Root-Zone Temperature Drives Coordinated Photosynthesis, Root Architecture, and Metabolism Responses in Schisandra chinensis (Trucz.) Baill
Abstract
1. Introduction
2. Results and Analysis
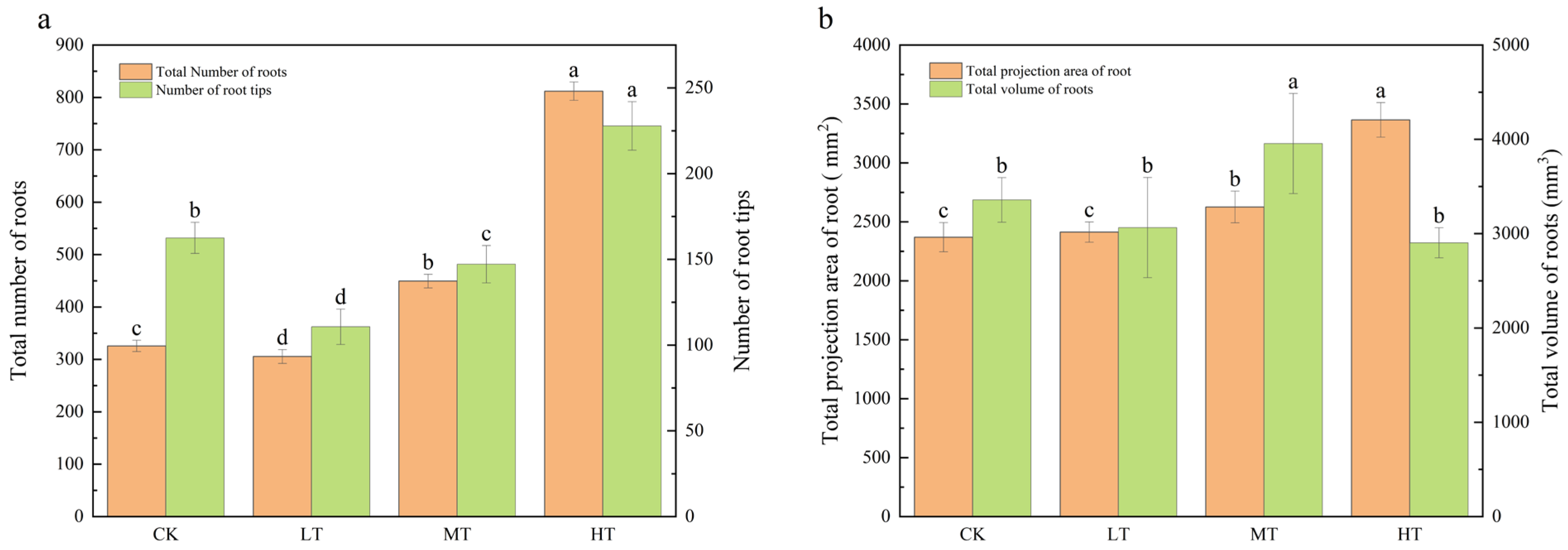
2.1. Effects on Plant Growth of Different RZT Treatments
2.2. Response of Gas Exchange Under Different RZTs
2.3. Response of Chlorophyll Fluorescence Level Under Different RZT Conditions
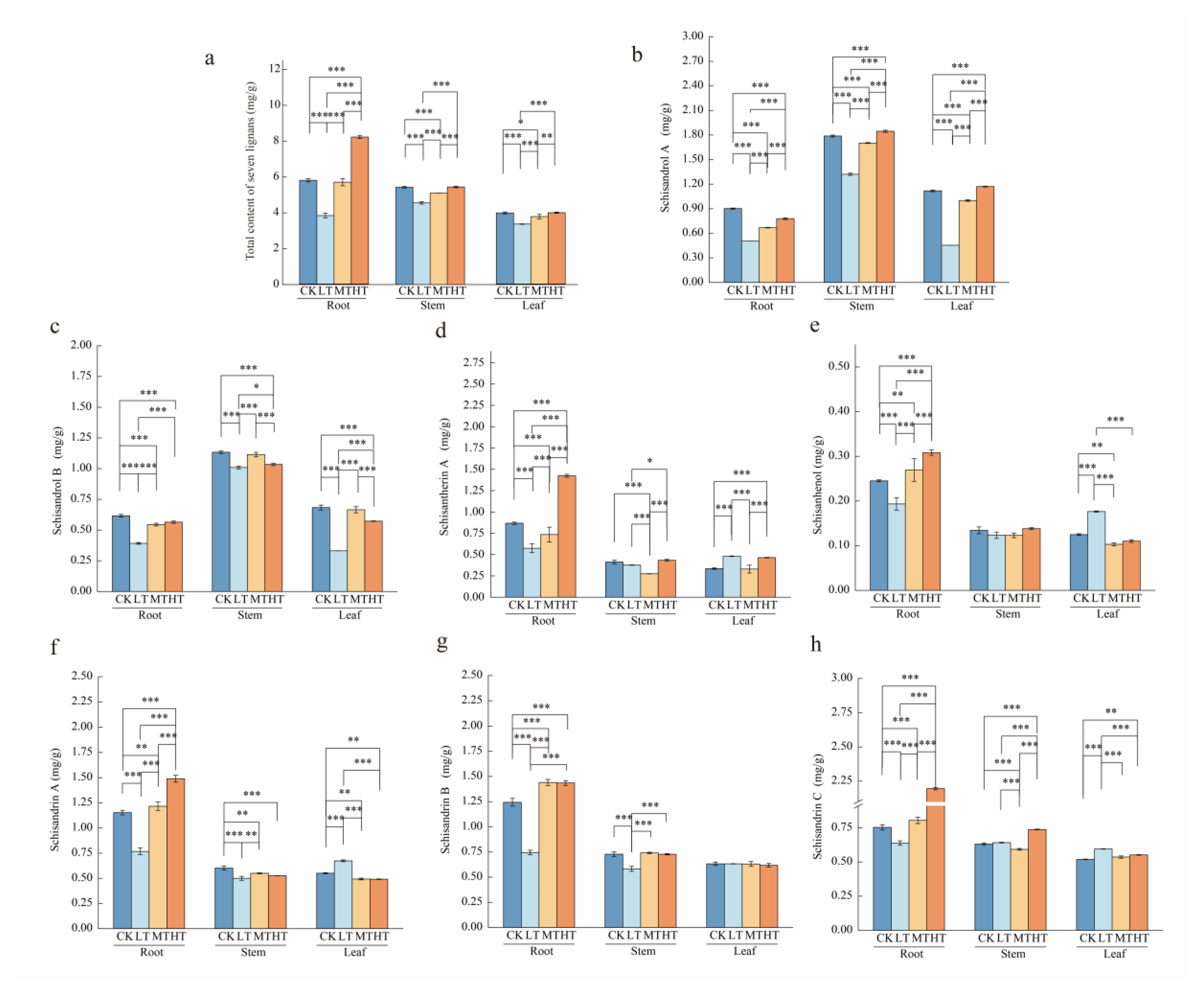
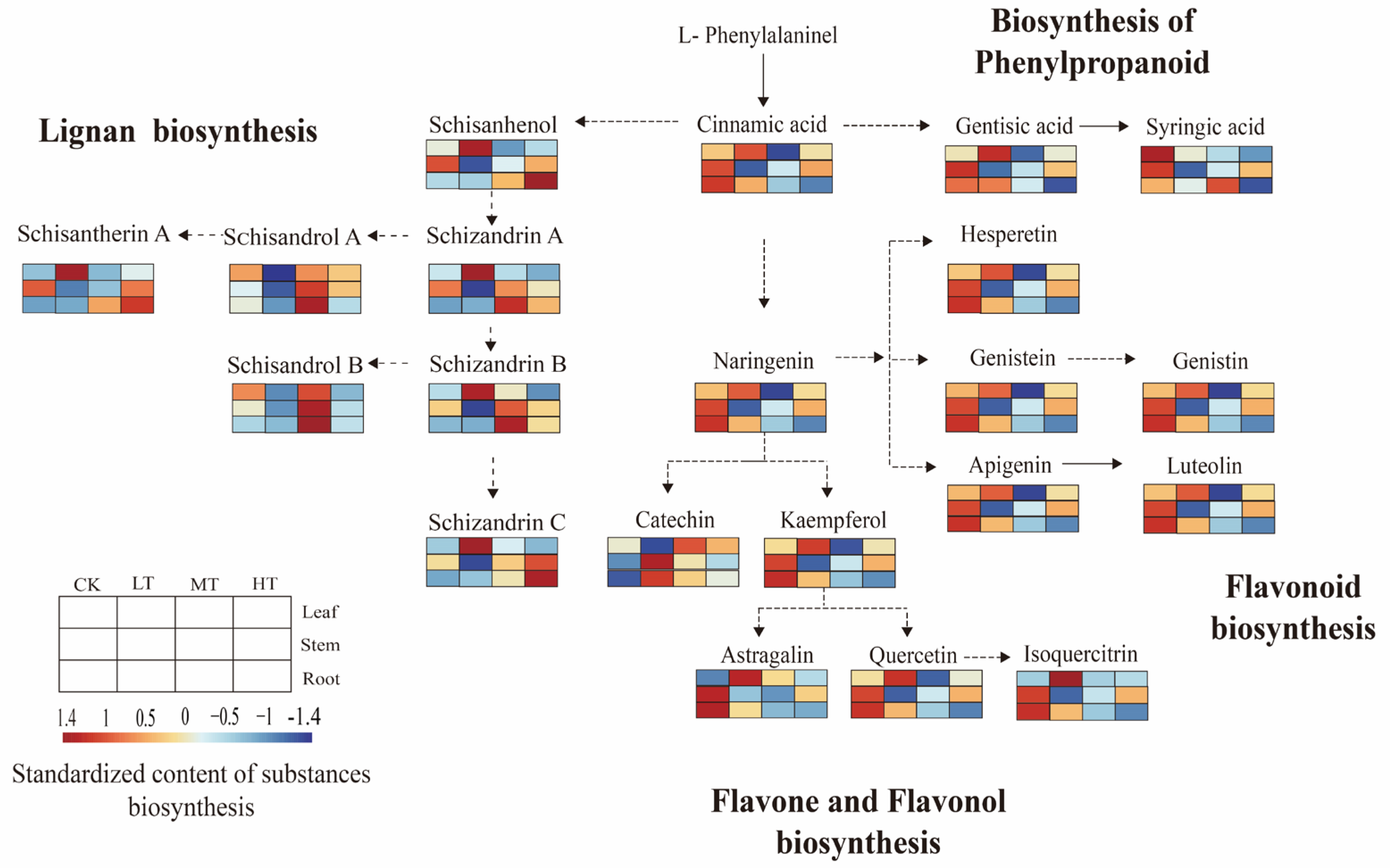
2.4. Response of Lignan Content Under Different RZT Conditions
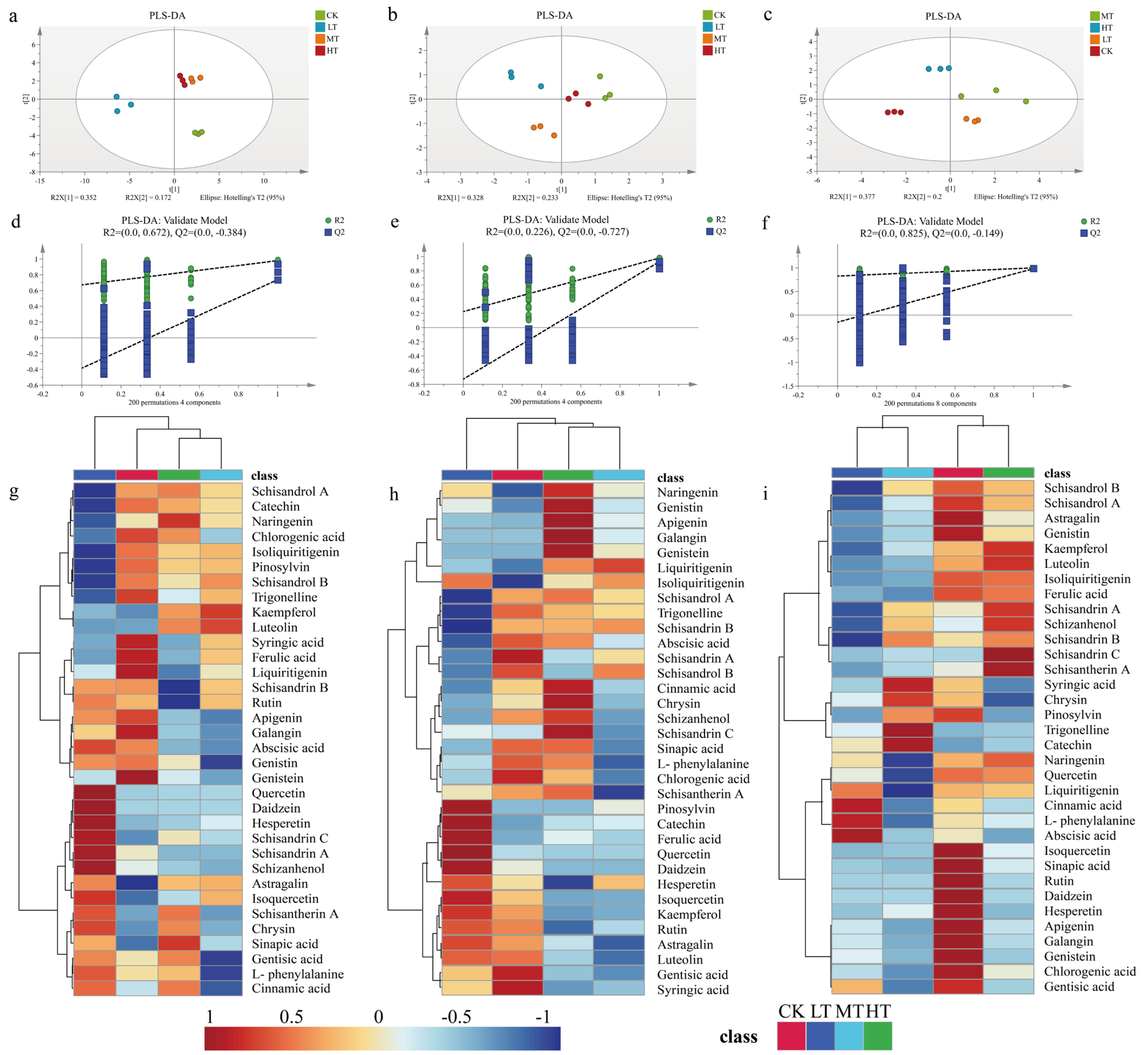
2.5. PLS-DA Analysis of Secondary Metabolites
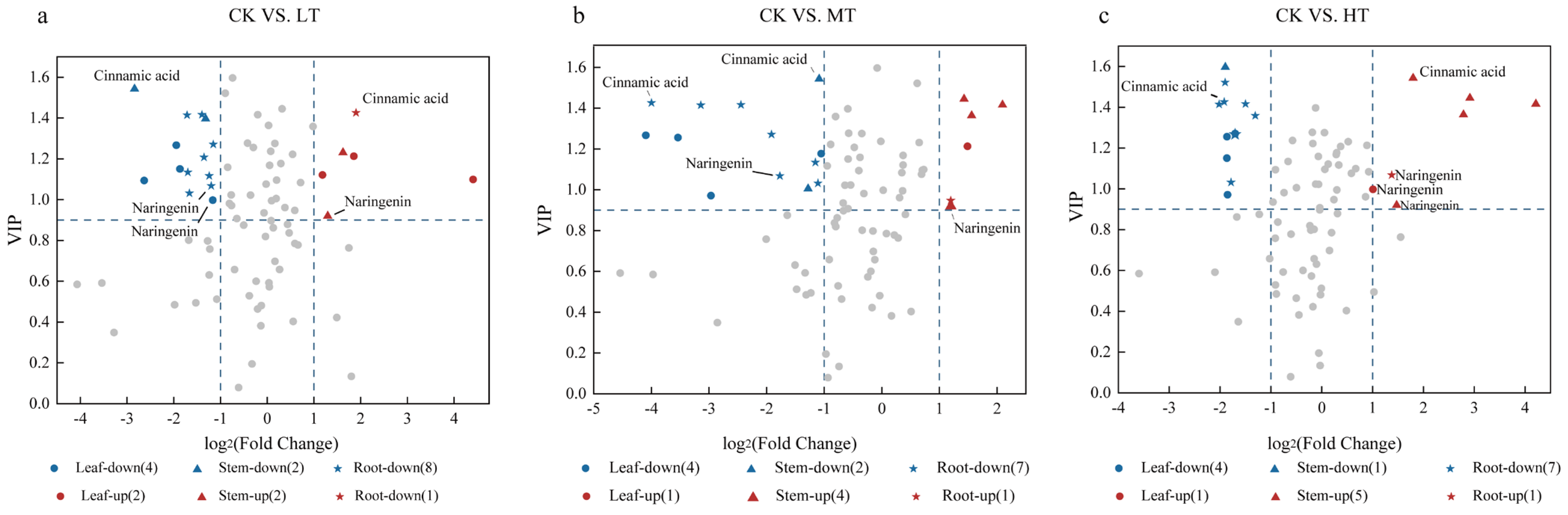
2.6. Screening of Phenolic Differentially Expressed Metabolites (DEMs)
2.7. Structural Analysis of Secondary Metabolism Network
3. Discussion
4. Materials and Methods
4.1. Plant Material and Root Zone Temperature (RZT) Treatments
4.2. Determination of Chlorophyll Content and Photosynthetic Parameters
4.3. Analysis of Secondary Metabolites
4.3.1. Determination of Lignan Content
4.3.2. LC-MS Analysis of Phenolic Compounds
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2016, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yuan, C. Schisandra chinensis: A comprehensive review on its phytochemicals and biological activities. Arab. J. Chem. 2021, 14, 103310. [Google Scholar] [CrossRef]
- Fu, K.; Zhou, H.; Wang, C.; Gong, L.; Ma, C.; Zhang, Y.; Li, Y. A review: Pharmacology and pharmacokinetics of Schisandrin A. Phytother. Res. 2022, 36, 2375–2393. [Google Scholar] [CrossRef]
- Yang, K.; Qiu, J.; Huang, Z.; Yu, Z.; Wang, W.; Hu, H.; You, Y. A comprehensive review of ethnopharmacology, phytochemistry, pharmacology, and pharmacokinetics of Schisandra chinensis (Turcz.) Baill. and Schisandra sphenanthera Rehd. et Wils. J. Ethnopharmacol. 2022, 284, 114759. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Sun, Y.; Li, X.; Xu, Z.; Jiang, P.; Rong, X.; Yang, B.; Kuang, H. Lignans and Terpenoids from the Leaves of Schisandra chinensis. Chem. Biodivers. 2020, 17, e2000035. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J. Antioxidant Effects of Schisandra chinensis Fruits and Their Active Constituents. Antioxidants 2021, 10, 620. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Zhang, D.-Y.; Kuang, H.-X.; Xia, Y.-G. Low-polymerization compositional fingerprinting for characterization of Schisandra polysaccharides by hydrophilic interaction liquid chromatography-electrospray mass spectrometry. Int. J. Biol. Macromol. 2021, 185, 983–996. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of the People ‘s Republic of China: Volume I.; Pharmaceutical Science and Technology Press: Beijing, China, 2020; pp. 13–14. [Google Scholar]
- He, D.; Pei, X.; Liu, B.; Li, J.; Dong, J.; Efferth, T.; Ma, P. Lignan contents of Schisandra chinensis (Turcz.) Baill. from different origins—A new model for evaluating the content of prominent components of Chinese herbs. Phytomedicine 2024, 128, 155361. [Google Scholar] [CrossRef]
- Wu, Z.; Jia, M.; Zhao, W.; Huang, X.; Yang, X.; Chen, D.; Qiaolongbatu, X.; Li, X.; Wu, J.; Qian, F.; et al. Schisandrol A, the main active ingredient of Schisandrae Chinensis Fructus, inhibits pulmonary fibrosis through suppression of the TGF-β signaling pathway as revealed by UPLC-Q-TOF/MS, network pharmacology and experimental verification. J. Ethnopharmacol. 2022, 289, 115031. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mu, X.; Liang, J.; Zhang, J.; Qiang, T.; Li, H.; Li, B.; Liu, H.; Zhang, B. Metabolic profiling on the analysis of different parts of Schisandra chinensis based on UPLC-QTOF-MS with comparative bioactivity assays. Front. Plant Sci. 2022, 13, 970535. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Liu, S.-Y.; Yan, Y.; Yin, L.; DI, P.; Liu, H.-M.; Liu, H.-Z. Candidate genes involved in the biosynthesis of lignan in Schisandra chinensis fruit based on transcriptome and metabolomes analysis. Chin. J. Nat. Med. 2020, 18, 684–695. [Google Scholar] [CrossRef]
- Ford, J.D.; Huang, K.-S.; Wang, H.-B.; Davin, L.B.; Lewis, N.G. Biosynthetic pathway to the cancer chemopreventive secoisolariciresinol diglucoside−hydroxymethyl glutaryl ester-linked lignan oligomers in flax (Linum usitatissimum) Seed. J. Nat. Prod. 2001, 64, 1388–1397. [Google Scholar] [CrossRef]
- Suzuki, S.; Umezawa, T. Biosynthesis of lignans and norlignans. J. Wood Sci. 2007, 53, 273–284. [Google Scholar] [CrossRef]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Chua, K.; Coggins, J.; Mohtadi, H. Heat Waves, Climate Change, and Economic Output. J. Eur. Econ. Assoc. 2021, 19, 2658–2694. [Google Scholar] [CrossRef]
- Su, H.; Li, L.; Ma, H.; Lyu, D.; Sun, J. Calcium Alleviates Temperature Stress by Regulating Nitrogen and Respiratory Metabolism in Malus baccata Roots. Int. J. Agric. Biol. 2016, 18, 286–292. [Google Scholar] [CrossRef]
- Guo, E.; Liu, X.; Zhang, J.; Wang, Y.; Wang, C.; Wang, R.; Li, D. Assessing spatiotemporal variation of drought and its impact on maize yield in Northeast China. J. Hydrol. 2017, 553, 231–247. [Google Scholar] [CrossRef]
- Ambebe, T.F.; Dang, Q.-L.; Li, J. Low soil temperature inhibits the effect of high nutrient supply on photosynthetic response to elevated carbon dioxide concentration in white birch seedlings. Tree Physiol. 2009, 30, 234–243. [Google Scholar] [CrossRef]
- Lee, S.; Wang, W.; Huq, E. Spatial regulation of thermomorphogenesis by HY5 and PIF4 in Arabidopsis. Nat. Commun. 2021, 12, 3656. [Google Scholar] [CrossRef]
- Bai, L.; Deng, H.; Zhang, X.; Yu, X.; Li, Y.; Cheng, Z. Gibberellin Is Involved in Inhibition of Cucumber Growth and Nitrogen Uptake at Suboptimal Root-Zone Temperatures. PLoS ONE 2016, 11, e0156188. [Google Scholar] [CrossRef]
- Wahid, A.; Close, T.J. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Gaillochet, C.; Burko, Y.; Platre, M.P.; Zhang, L.; Simura, J.; Willige, B.C.; Kumar, S.V.; Ljung, K.; Chory, J.; Busch, W. HY5 and phytochrome activity modulate shoot-to-root coordination during thermomorphogenesis in Arabidopsis. Development 2020, 147, dev192625. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G. The environment temperature affects post-germination growth and root system architecture of pea (Pisum sativum L.) plants. Sci. Hortic. 2021, 278, 109858. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Li, J.; Yan, X.; He, H.; Gao, X.; Jia, G. High temperature in the root zone repressed flowering in Lilium × formolongi by disturbing the photoperiodic pathway and reconfiguring hormones and primary metabolism. Environ. Exp. Bot. 2021, 192, 104644. [Google Scholar] [CrossRef]
- Chen, Y.; Lo, S.; Sun, P.; Lu, C.; Ho, T.D.; Yu, S. A late embryogenesis abundant protein HVA1 regulated by an inducible promoter enhances root growth and abiotic stress tolerance in rice without yield penalty. Plant Biotechnol. J. 2014, 13, 105–116. [Google Scholar] [CrossRef]
- Inoue, T.; Akaji, Y.; Noguchi, K. Distinct responses of growth and respiration to growth temperatures in two mangrove species. Ann. Bot. 2021, 129, 15–28. [Google Scholar] [CrossRef]
- Fanello, D.D.; Kelly, S.J.; Bartoli, C.G.; Cano, M.G.; Alonso, S.M.; Guiamet, J.J. Plasticity of root growth and respiratory activity: Root responses to above-ground senescence, fruit removal or partial root pruning in soybean. Plant Sci. 2020, 290, 110296. [Google Scholar] [CrossRef]
- Gonzalez-Fuentes, J.A.; Shackel, K.; Lieth, J.H.; Albornoz, F.; Benavides-Mendoza, A.; Evans, R.Y. Diurnal root zone temperature variations affect strawberry water relations, growth, and fruit quality. Sci. Hortic. 2016, 203, 169–177. [Google Scholar] [CrossRef]
- Yan, Q.; Duan, Z.; Mao, J.; Li, X.; Dong, F. Effects of root-zone temperature and N, P, and K supplies on nutrient uptake of cucumber (Cucumis sativus L.) seedlings in hydroponics. Soil Sci. Plant Nutr. 2012, 58, 707–717. [Google Scholar] [CrossRef]
- Yan, Q.-Y.; Duan, Z.-Q.; Mao, J.-D.; Li, X.; Dong, F. Low Root Zone Temperature Limits Nutrient Effects on Cucumber Seedling Growth and Induces Adversity Physiological Response. J. Integr. Agric. 2013, 12, 1450–1460. [Google Scholar] [CrossRef]
- Xia, Z.Q.; Si, L.Y.; Jin, Y.; Fu, Y.F.; Wang, Q.; Lu, H.D. Effects of Root Zone Temperature Increase on Physiological Indexes and Photosynthesis of Different Genotype Maize Seedlings. Russ. J. Plant Physiol. 2021, 68, 169–178. [Google Scholar] [CrossRef]
- Bai, L.; Han, G.; Jiang, L.; Crowther, T.W.; Zohner, C.M.; Yu, K.; Xu, Z.; Wang, Z.; Wu, Q.; Zhu, Y.; et al. Contrasting Responses of Flowering Phenology in C3 and C4 Plants Shape Grassland Community Structure under Global Change. Ecology 2025, 106, e70139. [Google Scholar] [CrossRef]
- Nagel, K.A.; Kastenholz, B.; Jahnke, S.; van Dusschoten, D.; Aach, T.; Mühlich, M.; Truhn, D.; Scharr, H.; Terjung, S.; Walter, A.; et al. Temperature responses of roots: Impact on growth, root system architecture and implications for phenotyping. Funct. Plant Biol. 2009, 36, 947–959. [Google Scholar] [CrossRef]
- González-García, M.P.; Conesa, C.M.; Lozano-Enguita, A.; Baca-González, V.; Simancas, B.; Navarro-Neila, S.; Sánchez-Bermúdez, M.; Salas-González, I.; Caro, E.; Castrillo, G.; et al. Temperature changes in the root ecosystem affect plant functionality. Plant Commun. 2022, 4, 100514. [Google Scholar] [CrossRef]
- Wang, J.; Jin, D.; Deng, Z.; Zheng, L.; Guo, P.; Ji, Y.; Song, Z.; Zeng, H.Y.; Kinoshita, T.; Liao, Z.; et al. The apoplastic pH is a key determinant in the hypocotyl growth response to auxin dosage and light. Nat. Plants 2025, 11, 279–294. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Deng, S.; Jin, C.; Shang, Z.; Chang, L.; Wang, J.; Han, X.; Wang, A.; Jin, D.; Wang, Y.; et al. Origin and evolution of auxin-mediated acid growth. Proc. Natl. Acad. Sci. USA 2024, 121, e2412493121. [Google Scholar] [CrossRef]
- Wu, B.; Zuo, J.; Niu, H.; Jia, R.; Li, Q.; Chen, X.; Yang, Z.; Zhang, C.; Sun, F.; Xi, Y. Transcriptome and metabolome analyses reveal the potential mechanism of root zone temperature regulates the growth rhythm of switchgrass (Panicum virgatum L.) seedlings. Ind. Crop. Prod. 2025, 230, 121041. [Google Scholar] [CrossRef]
- Luo, Z.R. Relationship between plant hormones and cold resistance. Plant Physiol. Commun. 1989, 3, 1–5. [Google Scholar] [CrossRef]
- Kim, N.; Jayakodi, M.; Lee, S.; Choi, B.; Jang, W.; Lee, J.; Kim, H.H.; Waminal, N.E.; Lakshmanan, M.; van Nguyen, B.; et al. Genome and evolution of the shade-requiring medicinal herb Panax ginseng. Plant Biotechnol. J. 2018, 16, 1904–1917. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, X.; Jia, Z.; Li, C.; Ma, J.; Zhang, J. The Effects of Ecological Factors on the Main Medicinal Components of Dendrobium officinale under Different Cultivation Modes. Forests 2020, 11, 94. [Google Scholar] [CrossRef]
- Yu, H.; Liao, J.; Jiang, Y.; Zhong, M.; Tao, S.; Chai, S.; Wang, L.; Lin, L.; Yang, R.; Deng, X.; et al. Ecotype-specific phenolic acid accumulation and root softness in Salvia miltiorrhiza are driven by environmental and genetic factors. Plant Biotechnol. J. 2025, 23, 2224–2241. [Google Scholar] [CrossRef]
- Zhou, Y.; Men, L.; Sun, Y.; Wei, M.; Fan, X. Pharmacodynamic effects and molecular mechanisms of lignans from Schisandra chinensis Turcz. (Baill.), a current review. Eur. J. Pharmacol. 2021, 892, 173796. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, A.; Choudhary, K.K. Revisiting the role of phenylpropanoids in plant defense against UV-B stress. Plant Stress 2023, 7, 100143. [Google Scholar] [CrossRef]
- Soyk, S.; Lemmon, Z.H.; Sedlazeck, F.J.; Jiménez-Gómez, J.M.; Alonge, M.; Hutton, S.F.; Van Eck, J.; Schatz, M.C.; Lippman, Z.B. Duplication of a domestication locus neutralized a cryptic variant that caused a breeding barrier in tomato. Nat. Plants 2019, 5, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Qiang, T.-Y.; Liu, J.-S.; Dong, Y.-Q.; Mu, X.-L.; Chen, Y.; Luo, H.-M.; Zhang, B.-G.; Liu, H.-T. Identification, Molecular Cloning, and Functional Characterization of a Coniferyl Alcohol Acyltransferase Involved in the Biosynthesis of Dibenzocyclooctadiene Lignans in Schisandra chinensis. Front. Plant Sci. 2022, 13, 881342. [Google Scholar] [CrossRef]
- Guo, L.; Tan, J.; Deng, X.; Mo, R.; Pan, Y.; Cao, Y.; Chen, D. Integrated analysis of metabolome and transcriptome reveals key candidate genes involved in flavonoid biosynthesis in Pinellia ternata under heat stress. J. Plant Res. 2023, 136, 359–369. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Austen, N.; Walker, H.J.; Lake, J.A.; Phoenix, G.K.; Cameron, D.D. The Regulation of Plant Secondary Metabolism in Response to Abiotic Stress: Interactions Between Heat Shock and Elevated CO2. Front. Plant Sci. 2019, 10, 1463. [Google Scholar] [CrossRef]
- Ri, I.; Pak, S.; Pak, U.; Yun, C.; Tang, Z. How does UV-B radiation influence the photosynthesis and secondary metabolism of Schisandra chinensis leaves? Ind. Crop. Prod. 2024, 208, 117832. [Google Scholar] [CrossRef]
- Wang, W.-J.; He, H.S.; Yu, G.; Wen-Xin, L.I.; Zhong-Hua, Z.; Yuan-Gang, Z.U. Methodological Comparison of Chlorophyll and Carotenoids Contents of Plant Species Measured by DMSO and Acetone-extraction Methods. Bull. Bot. Res. 2009, 29, 224–229. [Google Scholar]
- Mocan, A.; Schafberg, M.; Crișan, G.; Rohn, S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Foods 2016, 24, 579–594. [Google Scholar] [CrossRef]
- Shi, C.; Gao, C.; Guo, X.R.; Wang, H.Z.; Xu, M.Y.; Liu, Y.; Zhang, J.; Xing, K.X.; Tang, Z.H. Optimization of ultrasonic extraction process of lignans from Schisandra chinensis vine stem by analytic hierarchy process-entropy weight method combined with response surface methodology. J. Food Saf. Qual. 2023, 14, 293–302. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Wang, Y.; Abozeid, A.; Zu, Y.-G.; Tang, Z.-H. The integration of GC–MS and LC–MS to assay the metabolomics profiling in Panax ginseng and Panax quinquefolius reveals a tissue- and species-specific connectivity of primary metabolites and ginsenosides accumulation. J. Pharm. Biomed. Anal. 2017, 135, 176–185. [Google Scholar] [CrossRef]



| Pn (μmol m−2 s−1) | Gs (μmol m−2 s−1) | Ci (mmol m−2 s−1) | Tr (mmol m−2 s−1) | Chla (mg/g) | Chlb (mg/g) | Car (mg/g) | Chla + chlb | |
|---|---|---|---|---|---|---|---|---|
| CK | 2.13 ± 0.21d | 0.035 ± 0.0041b | 302.4 ± 20.82a | 0.87 ± 0.13c | 0.76 ± 0.14c | 0.47 ± 0.05b | 0.24 ± 0.02c | 1.23 ± 0.09c |
| LT | 3.72 ± 0.32c | 0.037 ± 0.0042b | 213.8 ± 36.49b | 1.16 ± 0.13b | 1.45 ± 0.01ab | 0.52 ± 0.05b | 0.39 ± 0.01b | 1.97 ± 0.05b |
| MT | 5.87 ± 0.28b | 0.050 ± 0.0039a | 291.6 ± 49.93a | 1.72 ± 0.13a | 1.41 ± 0.10b | 0.53 ± 0.05b | 0.39 ± 0.03b | 1.94 ± 0.15b |
| HT | 6.71 ± 0.27a | 0.037 ± 0.0038b | 218.4 ± 27.54b | 1.18 ± 0.09b | 1.60 ± 0.06a | 0.62 ± 0.03a | 0.45 ± 0.02a | 2.22 ± 0.09a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Song, X.; Jin, L.; Zhang, W.; Zheng, J.; Zhang, L.; Yu, Q.; Shi, Y.; Guan, X.; Zhang, Z.; et al. Root-Zone Temperature Drives Coordinated Photosynthesis, Root Architecture, and Metabolism Responses in Schisandra chinensis (Trucz.) Baill. Plants 2025, 14, 2595. https://doi.org/10.3390/plants14162595
Tang H, Song X, Jin L, Zhang W, Zheng J, Zhang L, Yu Q, Shi Y, Guan X, Zhang Z, et al. Root-Zone Temperature Drives Coordinated Photosynthesis, Root Architecture, and Metabolism Responses in Schisandra chinensis (Trucz.) Baill. Plants. 2025; 14(16):2595. https://doi.org/10.3390/plants14162595
Chicago/Turabian StyleTang, Huimin, Xiaoqian Song, Lu Jin, Weisan Zhang, Jie Zheng, Lu Zhang, Qiuyu Yu, Yu Shi, Xin Guan, Zhonghua Zhang, and et al. 2025. "Root-Zone Temperature Drives Coordinated Photosynthesis, Root Architecture, and Metabolism Responses in Schisandra chinensis (Trucz.) Baill" Plants 14, no. 16: 2595. https://doi.org/10.3390/plants14162595
APA StyleTang, H., Song, X., Jin, L., Zhang, W., Zheng, J., Zhang, L., Yu, Q., Shi, Y., Guan, X., Zhang, Z., Zheng, C., & Tang, Z. (2025). Root-Zone Temperature Drives Coordinated Photosynthesis, Root Architecture, and Metabolism Responses in Schisandra chinensis (Trucz.) Baill. Plants, 14(16), 2595. https://doi.org/10.3390/plants14162595







