Enhanced Recovery of Bioactive Compounds from Cagaita and Mamacadela Fruits Using Natural Deep Eutectic Solvents (NADES) and Ethanol: A Comparative Study
Abstract
1. Introduction
2. Results
2.1. Coloration and Water Activity of Extracts
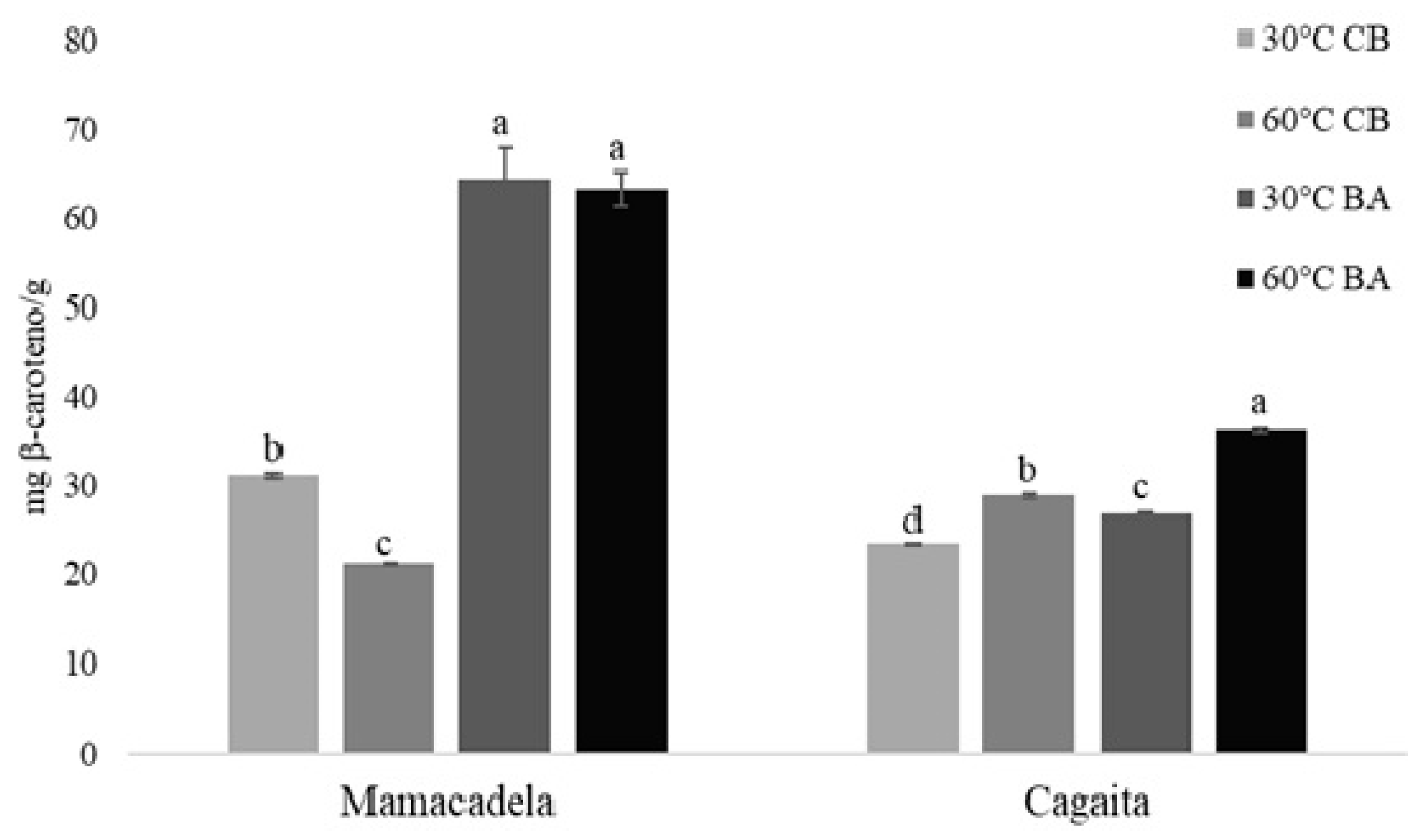
2.2. Bioactive Compounds of Extracts
2.3. Mass Spectrometry of the Different Extracts
3. Discussion
3.1. Coloration and Water Activity of Extracts
3.2. Bioactive Compounds of Extracts
3.3. Mass Spectrometry of Different Extracts
4. Materials and Methods
4.1. Preparation of Deep Eutectic Solvents
4.2. Materials
4.3. Obtaining the Extract
4.4. Analysis of Color and Water Activity
4.5. Quantification of Bioactive Compounds
4.5.1. Total Phenolic Compounds
4.5.2. DPPH Assay
4.5.3. ABTS Assay
4.5.4. Ferric Reducing Antioxidant Power (FRAP)
4.5.5. Flavonoids
4.5.6. Total Carotenoid Content
4.6. Electrospray Ionization with Triple Quadrupolar Mass Spectrometry (DI-ESI-MS)
4.7. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pompeu, J.; Assis, T.O.; Ometto, J.P. Landscape Changes in the Cerrado: Challenges of Land Clearing, Fragmentation and Land Tenure for Biological Conservation. Sci. Total Environ. 2024, 906, 167581. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Santana, J.C.; Simon, M.F. Plant Diversity Conservation in an Agricultural Frontier in the Brazilian Cerrado. Biodivers. Conserv. 2022, 31, 667–681. [Google Scholar] [CrossRef]
- Arruda, H.S.; Araújo, M.V.L.; Marostica Junior, M.R. Underexploited Brazilian Cerrado Fruits as Sources of Phenolic Compounds for Diseases Management: A Review. Food Chem Mol. Sci. 2022, 5, 100148. [Google Scholar] [CrossRef] [PubMed]
- de Britto Policarpi, P.; Turcatto, L.; Demoliner, F.; Ferrari, R.A.; Bascuñan, V.L.A.F.; Ramos, J.C.; Jachmanián, I.; Vitali, L.; Micke, G.A.; Block, J.M. Nutritional Potential, Chemical Profile and Antioxidant Activity of Chichá (Sterculia striata) Nuts and Its by-Products. Food Res. Int. 2018, 106, 736–744. [Google Scholar] [CrossRef]
- da Silva, E.K.A.; Rial, R.C. Fatty Acid Profile, Nutritional and Therapeutic Properties of Vegetable Oils from the Brazilian Cerrado. J. Food Compos. Anal. 2025, 137, 106819. [Google Scholar] [CrossRef]
- Pompeu, J. Performance of an Automated Conservation Status Assessment for the Megadiverse Vascular Flora of Brazil. J. Nat. Conserv. 2022, 70, 126272. [Google Scholar] [CrossRef]
- Strassburg, B.B.N.; Brooks, T.; Feltran-Barbieri, R.; Iribarrem, A.; Crouzeilles, R.; Loyola, R.; Latawiec, A.E.; Oliveira Filho, F.J.B.; De Scaramuzza, C.A.M.; Scarano, F.R.; et al. Moment of Truth for the Cerrado Hotspot. Nat. Ecol. Evol. 2017, 1, 0099. [Google Scholar] [CrossRef]
- Colli, G.R.; Vieira, C.R.; Dianese, J.C. Biodiversity and Conservation of the Cerrado: Recent Advances and Old Challenges. Biodivers. Conserv. 2020, 29, 1465–1475. [Google Scholar] [CrossRef]
- De Marco, P.; Villén, S.; Mendes, P.; Nóbrega, C.; Cortes, L.; Castro, T.; Souza, R. Vulnerability of Cerrado Threatened Mammals: An Integrative Landscape and Climate Modeling Approach. Biodivers. Conserv. 2018, 29, 1637–1658. [Google Scholar] [CrossRef]
- Trigueiro, W.R.; Nabout, J.C.; Tessarolo, G. Uncovering the Spatial Variability of Recent Deforestation Drivers in the Brazilian Cerrado. J. Environ. Manag. 2020, 275, 111243. [Google Scholar] [CrossRef]
- Bortolotto, I.M.; Hiane, P.A.; Ishii, I.H.; de Souza, P.R.; Campos, R.P.; Gomes, R.J.B.; Farias, C.S.; Leme, F.M.; Arruda, R.C.O.; da Costa, L.B.L.C.; et al. A knowledge network to promote the use and valorization of wild food plants in the Pantanal and Cerrado, Brazil. Reg. Environ. Change 2017, 17, 1329–1341. [Google Scholar] [CrossRef]
- dos Santos, P.P.A.; Ferrari, G.d.S.; Rosa, M.d.S.; Almeida, K.; Araújo, L.d.A.d.; Pereira, M.H.C.; Wanderley, M.E.F.; Neder Morato, P. Development and characterization of high protein functional ice cream with ora-pro-nóbis (Pereskia aculeata Miller) and inulin. Braz. J. Food Technol. 2022, 25, e2020129. [Google Scholar] [CrossRef]
- Huo, D.; Liu, F.; Jiao, C.; Feng, H.; Yang, C.; Cai, Q.; Xie, L. Effects of Different Extraction Solvents on the Major Chemical Compounds and in Vitro Biological Activities of Dendrobium Chrysotoxum Lindl. Stems. Ind. Crops Prod. 2024, 222, 119678. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, J.; Li, Q.; Liu, C.; Yue, R.; Zhang, Y.; Niu, F.; Zhu, H.; Ma, C.; Deng, S. Effects of Different Extraction Solvents on the Compositions, Primary Structures, and Anti-Inflammatory Activity of Pectin from Sweet Potato Processing by-Products. Carbohydr. Polym. 2025, 347, 122766. [Google Scholar] [CrossRef]
- Erdoğan, Ü.; Han Öztürk, T.; Önder, S.; Tonguç, M. Eco-Friendly Extraction of Six Different Rosemary (Rosmarinus officinalis L.) Genotypes-Using Natural Deep Eutectic Solvents: Optimization and Modeling via Response Surface Methodology (RSM). J. Mol. Liq. 2024, 407, 125167. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Huang, T.; Ren, Y.; Gong, W.; Guo, Y.; Wang, J.; Tu, Q. Preparation of Sodium Hyaluronate/Dopamine/AgNPs Hydrogel Based on the Natural Eutetic Solvent as an Antibaterial Wound Dressing. Int. J. Biol. Macromol. 2021, 191, 60–70. [Google Scholar] [CrossRef]
- Li, Z.; Cui, Y.; Li, C.; Shen, Y. Deep Desulfurization of Fuels Based on Deep Eutectic Theory. Sep. Purif. Technol. 2019, 219, 9–15. [Google Scholar] [CrossRef]
- Xu, T.; Sui, X.; Meng, Y.; Li, D.; Liu, C.; Ge, P.; Liu, J.; Yuan, C.; Liu, T. Application of Circulating and Pulsating Ultrasonic Extraction of Lignans from Schisandra Chinensis Baill Fruits Using Deep Eutetic Solvents. Ind. Crops Prod. 2024, 214, 118466. [Google Scholar] [CrossRef]
- Yusuf, J.Y.; Soleimani, H.; Chuan, L.K.; Soleimani, H.; Sulaimon, A.A.; Balogun, B.B.; Adam, A.A.; Balogun, A.I. Eco-Friendly Functionalization of MWCNTs with Deep Eutectic Solvents. Inorg. Chem. Commun. 2024, 163, 112282. [Google Scholar] [CrossRef]
- Alasalvar, H. Ultrasound Technology and Eco-Friendly Solvents for Extracting Antioxidant Phenolic Compounds from Immortelle (Helichrysum Italicum) Flowers: A Comparative Study on Conventional and Natural Deep Eutectic Solvents. Microchem. J. 2025, 208, 112390. [Google Scholar] [CrossRef]
- Martinez-Correa, H.A.; Paula, J.T.; Kayano, A.C.A.V.; Queiroga, C.L.; Magalhães, P.M.; Costa, F.T.M.; Cabral, F.A. Composition and Antimalarial Activity of Extracts of Curcuma longa L. Obtained by a Combination of Extraction Processes Using Supercritical CO2, Ethanol and Water as Solvents. J. Supercrit. Fluids 2017, 119, 122–129. [Google Scholar] [CrossRef]
- Pereira, T.C.; Souza, V.P.; Padilha, A.P.F.; Duarte, F.A.; Flores, E.M. Trends and Perspectives on the Ultrasound-Assisted Extraction of Bioactive Compounds Using Natural Deep Eutectic Solvents. Curr. Opin. Chem. Eng. 2025, 47, 101088. [Google Scholar] [CrossRef]
- Ghazal, A.F.; Zhang, M.; Liu, Z. Spontaneous Color Change of 3D Printed Healthy Food Product over Time After Printing as a Novel Application for 4D Food Printing. Food Bioproc. Technol. 2019, 12, 1627–1645. [Google Scholar] [CrossRef]
- Andruch, V.; Vojteková, V.; Kalyniukova, A.; Zengin, G.; Hagarová, I.; Yordanova, T. Application of Deep Eutectic Solvents for the Determination of Inorganic Analytes. Adv. Sample Prep. 2025, 13, 100158. [Google Scholar] [CrossRef]
- Vilková, M.; Płotka-Wasylka, J.; Andruch, V. The Role of Water in Deep Eutectic Solvent-Base Extraction. J. Mol. Liq. 2020, 304, 112747. [Google Scholar] [CrossRef]
- Fassi Fihri, R.; Ez-Zoubi, A.; Mbarkiou, L.; Amar, A.; Farah, A.; Bouchamma, E.O. Antibacterial and Antioxidant Activities of Chlorella Vulgaris and Scenedesmus Incrassatulus Using Natural Deep Eutectic Solvent under Microwave Assisted by Ultrasound. Heliyon 2024, 10, e35071. [Google Scholar] [CrossRef]
- Soukaina, K.; Safa, Z.; Soukaina, H.; Hicham, C.; Bouchra, C. Choline Chloride-Based Deep Eutectic Solvents (NADES): Potential Use as Green Extraction Media for Polyphenols from Mentha Pulegium, Antioxidant Activity, and Antifungal Activity. Microchem. J. 2024, 199, 110174. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Mass Transfer Process during Extraction of Phenolic Compounds from Milled Berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Masmoudi, M.; Besbes, S.; Ben Thabet, I.; Blecker, C.; Attia, H. Pectin Extraction from Lemon By-Product with Acidified Date Juice: Rheological Properties and Microstructure of Pure and Mixed Pectin Gels. Food Sci. Technol. Int. 2010, 16, 105–114. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Núñez, N.; Saurina, J.; Núñez, O. Polyphenolic Profiling of Coffee Beverages by Liquid Chromatography-High-Resolution Mass Spectrometry for Classification and Characterization. Microchem. J. 2024, 207, 111770. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Alharbi, H.O.A.; Khan, A.A.; Rahmani, A.H. Effects and Mechanisms of Luteolin, a Plant-Based Flavonoid, in the Prevention of Cancers via Modulation of Inflammation and Cell Signaling Molecules. Molecules 2024, 29, 1093. [Google Scholar] [CrossRef]
- Blunder, M.; Orthaber, A.; Bauer, R.; Bucar, F.; Kunert, O. Efficient Identification of Flavones, Flavanones and Their Glycosides in Routine Analysis via off-Line Combination of Sensitive NMR and HPLC Experiments. Food Chem. 2017, 218, 600–609. [Google Scholar] [CrossRef]
- Deng, Z.; Hassan, S.; Rafiq, M.; Li, H.; He, Y.; Cai, Y.; Kang, X.; Liu, Z.; Yan, T. Pharmacological Activity of Eriodictyol: The Major Natural Polyphenolic Flavanone. Evid. Based Complement. Altern. Med. 2020, 2020, 6681352. [Google Scholar] [CrossRef]
- Khan, F.A.; Irshad, R.; Tanveer, N.; Yaqoob, S.; Razaullah; Ali, R.; Ali, N.; Saifullah, J.; Ali Hasan, K.; Naz, S.; et al. Unleashing the Potential of Vanillic Acid: A New Twist on Nature’s Recipe to Fight Inflammation and Circumvent Azole-Resistant Fungal Infections. Bioorg. Chem. 2024, 145, 107254. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Song, B.; Hao, M.; Zhang, S.; Niu, W.; Li, Y.; Chen, Q.; Li, S.; Tong, C. Comprehensive Review of Hesperetin: Advancements in Pharmacokinetics, Pharmacological Effects, and Novel Formulations. Fitoterapia 2024, 179, 106206. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; Hou, D.X.; Wu, S. The Effects and Mechanisms of Cyanidin-3-Glucoside and Its Phenolic Metabolites in Maintaining Intestinal Integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, J.; Yang, J.; Qi, X.; Ramakrishna, R.; Li, Q.; Guo, W.; Li, C.; Liu, L.; Du, P.; et al. Unlocking the Mystery of Tibetan Yak Butter and Its Byproducts: Processing, Physicochemical Characteristics, Functional Benefits, and Applications. Trends Food Sci. Technol. 2024, 147, 104484. [Google Scholar] [CrossRef]
- Yap, K.M.; Sekar, M.; Wu, Y.S.; Gan, S.H.; Rani, N.N.I.M.; Seow, L.J.; Subramaniyan, V.; Fuloria, N.K.; Fuloria, S.; Lum, P.T. Hesperidin and Its Aglycone Hesperetin in Breast Cancer Therapy: A Review of Recent Developments and Future Prospects. Saudi J. Biol. Sci. 2021, 28, 6730–6747. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Gao, Z.; Hu, M. Anti-Tumor Role and Molecular Mechanism of Vanillic Acid. Discov. Oncol. 2025, 16, 20. [Google Scholar] [CrossRef]
- Cid-Ortega, S.; Monroy-Rivera, J.A. Extraction of Kaempferol and Its Glycosides Using Supercritical Fluids from Plant Sources: A Review. Food Technol. Biotechnol. 2018, 56, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and Inflammation: From Chemistry to Medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, S.U. Recent Studies on Kaempferol and Its Biological and Pharmacological Activities. EXCLI J. 2020, 19, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential Applications of Ferulic Acid from Natural Sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic Potential Through Its Antioxidant Property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Antioxidant Properties, Metabolism, Application and Mechanism of Ferulic Acid in Medicine, Food, Cosmetics, Livestock and Poultry. Antioxidants 2024, 13, 853. [Google Scholar] [CrossRef]
- Li, D.; Luo, Y.; Shang, P. Luteolin: A Flavonoid That Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef]
- Singh Tuli, H.; Rath, P.; Chauhan, A.; Sak, K.; Aggarwal, D.; Choudhary, R.; Sharma, U.; Vashishth, K.; Sharma, S.; Kumar, M.; et al. Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives. Cancers 2022, 14, 5373. [Google Scholar] [CrossRef]
- Hajeb, P.; Zhu, L.; Bossi, R.; Vorkamp, K. Sample Preparation Techniques for Suspect and Non-Target Screening of Emerging Contaminants. Chemosphere 2022, 287, 132306. [Google Scholar] [CrossRef]
- Wang, L.; Chen, P.; Aikemu, A.; Zhang, H.; Tian, S. Study on Ultrasonic-Assisted Deep Eutectic Solvent Extraction Process and In Vitro Antioxidant of Anchusa italica Retz. Flowers. Ultrason. Sonochem. 2024, 111, 107127. [Google Scholar] [CrossRef]
- Foroutani, Z.; Afshar Mogaddam, M.R.; Ghasempour, Z.; Ghareaghajlou, N. Application of Deep Eutectic Solvents in the Extraction of Anthocyanins: Stability, Bioavailability, and Antioxidant Property. Trends Food Sci. Technol. 2024, 144, 104324. [Google Scholar] [CrossRef]
- Liu, X.; Cao, L.; Jiang, C.; Wang, H.; Zhang, X.; Liu, Q.; Li, H.; Tang, Y.; Feng, Y. Fabrication of Multifunctional Hybrid Pigment for Color Cosmetics Based on Chitosan-Modified Palygorskite and Sappanwood Extract. Int. J. Biol. Macromol. 2024, 279, 135259. [Google Scholar] [CrossRef]
- Mansur, A.R.; Song, N.E.; Jang, H.W.; Lim, T.G.; Yoo, M.; Nam, T.G. Optimizing the Ultrasound-Assisted Deep Eutectic Solvent Extraction of Flavonoids in Common Buckwheat Sprouts. Food Chem. 2019, 293, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Row, K.H. Design and Evaluation of Polarity Controlled and Recyclable Deep Eutectic Solvent Based Biphasic System for the Polarity Driven Extraction and Separation of Compounds. J. Clean. Prod. 2020, 268, 122306. [Google Scholar] [CrossRef]
- Wan Mahmood, W.M.A.; Lorwirachsutee, A.; Theodoropoulos, C.; Gonzalez-Miquel, M. Polyol-Based Deep Eutectic Solvents for Extraction of Natural Polyphenolic Antioxidants from Chlorella vulgaris. ACS Sustain. Chem. Eng. 2019, 7, 5018–5026. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of Phenolic Compounds from Olive Pomace by Using Natural Deep Eutectic Solvents and Innovative Extraction Techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Wei, Z.; Qi, X.; Li, T.; Luo, M.; Wang, W.; Zu, Y.; Fu, Y. Application of Natural Deep Eutectic Solvents for Extraction and Determination of Phenolics in Cajanus Cajan Leaves by Ultra Performance Liquid Chromatography. Sep. Purif. Technol. 2015, 149, 237–244. [Google Scholar] [CrossRef]
- Silva, J.F.; da Silva, L.A.; Madrona, G.S.; Rossoni, D.F.; da Silva Scapim, M.R. Otimização No Processo Sustentável de Extração de Antioxidantes Naturais de Fruto Do Cerrado: Estudo de Caso Da Cagaita (Eugenia Dysenterica). Rev. Principia 2024, 61, 988–1006. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2012, 6, 36–60. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Artilha-Mesquita, C.A.F.; Stafussa, A.P.; Santos, P.D.S.d.; Santos, O.d.O.; da Costa, S.C.; Madrona, G.S. Extraction of Bioactive Compounds from the Fruits of Jambolan (Syzygium cumini (L.)) Using Alternative Solvents. Plants 2024, 13, 2065. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.S.M.; Fernandes, F.A.N.; Alves, R.E.; de Brito, E.S. Free Radical-Scavenging Behaviour of Some North-East Brazilian Fruits in a DPPH System. Food Chem. 2009, 114, 693–695. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant Capacity and Phenolic Content of Selected Tropical Fruits from Malaysia, Extracted with Different Solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis; International Food Policy Research Institute (IFPRI): Washington, DC, USA; International Center for Tropical Agriculture (CIAT): Cali, Colombia, 2004. [Google Scholar]
- Nardino, D.A.; Aranha, A.C.R.; Tonin, L.T.D.; Defendi, R.O.; Ishikawa, S.; Bressiani, P.A.; Santana, A.B.S.; Dusman, E.; Yonekawa, M.K.A.; Jaques, J.A.S.; et al. Optimization of the Extraction Process of Bioactive Compounds from Zingiber Officinale Roscoe, Evaluation of Acetylcholinesterase Enzyme Inhibition and Cytotoxic Activity of the Free and Encapsulated Extract. J. Braz. Chem. Soc. 2024, 35, e-20230186. [Google Scholar] [CrossRef]
- Akamine, L.A.; Vargas Medina, D.A.; Lanças, F.M. Magnetic Solid-Phase Extraction of Gingerols in Ginger Containing Products. Talanta 2021, 222, 121683. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Sobeh, M.; Rezq, S.; El-Shazly, A.M.; Mahmoud, M.F.; Wink, M. HPLC-ESI-MS/MS Profiling of Polyphenolics of a Leaf Extract from Alpinia Zerumbet (Zingiberaceae) and Its Anti-Inflammatory, Anti-Nociceptive, and Antipyretic Activities In Vivo. Molecules 2018, 23, 3238. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, A.V.; Dabić, D.Č.; Momirović, N.M.; Dojčinović, B.P.; Milojković-Opsenica, D.M.; Tešić, Ž.L.; Natić, M.M. Chemical Composition of Two Different Extracts of Berries Harvested in Serbia. J. Agric. Food Chem. 2013, 61, 4188–4194. [Google Scholar] [CrossRef] [PubMed]
- Sinosaki, N.B.M.; Tonin, A.P.P.; Ribeiro, M.A.S.; Poliseli, C.B.; Roberto, S.B.; da Silveira, R.; Visentainer, J.V.; Santos, O.O.; Meurer, E.C. Structural Study of Phenolic Acids by Triple Quadrupole Mass Spectrometry with Electrospray Ionization in Negative Mode and H/D Isotopic Exchange. J. Braz. Chem. Soc. 2020, 31, 402–408. [Google Scholar] [CrossRef]
- Tao, Y.I.; Li, W.; Liang, W.; Van Breemen, R.B. Identification and Quantification of Gingerols and Related Compounds in Ginger Dietary Supplements Using High-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2009, 57, 10014–10021. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.F. Sisvar: A Guide for Its Bootstrap Procedures in Multiple Comparisons. Ciênc Agrotecnol. 2014, 38, 109–112. [Google Scholar] [CrossRef]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs. Braz. J. Biomet. 2019, 37, 529–535. [Google Scholar] [CrossRef]
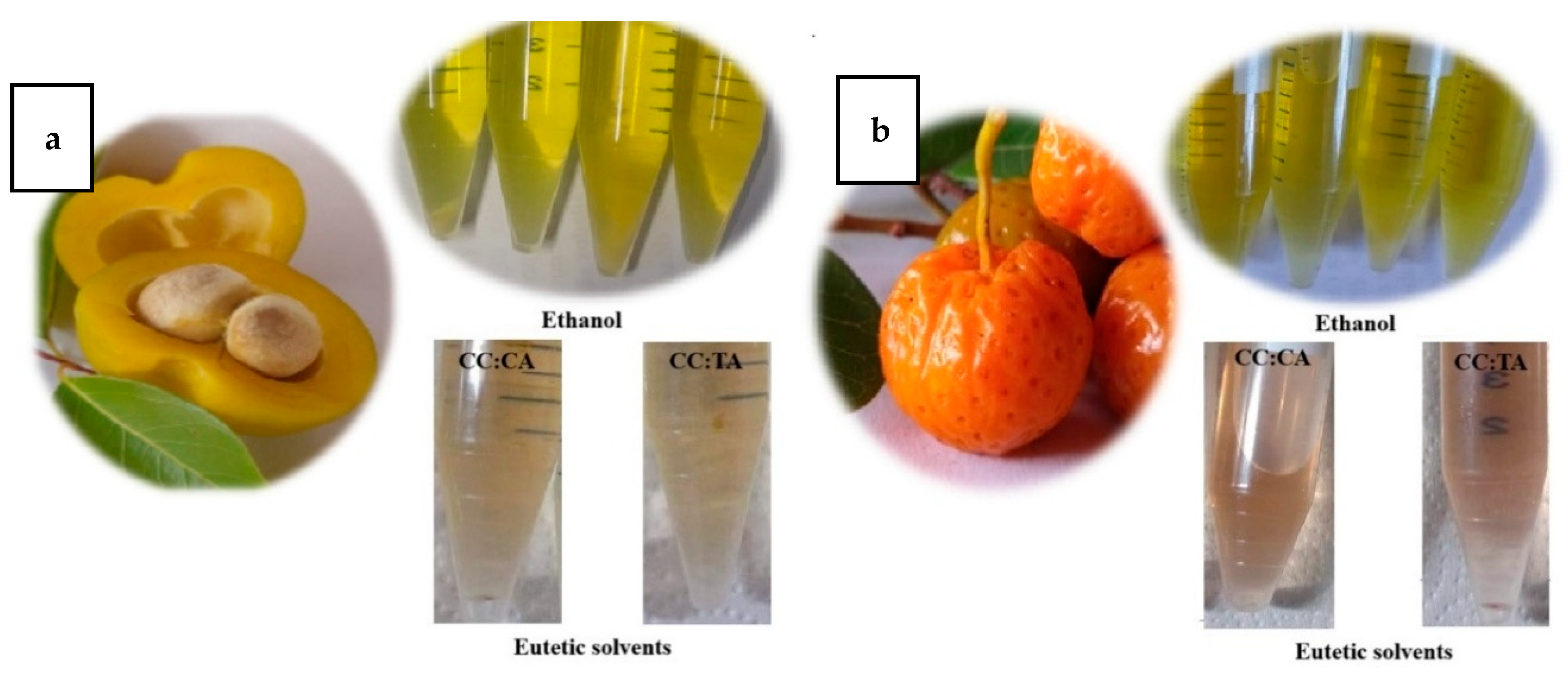
| Mamacadela | ||||||
| Extracts | L* | a* | b* | Wi | Yi | AW |
| CC:TA 30 °C (CB) | 52.34 ± 0.92 aAB | 4.28 ± 0.13 bB | 19.68 ± 0.83 abDE | 48.25 ± 0.54 aA | 37.58 ± 0.97 dE | 0.50 ± 0.00 cD |
| CC:CA 30 °C (CB) | 51.01 ± 0.58 aAB | 4.70 ± 0.07 bB | 22.26 ± 0.25 abDE | 45.98 aA ± 0.41 aA | 43.65 ± 0.14 cdDE | 0.45 ± 0.07 cDE |
| Ethanol 30 °C (CB) | 29.88 ± 2.15 bC | 0.12 ± 2.46 cC | 13.39 ± 3.29 cE | 28.52 ± 1.55 cC | 44.53 ± 8.20 bcdCD | 0.97 ± 0.00 aA |
| Ethanol 60 °C (CB) | 29.42 ± 1.40 bC | –1.78 ± 0.51 cC | 14.80 ± 1.78 cE | 27.84 ± 0.98 cC | 50.21 ± 3.61 abcC | 0.94 ± 0.02 aA |
| Ethanol 30 °C (BA) | 49.01 ± 7.79 aAB | 11.16 ± 0.86 aA | 28.13 ± 11.61 abCD | 39.65 ± 2.82 bB | 55.77 ± 16.69 abBC | 0.93 ± 0.02 aA |
| Ethanol 60 °C (BA) | 43.96 ± 5.49 aB | 9.55 ± 1.91 aA | 29.96 ± 5.23 aBCD | 35.49 ± 3.51 bB | 68.11 ± 7.72 aAB | 0.84 ± 0.00 bBC |
| Cagaita | ||||||
| Extracts | L* | a* | b* | Wi | Yi | AW |
| CC:TA 30 °C (CB) | 53.73 ± 3.05 aA | –1.23± 0.09 aC | 22.50 ± 1.31 cDE | 48.50 ± 2.20 aA | 41.88 ± 0.06 cDE | 0.40 ± 0.06 aE |
| CC:CA 30 °C (CB) | 54.36 ± 2.81 aA | –1.61 ± 0.08 aC | 20.77 ± 1.48 cDE | 49.79 ± 1.96 aA | 38.19 ± 0.76 cDE | 0.50 ± 0.00 aD |
| Ethanol 30 °C (CB) | 53.46 ± 0.80 aA | –8.81 ± 0.41 bcD | 37.77 ± 2.34 bABC | 39.38 ± 0.87 bB | 70.62 ± 3.36 bAB | 0.93 ± 0.00 aA |
| Ethanol 60 °C (CB) | 52.88 a ± 0.99 aAB | –8.08 ± 0.28 bcD | 40.74 ± 1.26 abA | 37.42 ± 0.22 bcB | 77.02 ± 1.04 aA | 0.91 ± 0.00 aAB |
| Ethanol 30 °C (BA) | 51.93 ± 0.95 aAB | –8.90 ± 0.39 cD | 37.39 ± 1.90 bABC | 38.42 ± 0.44 bcB | 71.97 ± 2.36 bAB | 0.82 ± 0.00 aC |
| Ethanol 60 °C (BA) | 52.60 ± 0.34 aAB | –8.32 ± 0.05 bD | 42.94 ± 0.43 aAB | 35.50 ± 0.18 cB | 81.63 ± 0.59 aA | 0.89 ± 0.00 aABC |
| Mamacadela | |||||
| Extracts | TPC | Flavonoids | ABTS•+ | FRAP | DPPH• |
| CC:TA 30 °C (CB) | 144.21 ± 6.37 aA | 18.32 ± 7.03 cC | 26.01 ± 11.43 cC | 246.86 ± 4.98 aB | 349.90 ± 70.77 aCD |
| CC:CA 30 °C (CB) | 141.91 ± 21.15 aA | 22.03 ± 0.69 cC | 35.49 ± 0.00 cC | 102.92 ± 7.60 bCDE | 255.66 ± 59.83 aDE |
| Ethanol 30 °C (CB) | 1.40 ± 0.70 bE | 81.39 ± 6.82 bB | 2.39 ± 2.01 cC | 156.22 ± 76.88 bCDE | ND |
| Ethanol 60 °C (CB) | 8.30 ± 2.49 bDE | 143.53 ± 5.75 abAB | 138.73 ± 21.62 bBC | 123.04 ± 17.55 bBCDE | ND |
| Ethanol 30 °C (BA) | 5.74 ± 3.84 bE | 169.04 ± 5.11 aA | 109.10 ± 10.26 bC | 163.90 ± 54.69 abBCD | ND |
| Ethanol 60 °C (BA) | 11.65 ± 5.59 bDE | 135.18 ± 3.98 abAB | 190.88 ± 20.83 aBC | 153.32 ± 7.47 abCD | ND |
| Cagaita | |||||
| Extracts | TPC | Flavonoids | ABTS•+ | FRAP | DPPH• |
| CC:TA 30 °C (CB) | 64.15 ± 9.38 bB | 20.64 ± 0.00 aC | 131.62 ± 19.80 cBC | 198.75 ± 10.72 bBC | 652.07 ± 7.12 aA |
| CC:CA 30 °C (CB) | 171.32 ± 21.52 aA | 21.57 ± 0.80 aC | 41.54 cC ± 32.26 cC | 53.56 ± 4.04 cE | 659.65 ± 35.70 aA |
| Ethanol 30 °C (CB) | 22.52 ± 2.24 cCDE | 12.29 ± 3.87 bcC | 1219.65 ± 448.70 abA | 510.68 ± 58.18 aA | 312.34 ± 2.48 cdCDE |
| Ethanol 60 °C (CB) | 43.82 ± 6.96 bcBC | 1.62 ± 0.80 dC | 1120.09 ± 322.21 abA | 81.77 ± 10.99 cDE | 493.57 ± 38.36 bB |
| Ethanol 30 °C (BA) | 23.49 ± 4.83 cCDE | 14.84 ± 1.06 bC | 1435.36 ± 70.60 aA | 508.40 ± 46.69 aA | 236.55 ± 38.86 dE |
| Ethanol 60 °C (BA) | 37.86 ± 8.03 bcBCD | 8.58 ± 0.80 cC | 597.41 ± 49.78 bcB | 63.51 cE ± 3.64 cE | 375.93 ± 17.01 cC |
| Bioactive Compound | [M − H]− (m/z) | Fragmentation (m/z) | Mamacadela (a.u.) | |||||
|---|---|---|---|---|---|---|---|---|
| CC:AT | CC:AC | Ethanol | ||||||
| 30 °C CB | 60 °C CB | 30 °C BA | 60 °C BA | |||||
| Trans-cinnamic acid | 147 | 103 | 243.47 ± 73.19 | 26.00 ± 5.56 | 8.97 ± 3.71 | 6.76 ± 1.31 | 6.30 ± 3.25 | 18.18 ± 17.72 |
| p-Coumaric acid | 163 | 119 | 141.00 ± 33.51 | 4.00 ± 1.73 | 6.87 ± 5.08 | 6.41 ± 2.69 | 12.41 ± 1.28 | 8.529 ± 4.37 |
| Propoccecuic acid | 153 | 109 | ND | 4.67 ± 2.08 | 4.14 ± 1.27 | 8.03 ± 5.60 | 7.98 ± 2.91 | 8.57 ± 5.41 |
| Gallic acid | 169 | 125 | ND | 16.33 ± 8.38 | 18.87 ± 12.35 | 26.89 ± 13.30 | 23.34 ± 11.05 | 34.24 ± 25.31 |
| p-Hydroxybenzoic acid | 137 | 93 | 115.00 ± 10.26 | ND | 10.98 ± 3.25 | 25.81 ± 14.3 | 15.63 ± 3.66 | 36.98 ± 32.98 |
| Caffeic acid | 179 | 135 | 157.00 ± 50.89 | 9.33 ± 2.08 | 4.98 ± 2.59 | 15.72 ± 2.66 | 5.34 ± 2.05 | 18.14 ± 7.25 |
| Vanillic acid | 167 | 108 | 137.50 ± 41.72 | ND | ND | ND | ND | ND |
| Syringic acid | 197 | 182 | 189.50 ± 10.60 | ND | ND | ND | ND | ND |
| Catechin | 289 | 245 | 267.00 ± 76.89 | 4.00 ± 1.73 | ND | ND | ND | 14.97 ± 6.98 |
| Protocatechuic acid glucoside | 315 | 153 | 234.00 ± 117.37 | ND | ND | ND | ND | ND |
| Isoramnetine | 315 | 300 | 307.50 ± 10.60 | ND | ND | ND | ND | ND |
| Mircetina | 317 | 151 | 234.00 ± 117.38 | ND | ND | ND | ND | ND |
| Chlorogenic acid | 353 | 135 | 244.00 ± 154.14 | ND | 3.79 ± 1.13 | ND | ND | ND |
| 3-O and 5-O Caffeoylquimic-acid | 353.2 | 191 | 272.10 ± 114.69 | 27.00 ± 16.70 | ND | ND | 4.55 ± 1.34 | ND |
| Chlorogenic acid | 353.4 | 191 | 272.20 ± 114.83 | 15.00 ± 9.16 | 8.25 ± 3.99 | ND | 4.12 ± 1.92 | ND |
| Rosmarinic acid | 359 | 161 | 260.00 ± 198.34 | ND | ND | ND | ND | ND |
| Salvianolic Acid A | 493 | 295 | 394.00 ± 210.23 | ND | ND | 4.08 ± 0.97 | ND | 3.68 ± 1.18 |
| Salvianolic Acid H | 537 | 339 | 438.00 ± 120.56 | 4.00 ± 1.00 | ND | ND | ND | ND |
| Eriordictyol-O-Rutinoside | 595 | 287 | 441.00 ± 217.79 | 7.00 ± 5.29 | ND | 3.85 ± 1.46 | ND | 3.42 ± 0.58 |
| Salvianolic Acid B | 717 | 393 | 555.00 ± 229.10 | 7.67 ± 4.16 | ND | ND | ND | ND |
| Salvianolic acid E | 717 | 537 | 627.00 ± 127.27 | 3.00 ± 0.00 | ND | ND | ND | ND |
| Kaempferol | 258 | 161 | 209.50 ± 68.59 | ND | ND | 3.67 ± 1.15 | 3.67 ± 0.57 | ND |
| Luteolina | 285 | 151 | 218.00 ± 94.75 | ND | ND | ND | ND | 4.96 ± 1.51 |
| 5,7,3′,41-Flavan-3-OL (quercetin) | 301 | 151 | 226.00 ± 106.06 | 9.00 ± 5.57 | 8.13 ± 5.37 | 10.15 ± 4.19 | 5.32 ± 1.62 | 6.76 ± 3.40 |
| Hiesperetina | 301 | 286 | 293.50 ± 10.60 | ND | ND | ND | ND | ND |
| 5,7,3′,41-Flavan-3-OL (quercetin) | 301 | 299 | 300.00 ± 1.41 | ND | ND | ND | ND | ND |
| Cyanidine-3-O-arabinoside | 418 | 287 | 352.50 ± 92.63 | 60.33 ± 32.12 | 71.47 ± 4.54 | 23.77 ± 13.47 | 30.32 ± 18.90 | 31.41 ± 6.05 |
| Cyanidine-3-O-Glucoside | 448 | 287 | 367.50 ± 113.84 | 5.33 ± 2.51 | ND | ND | ND | 4.33 ± 1.15 |
| Luteolin-7-O-Glucuronide | 461 | 285 | 373.00 ± 124.45 | 35.33 ± 11.54 | 4.44 ± 1.33 | 5.67 ± 1.85 | 6.54 ± 2.96 | ND |
| Kaempferol-3-malonihexoside | 533 | 285 | 409.00 ± 175.36 | 77.00 ± 32.23 | ND | ND | 8.50 ± 3.70 | 5.00 ± 1.85 |
| Naringerin-O-Rutinoside | 579 | 271 | 425.00 ± 217.78 | 3.33 ± 0.58 | ND | ND | ND | ND |
| Hesperetin-O-Rutinoside | 609 | 301 | ND | 3.00 ± 0.00 | ND | ND | ND | ND |
| Catechin | 289 | 245 | ND | ND | ND | 4.38 ± 0.69 | ND | 7.55 ± 6.90 |
| Narigenina | 217 | 151 | ND | ND | ND | 6.06 ± 5.30 | ND | ND |
| Apigenin-O-Rutinoside | 577 | 269 | ND | ND | ND | ND | 6.46 ± 0.80 | ND |
| Diosmin | 607 | 299 | ND | ND | 4.00 ± 0.89 | ND | ND | ND |
| Cyanidine-3,5,O-Dihexiside | 610 | 287 | ND | ND | ND | 4.53 ± 2.20 | ND | 4.72 ± 2.13 |
| Cyanidine-3,5,O-Dihexiside | 610 | 448 | ND | ND | 8.97 ± 3.71 | 6.76 ± 1.31 | 6.30 ± 3.25 | 18.18 ± 17.72 |
| Bioactive Compound | [M − H]− (m/z) | Fragmentation (m/z) | Cagaita (a.u.) | |||||
|---|---|---|---|---|---|---|---|---|
| CC:AT | CC:AC | Ethanol | ||||||
| 30 °C CB | 60 °C CB | 30 °C BA | 60 °C BA | |||||
| Trans-cinnamic acid | 147 | 103 | 107.00 ± 21.51 | 31.33 ± 20.13 | 10.10 ± 0.88 | 8.59 ± 4.24 | 8.64 ± 4.74 | 6.69 ± 2.66 |
| p-Coumaric acid | 163 | 119 | 10.00 ± 7.81 | 7.67 ± 7.23 | 6.86 ± 4.16 | 5.07 ± 1.86 | 4.95 ± 1.69 | 4.84 ± 1.69 |
| Propocaceic acid | 153 | 109 | 4.33 ± 0.58 | 8.67 ± 3.78 | 4.60 ± 0.62 | ND | 4.16 ± 1.14 | 4.25 ± 1.57 |
| Gallic acid | 169 | 125 | 19.67 ± 9.01 | 7.00 ± 2.64 | 27.08 ± 8.87 | 34.70 ± 15.56 | 19.26 ± 9.81 | 15.43 ± 8.40 |
| p-Hydroxybenzoic acid | 137 | 93 | 54.00 ± 30.31 | 25.67 ± 8.38 | 20.52 ± 2.25 | 17.96 ± 10.64 | 12.50 ± 4.22 | 21.79 ± 6.46 |
| Caffeic acid | 179 | 135 | 6.33 ± 3.05 | 4.33 ± 1.52 | 10.13 ± 4.20 | 9.74 ± 5.43 | 5.82 ± 3.29 | 13.50 ± 5.38 |
| Ferrulic acid | 193 | 134 | 5.00 ± 3.46 | ND | ND | ND | 5.33 ± 2.51 | ND |
| Siringic acid | 197 | 182 | 5.67 ± 3.78 | 5.33 ± 2.51 | 4.38 ± 2.37 | ND | ND | ND |
| Catechin | 289 | 245 | 5.33 ± 4.04 | ND | 4.43 ± 1.63 | 5.64 ± 1.47 | 8.10 ± 5.73 | ND |
| Protocatechuic acid glucoside | 315 | 153 | ND | 8.67 ± 6.42 | ND | ND | 4.26 ± 1.44 | ND |
| Isoramnetine | 315 | 300 | 4.00 ± 1.73 | ND | ND | ND | ND | ND |
| Mircetina | 317 | 151 | 7.67 ± 3.51 | 6.33 ± 0.57 | ND | 4.27 ± 2.20 | ND | 4.00 ± 0.18 |
| Chlorogenic acid | 353 | 135 | 9.33 ± 5.03 | 6.67 ± 3.21 | 5.13 ± 3.16 | ND | ND | 4.85 ± 0.41 |
| 3-O and 5-O Caffeoylquimic-acid | 353.2 | 191 | ND | 57.33 ± 39.92 | 4.30 ± 1.14 | ND | 9.14 ± 2.61 | 4.59 ± 1.66 |
| Chlorogenic acid | 353.4 | 191 | ND | 11.67 ± 10.69 | 3.82 ± 0.79 | 5.84 ± 2.45 | ND | 6.94 ± 3.74 |
| Rosmarinic acid | 359 | 161 | ND | ND | 3.63 ± 0.52 | ND | ND | ND |
| Salvianolic Acid A | 493 | 295 | ND | ND | 3.25 ± 0.35 | ND | ND | 4.00 ± 0.8 |
| Salvianolic Acid H | 537 | 339 | 4.00 ± 1.00 | 5.00 ± 2.00 | ND | ND | ND | ND |
| Salvianolic Acid B | 717 | 393 | 5.33 ± 4.04 | 14.33 ± 10.06 | ND | ND | ND | ND |
| Salvianolic acid E | 717 | 537 | 3.67 ± 1.15 | 3.67 ± 1.15 | 6.35 ± 2.68 | ND | ND | ND |
| Kaempferol | 258 | 161 | ND | ND | ND | 3.67 ± 1.15 | ND | ND |
| Luteolina | 285 | 151 | 6.33 ± 5.77 | 8.67 ± 7.23 | 4.93 ± 2.45 | 7.35 ± 5.76 | 4.82 ± 1.56 | 7.71 ± 3.23 |
| Luteolina | 285 | 259 | 3.33 ± 0.58 | 3.33 ± 0.57 | ND | ND | ND | ND |
| 5,7,3′,41-Flavan-3-OL (quercetin) | 301 | 151 | ND | ND | 8.40 ± 5.01 | 15.34 ± 8.38 | ND | 12.01 ± 6.10 |
| 5,7,3′,41-Flavan-3-OL (quercetin) | 301 | 299 | ND | ND | ND | 4.03 ± 1.52 | ND | ND |
| Cyanidine-3-O-Arabinoside | 418 | 287 | 71.67 ± 40.70 | 89.33 ± 7.63 | 48.21 ± 25.04 | 63.94 ± 17.81 | 101.09 ± 54.49 | 73.06 ± 16.56 |
| Luteolin-7-O-glucuronide | 461 | 285 | 3.33 ± 0.58 | 5.00 ± 2.00 | 6.49 ± 1.79 | 8.68 ± 4.83 | 7.86 ± 4.70 | ND |
| Kaempferol-3-malonihexoside | 533 | 285 | 27.33 ± 9.81 | ND | 5.38 ± 3.99 | 4.07 ± 0.93 | ND | ND |
| Naringerin-O-Rutinoside | 579 | 271 | 16.33 ± 10.01 | 78.67 ± 10.21 | ND | ND | ND | 21.19 ± 12.90 |
| Hesperetin-O-Rutinoside | 609 | 301 | ND | ND | ND | ND | ND | 6.00 ± 2.64 |
| Quercitin-3-O-Rutinoside (RUTIN) | 609 | 300 | 3.00 ± 0.00 | ND | ND | ND | ND | ND |
| Luteolin-O-Diglucuronide | 637 | 285 | 4.00 ± 1.73 | ND | ND | ND | ND | ND |
| Catechin | 289 | 245 | 5.33 ± 4.04 | ND | 8.97 ± 0.94 | ND | 8.60 ± 4.29 | ND |
| Narigenina | 217 | 151 | 4.67 ± 2.08 | 15.00 ± 6.08 | 4.11 ± 1.93 | 6.57 ± 3.73 | ND | 7.52 ± 5.44 |
| Myrcetin-O-Glucoside | 479 | 317 | 12.33 ± 7.50 | 6.33 ± 4.04 | 7.61 ± 3.54 | ND | ND | ND |
| Apigenin-O-Rutinoside | 577 | 269 | 5.33 ± 4.04 | 3.33 ± 0.57 | ND | 4.80 ± 0.67 | 3.30 ± 0.37 | 5.086 ± 1.23 |
| Kaenferol | 285 | 145 | ND | ND | 6.16 ± 3.14 | ND | ND | ND |
| Diosmin | 607 | 299 | ND | ND | 4.00 ± 0.89 | ND | ND | 5.30 ± 3.10 |
| Eriordictyol-O-Hexoside | 499 | 287 | ND | ND | ND | ND | 3.96 ± 0.80 | ND |
| Kaempferol-Hexoside | 447 | 285 | ND | ND | ND | ND | ND | 4.86 ± 2.44 |
| Cyanidine-3,5,O-Dihexisid | 610 | 287 | ND | ND | ND | 4.95 ± 2.92 | ND | 4.19 ± 1.42 |
| Cyanidine-3,5,O-Dihexisid | 610 | 448 | ND | ND | 10.10 ± 0.88 | 8.59 ± 4.24 | 8.64 ± 4.74 | 6.69 ± 2.66 |
| Component | Condition 1 | Condition 2 |
|---|---|---|
| Desolvation gas temperature/°C | 200 | 250 |
| Source gas temperature/°C | 110 | 110 |
| Ionization mode | Negative | Negative |
| Capillary voltage/kV | 2.0 | 2.5 |
| Cone voltage/V | 20.0 | 40.0 |
| Collision energy/V | 15.0 | 30.0 |
| Collision gas pressure/mmHg | 3.5 × 10−3 | 3.5 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.F.; Guedes, C.T.; Alves, E.d.S.; Oliveira, É.L.d.; Meurer, E.C.; Santos, S.S.d.; Scapim, M.R.d.S.; Madrona, G.S. Enhanced Recovery of Bioactive Compounds from Cagaita and Mamacadela Fruits Using Natural Deep Eutectic Solvents (NADES) and Ethanol: A Comparative Study. Plants 2025, 14, 2596. https://doi.org/10.3390/plants14162596
Silva JF, Guedes CT, Alves EdS, Oliveira ÉLd, Meurer EC, Santos SSd, Scapim MRdS, Madrona GS. Enhanced Recovery of Bioactive Compounds from Cagaita and Mamacadela Fruits Using Natural Deep Eutectic Solvents (NADES) and Ethanol: A Comparative Study. Plants. 2025; 14(16):2596. https://doi.org/10.3390/plants14162596
Chicago/Turabian StyleSilva, Jaqueline Ferreira, Carmen Torres Guedes, Eloize da Silva Alves, Évelin Lemos de Oliveira, Eduardo Cesar Meurer, Suelen Siqueira dos Santos, Mônica Regina da Silva Scapim, and Grasiele Scaramal Madrona. 2025. "Enhanced Recovery of Bioactive Compounds from Cagaita and Mamacadela Fruits Using Natural Deep Eutectic Solvents (NADES) and Ethanol: A Comparative Study" Plants 14, no. 16: 2596. https://doi.org/10.3390/plants14162596
APA StyleSilva, J. F., Guedes, C. T., Alves, E. d. S., Oliveira, É. L. d., Meurer, E. C., Santos, S. S. d., Scapim, M. R. d. S., & Madrona, G. S. (2025). Enhanced Recovery of Bioactive Compounds from Cagaita and Mamacadela Fruits Using Natural Deep Eutectic Solvents (NADES) and Ethanol: A Comparative Study. Plants, 14(16), 2596. https://doi.org/10.3390/plants14162596







