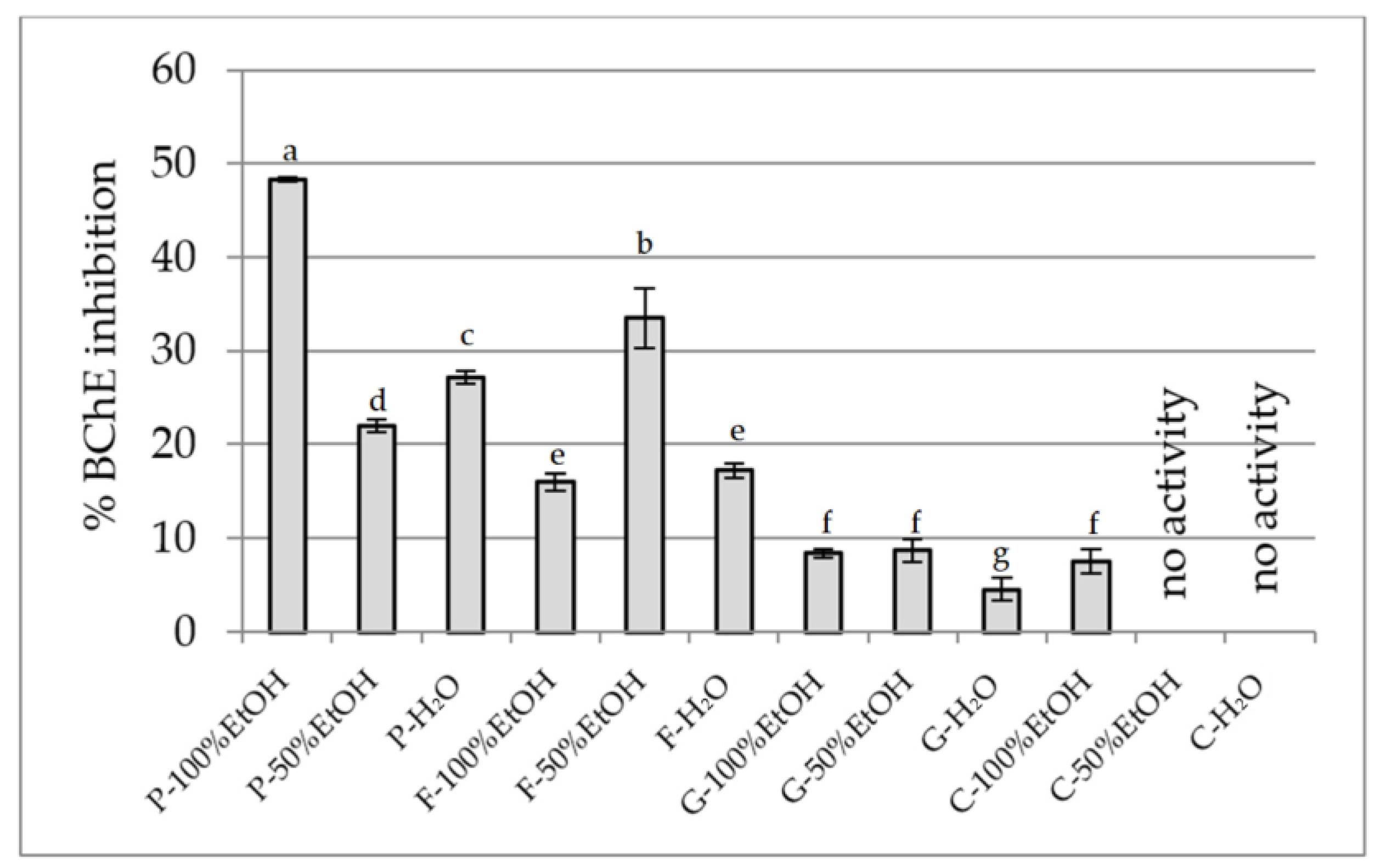
2.2.4. Anti-Cholinesterase Activity Assay
3. Materials and Methods
3.1. Chemical Reagents
3.2. Plant Material
3.3. Extraction Process
3.4. Phytochemical Studies of the Extracts
3.4.1. Total Polyphenol Content
3.4.2. Total Flavonoid Content
3.4.3. Standardisation of the Extract Using High-Performance Liquid Chromatography (HPLC)
3.4.4. GC-MS Analysis
3.5. Studies of the Biological Activity of Extracts
3.5.1. Antioxidant Activity Assay
DPPH Assay
ABTS Assay
CUPRAC Assay
FRAP Assay
Chelation Power on Ferrous (Fe2+) Ions Assay
3.5.2. Inhibition of Carbohydrate-Hydrolysing Enzymes
α-Glucosidase Inhibition Assay
α-Amylase Inhibition Assay
3.5.3. Anti-Inflammatory Activity Assay
Hyaluronidase Inhibition Assay
Determination of Anti-Inflammatory Potential in RAW 264.7 Model
3.5.4. Anti-Cholinesterase Activity Assay
AChE and BChE Inhibition Activity Assays
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Guzman-Vilca, W.C.; Carrillo-Larco, R.M. Number of People with Type 2 Diabetes Mellitus in 2035 and 2050: A Modelling Study in 188 Countries. Curr. Diabetes Rev. 2025, 21, E120124225603. [Google Scholar] [CrossRef]
- Niedowicz, D.M.; Daleke, D.L. The Role of Oxidative Stress in Diabetic Complications. Cell Biochem. Biophys. 2005, 43, 289–330. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.A. Developmental Origins of Diabetes: The Role of Oxidative Stress. Free Radic. Biol. Med. 2006, 40, 917–922. [Google Scholar] [CrossRef]
- Ikegami-Kawai, M.; Suzuki, A.; Karita, I.; Takahashi, T. Increased Hyaluronidase Activity in the Kidney of Streptozotocin-Induced Diabetic Rats. J. Biochem. 2003, 134, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.A.T.; Bitencourt, C.S.; Reis, M.B.; Frantz, F.G.; Sorgi, C.A.; Souza, C.O.S.; Silva, C.L.; Gardinassi, L.G.; Faccioli, L.H. Immunomodulatory Activity of Hyaluronidase Is Associated with Metabolic Adaptations during Acute Inflammation. Inflamm. Res. 2020, 69, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Dogné, S.; Rath, G.; Jouret, F.; Caron, N.; Dessy, C.; Flamion, B. Hyaluronidase 1 Deficiency Preserves Endothelial Function and Glycocalyx Integrity in Early Streptozotocin-Induced Diabetes. Diabetes 2016, 65, 2742–2753. [Google Scholar] [CrossRef]
- Rao, A.A.; Sridhar, G.R.; Das, U.N. Elevated Butyrylcholinesterase and Acetylcholinesterase May Predict the Development of Type 2 Diabetes Mellitus and Alzheimer’s Disease. Med. Hypotheses 2007, 69, 1272–1276. [Google Scholar] [CrossRef]
- Mushtaq, G.; H Greig, N.; A Khan, J.; A Kamal, M. Status of Acetylcholinesterase and Butyrylcholinesterase in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS Neurol. Disord. Targets (Formerly Curr. Drug Targets CNS Neurol. Disord.) 2014, 13, 1432–1439. [Google Scholar] [CrossRef]
- Phung, O.J.; Sobieraj, D.M.; Engel, S.S.; Rajpathak, S. Early Combination Therapy for the Treatment of Type 2 Diabetes Mellitus: Systematic Review and Meta-analysis. Diabetes Obes. Metab. 2014, 16, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Shetty, R.; Basheer, F.T.; Poojari, P.G.; Thunga, G.; Chandran, V.P.; Acharya, L.D. Adverse Drug Reactions of GLP-1 Agonists: A Systematic Review of Case Reports. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102427. [Google Scholar] [CrossRef]
- Halimi, S.; Vergès, B. Adverse Effects and Safety of SGLT-2 Inhibitors. Diabetes Metab. 2014, 40, S28–S34. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Murtaza, G.; Liu, G.; Rahu, N.; Kalhoro, M.S.; Kalhoro, D.H.; Adebowale, T.O.; Mazhar, M.U.; ur Rehman, Z. Flavonoids and Type 2 Diabetes: Evidence of Efficacy in Clinical and Animal Studies and Delivery Strategies to Enhance Their Therapeutic Efficacy. Pharmacol. Res. 2020, 152, 104629. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Tudoreanu, L.; Ștefan, G. Plant Polyphenols Mechanisms of Action on Insulin Resistance and against the Loss of Pancreatic Beta Cells. Crit. Rev. Food Sci. Nutr. 2021, 62, 325–352. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Mistry, P.S.; Chorawala, M.R.; Sivamaruthi, B.S.; Prajapati, B.G.; Kumar, A.; Chaiyasut, C. The Role of Dietary Anthocyanins for Managing Diabetes Mellitus-Associated Complications. Curr. Diabetes Rev. 2025, 21, 76–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Liu, H.; Fan, Y.; Liu, H.H. The Application of Procyanidins in Diabetes and Its Complications: A Review of Preclinical Studies. Front. Pharmacol. 2025, 16, 1532246. [Google Scholar] [CrossRef]
- Mao, T.; Akshit, F.N.U.; Mohan, M.S. Effects of Anthocyanin Supplementation in Diet on Glycemic and Related Cardiovascular Biomarkers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2023, 10, 1199815. [Google Scholar] [CrossRef]
- Yang, L.; Ling, W.; Qiu, Y.; Liu, Y.; Wang, L.; Yang, J.; Wang, C.; Ma, J. Anthocyanins Increase Serum Adiponectin in Newly Diagnosed Diabetes but Not in Prediabetes: A Randomized Controlled Trial. Nutr. Metab. 2020, 17, 78. [Google Scholar] [CrossRef]
- Adel-Mehraban, M.S.; Tabatabaei-Malazy, O.; Manayi, A.; Alatab, S.; Mohseni, S.; Fana, S.E.; Asili, P.; Bahramsoltani, R.; Esmaeili, F.; Azizi, B. Antioxidative and Anti-Inflammatory Effects of Plant-Derived Hypoglycemic Medicines: An In Vivo/In Vitro Systematic Review. Curr. Top. Med. Chem. 2024, 24, 1408–1450. [Google Scholar] [CrossRef] [PubMed]
- Nobel, S.; Baker, C.; Loullis, C. Crave Crush TM Lozenges Containing Gymnemic Acids Reduce Consumption of High Sugar Foods. Adv. Med. Plant Res. 2017, 5, 63–67. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Vareltzis, P.; Floros, S.; Androutsos, O.; Bargiota, A.; Gortzi, O. Development of a Functional Acceptable Diabetic and Plant-Based Snack Bar Using Mushroom (Coprinus comatus) Powder. Foods 2023, 12, 2702. [Google Scholar] [CrossRef]
- Barrea, L.; Vetrani, C.; Verde, L.; Frias-Toral, E.; Ceriani, F.; Cernea, S.; Docimo, A.; Graziadio, C.; Tripathy, D.; Savastano, S.; et al. Comprehensive Approach to Medical Nutrition Therapy in Patients with Type 2 Diabetes Mellitus: From Diet to Bioactive Compounds. Antioxidants 2023, 12, 904. [Google Scholar] [CrossRef]
- Miñambres, I.; Cuixart, G.; Gonçalves, A.; Corcoy, R. Effects of Inositol on Glucose Homeostasis: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2019, 38, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Bule, M.; Albelbeisi, A.H.; Nikfar, S.; Amini, M.; Abdollahi, M. The Antidiabetic and Antilipidemic Effects of Hibiscus sabdariffa: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Food Res. Int. 2020, 130, 108980. [Google Scholar] [CrossRef]
- Carboni, A.D.; Di Renzo, T.; Nazzaro, S.; Marena, P.; Puppo, M.C.; Reale, A. A Comprehensive Review of Edible Flowers with a Focus on Microbiological, Nutritional, and Potential Health Aspects. Foods 2025, 14, 1719. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Anti-Hyperglycemic and Anticholinergic Effects of Natural Antioxidant Contents in Edible Flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef]
- Gupta, G.; Chahal, J.; Bhatia, M.S. Clitoria ternatea (L.): Old and New Aspects. J. Pharm. Res. 2010, 3, 2610–2614. [Google Scholar]
- Tarnam, Y.A.; Ilyas, M.H.M.; Begum, T.N. Biological Potential and Phytopharmacological Screening of Gomphrena Species. Int. J. Pharma. Res. Rev. 2014, 3, 58–66. [Google Scholar]
- Zhou, H.; Li, T.; Li, B.; Sun, S. Skin Health Properties of Paeonia Lactiflora Flower Extracts and Tyrosinase Inhibitors and Free Radical Scavengers Identified by HPLC Post-Column Bioactivity Assays. Heliyon 2023, 9, e18569. [Google Scholar] [CrossRef]
- Michalak, B.; Filipek, A.; Chomicki, P.; Pyza, M.; Woźniak, M.; Żyżyńska-Granica, B.; Piwowarski, J.P.; Kicel, A.; Olszewska, M.A.; Kiss, A.K. Lignans from Forsythia x Intermedia Leaves and Flowers Attenuate the Pro-Inflammatory Function of Leukocytes and Their Interaction with Endothelial Cells. Front. Pharmacol. 2018, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Rivas-García, L.; Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Forbes-Hernández, T.Y.; Varela-López, A.; Llopis, J.; Sánchez-González, C.; Quiles, J.L. Edible Flowers as a Health Promoter: An Evidence-Based Review. Trends Food Sci. Technol. 2021, 117, 46–59. [Google Scholar] [CrossRef]
- Vujanović, M.; Majkić, T.; Zengin, G.; Beara, I.; Tomović, V.; Šojić, B.; Đurović, S.; Radojković, M. Elderberry (Sambucus nigra L.) Juice as a Novel Functional Product Rich in Health-Promoting Compounds. RSC Adv. 2020, 10, 44805–44814. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Kicel, A.; Owczarek, A.; Michel, P.; Skalicka-Woźniak, K.; Kiss, A.K.; Olszewska, M.A. Application of HPCCC, UHPLC-PDA-ESI-MS3 and HPLC-PDA Methods for Rapid, One-Step Preparative Separation and Quantification of Rutin in Forsythia Flowers. Ind. Crops Prod. 2015, 76, 86–94. [Google Scholar] [CrossRef]
- Lee, S.; Park, N.I.; Park, Y.; Heo, K.; Kwon, Y.; Kim, E.S.; Son, Y.K.; Lee, K.J.; Choi, S.Y.; Choi, B.S.; et al. Distribution of Flavonoids in Paeonia suffruticosa and Analyses of the Genes Involved in Flavonoid Biosynthesis in Plants. Plant Gene 2025, 41, 100490. [Google Scholar] [CrossRef]
- Gonçalves, G.C.P.; Rosas, A.L.G.; de Sousa, R.C.; Vieira, T.R.R.; de Albuquerque Sousa, T.C.; Ramires, T.; da Silveira, T.F.F.; Barros, L.; da Silva, W.P.; Dias, Á.R.G.; et al. A Green Method for Anthocyanin Extraction from Clitoria ternatea Flowers Cultivated in Southern Brazil: Characterization, in Vivo Toxicity, and Biological Activity. Food Chem. 2024, 435, 137575. [Google Scholar] [CrossRef]
- Bujak, T.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Lal, K.; Wasilewski, T.; Hordyjewicz-Baran, Z. Flower Extracts as Multifunctional Dyes in the Cosmetics Industry. Molecules 2022, 27, 922. [Google Scholar] [CrossRef]
- Bujak, T.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Wasilewski, T.; Hordyjewicz-Baran, Z. Antioxidant and Cytoprotective Properties of Plant Extract from Dry Flowers as Functional Dyes for Cosmetic Products. Molecules 2021, 26, 2809. [Google Scholar] [CrossRef]
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A Comprehensive Review on Traditional Uses, Phytochemistry, Pharmacological Activities, Clinical Application, and Toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef] [PubMed]
- Demasi, S.; Caser, M.; Donno, D.; Ravetto Enri, S.; Lonati, M.; Scariot, V. Exploring Wild Edible Flowers as a Source of Bioactive Compounds: New Perspectives in Horticulture. Folia Hortic. 2021, 33, 27–48. [Google Scholar] [CrossRef]
- Khatoon, S.; Irshad, S.; Rawat, A.K.S.; Misra, P.K. Comparative Pharmacognostical Studies of Blue and White Flower Varieties of Clitoria ternatea L. J. Pharmacogn. Nat. Prod. 2015, 1, 1. [Google Scholar]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Scientific Validation of Synergistic Antioxidant Effects in Commercialised Mixtures of Cymbopogon citratus and Pterospartum tridentatum or Gomphrena globosa for Infusions Preparation. Food Chem. 2015, 185, 16–24. [Google Scholar] [CrossRef][Green Version]
- Roshan, H.; Nikpayam, O.; Sedaghat, M.; Sohrab, G. Effects of Green Coffee Extract Supplementation on Anthropometric Indices, Glycaemic Control, Blood Pressure, Lipid Profile, Insulin Resistance and Appetite in Patients with the Metabolic Syndrome: A Randomised Clinical Trial. Br. J. Nutr. 2018, 119, 250–258. [Google Scholar] [CrossRef]
- Zuñiga, L.Y.; Aceves-de la Mora, M.C.A.; González-Ortiz, M.; Ramos-Núñez, J.L.; Martínez-Abundis, E. Effect of Chlorogenic Acid Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J. Med. Food 2017, 21, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi, A.; Martins, A.; Hsieh, T.J.; Seres, A.; Zupkó, I. Chlorogenic Acid and Rutin Play a Major Role in the In Vivo Anti-Diabetic Activity of Morus Alba Leaf Extract on Type II Diabetic Rats. PLoS ONE 2012, 7, e50619. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic Acid Attenuates High-Fat Diet Fed-Streptozotocin-Induced Insulin Resistance via Partial Agonism of PPARγ in Experimental Type 2 Diabetic Rats and Enhances Glucose Uptake through Translocation and Activation of GLUT4 in PI3K/p-Akt Signaling Pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic Effects of Simple Phenolic Acids: A Comprehensive Review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Huang, D.W.; Chang, W.C.; Wu, J.S.B.; Shih, R.W.; Shen, S.C. Gallic Acid Ameliorates Hyperglycemia and Improves Hepatic Carbohydrate Metabolism in Rats Fed a High-Fructose Diet. Nutr. Res. 2016, 36, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, G.; Zhang, C.; Wang, N.; Feng, Y. Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress. Molecules 2021, 26, 7115. [Google Scholar] [CrossRef]
- Ganjayi, M.S.; Karunakaran, R.S.; Gandham, S.; Meriga, B. Quercetin-3-O-Rutinoside from Moringa Oleifera Downregulates Adipogenesis and Lipid Accumulation and Improves Glucose Uptake by Activation of AMPK/Glut-4 in 3T3-L1 Cells. Rev. Bras. Farmacogn. 2023, 33, 334–343. [Google Scholar] [CrossRef]
- Kappel, V.D.; Frederico, M.J.S.; Postal, B.G.; Mendes, C.P.; Cazarolli, L.H.; Silva, F.R.M.B. The Role of Calcium in Intracellular Pathways of Rutin in Rat Pancreatic Islets: Potential Insulin Secretagogue Effect. Eur. J. Pharmacol. 2013, 702, 264–268. [Google Scholar] [CrossRef]
- Arora, S.K.; Verma, P.R.; Itankar, P.R.; Prasad, S.K.; Nakhate, K.T. Evaluation of Pancreatic Regeneration Activity of Tephrosia Purpurea Leaves in Rats with Streptozotocin-Induced Diabetes. J. Tradit. Complement. Med. 2021, 11, 435–445. [Google Scholar] [CrossRef]
- Dias, D.T.M.; Palermo, K.R.; Motta, B.P.; Kaga, A.K.; Lima, T.F.O.; Brunetti, I.L.; Baviera, A.M. Rutin Inhibits the in Vitro Formation of Advanced Glycation Products and Protein Oxidation More Efficiently than Quercetin. Rev. Ciências Farm. Básica Apl. 2021, 42, 1–13. [Google Scholar]
- Tian, R.; Yang, W.; Xue, Q.; Gao, L.; Huo, J.; Ren, D.; Chen, X. Rutin Ameliorates Diabetic Neuropathy by Lowering Plasma Glucose and Decreasing Oxidative Stress via Nrf2 Signaling Pathway in Rats. Eur. J. Pharmacol. 2016, 771, 84–92. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Pham, B.; Le, L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Tsagogiorgas, C.; Otto, M. Semifluorinated Alkanes as New Drug Carriers—An Overview of Potential Medical and Clinical Applications. Pharmaceutics 2023, 15, 1211. [Google Scholar] [CrossRef]
- Shahidi, F.; Danielski, R. Review on the Role of Polyphenols in Preventing and Treating Type 2 Diabetes: Evidence from in Vitro and in Vivo Studies. Nutrients 2024, 16, 3159. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, X.; Li, Y.; Zhang, S.; Xie, H. Association between Dietary Antioxidant Levels and Diabetes: A Cross-Sectional Study. Front. Nutr. 2024, 11, 1478815. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, C. Oxidative Stress: A Culprit in the Progression of Diabetic Kidney Disease. Antioxidants 2024, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial Dysfunction in Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Pakdeepromma, S.; Pintha, K.; Tantipaiboonwong, P.; Thephinlap, C.; Suttajit, M.; Kaowinn, S.; Kangwan, N.; Suwannaloet, W.; Pangjit, K. Assessing the Antioxidant, Hepatoprotective, and Iron-Chelating Potential of Perilla frutescens Seed. Biomedicines 2025, 13, 851. [Google Scholar] [CrossRef] [PubMed]
- Dienaitė, L.; Pukalskienė, M.; Pukalskas, A.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Isolation of Strong Antioxidants from Paeonia officinalis Roots and Leaves and Evaluation of Their Bioactivities. Antioxidants 2019, 8, 249. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Wu, K.; Wang, M.; Liu, P.; Wang, X.; Deng, R. Antioxidant Activities and Chemical Constituents of Flavonoids from the Flower of Paeonia ostii. Molecules 2016, 22, 5. [Google Scholar] [CrossRef]
- Yang, X.N.; Kang, S.C. In Vitro Antioxidant Activity of the Water and Ethanol Extracts of Forsythia koreana Flowers. Nat. Prod. Res. 2012, 26, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Lin, J.; Zhou, J.; Du, H.; Chen, X.; Liu, M.; Qu, Q.; Lv, W.; Guo, S. Polyphenol Rich Forsythia suspensa Extract Alleviates DSS-Induced Ulcerative Colitis in Mice through the Nrf2-NLRP3 Pathway. Antioxidants 2022, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Riyaphan, J.; Jhong, C.H.; Tsai, M.J.; Lee, D.N.; Leong, M.K.; Weng, C.F. Potent Natural Inhibitors of Alpha-Glucosidase and Alpha-Amylase against Hyperglycemia in Vitro and in Vivo. Preprints 2017, 2017030116. [Google Scholar] [CrossRef]
- Escher, G.B.; Marques, M.B.; do Carmo, M.A.V.; Azevedo, L.; Furtado, M.M.; Sant’Ana, A.S.; da Silva, M.C.; Genovese, M.I.; Wen, M.; Zhang, L.; et al. Clitoria ternatea L. Petal Bioactive Compounds Display Antioxidant, Antihemolytic and Antihypertensive Effects, Inhibit α-Amylase and α-Glucosidase Activities and Reduce Human LDL Cholesterol and DNA Induced Oxidation. Food Res. Int. 2020, 128, 108763. [Google Scholar] [CrossRef]
- Tang, S.R.; Sun, Y.X.; Gu, T.T.; Cao, F.F.; Shen, Y.B.; He, J.P.; Xie, Z.X.; Li, C. Phenolic Compounds from Gomphrena globosa L.: Phytochemical Analysis, Antioxidant, Antimicrobial, and Enzyme Inhibitory Activities in Vitro. CyTA J. Food 2022, 20, 218–227. [Google Scholar] [CrossRef]
- Li, C.; Tang, W.; Chen, S.; He, J.; Li, X.; Zhu, X.; Li, H.; Peng, Y. Phytochemical Properties and In Vitro Biological Activities of Phenolic Compounds from Flower of Clitoria ternatea L. Molecules 2022, 27, 6336. [Google Scholar] [CrossRef]
- Chusak, C.; Henry, C.J.; Chantarasinlapin, P.; Techasukthavorn, V.; Adisakwattana, S. Influence of Clitoria ternatea Flower Extract on the In Vitro Enzymatic Digestibility of Starch and Its Application in Bread. Foods 2018, 7, 102. [Google Scholar] [CrossRef]
- Lucianus, J.; Darsono, L.; Widowati, W.; Rusmana, D.; Tiono, H.; Onggowidjaja, P.; Tjokropranoto, R.; Kusuma, H.S.W.; Nindya, F.S.; Hadiprasetyo, D.S. Anti-Diabetes Mellitus Potential of Butterfly Pea Flower (Clitoria ternatea L.) Extract Based on Antioxidant and Inhibition of α-Amylase and α-Glucosidase Activities. Med. Plants—Int. J. Phytomed. Relat. Ind. 2024, 16, 267–273. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In Vitro Inhibitory Effects of Plant-Based Foods and Their Combinations on Intestinal α-Glucosidase and Pancreatic α-Amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, H.; Zhang, L.; Liu, Z.; Huang, Y.; Liu, Q.; Jin, L.; Zhu, M.; Zhang, L. Inflammation in Diabetes Complications: Molecular Mechanisms and Therapeutic Interventions. MedComm 2024, 5, e516. [Google Scholar] [CrossRef] [PubMed]
- Zamani-Garmsiri, F.; Emamgholipour, S.; Rahmani Fard, S.; Ghasempour, G.; Jahangard Ahvazi, R.; Meshkani, R. Polyphenols: Potential Anti-Inflammatory Agents for Treatment of Metabolic Disorders. Phyther. Res. 2022, 36, 415–432. [Google Scholar] [CrossRef]
- Senobari, F.; Abolmaali, S.S.; Farahavr, G.; Tamaddon, A.M. Targeting Inflammation with Hyaluronic Acid-Based Micro- and Nanotechnology: A Disease-Oriented Review. Int. J. Biol. Macromol. 2024, 280, 135923. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic Acid and Its Biomedical Applications: A Review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Moseley, R.; Leaver, M.; Walker, M.; Waddington, R.J.; Parsons, D.; Chen, W.Y.J.; Embery, G. Comparison of the Antioxidant Properties of HYAFF-11p75, AQUACEL and Hyaluronan towards Reactive Oxygen Species in Vitro. Biomaterials 2002, 23, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Mat Zian, N.F.A.; Swain, P.; Mohd Faudzi, S.M.; Zakaria, N.; Wan Ibrahim, W.N.; Abu Bakar, N.; Shaari, K.; Stanslas, J.; Choi, T.I.; Kim, C.H. Mapping Molecular Networks within Clitoria ternatea Linn. against LPS-Induced Neuroinflammation in Microglial Cells, with Molecular Docking and In Vivo Toxicity Assessment in Zebrafish. Pharmaceuticals 2022, 15, 467. [Google Scholar] [CrossRef]
- Tian, C.; Guo, Y.; Chang, Y.; Zhao, J.; Cui, C.; Liu, M. Dose-Effect Relationship on Anti-Inflammatory Activity on LPS Induced RAW 264.7 Cells and Antioxidant Activity of Rutin in Vitro. Acta Pol. Pharm. Res. 2019, 76, 511–522. [Google Scholar] [CrossRef]
- Hassani, A.; Azarian, M.M.S.; Ibrahim, W.N.; Hussain, S.A. Preparation, Characterization and Therapeutic Properties of Gum Arabic-Stabilized Gallic Acid Nanoparticles. Sci. Rep. 2020, 10, 17808. [Google Scholar] [CrossRef]
- Kim, N.Y.; Kang, T.H.; Song, E.K.; Pae, H.O.; Chung, H.T.; Kim, Y.C. Inhibitory Effects of Butanol Fraction of the Aqueous Extract of Forsythia koreana on the Nitric Oxide Production by Murine Macrophage-like RAW 264.7 Cells. J. Ethnopharmacol. 2000, 73, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a Risk Factor for Dementia and Mild Cognitive Impairment: A Meta-Analysis of Longitudinal Studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.; Ekavali. A Review on Alzheimer’s Disease Pathophysiology and Its Management: An Update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Silva, L.R.; Valentão, P.; Faria, J.; Ferreres, F.; Sousa, C.; Gil-Izquierdo, A.; Pinho, B.R.; Andrade, P.B. Phytochemical Investigations and Biological Potential Screening with Cellular and Non-Cellular Models of Globe Amaranth (Gomphrena globosa L.) Inflorescences. Food Chem. 2012, 135, 756–763. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Tosun, F.; Sener, B. Screening of Various Phenolic Acids and Flavonoid Derivatives for Their Anticholinesterase Potential. Z. Naturforsch. C 2007, 62, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid. Based Complement. Altern. Med. 2016, 21, NP11–NP17. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Galanty, A.; Gościniak, A.; Wieczorek, M.; Kłaput, M.; Dudek-Makuch, M.; Cielecka-Piontek, J. Herbal Infusions as a Valuable Functional Food. Nutrients 2021, 13, 4051. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Bulicz, M.; Henkel, M.; Rosiak, N.; Paczkowska-Walendowska, M.; Szwajgier, D.; Baranowska-Wójcik, E.; Korybalska, K.; Cielecka-Piontek, J. Pleiotropic Potential of Evernia Prunastri Extracts and Their Main Compounds Evernic Acid and Atranorin: In Vitro and In Silico Studies. Molecules 2023, 29, 233. [Google Scholar] [CrossRef] [PubMed]
- Chanaj-Kaczmarek, J.; Osmałek, T.; Szymańska, E.; Winnicka, K.; Karpiński, T.M.; Dyba, M.; Bekalarska-Dębek, M.; Cielecka-Piontek, J. Development and Evaluation of Thermosensitive Hydrogels with Binary Mixture of Scutellariae baicalensis Radix Extract and Chitosan for Periodontal Diseases Treatment. Int. J. Mol. Sci. 2021, 22, 11319. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Paczkowska-Walendowska, M.; Kledzik, J.; Galanty, A.; Gościniak, A.; Szulc, P.; Korybalska, K.; Cielecka-Piontek, J. Antidiabetic Potential of Black Elderberry Cultivars Flower Extracts: Phytochemical Profile and Enzyme Inhibition. Molecules 2024, 29, 5775. [Google Scholar] [CrossRef] [PubMed]
- Tiveron, A.P.; Melo, P.S.; Bergamaschi, K.B.; Vieira, T.M.F.S.; Regitano-d’Arce, M.A.B.; Alencar, S.M. Antioxidant Activity of Brazilian Vegetables and Its Relation with Phenolic Composition. Int. J. Mol. Sci. 2012, 13, 8943–8957. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Dudek-Makuch, M.; Chanaj-Kaczmarek, J.; Czepulis, N.; Korybalska, K.; Rutkowski, R.; Łuczak, J.; Grabowska, K.; Bylka, W.; Witowski, J. Anti-Inflammatory Activity and Phytochemical Profile of Galinsoga parviflora Cav. Molecules 2018, 23, 2133. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Bańdurska, M.; Rosiak, N.; Szwajgier, D.; Baranowska-Wójcik, E.; Szymański, M.; Gruszka, W.; Cielecka-Piontek, J. Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia glauca within the Nervous System? Antioxidants 2022, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Żarowski, M.; Plech, T.; Cielecka-Piontek, J. Permeability of Hypogymnia Physodes Extract Component—Physodic Acid through the Blood–Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]

| Extract | Compound Detected Amount [mg/100 g DW] | ||||
|---|---|---|---|---|---|
| Gallic Acid | Chlorogenic Acid | p-Coumaric Acid | Ferulic Acid | Rutin | |
| P-100% EtOH | 493.31 ± 18.43 b | nd | nd | nd | 239.59 ± 29.66 c |
| P-50% EtOH | 390.27 ± 15.48 c | nd | nd | nd | 167.65 ± 0.56 d |
| P-H2O | 603.67 ± 10.10 a | nd | nd | nd | 207.56 ± 0.41 c,d |
| F-100% EtOH | 1.42 ± 0.07 d | 319.79 ± 4.21 b | nd | 6.37 ± 0.64 e | 2828.71 ± 41.11 b |
| F-50% EtOH | 19.87 ± 3.36 d | 796.03 ± 2.4 a | 1.11 ± 0.09 c,d | 8.80 ± 1.12 d | 4657.71 ± 66.37 a |
| F-H2O | 4.82 ± 0.54 d | 0.31 ± 0.28 c | 1.11 ± 0.38 c,d | 0.33 ± 0.30 h | 0.90 ± 0.31 f |
| G-100% EtOH | 0.08 ± 0.06 d | 2.83 ± 0.37 c | 0.88 ± 0.06 d | 4.53 ± 1.18 f | 34.90 ± 0.38 f |
| G-50% EtOH | 0.05 ± 0.07 d | 0.70 ± 0.47 c | 0.86 ± 0.09 d | 6.05 ± 1.10 | 38.44 ± 0.37 f |
| G-H2O | 0.17 ± 0.10 d | 2.55 ± 0.19 c | 5.07 ± 0.10 a | 12.18 ± 0.23 c | 23.39 ± 0.27 f |
| C-100% EtOH | nd | 0.17 ± 0.04 c | 0.06 ± 0.02 e | 3.17 ± 0.01 g | 11.69 ± 0.89 f |
| C-50% EtOH | nd | 1.93 ± 0.83 c | 1.32 ± 0.08 c | 85.95 ± 1.02 a | 160.35 ± 0.99 d |
| C-H2O | nd | 1.59 ± 0.16 c | 1.70 ± 0.35 b | 57.76 ± 0.69 b | 91.87 ± 4.47 e |
| Plant | Rt (min) | Quantity (%) | Compound Name | Formula | Main Characteristic m/z |
|---|---|---|---|---|---|
| Peonia officinalis | 15.75 | 1.53 | Propanoic acid, 2-(3-acetoxy-4,4,14-trimethylandrost-8-en-17-yl)- | C27H42O4 | 73, 147, 207, 281, 355, 444 |
| 22.42 | 8.83 | Octadecane, 3-ethyl-5-(2-ethylbutyl)- | C26H54 | 57, 71, 85, 99, 141, 207 | |
| 23.236 | 7.62 | Oleic acid, 3-(octadecyloxy)propyl ester | C39H76O3 | 57, 207, 281, 407, 462, 519 | |
| 24.022 | 47.31 | Pentacosane | C25H52 | 57, 71, 85, 99, 127, 141, 207, 225, 281, 322 | |
| 25.552 | 29.15 | Tetratetracontane | C44H90 | 57, 71, 85, 97, 207, 281, 326, 372., 423, 533 | |
| 26.191 | 5.56 | Spirost-8-en-11-one, 3-hydroxy-, (3ß,5a,14ß,20ß,22ß,25R)- | C27H40O4 | 57, 69, 96, 207, 281, 355, 415 | |
| Forsythia × intermedia | 22.188 | 1.462 | 2-Hexadecanol | C16H34O | 55, 69, 97, 207 |
| 22.292 | 0.349 | Oleic Acid | C18H34O2 | 55, 69, 83, 97, 207, 237, 281 | |
| 22.405 | 3.931 | Hexadecane, 2,6,10,14-tetramethyl- | C20H42 | 57, 71, 85, 99, 113, 141, 207, 238, 266 | |
| 23.069 | 1.209 | 9-Octadecenamide | C18H35NO | 55, 59, 72, 83, 154, 207, 281 | |
| 23.724 | 2.102 | Heptacosane | C27H56 | 57, 71, 85, 99, 127, 141, 183, 207, 239, 388 | |
| 23.824 | 1.177 | 17-Pentatriacontene | C35H70 | 57, 69, 83, 97, 207, 281, 350, 379, 445 | |
| 24.017 | 3.915 | Hentriacontane | C31H64 | 57, 71, 85, 99, 127, 155, 183, 207, 239, 265, 295, 323, 352 | |
| 25.544 | 4.212 | Tetratetracontane | C44H90 | 57, 71, 85, 97, 133, 207, 281, 337, 383, 575, 624 | |
| 27.07 | 0.370 | Ergosta-5,22-dien-3-ol, acetate, (3ß,22E)- | C30H48O2 | 57, 67, 91, 133, 145, 207, 281, 341, 393 | |
| 27.486 | 1.602 | Octadecane, 3-ethyl-5-(2-ethylbutyl)- | C26H54 | 57, 71, 85, 99, 127, 207, 281 | |
| 30.858 | 79.670 | Dihydrofuran-2-one, 4-(3,4-dimethoxybenzyl)-3-(4-hydroxy-3-methoxybenzyl)- | C21H24O6 | 77, 122, 137, 151, 177, 189, 207, 235, 265, 293, 321, 372 | |
| Gomphrena globosa | 21.838 | 7.208 | 3,10B-Dihydrofluoranthene | C16H12 | 101, 202, 282 |
| 23.114 | 3.658 | 5,7,9(11)-Androstatriene, 3-hydroxy-17-oxo- | C19H24O2 | 59, 77, 115, 133, 195, 207, 284 | |
| 24.03 | 1.075 | Oleic acid, 3-(octadecyloxy)propyl ester | C39H76O3 | 57, 133, 207, 281, 309, 403 | |
| 25.551 | 4.978 | Heptacosane | C27H56 | 57, 71, 85, 99, 113, 207, 281, 341, 389 | |
| 27.492 | 41.740 | Tetratetracontane | C44H90 | 57, 71, 85, 97, 127, 207, 281, 377, 429 | |
| 30.315 | 30.131 | Hexatriacontane | C36H74 | 57, 71, 85, 99, 127, 155, 207, 281, 322, 391, 436 | |
| 34.609 | 11.210 | Tetracosane, 11-decyl- | C34H70 | 57, 71, 85, 99, 207, 281, 355, 402 | |
| Clitoria ternatea | 11.612 | 0.917 | Spironolactone | C24H32O4S | 55, 73, 91, 147, 207, 251, 341, 429 |
| 13.807 | 1.117 | Octadecane, 1,1′-[1,3-propanediylbis(oxy)]bis- | C39H80O2 | 55, 73, 85, 97, 147, 207, 251, 281, 327, 415, 504 | |
| 18.137 | 4.952 | 17-Octadecynoic acid | C18H32O2 | 55, 68, 81, 95, 123, 207, 281 | |
| 18.254 | 2.652 | 2-Hexadecanol | C16H34O | 55, 69, 79, 95, 123, 207, 237 | |
| 18.389 | 2.459 | Ethanol, 2-(9-octadecenyloxy)-, (Z)- | C20H40O2 | 55, 69, 81, 96, 207, 250, 281, 325 | |
| 18.585 | 3.424 | 13-Heptadecyn-1-ol | C17H32O | 55, 67, 81, 95, 123, 207, 278 | |
| 25.549 | 4.120 | Heptacosane | C27H56 | 57, 71, 85, 99, 133, 207, 281, 355, 402 | |
| 27.489 | 14.287 | Tetratetracontane | C44H90 | 57, 71, 85, 97, 127, 207, 281, 306, 347, 391, 439, 481, 535 | |
| 30.311 | 49.099 | Octadecane, 3-ethyl-5-(2-ethylbutyl)- | C26H54 | 57, 71, 85, 97, 127, 155, 207, 281, 322, 355 | |
| 34.602 | 16.309 | Hexatriacontane | C36H74 | 57, 71, 85, 95, 207, 281, 341 | |
| 36.634 | 0.662 | Benz[e]azulene-3,8-dione, 3a,4,6a 7,9,10,10a,10b-octahydro-3a,10a-dihydroxy-5-(hydroxymethyl)-7-(1-hydroxy-1-methylethyl)-2,10-dimethyl-, [3aR-(3aa,6aa,7a,10ß,10aß,10bß)]- | C20H28O6 | 53, 69, 73, 69, 133, 179, 207, 281, 314 |
| DPPH [%] | ABTS [%] | Fe2+ chelation [%] | CUPRAC [mg TE/g DW] | FRAP [mg TE/g DW] | |
|---|---|---|---|---|---|
| P-100% EtOH | 97.65 ± 0.60 a | 100.00 ± 0.99 a | nd | 175.06 ± 1.04 a | 144.06 ± 4.56 a |
| P-50% EtOH | 90.42 ± 0.89 c | 99.51 ± 0.41 a | 73.78 ± 2.58 c | 151.44 ± 2.18 c | 140.29 ± 1.10 b |
| P-H2O | 91.01 ± 0.41 c | 99.78 ± 0.18 a | 83.36 ± 0.44 a | 162.04 ± 0.01 b | 137.52 ± 3.09 c |
| F-100% EtOH | 95.48 ± 0.27 b | 65.09 ± 1.02 b | nd | 72.58 ± 0.87 e | 51.94 ± 0.21 e |
| F-50% EtOH | 89.28 ± 0.64 d | 99.10 ± 0.53 a | 52.76 ± 1.32 e | 109.30 ± 2.82 d | 81.30 ± 0.76 d |
| F-H2O | 28.44 ± 0.49 e | 43.26 ± 0.91 e | 77.38 ± 0.56 b | 24.24 ± 0.49 f | 20.36 ± 0.33 f |
| G-100% EtOH | 10.56 ± 0.93 i | 10.50 ± 0.32 i | nd | 6.32 ± 0.05 i | 5.36 ± 0.02 f |
| G-50% EtOH | 6.89 ± 0.84 j | 15.82 ± 0.90 h | 52.68 ± 4.27 e | 7.81 ± 0.31 h,i | 7.36 ± 0.07 g |
| G-H2O | 12.34 ± 0.37 h | 29.63 ± 0.94 f | 80.81 ± 0.90 a | 8.56 ± 0.49 h | 8.37 ± 0.04 g |
| C-100% EtOH | 15.87 ± 1.78 g | 20.30 ± 0.76 g | nd | 8.90 ± 0.35 h | 5.83 ± 0.04 g |
| C-50% EtOH | 21.83 ± 0.77 f | 54.36 ± 1.60 c | 58.62 ± 6.27 d | 23.41 ± 0.40 f | 20.30 ± 0.24 f |
| C-H2O | 22.06 ± 0.72 f | 47.43 ± 1.71 d | 71.28 ± 3.24 c | 21.70 ± 0.12 g | 19.46 ± 0.17 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Książkiewicz, M.; Karczewska, M.; Nawrot, F.; Grabowska, K.; Szymański, M.; Cielecka-Piontek, J.; Studzińska-Sroka, E. Edible Flowers as Bioactive Food Ingredients with Antidiabetic Potential: A Study on Paeonia officinalis L., Forsythia × intermedia, Gomphrena globosa L., and Clitoria ternatea L. Plants 2025, 14, 2603. https://doi.org/10.3390/plants14162603
Książkiewicz M, Karczewska M, Nawrot F, Grabowska K, Szymański M, Cielecka-Piontek J, Studzińska-Sroka E. Edible Flowers as Bioactive Food Ingredients with Antidiabetic Potential: A Study on Paeonia officinalis L., Forsythia × intermedia, Gomphrena globosa L., and Clitoria ternatea L. Plants. 2025; 14(16):2603. https://doi.org/10.3390/plants14162603
Chicago/Turabian StyleKsiążkiewicz, Maciej, Michalina Karczewska, Filip Nawrot, Karolina Grabowska, Marcin Szymański, Judyta Cielecka-Piontek, and Elżbieta Studzińska-Sroka. 2025. "Edible Flowers as Bioactive Food Ingredients with Antidiabetic Potential: A Study on Paeonia officinalis L., Forsythia × intermedia, Gomphrena globosa L., and Clitoria ternatea L." Plants 14, no. 16: 2603. https://doi.org/10.3390/plants14162603
APA StyleKsiążkiewicz, M., Karczewska, M., Nawrot, F., Grabowska, K., Szymański, M., Cielecka-Piontek, J., & Studzińska-Sroka, E. (2025). Edible Flowers as Bioactive Food Ingredients with Antidiabetic Potential: A Study on Paeonia officinalis L., Forsythia × intermedia, Gomphrena globosa L., and Clitoria ternatea L. Plants, 14(16), 2603. https://doi.org/10.3390/plants14162603









